Published online Sep 19, 2023. doi: 10.5498/wjp.v13.i9.620
Peer-review started: May 19, 2023
First decision: June 1, 2023
Revised: July 4, 2023
Accepted: July 28, 2023
Article in press: July 28, 2023
Published online: September 19, 2023
Processing time: 119 Days and 0.7 Hours
Autophagy is associated with hippocampal injury following status epilepticus (SE) and is considered a potential therapeutic mechanism. Baicalin, an emerging multitherapeutic drug, has shown neuroprotective effects in patients with nervous system diseases due to its antioxidant properties.
To investigate the potential role of autophagy in LiCl-pilocarpine-induced SE.
The drugs were administered 30 min before SE. Nissl staining showed that Baicalin attenuated hippocampal injury and reduced neuronal death in the hippocampus. Western blotting and terminal deoxynucleotidyl transferase dUTP nick end labeling assay confirmed that Baicalin reversed the expression intensity of cleaved caspase-3 and apoptosis in hippocampal CA1 following SE. Fur-thermore, western blotting and immunofluorescence staining were used to measure the expression of autophagy markers (p62/SQSTM1, Beclin 1, and LC3) and apoptotic pathway markers (cleaved caspase-3 and Bcl-2).
Baicalin significantly upregulated autophagic activity and downregulated mitochondrial apoptotic pathway markers. Conversely, 3-methyladenine, a commonly used autophagy inhibitor, was simultaneously administered to inhibit the Baicalin-induced autophagy, abrogating the protective effect of Baicalin on the mitochondrial apoptotic level.
We illustrated that Baicalin-induced activation of autophagy alleviates apoptotic death and protects the hippocampus of SE rats.
Core Tip: This study established the rat model of status epilepticus by intraperitoneally injecting LiCl-pilocarpine. Then, Baicalin was administered to the rats for treatment. The pathological changes of hippocampal were observed. Western blotting and terminal deoxynucleotidyl transferase dUTP nick end labeling assays were used to verify the inhibitory effect of Baicalin on apoptosis of rat hippocampal neuronal cells. We have drawn the conclusion that Baicalin protects the hippocampus from apoptosis.
- Citation: Yang B, Wen HY, Liang RS, Lu TM, Zhu ZY, Wang CH. Hippocampus protection from apoptosis by Baicalin in a LiCl-pilocarpine-induced rat status epilepticus model through autophagy activation. World J Psychiatry 2023; 13(9): 620-629
- URL: https://www.wjgnet.com/2220-3206/full/v13/i9/620.htm
- DOI: https://dx.doi.org/10.5498/wjp.v13.i9.620
It has been reported that 1% of the global population is affected by epilepsy, which has diverse etiologies and is characterized by recurrent and spontaneous seizures[1,2]. The most notable kind of epilepsy is temporal lobe epilepsy, characterized by a typical seizure that originates from the hippocampus-a structure located in the mesial temporal lobe[3-5]. Hippocampal injury following status epilepticus (SE) is due to oxidant damage. The accumulation of reactive oxygen species can harm hippocampal neurons, inducing cell death through an apoptotic or necrotic pathway, as hippocampal cells are highly sensitive to oxidative stress[6].
Autophagy is a highly conserved intracellular process that can be categorized into three classes: Macroautophagy, chaperone-mediated autophagy, and microautophagy. As the major type of autophagy, macroautophagy (hereinafter referred to as “autophagy”) is crucial for eliminating cytoplasmic materials and maintaining intracellular homeostasis under pathological conditions[7,8]. This process of degrading long-lived proteins and cytoplasmic organelles is associated with SE, PD, and other neurodegenerative diseases[9]. Indications of autophagy variation have been observed in several neuroprotective drugs (e.g., 17-allylamino-demethoxygeldanamycin and Tanshinone IIA), which simultaneously confirmed their ability to ameliorate SE-induced hippocampal neuronal death by upregulating autophagy[10,11]. Baicalin, as a natural extract, undergoes a safe and established preparation process and offers several significant advantages, including minimal side effects.
Baicalin-a traditional Chinese medicine-is among the main flavonoid compounds isolated from Scutellaria baicalensis Georgi and possesses multiple pharmacological properties, including neuroprotective[12], anti-inflammatory[13], antiapoptotic[14], and antioxidant[15] effects. Baicalin can freely cross the blood-brain barrier[14,16]; thus, it has been used to treat many nervous system diseases. Several studies have explored the relationship between autophagy and Baicalin. For example, Baicalin has been found to induce autophagy in tubercle bacillus-infected macrophages through the PI3K/Akt/mTOR signaling pathway, indicating its potential to alter disease progression by regulating autophagy activity[17]. Baicalin has also been shown to exert anticancer and anti-inflammatory effects by activating autophagy in pathogenic cells, such as human bladder cancer T24 cells, human hepatocellular carcinoma SMMC-7721 cells[18], and Mycobacterium tuberculosis-infected macrophages. A much-debated issue is whether Baicalin exerts neuroprotective effects while regulating autophagy. However, the mechanism by which Baicalin activates autophagy in the hippocampus following SE remains unclear. Thus, we aim to investigate the emerging role of autophagy in the hippocampus during SE and elucidate the precise mechanism underlying the neuroprotective effects of Baicalin.
Ninety-six pathogen-free Wistar rats (male, 180-220 g) were purchased from Shanghai Laboratory Animal Center. The rats were raised under controlled conditions with a 24 °C ± 1 °C temperature and a 12-h light/dark cycle. All rats had free access to water and food.
The rat model of SE was induced by intraperitoneally injecting adult Wistar rats with LiCl-pilocarpine[19]. The rats were lightly anesthetized through isoflurane inhalation and then intraperitoneally injected with 0.2 mL (127 mg/kg) of lithium chloride; Pilocarpine (30 mg/kg) was injected 16 h after LiCl administration. Rats that did not develop SE after the injection were excluded. The rats were randomly divided into four groups: Control, SE, SE + B100, and SE + B200 (Figure 1).
For the SE group, pilocarpine (25 mg/kg) was injected intraperitoneally 30 min after intraperitoneal injection of atropine methyl nitrate (2 mg/kg) to ameliorate peripheral cholinergic signs. The control group received a physiological salt solution instead of pilocarpine. The SE + B100 and SE + B200 groups were intraperitoneally injected with 100 mg/kg and 200 mg/kg of Baicalin, respectively, after the pilocarpine injection. The drug dosages used in this study were based on previous reports[20].
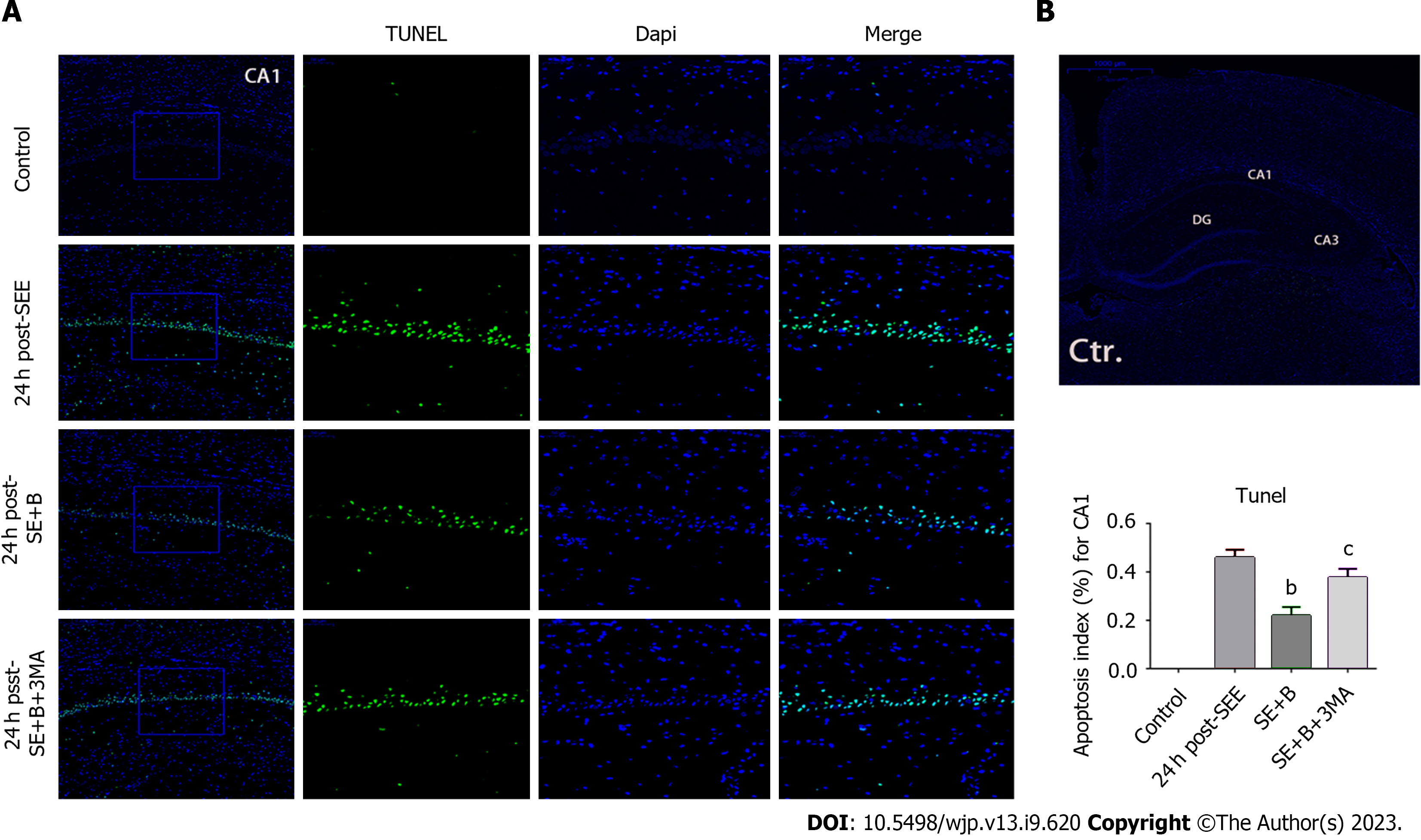
3-Methyladenine (3-MA) was used to inhibit autophagy. Subsequently, the rats were randomly divided into the following groups: Control, SE, SE + Baicalin, and SE + Baicalin + 3-MA. In the SE + Baicalin + 3-MA group, rats were injected with 400 nmoL 3-MA (2 μL) through an intracerebroventricular injection to the right lateral ventricle 1 h before SE induction, according to a previous study[21]. The SE + Baicalin and SE + Baicalin + 3-MA received a dose of 200 mg/kg of Baicalin. Isoflurane inhalation was used for anesthesia after SE induction. All rat brains were harvested for subsequent histological and biochemical studies.
Twenty-four rats were executed 24 h after the onset of SE. HE staining was performed to detect damaged neurons in the hippocampus (six per group) based on a previous study[22]. Damaged neurons were characterized by an abnormal neuronal morphology, a dried-up cytoplasm with vacuoles, and a shrunken- hyperchromatic nucleus, as reported in a previous study[23].
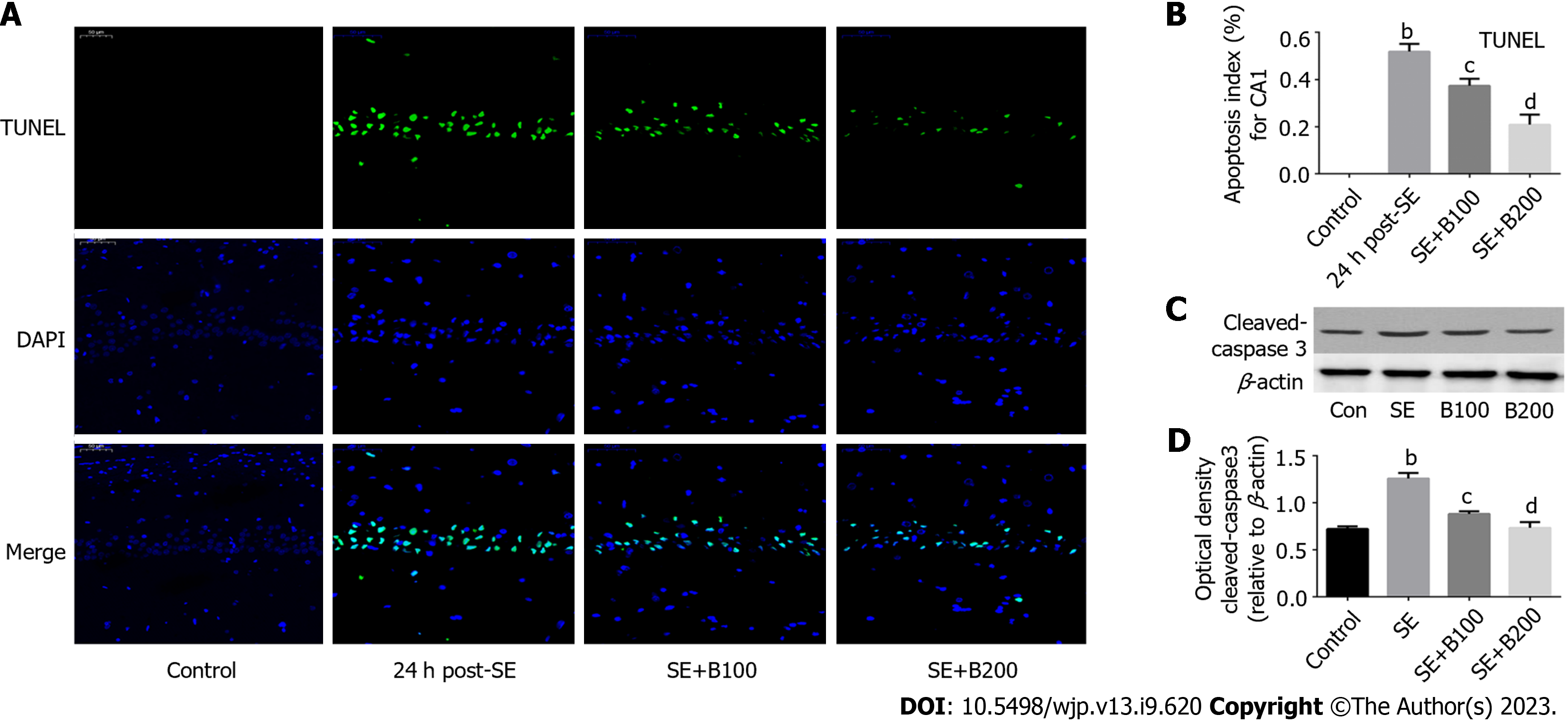
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining was conducted using an in situ cell death detection kit (Promega) to detect the cell apoptosis index. Briefly, rats were executed 24 h after the onset of SE, and 4-μm-thick coronal slices of the brain tissue were prepared using a paraffin slicer. Proteinase K (Nanjing Jianchen Co., Ltd.) was used to digest the coronal sections for 15 min. After deparaffinization, rehydration, and washing (with PBS), the sections were cultivated with a TdT reaction mix (Promega) containing TUNEL reaction fluid for 50 min at 37 °C (avoiding exposure to light from hereon). To stop the reaction, plastic coverslips were removed, and coronal slides were immersed in 2XSSC (Nanjing Jianchen Co., Ltd.) for 15 min. After three washes with 0.02 M TBS (5 min/wash), the liquid around the sections was dried, and the tablets were sealed with a seal containing DAPI fluorescent dye. Under a fluorescence microscope, localized apoptotic cells appeared green, and DAPI-stained nuclei appeared blue. Five slides per rat and five random fields per slide (scale bar = 50 μm) were selected to calculate the final average percentage and apoptosis index.
Hippocampal tissues were isolated, and hippocampus protein was extracted. The protein content was calculated using the BCA assay. Each quantity of protein per lane (30 μg) was separated on a 12% SDS-PAGE gel. The proteins were then electrotransferred onto a PVDF membrane (Millipore, United States). The membrane was blocked with 5% skim-fat milk prepared with TBST for 3 h. Primary antibody incubation was conducted with the following antibodies: LC3B (1:1000, Cell Signaling Technology), Beclin 1 (1:2000, Cell Signaling Technology), p62/SQSTM1 (1:500, Cell Signaling Technology), cleaved caspase-3 (1:500 dilution, Cell Signaling Technology), Bcl-2 (1:500 dilution, Cell Signaling Technology), and β-actin (1:2000, Bioworld Technology). Subsequently, secondary antibody incubation was conducted for 3 h at room temperature. The signals were visualized using an ECL reagent (Millipore), and band density was quantitatively analyzed using Quantity One software. The expression intensity was normalized to the loading control (β-actin).
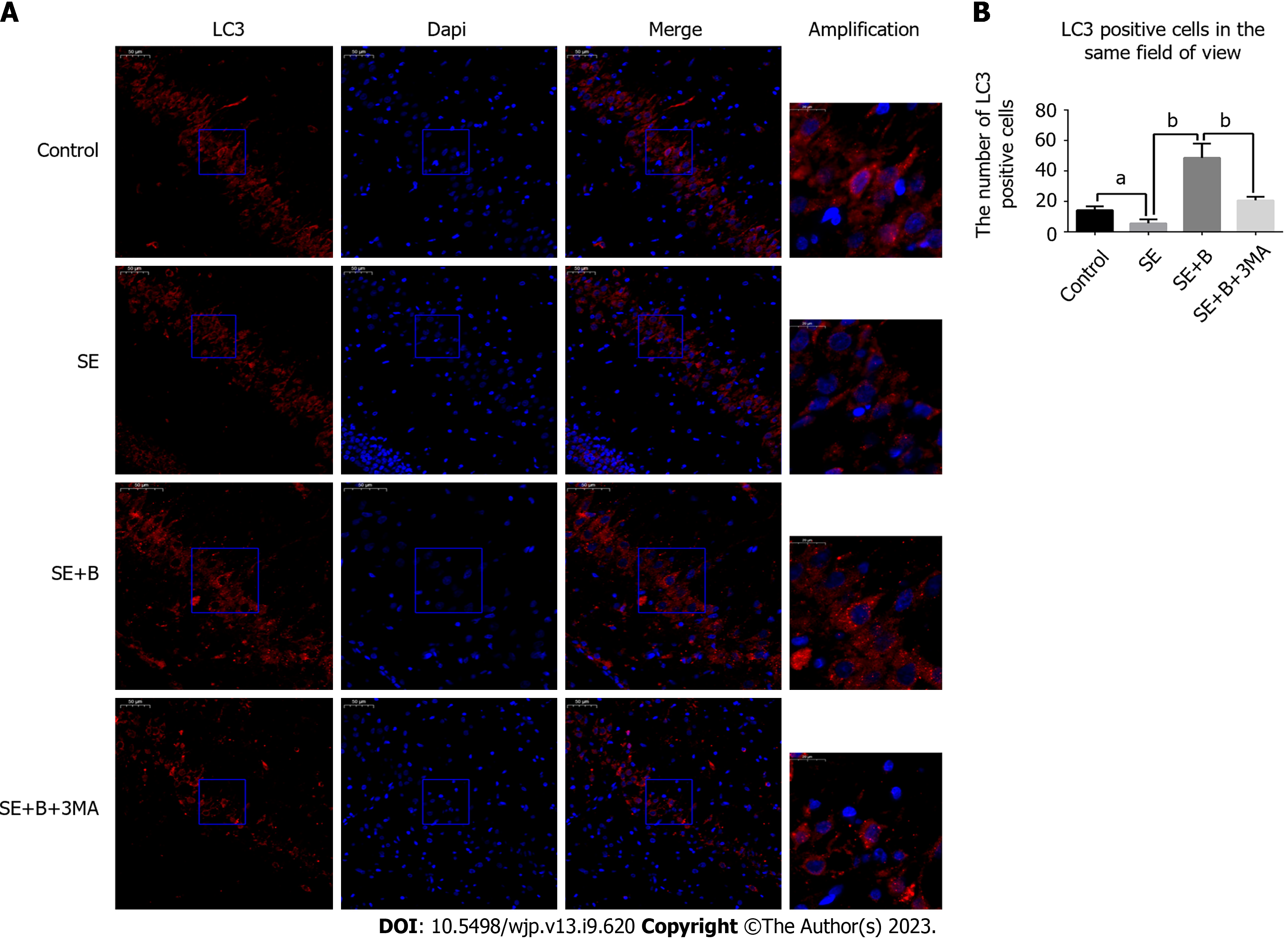
Immunofluorescence staining was performed to localize LC3 in the hippocampal cells. Brain sections from 24 rats (six per group) were prepared as described for the TUNEL staining procedure.
The slides were blocked with 5% normal donkey serum containing 0.01% Triton X-100 for 2 h. Primary antibody incubation was conducted with antibodies against LC3B (1:1000, Cell Signaling Technology) for 12 h at 4 °C. After washing three times with PBST, the sections were incubated with the secondary antibody (1:200, Alexa Fluor 594) for 2 h. DAPI treatment was applied for 1 min, and the sections were analyzed under a fluorescence microscope. We counted the cells with LC3 puncta, which appear as a result of LC3-II aggregation around the nucleus, and positive cells in the same field of view were quantified. In each coronal section, six fields (scale bar = 50 μm) around the hippocampus were randomly chosen to determine the average.
All results were represented as mean ± SD. The GraphPad Prism 6 software was used to analyze the data. Analysis of variance was used to compare data among multiple groups. Inspection level α = 0.05 and P < 0.05 was considered a statistical difference.
Because the neurons in hippocampal CA1 are sensitive to SE stress, toluidine blue staining was performed to detect neuronal loss. The number of surviving neurons was significantly lower in the SE group than in the control group. However, Baicalin significantly increased the number of surviving neurons after SE (Table 1). To further confirm whether Baicalin provides neuroprotection to the hippocampus in SE rats, we monitored the latency of seizures following pilocarpine administration. The latent period (the time from pilocarpine administration to the onset of a seizure above the grade of Racine IV) was 29.60 ± 6.603 min in the SE group, whereas it was 41.70 ± 10.93 min and 63.80 ± 11.73 min in the Baicalin intervention groups (SE + B100 and SE + B200 groups, respectively). Treatment with different doses of Baicalin significantly prolonged the latency, and these differences were statistically significant (P < 0.01). The seizure latency in the SE + B200 group exceeded that in the SE + B100 group (Table 2). These results indicate that both concentrations of Baicalin exerted neuroprotective effects on the hippocampus dose-dependently. Therefore, the dosage of Baicalin (200 mg/kg) that produced superior effects was selected and employed for the subsequent experiments.
SE induced significant apoptosis of neurons in hippocampal CA1 (P < 0.01; Figure 1A and B). However, after 24 h, the apoptosis percentage was considerably lower in the SE + B100 and SE + B200 groups than in the SE group, with the SE + B200 group showing a more significant decrease (Figure 1B).
The level of pro-apoptotic cleaved caspase-3 in the SE + B200 group was significantly lower than in the SE group (P < 0.01; Figure 1C and D). These findings confirmed that Baicalin has neuroprotective effects against SE-induced apoptosis in an SE rat (Figure 2).
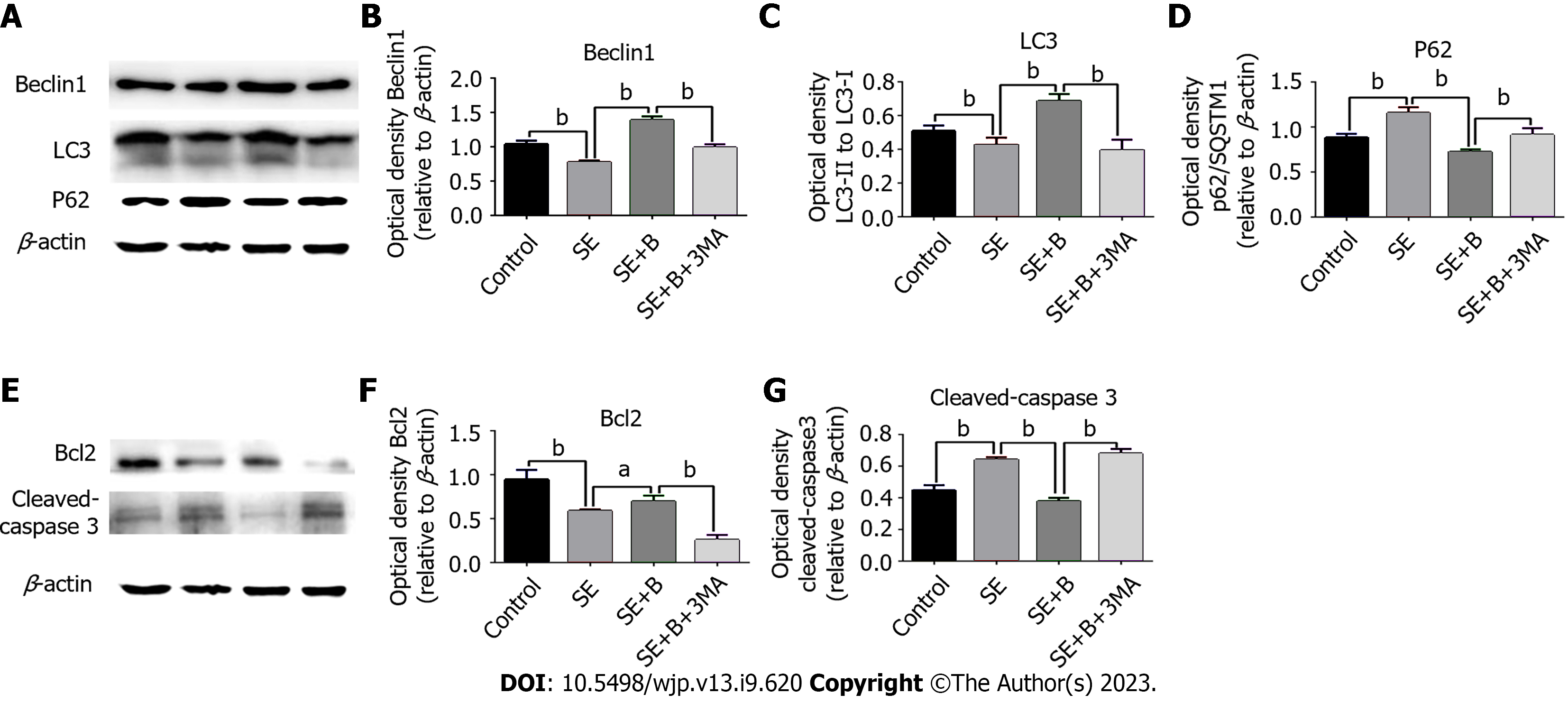
To further investigate the potential relationship between autophagy and the neuroprotective effect of Baicalin, we measured three highly related autophagy proteins (p62/SQSTM1, Beclin 1, and LC3) as markers of autophagy using western blotting[24]. Under normal conditions, LC3 exists in a cytosolic form (LC3-I), whereas during autophagy activation, LC3-I is cleaved, lipidated, and inserted into autophagosome membranes as LC3-II. Beclin 1, located in the trans-Golgi network, contributes to autophagosome formation and localizes autophagy-related proteins in the pre-autophagosome membrane. p62, an important factor in cytoplasmic material delivery, is also degraded by autophagy and thus frequently serves as an autophagy marker.
As shown in Figure 3A-D, Beclin 1 and LC3-II levels were lower in the SE group than in the control group, whereas the level of p62 was significantly higher in the SE group. These results indicate that SE insults can partially inhibit autophagy activity. However, Baicalin treatment significantly upregulated the levels of Beclin 1 and LC3-II and downregulated the level of SQSTM1/p62 compared with the SE group, indicating that Baicalin activates autophagy in the pilocarpine-induced SE rat model. The results of immunofluorescence staining are consistent with those of western blotting (Figure 3). The SE group exhibited a slight decrease in the number of cells with LC3 puncta (Figure 4A). Moreover, Baicalin significantly enhanced the number of LC3-positive cells compared to the group SE (Figure 4B).
To further explore the correlation between Baicalin-induced autophagy and apoptosis following SE, we applied 3-MA to detect alterations in autophagy proteins through western blotting. LC3-II and Beclin 1 levels were significantly lower in the SE + B + 3-MA group than in the SE + B group (Figure 3B and C). Conversely, the level of p62 was higher in the SE + B + 3-MA group than in the SE + B group (Figure 3D). These results indicate that normal baseline autophagy is possibly inhibited by SE and that Baicalin-induced autophagy is abolished by 3-MA.
Activated caspase-3 degrades the substrate, and the degradation products enhance mitochondrial permeability, ultimately resulting in apoptosis. Bcl-2 can reportedly suppress apoptosis by inhibiting the activation of caspase-3, which is released in the upstream and downstream apoptotic pathways. To further investigate the mechanism underlying the neuroprotective effect of Baicalin following SE, we evaluated mitochondrial apoptosis–related proteins using western blotting (Figure 3E-G).
The level of Bcl-2 was lower in the SE group than in the control group, whereas the level of cleaved caspase-3 was significantly higher in the SE group than in the control group (P < 0.01; Figure 3E-G). Therefore, SE stress could induce neuronal apoptosis in the hippocampus, possibly through the regulation of mitochondrial apoptosis-related proteins. Furthermore, Baicalin restored Bcl-2 levels and reduced the level of cleaved caspase-3 compared with the SE group, suggesting that Baicalin ameliorated SE-induced mitochondrial apoptosis. In addition, 3-MA significantly increased mitochondrial apoptosis by enhancing cleaved caspase-3 and abrogating the restoration of Bcl-2 induced by Baicalin. Overall, these results illustrate that 3-MA exacerbated mitochondrial apoptosis in SE and abrogated the neuroprotective effect of Baicalin against the apoptotic pathway.
SE is a complex pathophysiological process involving multiple mechanisms. Baicalin is a traditional herbal medicine with multitarget protective effects against seizures[25]. A previous study in our laboratory found that Baicalin has a neuroprotective effect on the hippocampus following SE through the antiapoptotic pathway. However, the correlation between autophagy and the effects of Baicalin remained unclear.
To further investigate the function of Baicalin after SE, we monitored seizure latency and apoptosis in this study. The most significant finding (using western blotting and immunofluorescence) was that Baicalin promoted autophagy activity and inhibited mitochondrial apoptosis following SE. Furthermore, the intervention of 3-MA inhibited Baicalin-induced autophagy and even abolished its neuroprotective effects against mitochondrial apoptosis. These findings have theoretical implications for the treatment of epilepsy by regulating autophagy.
In recent decades, the focus on alterations in autophagy after SE has increased due to the close relationship between autophagy activity and hippocampal injury. Different theories regarding the role of autophagy in nervous system diseases exist in the literature. The prevailing view is that activated autophagy has a protective role in rat models of ischemia-reperfusion[26-29], but there is still some ambiguity. A previous study focusing on autophagy has noted that autophagy dynamics in a rat’s hippocampus act as determinants for epileptogenesis, suggesting that applying autophagy inducers such as rapamycin, an mTOR inhibitor, to activate autophagy has an unambiguous effect on severe epileptic seizures[30,31]. Similarly, several studies have supported the view that activating autophagy through specific factors could provide neuroprotection in rat models of epilepsy[32], suggesting that autophagy induction plays a positive role in cell survival.
One significant finding is the activation of autophagy using recombinant human erythropoietin, which has been recently confirmed to exert neuroprotective effects and help ameliorate apoptosis in hippocampal neurons after SE[33]. In our study, we showed that Baicalin treatment significantly upregulated autophagy activity based on alterations in autophagy-related proteins and immunofluorescence staining of LC3-II puncta. In addition, decreased neuronal apoptosis and prolonged seizure latency were observed in Baicalin-treated groups, consistent with the findings of previous studies that support the neuroprotective effect of autophagy on the hippocampus in patients with seizures. A prior report concluded that impaired autophagy possibly contributes to epileptogenesis, which may be interesting as a potential therapeutic target for treating and preventing epilepsy[34]. Nevertheless, we could not elucidate whether autophagy inhibition promotes the occurrence of epilepsy, which is a limitation of our study.
There are possible situations where autophagy plays a dual role in epilepsy. The practical effect of autophagy depends on the degree of its activation due to the release of various deleterious factors following SE. The possible situations are as follows: (1) Inhibited autophagy potentially contributing to epileptogenesis, although a definitive causal relationship between autophagy and epileptogenesis in rat models has not been established; (2) Proper activation of autophagy benefiting the survival of hippocampal neurons by generating adenosine triphosphate; and (3) SE causing autophagic death and apoptosis in hippocampal neurons due to excessive autophagy[35].
It has not been determined whether administering neuroprotective drugs increases or decreases autophagic flow. For instance, we can only detect signs of decreased autophagy because the activation of autophagy promotes the clearance of autophagosome accumulation[36]. However, the amelioration of apoptosis by autophagy activation in epilepsy is complex and has not been fully elucidated.
Pro-apoptotic materials, such as damaged mitochondria and Bax accumulation induced by traumatic brain injury, can be eliminated by increased autophagy flux[37].
A previous study reported another possible mechanism involving autophagy activation and sequestration of abnormal proteins that trigger endoplasmic reticulum (ER) stress[38]. Hence, autophagy activation may inhibit ER stress in response to external stimuli and ameliorate apoptosis[39]. However, the role of autophagy in apoptosis after SE remains unknown.
In this study, we discovered that Baicalin induces autophagy activation and alleviates apoptosis in hippocampal neurons following SE. Significantly, the abolition of Baicalin-induced autophagy and its neuroprotective effects against the mitochondrial apoptotic pathway by 3-MA administration suggest that autophagy activation may reduce neuronal apoptosis by removing damaged mitochondria after SE.
These results further support previous studies that have linked mitophagy, apoptosis, and neuron survival[34] and reported that mitophagy exerts anti-apoptosis effects, which promote cell survival. In this research, only male rats were used to reduce the impact of gender differences on research results. However, further experiments need to be validated with female animals.
In conclusion, we demonstrated that Baicalin prolongs seizure latency, ameliorates hippocampal injury, increases the survival rate of hippocampal neurons, and reduces mitochondrial apoptosis following SE in rats through autophagy activation. This study contributes to the pharmacological effects of traditional Chinese herbs such as Baicalin. Furthermore, it provides a new way of regulating autophagy for treating SE.
Autophagy is associated with hippocampal injury after status epilepticus (SE), and is considered a potential mechanism with curative value. Baicalin, an emerging multi-therapeutic drug that has been demonstrated to exert neuroprotective effects in patients with nervous system diseases because of its antioxidant property.
We investigate the influence of Baicalin on the improvement of LiCl-Pilocarpine-induced rat SE.
We intended to investigate the potential role of autophagy in LiCl-pilocarpine-induced SE.
Nissl staining showed that Baicalin attenuates hippocampal injury and reduces the number of neuronal deaths in the hippocampus. Besides, the expression intensity of cleaved caspase-3 and apoptosis in hippocampal CA1 following SE were reversed by Baicalin, as proven by western blotting and terminal deoxynucleotidyl transferase dUTP nick end labelling assay. Furthermore, western blotting and immunofluorescence staining were used to measure the expression of autophagy markers (p62/SQSTM1, Beclin 1, and LC3) and apoptotic pathway markers (cleaved caspase-3 and Bcl-2).
Baicalin significantly upregulated autophagic activity and downregulated mitochondrial apoptotic pathway markers. Conversely, 3-methyladenine, a commonly used inhibitor of autophagy, was simultaneously administered to inhibit the autophagy induced by Baicalin, abrogating the latter’s protection on the mitochondria apoptotic level.
We illustrated that Baicalin induced activation of autophagy alleviates apoptotic death and protects the hippocampus of SE rats.
The improvement of LiCl-Pilocarpine-induced rat SE by Baicalin was validated.
| 1. | Dichter MA. Emerging insights into mechanisms of epilepsy: implications for new antiepileptic drug development. Epilepsia. 1994;35 Suppl 4:S51-S57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 47] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Sander JW. The epidemiology of epilepsy revisited. Curr Opin Neurol. 2003;16:165-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 485] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 3. | Téllez-Zenteno JF, Hernández-Ronquillo L. A review of the epidemiology of temporal lobe epilepsy. Epilepsy Res Treat. 2012;2012:630853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 203] [Cited by in RCA: 264] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 4. | Leite JP, Garcia-Cairasco N, Cavalheiro EA. New insights from the use of pilocarpine and kainate models. Epilepsy Res. 2002;50:93-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 201] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 5. | Wahab A, Albus K, Gabriel S, Heinemann U. In search of models of pharmacoresistant epilepsy. Epilepsia. 2010;51 Suppl 3:154-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 50] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Rowley S, Patel M. Mitochondrial involvement and oxidative stress in temporal lobe epilepsy. Free Radic Biol Med. 2013;62:121-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 197] [Cited by in RCA: 201] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 7. | Jing K, Lim K. Why is autophagy important in human diseases? Exp Mol Med. 2012;44:69-72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 109] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 8. | Glick D, Barth S, Macleod KF. Autophagy: cellular and molecular mechanisms. J Pathol. 2010;221:3-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2980] [Cited by in RCA: 2941] [Article Influence: 183.8] [Reference Citation Analysis (0)] |
| 9. | Bockaert J, Marin P. mTOR in Brain Physiology and Pathologies. Physiol Rev. 2015;95:1157-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 282] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 10. | Zhu Y, Tang Q, Wang G, Han R. Tanshinone IIA Protects Hippocampal Neuronal Cells from Reactive Oxygen Species Through Changes in Autophagy and Activation of Phosphatidylinositol 3-Kinase, Protein Kinas B, and Mechanistic Target of Rapamycin Pathways. Curr Neurovasc Res. 2017;14:132-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Li J, Yang F, Guo J, Zhang R, Xing X, Qin X. 17-AAG post-treatment ameliorates memory impairment and hippocampal CA1 neuronal autophagic death induced by transient global cerebral ischemia. Brain Res. 2015;1610:80-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Liao ZJ, Liang RS, Shi SS, Wang CH, Yang WZ. Effect of baicalin on hippocampal damage in kainic acid-induced epileptic mice. Exp Ther Med. 2016;12:1405-1411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Li L, Bao H, Wu J, Duan X, Liu B, Sun J, Gong W, Lv Y, Zhang H, Luo Q, Wu X, Dong J. Baicalin is anti-inflammatory in cigarette smoke-induced inflammatory models in vivo and in vitro: A possible role for HDAC2 activity. Int Immunopharmacol. 2012;13:15-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 14. | Zuo D, Lin L, Liu Y, Wang C, Xu J, Sun F, Li L, Li Z, Wu Y. Baicalin Attenuates Ketamine-Induced Neurotoxicity in the Developing Rats: Involvement of PI3K/Akt and CREB/BDNF/Bcl-2 Pathways. Neurotox Res. 2016;30:159-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 15. | Cheng F, Lu Y, Zhong X, Song W, Wang X, Sun X, Qin J, Guo S, Wang Q. Baicalin's Therapeutic Time Window of Neuroprotection during Transient Focal Cerebral Ischemia and Its Antioxidative Effects In Vitro and In Vivo. Evid Based Complement Alternat Med. 2013;2013:120261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Tarragó T, Kichik N, Claasen B, Prades R, Teixidó M, Giralt E. Baicalin, a prodrug able to reach the CNS, is a prolyl oligopeptidase inhibitor. Bioorg Med Chem. 2008;16:7516-7524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 17. | Zhang Q, Sun J, Wang Y, He W, Wang L, Zheng Y, Wu J, Zhang Y, Jiang X. Antimycobacterial and Anti-inflammatory Mechanisms of Baicalin via Induced Autophagy in Macrophages Infected with Mycobacterium tuberculosis. Front Microbiol. 2017;8:2142. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 98] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 18. | Zhang X, Tang X, Liu H, Li L, Hou Q, Gao J. Autophagy induced by baicalin involves downregulation of CD147 in SMMC-7721 cells in vitro. Oncol Rep. 2012;27:1128-1134. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Grabenstatter HL, Cogswell M, Cruz Del Angel Y, Carlsen J, Gonzalez MI, Raol YH, Russek SJ, Brooks-Kayal AR. Effect of spontaneous seizures on GABAA receptor α4 subunit expression in an animal model of temporal lobe epilepsy. Epilepsia. 2014;55:1826-1833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 20. | Fang J, Wang H, Zhou J, Dai W, Zhu Y, Zhou Y, Wang X, Zhou M. Baicalin provides neuroprotection in traumatic brain injury mice model through Akt/Nrf2 pathway. Drug Des Devel Ther. 2018;12:2497-2508. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 21. | Wang YQ, Wang L, Zhang MY, Wang T, Bao HJ, Liu WL, Dai DK, Zhang L, Chang P, Dong WW, Chen XP, Tao LY. Necrostatin-1 suppresses autophagy and apoptosis in mice traumatic brain injury model. Neurochem Res. 2012;37:1849-1858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 22. | Ding H, Wang H, Zhu L, Wei W. Ursolic Acid Ameliorates Early Brain Injury After Experimental Traumatic Brain Injury in Mice by Activating the Nrf2 Pathway. Neurochem Res. 2017;42:337-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 23. | Liang J, Wu S, Xie W, He H. Ketamine ameliorates oxidative stress-induced apoptosis in experimental traumatic brain injury via the Nrf2 pathway. Drug Des Devel Ther. 2018;12:845-853. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 49] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 24. | Zhang L, Wang H, Zhou Y, Zhu Y, Fei M. Fisetin alleviates oxidative stress after traumatic brain injury via the Nrf2-ARE pathway. Neurochem Int. 2018;118:304-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 80] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 25. | Liu W, Ge T, Pan Z, Leng Y, Lv J, Li B. The effects of herbal medicine on epilepsy. Oncotarget. 2017;8:48385-48397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 26. | Yang Y, Gao K, Hu Z, Li W, Davies H, Ling S, Rudd JA, Fang M. Autophagy upregulation and apoptosis downregulation in DAHP and triptolide treated cerebral ischemia. Mediators Inflamm. 2015;2015:120198. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 45] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 27. | Russo R, Varano GP, Adornetto A, Nazio F, Tettamanti G, Girardello R, Cianfanelli V, Cavaliere F, Morrone LA, Corasaniti MT, Cecconi F, Bagetta G, Nucci C. Rapamycin and fasting sustain autophagy response activated by ischemia/reperfusion injury and promote retinal ganglion cell survival. Cell Death Dis. 2018;9:981. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 28. | Sun Y, Zhang T, Zhang Y, Li J, Jin L, Sun Y, Shi N, Liu K, Sun X. Ischemic Postconditioning Alleviates Cerebral Ischemia-Reperfusion Injury Through Activating Autophagy During Early Reperfusion in Rats. Neurochem Res. 2018;43:1826-1840. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 29. | Li Q, Zhang T, Wang J, Zhang Z, Zhai Y, Yang GY, Sun X. Rapamycin attenuates mitochondrial dysfunction via activation of mitophagy in experimental ischemic stroke. Biochem Biophys Res Commun. 2014;444:182-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 172] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 30. | McMahon J, Huang X, Yang J, Komatsu M, Yue Z, Qian J, Zhu X, Huang Y. Impaired autophagy in neurons after disinhibition of mammalian target of rapamycin and its contribution to epileptogenesis. J Neurosci. 2012;32:15704-15714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 130] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 31. | Giorgi FS, Biagioni F, Lenzi P, Frati A, Fornai F. The role of autophagy in epileptogenesis and in epilepsy-induced neuronal alterations. J Neural Transm (Vienna). 2015;122:849-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 32. | Ali SO, Shahin NN, Safar MM, Rizk SM. Therapeutic potential of endothelial progenitor cells in a rat model of epilepsy: Role of autophagy. J Adv Res. 2019;18:101-112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 33. | Li Q, Han Y, Du J, Jin H, Zhang J, Niu M, Qin J. Recombinant human erythropoietin protects against brain injury through blunting the mTORC1 pathway in the developing brains of rats with seizures. Life Sci. 2018;194:15-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 34. | Wong M. Cleaning up epilepsy and neurodegeneration: the role of autophagy in epileptogenesis. Epilepsy Curr. 2013;13:177-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Rubinsztein DC, DiFiglia M, Heintz N, Nixon RA, Qin ZH, Ravikumar B, Stefanis L, Tolkovsky A. Autophagy and its possible roles in nervous system diseases, damage and repair. Autophagy. 2005;1:11-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 318] [Cited by in RCA: 344] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 36. | Lipinski MM, Wu J, Faden AI, Sarkar C. Function and Mechanisms of Autophagy in Brain and Spinal Cord Trauma. Antioxid Redox Signal. 2015;23:565-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 179] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 37. | Tan X, Azad S, Ji X. Hypoxic Preconditioning Protects SH-SY5Y Cell against Oxidative Stress through Activation of Autophagy. Cell Transplant. 2018;27:1753-1762. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 38. | Fernández A, Ordóñez R, Reiter RJ, González-Gallego J, Mauriz JL. Melatonin and endoplasmic reticulum stress: relation to autophagy and apoptosis. J Pineal Res. 2015;59:292-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 418] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 39. | Lee WS, Yoo WH, Chae HJ. ER Stress and Autophagy. Curr Mol Med. 2015;15:735-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 155] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gawrych M, Poland; Mensah HK, Ghana S-Editor: Fan JR L-Editor: A P-Editor: Chen YX