Published online Aug 19, 2023. doi: 10.5498/wjp.v13.i8.583
Peer-review started: June 20, 2023
First decision: July 7, 2023
Revised: July 17, 2023
Accepted: July 19, 2023
Article in press: July 19, 2023
Published online: August 19, 2023
Processing time: 57 Days and 22.2 Hours
The efficacy of cognitive behavioral group therapy (CBGT) for cognitive dys-function and negative symptoms of schizophrenia is established, but more evidence is required.
To assess the effectiveness of CBGT combined with mental health education as a treatment for schizophrenia compared with mental health education alone.
In all, 120 schizophrenia out-patients were randomized into CBGT combined with mental health education or single mental health education. The primary outcomes were positive and negative symptoms, cognitive function, excitatory factor, anxiety and depression symptom improvements on the positive and negative syndrome scale score. Secondary outcome measures included social function and drug compliance.
There were significant differences between CBGT combined with mental health education and single mental health education on measures of positive and negative symptoms, cognitive functions, excitatory factor, anxiety and depression symptoms, and social functions. No other significant difference in outcomes was observed.
CBGT combined with mental health education may be relevant beneficial treatment method in reducing symptoms, cognitive and social functions of patients with schizophrenia.
Core Tip: Psychological therapies for schizophrenia still deserves to be explored due to its advantages of having comparatively fewer side effects. As one of the different psychological approaches, cognitive behavioral therapy has been proven to be effective in the treatment of schizophrenia with sound evidence. This study provides new insights by adding cognitive behavioral group therapy to the conventional pharmaceutical therapy plus mental health education for the treatment of schizophrenia. It also provides some guidance on developing community rehabilitation models in patients with mental illness.
- Citation: Chen XL, Deng XT, Sun FG, Huang QJ. Effect of cognitive behavioral group therapy on rehabilitation of community patients with schizophrenia: A short-term randomized control trial. World J Psychiatry 2023; 13(8): 583-592
- URL: https://www.wjgnet.com/2220-3206/full/v13/i8/583.htm
- DOI: https://dx.doi.org/10.5498/wjp.v13.i8.583
Schizophrenia is a serious mental disorder, which can lead to loss of personality, decline in social function and the state of mental disability in various levels. Negative symptoms and cognitive impairment have been found to have a profound impact on long-term outcomes of schizophrenia, but current treatment options are limited. Recent studies have shown that the combination of cognitive behavioral group therapy (CBGT) can improve patient compliance with drug therapy, alleviate psychotic symptoms, reduce recurrence and rehospitalization rates, and improve social function and quality of life in schizophrenia.
This study aims to evaluate the impact of CBGT combined with mental health education on the clinical symptoms, cognitive and social functions and psychological status in patients with schizophrenia on the basis of conventional drug treatment, so as to provide evidence for its application to community rehabilitation for patients with schizophrenia.
This was a randomized control trial approved by the Biomedical Ethics Review Committee of Longhua District Chronic Disease Prevention and Treatment Center (Mental Health Center), and obtained the informed consent of family members of the research patients. Participants were recruited from outpatients with schizophrenia. The inclusion criteria included diagnosis of schizophrenia (ICD-10 codes F20.0 to F20.9); 1 to 5 years of disease course with a stable condition; presence of dangerous behavior involving verbal threats; no obvious vandalism; partial recovery of self-knowledge; positive score for positive and negative symptom scale (PANSS) ≤ 49 or negative scale score ≤ 49; social function deficiency screening scale (SDSS) score ≥ 2; aged 19 to 60 years old; higher levels of education than primary school; with at least one guardian; taking second-generation antipsychotics for ≥ 1 year. Inclusion criteria for family members was those who have no mental history. Patients with co-morbidities, other psychiatric disorders (e.g., organic disorders, psychoactive substance-induced psychiatric disorders) or serious physical disorders affecting cognitive functions and family members with speech impairment were excluded from this trial.
Randomization was conducted using computer-generated random numbers with 120 participants allocated in blocks. These patients were randomly allocated to an intervention group and a control group with 60 patients in each group. The intervention group was subdivided into four groups with 15 patients and their family members in each group receiving CBGT combined with mental health education for 8 wk. The interventions in each group were led by an experienced psychiatrist or psychotherapist trained in CBGT. The control group was only given mental health education courses for 8 wk. The study was reviewed and approved by the Biomedical Ethics Review Committee of the Shenzhen Longhua District Chronic Disease Control Centre (Mental Health Centre) and informed consent was obtained from patients’ families.
CBGT intervention is divided into four stages: (1) Stage 1 (the first time): The initial stage. The basic task is acceptance and commitment. Members and team leaders get to know each other, understand the troubles in each other’s life and communicate with each other, normalize problems, introduce CBGT to group members, set group contracts, set group goals and enhance group cohesiveness; (2) Stage 2 (2nd-3rd): i.e., transition stage. Assist group members to deal with their emotional reactions and conflicts, and promote trust and relationship building. Identify the situations that induce stress and emotional conflicts, and perceive the related assumptions of situations, ideas, emotions and behaviors constructed under the stress situation; Identify and evaluate symptoms and negative cognitions; Examine the evidence to repair negative cognitions, core beliefs and dysfunctional assumptions. In addition, this stage also includes relaxation training, role play, imitation demonstration, resolving emotional conflicts such as anxiety and depression, seeking resource support and increasing self-confidence; (3) Stage 3 (4th-7th): i.e., working stage. Further explore problems and take effective actions to promote the change of members’ behaviors. Through psychological education and social skill training, encourage members to observe and imitate, consolidate self-monitoring and self-guidance training during rehabilitation, change distorted thinking patterns and improve self-esteem; and (4) Stage 4 (8th): The end stage. Summarize group experiences, consolidate achievements, encourage team members to use learning experiences in life, solve problems and emotional conflicts, enhance self-confidence, inject hope, and deal with parting emotions.
The participants were randomly divided into an intervention group (60 cases) and a control group (60 cases). Each patient had his/her one family member to participate in the trial. In addition to antipsychotics and mental health education implemented in the community, patients in the intervention group received CBGT together with their guardians for 8 wk. Patients in the control group only received antipsychotic drugs and mental health. Patient social functions, symptoms of schizophrenia and activities of daily living (ADL) were evaluated by SDSS, PANSS and ADL scale before CBGT treatment, 8 wk and 4 wk after the treatment (i.e., before the intervention, after the intervention and 4 wk after the intervention, respectively). According to the evaluation results of the scale at three time points, the intervention effects of CBGT on patient symptoms and social functions were analyzed.
Primary outcome measures include improvements in symptoms measured by the scale of the PANSS. Using the positive and negative symptom scale, five-factor dimensions of Chinese norm were evaluated: Positive factor, negative factor, cognitive factor, excitatory factor, anxiety and depression factor. Social function is evaluated by SDSS scale.
Two psychiatrists scored 10 patients using PANSS scale, and calculated kappa coefficient = 0.822 through SPSS 22.0. The evaluation of the two doctors is in good agreement and has high consistency.
The ability to live was assessed by the ability of daily living scale. Medication compliance was evaluated from three types: (1) Complete compliance: Completely follow the doctor’s advice for one month; (2) part compliance: Taking medicine for more than 33% course of treatment in a month; and (3) non-compliance: 33% course of treatment in a month with no medication.
Statistical analysis was conducted using the SPSS 22.0 software. The rank-sum test and sample independent t-test were used for the variable data, and the chi square test was used for the categorical data. Repeated measures ANOVA was used to determine the effects of different intervention modalities on patients’ clinical symptoms, social functioning, abilities to perform daily living activities and medication adherence at three different time points: Pre-intervention, post-intervention and 4 wk post-intervention. By analysis of studentized residuals, the data in each group had no outliers (≥ ± 3SD) and followed a normal distribution (P > 0.05) according to the Kolmogorov-Smirnov test. The conditions for the sphericity test must be satisfied so that the data could be tested for normality and symmetry with sphericity, and in case the assumption for sphericity was not met, the method of Greenhouse-Geisser (G-G) for correction was used. The test level of P < 0.05 was considered statistically significant. It was then determined whether there was an interaction between factors (groups and time points) in the intervention group and the control group. If there was an interaction, t-test and one-way ANOVA were required to determine whether there was a separate effect. When a separate effect test was required, test level of P < 0.05 was considered statistically significant. If there was no interaction, a main effect test was required to determine whether there was a main effect, with a test level of P < 0.05 being statistically significant. A two-by-two comparison was carried out. We used Bonferroni correction for multiple repeated ANOVA testing.
After the treatment and at 4-wk follow-up, none of the 120 patients dropped out.
Patients with schizophrenia in both groups had a disease course of 1 to 5 years and were in stable condition. They were treated with second-generation antipsychotics in the community. General data show that the average age of them is 38 years old. There were slightly more women (53.33%) than men (46.67%) in the intervention group, and there were more men (61.67%) than women (38.33%) in the control group. The educational background for the participants is mainly below junior high school. There was no significant difference in age, gender, education level, medical burden and family income between the intervention group and the control group. The baseline characteristics of the participants are presented in Table 1.
| Variables | Control group (n = 60) | Intervention group (n = 60) | t/χ2 | P value |
| Age (yr) | 37.95 ± 10.33 | 38.47 ± 10.68 | 0.269 | 0.788 |
| Gender | 2.719 | 0.099 | ||
| Male | 37 (61.67) | 28 (46.67) | ||
| Female | 23 (38.33) | 32 (53.33) | ||
| Educational level | 5.967 | 0.113 | ||
| Primary school | 10 (16.67) | 13 (21.67) | ||
| Junior high school | 40 (66.67) | 29 (48.33) | ||
| Technical secondary school/high school | 8 (13.33) | 10 (16.67) | ||
| College/undergraduate | 2 (3.33) | 8 (13.33) | ||
| Medical burden (CNY) | 3300 ± 2700 | 3000 ± 1600 | -0.865 | 0.389 |
| Annual household income (CNY) | 45200 ± 32800 | 38500 ± 29200 | -1.178 | 0.241 |
The general data (age, gender, education level, medical burden, annual family income) of the family members were analyzed and compared in the two groups. There was no significant difference in general data between the two groups (P > 0.05). The baseline characteristics of the sample are presented in Table 2.
| Variables | Control group (n = 60) | Intervention group (n = 60) | t/χ2 | P value |
| Age (yr) | 43.02 ± 10.44 | 42.38 ± 9.40 | -0.349 | 0.727 |
| Gender | 2.344 | 0.126 | ||
| Male | 35 (58.33) | 43 (71.67) | ||
| Female | 25 (41.67) | 17 (28.33) | ||
| Educational level | 9.226 | 0.026 | ||
| Primary school | 3 (5.00) | 13(21.67) | ||
| Junior high school | 40 (66.67) | 32 (53.33) | ||
| Technical secondary school/high school | 3 (5.00) | 6 (10.00) | ||
| College/undergraduate | 14 (23.33) | 9 (15.00) | ||
| Medical burden (CNY) | 3300 ± 2700 | 3000 ± 1600 | -0.865 | 0.389 |
| Annual household income (CNY) | 45200 ± 32800 | 38500 ± 29200 | -1.178 | 0.241 |
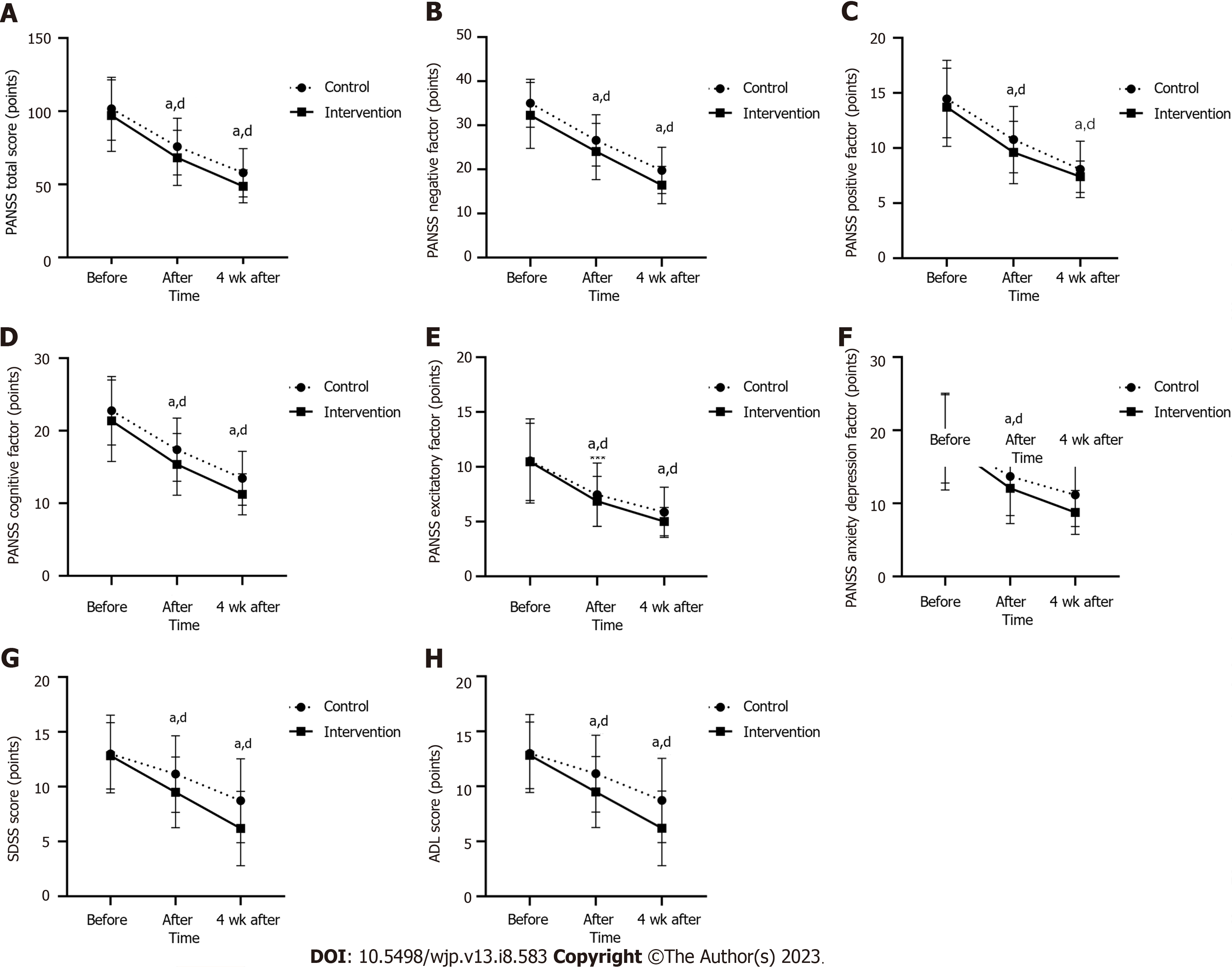
The overall PANSS score, positive factor score, cognitive factor score, excitatory factor score, anxiety and depression factor score and SDSS score decreased successively in the two groups at three time points. After the intervention and at four weeks after the intervention, the overall PANSS score (P < 0.05 and P < 0.001, respectively), positive factor score (P > 0.05 and P < 0.001, respectively), cognitive factor score (P < 0.05 and P < 0.001, respectively), excitatory factor score (P > 0.05 and P < 0.001, respectively), the scores of anxiety and depression factors (P > 0.05 and P < 0.001, respectively) and the overall score of SDSS (P < 0.01 and P < 0.01, respectively) were significantly different from those before the intervention. After repeated measurement analysis of variance, there was interaction between group and time points in the total overall score of SDSS (P < 0.05). After the intervention, there were significant differences between the intervention group and the control group in PANSS total score (P < 0.05), positive factor score (P < 0.05), cognitive factor score (P < 0.05) and SDSS total score (P < 0.01). At four weeks after the intervention, there were significant differences between the intervention group and the control group in PANSS total score (P < 0.01), excitation factor score (P < 0.05), cognitive factor score (P < 0.01), anxiety and depression factor score (P < 0.01) and SDSS total score (P < 0.01). Outcomes are shown in Table 3 and Figure 1.
| Variables | Control group (n = 60), mean ± SD | Intervention group (n = 60), mean ± SD | Repeated measurement, F-test | ||||||
| Before intervention | After intervention | 4 wk after intervention | Before intervention | After intervention | 4 wk after intervention | Main effect for groups (F, P value, η2partial) | Main effect for time points (F, P value, η2partial) | Group by time point interaction (F, P value, η2partial) | |
| Overall score | 101.67 ± 21.48 | 75.78 ± 19.36 | 57.90 ± 16.52 | 97.02 ± 24.40 | 68.15 ± 18.85 | 48.72 ± 11.28 | F = 5.617, P = 0.019, | F = 344.924, P = 0.000, | F = 0.829, P = 0.439, |
| Negative factor | 35.00 ± 5.42 | 26.58 ± 5.83 | 19.77 ± 5.26 | 32.27 ± 7.48 | 24.05 ± 6.37 | 16.45 ± 4.22 | F = 9.777, P = 0.002, | F = 452.858, P = 0.000, | F = 0.451, P = 0.638, |
| Positive factor | 14.45 ± 3.50 | 10.77 ± 3.01 | 8.07 ± 2.56 | 13.70 ± 3.53 | 9.60 ± 2.83 | 7.40 ± 1.43 | F = 3.766, P = 0.055, | F = 243.801, P = 0.000, | F = 0.873, P = 0.420, |
| Excitatory factor | 10.53 ± 3.84 | 7.45 ± 2.89 | 5.85 ± 2.28 | 10.45 ± 3.52 | 6.85 ± 2.28 | 5.00 ± 1.29 | F = 1.437, P = 0.233, | F = 140.501, P = 0.000, | F = 0.801, P = 0.451, |
| Cognitive factor | 22.75 ± 4.72 | 17.38 ± 4.35 | 13.45 ± 3.72 | 21.37 ± 5.61 | 15.37 ± 4.25 | 11.23 ± 2.83 | F = 7.751, P = 0.006, | F = 275.171, P = 0.000, | F = 0.505, P = 0.605, |
| Anxiety and depression factor | 18.82 ± 6.04 | 13.70 ± 5.36 | 11.15 ± 4.31 | 18.45 ± 6.61 | 12.07 ± 4.82 | 8.77 ± 3.00 | F = 3.409, P = 0.067, | F = 153.796, P = 0.000, | F = 2.130, P = 0.123, |
| SDSS score | 12.98 ± 3.55 | 11.1 ± 3.50 | 8.72 ± 3.82 | 12.82 ± 3.03 | 9.48 ± 3.23 | 6.18 ± 3.40 | F = 5.798, P = 0.018, | F = 593.573, P = 0.000, | F = 30.062, P = 0.000, |
| ADL score | 20.13 ± 8.49 | 16.78 ± 3.95 | 16.25 ± 3.23 | 21.05 ± 8.94 | 16.90 ± 4.00 | 16.23 ± 2.99 | F = 0.134, P = 0.715, | F = 28.065, P = 0.000, | F = 0.323, P = 0.724, |
χ2-test was performed for medication compliance in the two groups at three time points. The results showed that there was no significant difference between the intervention group and the control group before the intervention (P > 0.05). There was no significant difference in medication compliance between the two groups after the intervention and at 4 wk after the intervention (P > 0.05). Compared with the control group, the proportion of complete compliance in the intervention group increased slightly (8.34%) after the intervention (83.33%) and at 4 wk after the intervention (91.67%) as presented in Table 4.
| Pre-intervention compliance | Post-intervention compliance | Compliance at 4 wk after intervention | ||||
| Complete compliance | Partial compliance | Complete compliance | Partial compliance | Complete compliance | Partial compliance | |
| Control group (n = 60) | 49 (81.67) | 11 (18.33) | 48 (80.00) | 12 (20.00) | 53 (88.33) | 7 (11.67) |
| Intervention group (n = 60) | 52 (86.67) | 8 (13.33) | 50 (83.33) | 10 (16.67) | 55 (91.67) | 5 (8.33) |
| χ2 | 0.563 | 0.223 | 0.370 | |||
| P value | 0.453 | 0.637 | 0.543 | |||
This is a study to evaluate the effect of CBGT combined with mental health education on community-based rehabilitation of patients with schizophrenia. The main results showed that: (1) Compared with the control group, patients in the intervention group had significantly lower PANSS total scores and five factor scores post-intervention, with negative factor and cognitive factor scores still showing reduction at 4 wk post-intervention; (2) SDSS scores in the intervention group significantly decreased; (3) ADL scores decreased in both groups after the intervention and at 4 wk after the intervention, and the difference was not significant; and (4) there was no significant difference in medication adherence between the intervention group and the control group.
Our findings confirm the ameliorative effect of CBGT on the psychotic symptoms of patients with schizophrenia. Combined with the current situation of community psychiatric rehabilitation management in Shenzhen, the participants of this study are migrant workers in Shenzhen, and as general information shows, this group of population has a low level of education, and nearly half of them do not purse education beyond junior high school with a household income of around 40000 CNY. The migrant workers have medical and social resources availability is restricted and have received relatively little social support. Therefore, the aim of this study is to explore a feasible community-based rehabilitation model for migrant workers. In this study, a comparative analysis of psychiatric symptoms using the five-factor dimension of the PANSS scale Chinese normative model found that 8 wk of CBGT combined with mental health education significantly improved the patients’ clinicopathological symptoms and remained effective in improving negative symptoms and cognitive dysfunction after 4 wk of follow-up.
Previous studies have reported inconsistent results on the effectiveness of CBGT in improving positive and negative symptoms of schizophrenia, possibly because different studies have used different evaluation criteria and intervention cycles[1-11]. This study was completed using a modified short-term CBGT format in patients with schizophrenia and their family members, and explored together with patients the reasons for their laziness and reluctance to think and communicate, which were often related to their automatic thoughts such as “I am a useless person”, “I will not be welcomed by anyone”, “things outside are not interesting”, etc. Behavioral tests are used, e.g. developing a behavioral plan to test the validity of one’s beliefs, e.g. encouraging one to recall his/her pre-morbid state, self-affirming exceptions and increase self-confidence. When distressing experiences such as hallucinations and delusions of “if I go out, I’ll get hurt”, coping strategies such as self-symptom monitoring logs and problem solving support are used to alleviate patient feelings of unease and surveillance towards the external environment. Patients are encouraged to think about how they perceive the attitudes and behaviors of others in a situation, and how their performance respond to the patient’s behaviors. Patients are encouraged to be friendly to group members (behavioral activation: Greeting, asking for help, etc.) and then to observe if the group members are interested in them. Friendly feedback from others encourages patients to be more positive in their interpersonal interactions. When prejudicial attributions and negative self-evaluations such as “I can’t do it”, “I’m useless”, “No one wants to help me”, “I’m not the same as others” occur, self-focus is enhanced through family companionship, empathic psychological support, imitative learning among group members, focusing on solving difficult problems, improving self-awareness and insight, and strengthening social skills training. Through positive and effective communication, expressing one’s real emotional needs, accessing resource support (family support, peer support and social support), coping with crisis, testing hypotheses in practice and feeling trusted. Recent research has also demonstrated the effectiveness of social skills training in improving negative symptoms[1,12]. Cognitive remediation has a small to moderate beneficial effect on negative symptoms enhancing a positive outcome for cognitive impairment[13,14].
The results showed that combined CBGT and mental health education significantly improved social functioning in patients with schizophrenia, but did not have a significant advantage over single mental health education in improving daily living skills[15]. Related studies found that there were more factors influencing daily living skills, such as executive function (21%), memory and abstract thinking (13.5%), negative symptoms (13%), age of onset and years of education (8%)[16]. This shows that the improvement in cognitive function facilitates the improvement of patient independent living in their communities and issue management in their daily lives. We also saw that the short duration of the intervention and the influence of other related factors, among others, suggest that further research is needed to reveal the effect of CBGT on other factors related to the abilities of schizophrenia patients in their everyday lives.
Furthermore, the results suggest that CBGT combined with mental health education did not have a significant impact on medication adherence in schizophrenia patients, and only slightly increased the proportion of patients who were fully adherent. We can see that outpatients’ medication adherence (duration of medication) was more stable under family medication supervision, but we speculate that this may be related to a number of factors influencing adherence, including severity of illness, level of awareness of illness, duration and dosage of medication, family factors, environmental factors, and doctor-patient relationship[17-19]. In future studies, we may need to optimize adherence assessment criteria and will further explore the correlation between adherence and other factors.
We know that treatment with CBGT can have a positive impact on the pathophysiology and cognitive control networks in functional areas of the brain such as the anterior cingulate cortex, dorsolateral striatum, dorsolateral prefrontal cortex, amygdala, basal ganglia, and thalamus, and this evidence provides the basis for a neurobiological brain mechanism for CBGT treatment to relieve negative symptoms and cognitive impairments[20-29]. This study has demonstrated that CBGT combined with mental health education intervention improved social functioning, enhanced work drive, interpersonal interactions and cognitive functioning (verbal communication), and reduced positive symptoms, and negative symptoms better than single mental health education in community-rehabilitated schizophrenia patients.
There are some limitations to this study. Firstly, the sample size of this study is relatively small and our findings are preliminary and need to be further validated in an expanded patient sample. Secondly, although the PANSS scale is a screening tool to assess psychopathological symptoms, it cannot further assess quantitative cognitive functions such as executive function, reasoning, attention, verbal-visual learning, and working memory. In future studies, we should select corresponding assessment tools to test the relationship between research CBGT and cognitive impairment. Thirdly, only the duration of medication was considered when evaluating medication adherence. We need to include other relevant factors (such as disease severity, medication duration and dosing) as measurements to effectively evaluate adherence in patients taking medication regularly in outpatient clinics. Fourthly, this study is a cross-sectional study and suffers from the general limitation of clinician and patient blinding settings that cannot be overcome by CBGT studies, which may lead to a high risk of bias in the assessment results. Further measurements of cognitive function, duration of illness, dosing of medication and other relevant factors are needed in the future to help support the clinical evidence.
In conclusion, our data support the view that CBGT combined with mental health education improves clinical symptoms and psychosocial rehabilitation outcomes in people with schizophrenia in the community. Good medication adherence lays the foundation for psychosocial recovery. The degree of family involvement in patient medication supervision has a significant impact on patient psychiatric recovery. Support systems such as family support, peer support and social support are important positive factors for community rehabilitation. Patients can gain social experiences in group-based activity exchanges, gain emotional support and recognition through mutual imitation and learning exchanges, enhance resilience and thus rebuild rational beliefs and strategies, and it also provides some guidance on the development of community rehabilitation models in patients with mental illness.
Cognitive behavioral therapy is an evidence-based adjunctive intervention for schizophrenia and has shown benefits.
Whether the combination therapy of cognitive behavioral group therapy (CBGT) and mental health education shows superior benefits to mental health education alone in the management of schizophrenia?
This study aimed to compare the efficacy of CBGT combined with mental health single mental health education and mental health education alone for schizophrenia.
A total of 120 patients with schizophrenia were enrolled and allocated to an intervention group (n = 60) and a control group (n = 60). Patients in the intervention group received CBGT therapy which was added as an adjunctive intervention to antipsychotics and mental health education. Patients in the control group only received antipsychotic drugs and mental health. The cycle of treatment is 8 wk. After a follow-up of four weeks, score for positive and negative symptom scale, social function, activities of daily living and medication compliance were measured.
It showed that 8 wk of CBGT combined with mental health education significantly alleviated symptoms and cognitive dysfunction and improved social functioning although it did not have a significant impact on medication adherence in patients with schizophrenia after 4 wk of follow-up.
The results of this study indicate that the combination therapy of CBGT and mental health education is a promising addition to antipsychotics.
To attain robust results, further large-scale studies should be conducted with the measurements of cognitive function, duration of illness, dosing of medication and other relevant factors are considered to support the clinical evidence.
| 1. | Turner DT, van der Gaag M, Karyotaki E, Cuijpers P. Psychological interventions for psychosis: a meta-analysis of comparative outcome studies. Am J Psychiatry. 2014;171:523-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 206] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 2. | Bechdolf A, Köhn D, Knost B, Pukrop R, Klosterkötter J. A randomized comparison of group cognitive-behavioural therapy and group psychoeducation in acute patients with schizophrenia: outcome at 24 months. Acta Psychiatr Scand. 2005;112:173-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 3. | Vesterager L, Christensen TØ, Olsen BB, Krarup G, Forchhammer HB, Melau M, Gluud C, Nordentoft M. Cognitive training plus a comprehensive psychosocial programme (OPUS) versus the comprehensive psychosocial programme alone for patients with first-episode schizophrenia (the NEUROCOM trial): a study protocol for a centrally randomised, observer-blinded multi-centre clinical trial. Trials. 2011;12:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 4. | Jones C, Hacker D, Cormac I, Meaden A, Irving CB. Cognitive behaviour therapy versus other psychosocial treatments for schizophrenia. Cochrane Database Syst Rev. 2012;4:CD008712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 5. | Bucci P, Piegari G, Mucci A, Merlotti E, Chieffi M, De Riso F, De Angelis M, Di Munzio W, Galderisi S. Neurocognitive individualized training versus social skills individualized training: a randomized trial in patients with schizophrenia. Schizophr Res. 2013;150:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Grant PM, Huh GA, Perivoliotis D, Stolar NM, Beck AT. Randomized trial to evaluate the efficacy of cognitive therapy for low-functioning patients with schizophrenia. Arch Gen Psychiatry. 2012;69:121-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 225] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 7. | Hazell CM, Hayward M, Cavanagh K, Strauss C. A systematic review and meta-analysis of low intensity CBT for psychosis. Clin Psychol Rev. 2016;45:183-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 8. | Jauhar S, McKenna PJ, Radua J, Fung E, Salvador R, Laws KR. Cognitive-behavioural therapy for the symptoms of schizophrenia: systematic review and meta-analysis with examination of potential bias. Br J Psychiatry. 2014;204:20-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 334] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 9. | Opoka SM, Lincoln TM. The Effect of Cognitive Behavioral Interventions on Depression and Anxiety Symptoms in Patients with Schizophrenia Spectrum Disorders: A Systematic Review. Psychiatr Clin North Am. 2017;40:641-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Müller H, Kommescher M, Güttgemanns J, Wessels H, Walger P, Lehmkuhl G, Kuhr K, Hamacher S, Lehmacher W, Müller K, Herrlich J, Wiedemann G, Stösser D, Klingberg S, Bechdolf A. Cognitive behavioral therapy in adolescents with early-onset psychosis: a randomized controlled pilot study. Eur Child Adolesc Psychiatry. 2020;29:1011-1022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Mortan Sevi O, Tekinsav Sutcu S, Yesilyurt S, Turan Eroglu S, Gunes B. Comparison of the Effectiveness of Two Cognitive-Behavioral Group Therapy Programs for Schizophrenia: Results of a Short-Term Randomized Control Trial. Community Ment Health J. 2020;56:222-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Turner DT, McGlanaghy E, Cuijpers P, van der Gaag M, Karyotaki E, MacBeth A. A Meta-Analysis of Social Skills Training and Related Interventions for Psychosis. Schizophr Bull. 2018;44:475-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 134] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 13. | Nijman SA, Veling W, van der Stouwe ECD, Pijnenborg GHM. Social Cognition Training for People With a Psychotic Disorder: A Network Meta-analysis. Schizophr Bull. 2020;46:1086-1103. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 60] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 14. | Cella M, Preti A, Edwards C, Dow T, Wykes T. Cognitive remediation for negative symptoms of schizophrenia: A network meta-analysis. Clin Psychol Rev. 2017;52:43-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 153] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 15. | Hyun MS, Nam KA, Kim MA. Randomized controlled trial of a cognitive-behavioral therapy for at-risk Korean male adolescents. Arch Psychiatr Nurs. 2010;24:202-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Lipskaya L, Jarus T, Kotler M. Influence of cognition and symptoms of schizophrenia on IADL performance. Scand J Occup Ther. 2011;18:180-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Drury V, Birchwood M, Cochrane R. Cognitive therapy and recovery from acute psychosis: a controlled trial. 3. Five-year follow-up. Br J Psychiatry. 2000;177:8-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 62] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 18. | Garety PA, Fowler DG, Freeman D, Bebbington P, Dunn G, Kuipers E. Cognitive--behavioural therapy and family intervention for relapse prevention and symptom reduction in psychosis: randomised controlled trial. Br J Psychiatry. 2008;192:412-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 177] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 19. | Haddock G, Tarrier N, Morrison AP, Hopkins R, Drake R, Lewis S. A pilot study evaluating the effectiveness of individual inpatient cognitive-behavioural therapy in early psychosis. Soc Psychiatry Psychiatr Epidemiol. 1999;34:254-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 93] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Barch DM, Ceaser A. Cognition in schizophrenia: core psychological and neural mechanisms. Trends Cogn Sci. 2012;16:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 541] [Cited by in RCA: 557] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 21. | Kimoto S, Makinodan M, Kishimoto T. Neurobiology and treatment of social cognition in schizophrenia: Bridging the bed-bench gap. Neurobiol Dis. 2019;131:104315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 22. | Lencz T, Smith CW, McLaughlin D, Auther A, Nakayama E, Hovey L, Cornblatt BA. Generalized and specific neurocognitive deficits in prodromal schizophrenia. Biol Psychiatry. 2006;59:863-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 286] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 23. | Wood SJ, Pantelis C, Velakoulis D, Yücel M, Fornito A, McGorry PD. Progressive changes in the development toward schizophrenia: studies in subjects at increased symptomatic risk. Schizophr Bull. 2008;34:322-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 136] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 24. | Robison AJ, Thakkar KN, Diwadkar VA. Cognition and Reward Circuits in Schizophrenia: Synergistic, Not Separate. Biol Psychiatry. 2020;87:204-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 71] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 25. | Sheffield JM, Barch DM. Cognition and resting-state functional connectivity in schizophrenia. Neurosci Biobehav Rev. 2016;61:108-120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 306] [Cited by in RCA: 283] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 26. | Guo JY, Ragland JD, Carter CS. Memory and cognition in schizophrenia. Mol Psychiatry. 2019;24:633-642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 186] [Article Influence: 26.6] [Reference Citation Analysis (0)] |
| 27. | Thomas EHX, Bozaoglu K, Rossell SL, Gurvich C. The influence of the glutamatergic system on cognition in schizophrenia: A systematic review. Neurosci Biobehav Rev. 2017;77:369-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 28. | Lewis DA, Cruz DA, Melchitzky DS, Pierri JN. Lamina-specific deficits in parvalbumin-immunoreactive varicosities in the prefrontal cortex of subjects with schizophrenia: evidence for fewer projections from the thalamus. Am J Psychiatry. 2001;158:1411-1422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 142] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 29. | Bègue I, Kaiser S, Kirschner M. Pathophysiology of negative symptom dimensions of schizophrenia - Current developments and implications for treatment. Neurosci Biobehav Rev. 2020;116:74-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Demmin DL, United States; Matvienko-Sikar K, Ireland S-Editor: Yan JP L-Editor: A P-Editor: Ji MX