Published online Jun 19, 2023. doi: 10.5498/wjp.v13.i6.340
Peer-review started: April 14, 2023
First decision: April 26, 2023
Revised: May 5, 2023
Accepted: May 22, 2023
Article in press: May 22, 2023
Published online: June 19, 2023
Processing time: 66 Days and 0 Hours
Insomnia is a disease where individuals cannot maintain a steady and stable sleep state or fail to fall asleep. Western medicine mainly uses sedatives and hypnotic drugs to treat insomnia, and long-term use is prone to drug resistance and other adverse reactions. Acupuncture has a good curative effect and unique advantages in the treatment of insomnia.
To explore the molecular mechanism of acupuncture at Back-Shu point for the treatment of insomnia.
We first prepared a rat model of insomnia, and then carried out acupuncture for 7 consecutive days. After treatment, the sleep time and general behavior of the rats were determined. The Morris water maze test was used to assess the learning ability and spatial memory ability of the rats. The expression levels of inflammatory cytokines in serum and the hippocampus were detected by ELISA. qRT-PCR was used to detect the mRNA expression changes in the ERK/NF-κB signaling pathway. Western blot and immunohistochemistry were carried out to evaluate the protein expression levels of RAF-1, MEK-2, ERK1/2 and NF-κB.
Acupuncture can prolong sleep duration, and improve mental state, activity, diet volume, learning ability and spatial memory. In addition, acupuncture increased the release of 1L-1β, 1L-6 and TNF-α in serum and the hippocampus and inhibited the mRNA and protein expression of the ERK/NF-κB signaling pathway.
These findings suggest that acupuncture at Back-Shu point can inhibit the ERK/NF-κB signaling pathway and treat insomnia by increasing the release of inflammatory cytokines in the hippo-campus.
Core Tip: In this study, insomnia was a condition that was unable to maintain a stable sleep state or to sleep. Western medicine mainly uses sedative and hypnotic drugs to treat insomnia, which easy to produce drug resistance and some adverse reactions. Acupuncture has excellent efficacy and unique advantages in the treatment of insomnia. This study aimed to investigate the molecular mechanism of Back-Shu acupuncture for insomnia.
- Citation: Zhang MM, Zhao JW, Li ZQ, Shao J, Gao XY. Acupuncture at Back-Shu point improves insomnia by reducing inflammation and inhibiting the ERK/NF-κB signaling pathway. World J Psychiatry 2023; 13(6): 340-350
- URL: https://www.wjgnet.com/2220-3206/full/v13/i6/340.htm
- DOI: https://dx.doi.org/10.5498/wjp.v13.i6.340
Insomnia is a common clinical disease. Sleeping difficulty and short sleep time are typical clinical symptoms of insomnia. Insomnia not only seriously affects patients' daily activities and work, but also leads to a reduction in their living standards. Some patients also have anxiety, depression, and other negative psychological emotions. Long-term sleep disorders can easily induce immunity and memory loss, increase the risk of cardiovascular and cerebrovascular diseases, and threaten the life and health of patients[1]. At present, sedatives, hypnotics, antidepressants, and other psychiatric drugs are mainly used to treat insomnia. However, high drug dependence and a high incidence of adverse reactions limit their clinical application[2]. Traditional Chinese medicine believes that insomnia is a type of restlessness caused by liver qi stagnation and spleen damage. Traditional Chinese medicine prescriptions and external treatment with traditional Chinese medicine have remarkable effects on this disease[3].
The pathogenesis of insomnia is still unclear and may be related to the dysfunction of neurotransmitters, inflammatory cytokines, neuroendocrine factors, melatonin, signal transduction pathways, neurotrophic factors, and intestinal flora in the brain. The ERK pathway belongs to the MAPK family and mediates various physiological and pathological processes such as cell proliferation, differentiation, apoptosis, oxidative stress, and immune inflammation. It plays an essential regulatory role in tumor pathogenesis, various inflammatory diseases, mental disorders, insomnia, and other diseases[4,5]. The ERK signaling pathway can be activated after receiving stimulation signals, which activates the downstream transcription factor NF-κB, affects the secretion of inflammatory cytokines [interleukin (1L)-1β, 1L-6, tumor necrosis factor (TNF)-α], and induces functional changes, thereby leading to insomnia and increasing the risk of chronic inflammatory diseases[6-8]. Abnormal activation of the ERK signaling pathway can also aggravate apoptosis and inflammatory injury in cardiomyocytes, leading to cardiac diseases. The administration of corresponding inhibitors can enhance myocardial reoxygenation capacity, inhibit apoptosis and the inflammatory response, reduce myocardial ischemia, and promote the recovery of cardiomyocyte function[9,10]. Clinical studies have found that acupuncture at Back-Shu point has an excellent therapeutic effect on insomnia, effectively improving depression, anxiety, memory loss, immunity decline and other clinical symptoms caused by insomnia[11]. Experimental studies have confirmed that the pathogenesis of insomnia is closely related to the ERK/NF-κB signaling pathway[12]. We hypothesize that inhibition of ERK/NF-κB signaling pathway activation may be a potential target in the treatment of insomnia.
In this study, a rat model of insomnia was established by intraperitoneal injection of p-chloro
Forty male specific pathogen free (SPF) Sprague Dawley (SD) rats aged 12 wk weighing 200 ± 20 g were selected and were given water and food ad libitum. The rats, purchased from Henan Laboratory Animal Center, were housed at a room temperature of 20-24 °C, and relative humidity of 40%-60%. The 40 rats were randomly divided into 4 groups: Control group (Con), model group (Model), acupuncture group (Acupuncture), and U0126 group (U0126), with 10 rats in each group. Rats in the Model group were continuously injected with PCPA suspension (1 mL/kg) by intraperitoneal injection. Rats in the Con group were injected with the same amount of normal saline. Rats in the Acupuncture group received acupuncture 7 d in advance, which was performed continuously for 7 d. Rats in the U0126 group were injected with U0126 (0.2 mg/kg/d) (Alfa Chemical, Zhengzhou, Henan, China) via the tail vein 7 d in advance, once a day.
Ultrapure water was heated to between 30-40 °C, sodium bicarbonate powder (Tianjin Hengxing Chemical Reagent, Tianjin, China) was slowly added to prepare a 5% NaHCO3 aqueous solution, and 0.9% physiological saline was added to dilute to a weak alkaline solution (pH 7-8). PCPA was then added and sonicated for 10 h to obtain a PCPA suspension.
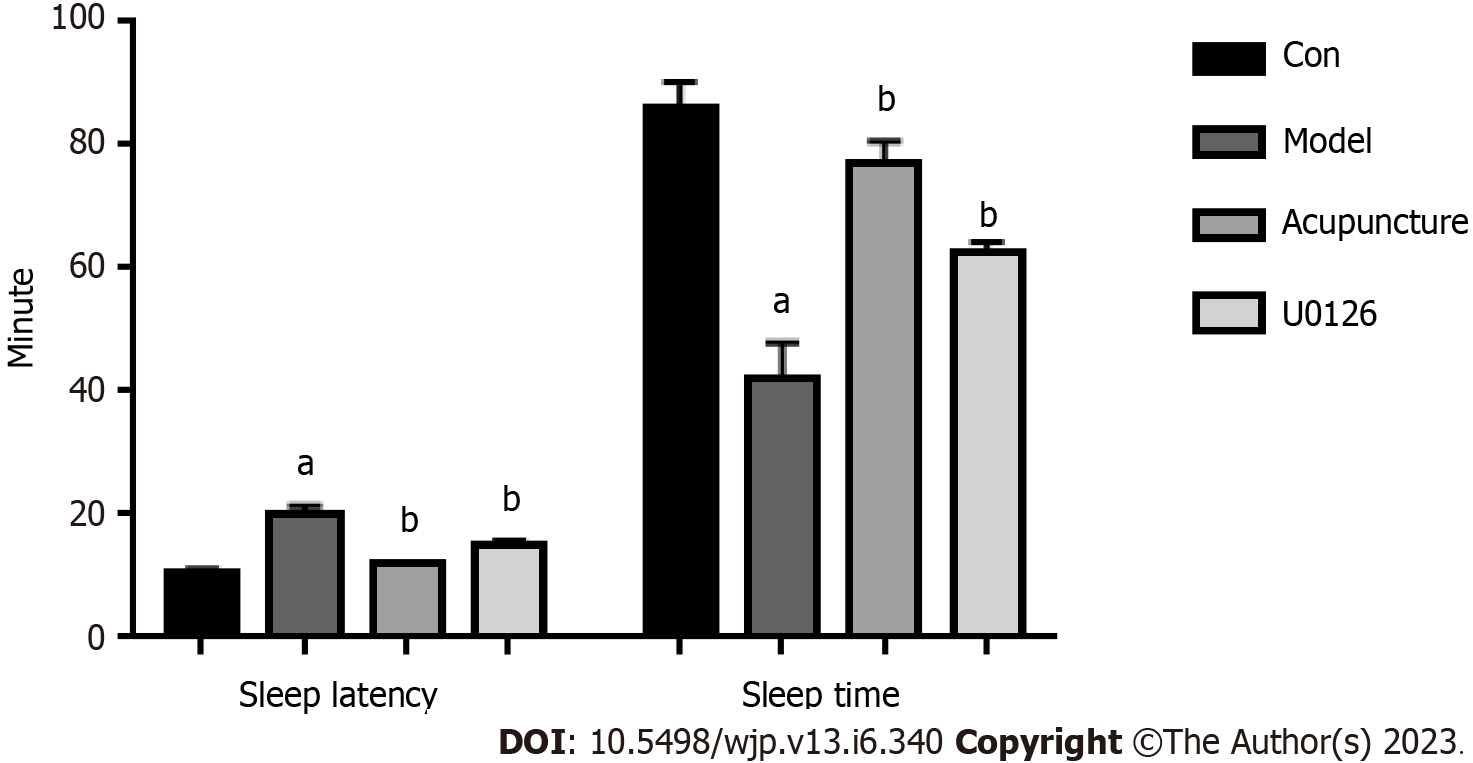
Twelve hours after PCPA suspension injection on day 3, three rats were randomly selected from the Con and Model groups to receive pentobarbital sodium (45 mg/kg). The rats were placed in the supine position on the experimental table to observe the righting reflex. The disappearance of the righting reflex for 1 min was considered the sleep latency, the time to recovery was the sleep time, and the rats varus 3 times within 30 s was the end index. The sleep latency and sleep time of rats were recorded, respectively.
The acupoints on both sides were "Xin Yu," "Gan Yu," "PI Yu," "Fei Yu," and "Shen Yu." Xin Yu: The 5th thoracic vertebra on the lower intercostal side; Pi Yu: The 12th thoracic vertebra on the lower intercostal side; Gan Yu: The spinous process of the 9th thoracic vertebra is opened 3 mm below; Fei Yu: The 3rd thoracic vertebra on the lower intercostal side; Shen Yu: The lower side of the 2nd lumbar spine. After disinfection, the operator held a millimeter needle (0.25 mm × 15 mm) in the right hand, which was quickly inserted into the acupoint to 6 mm. The needle was retained for 10 min, and then removed. Acupuncture began at 9:00 a.m. every day for 7 consecutive days.
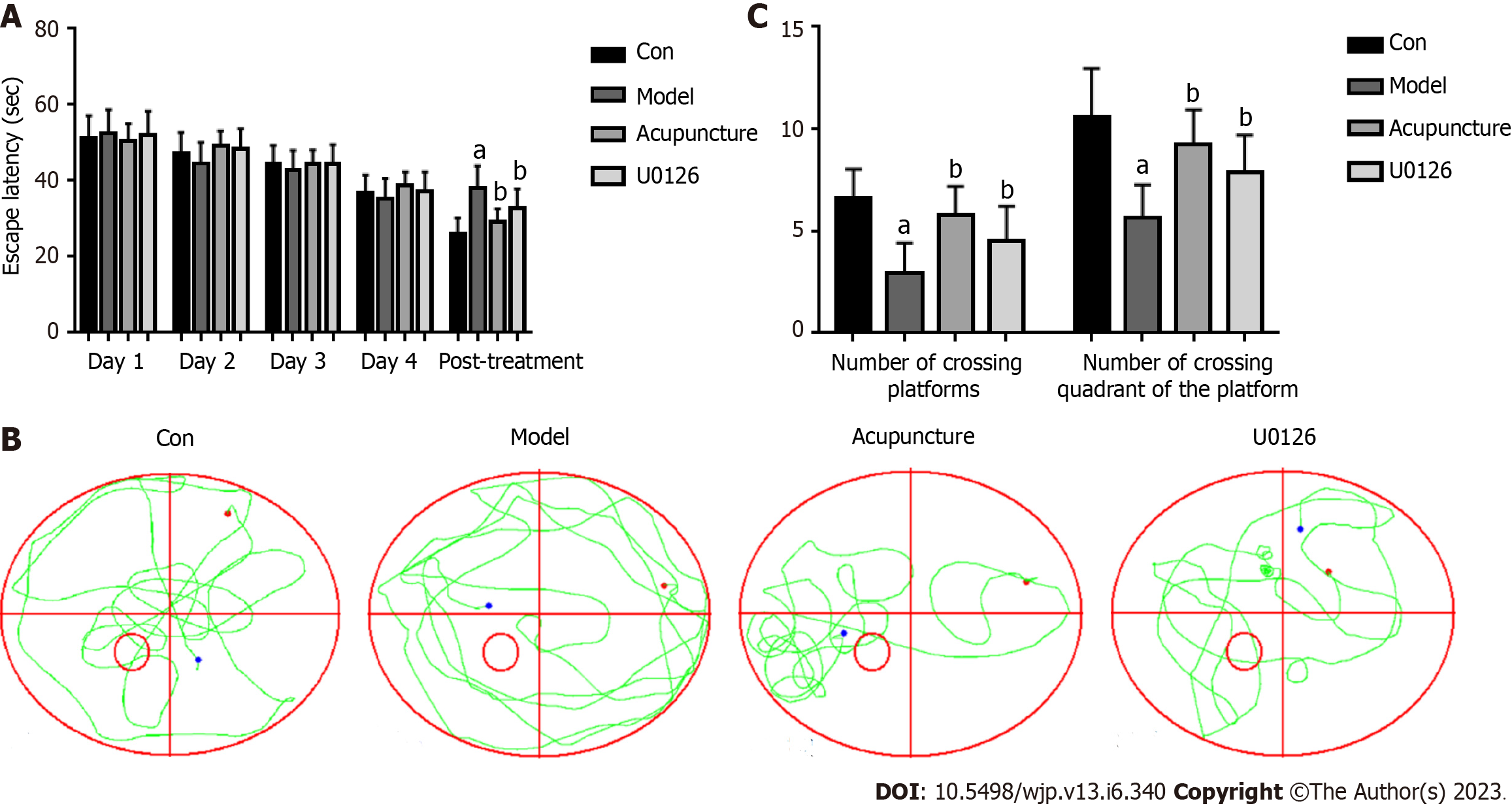
The rats in each group were placed into the water maze for adaptive training 1 d before the experiment. The positioning navigation experiment was used to train the learning and memory ability of the rats. The rats were placed in quadrants I, II, III, and IV daily. The test time for each quadrant was 70 s. The escape latency period was the time from placed in the water to finding the landing platform. If the platform was not found within the specified time, the animal was actively placed on the platform for 15 s to induce learning and memory ability. The SuperMaze software recorded the movement trajectory and escape latency using a camera. The spatial exploration experiment was used to evaluate the spatial memory ability of the rats. The rats were placed in the water from the contralateral side of quadrant III and allowed to freely explore for 60 s. The SuperMaze software recorded the movement trajectory of the rats using a camera.
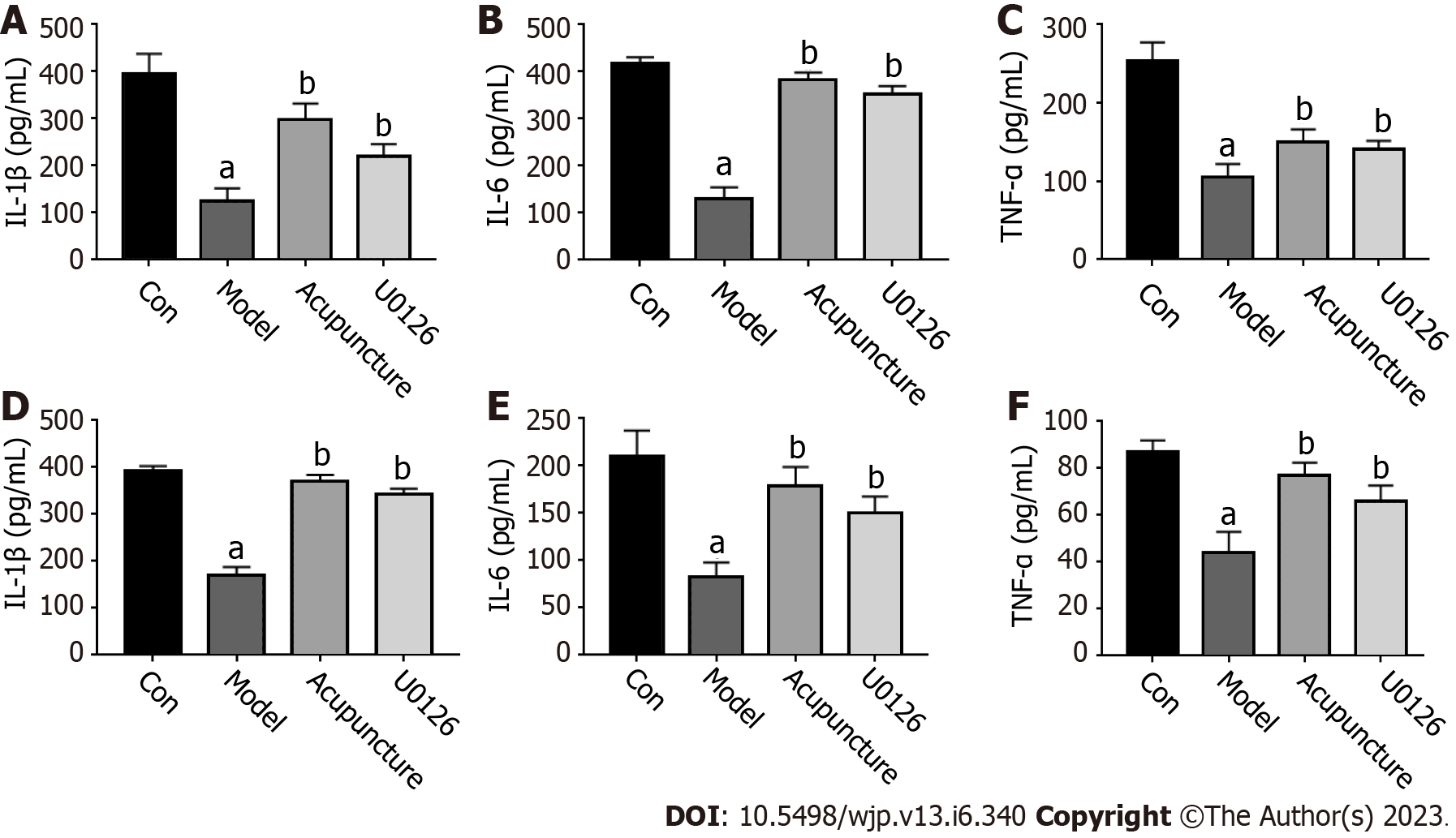
After the last behavioral experiment, the rats were anesthetized by intraperitoneal injection of 10% chloral hydrate (30 mg/kg), placed supine on the rat board, blood was collected from the abdominal aorta, the animal was decapitated, and the hippocampus was isolated, homogenized, centrifuged, and the supernatant obtained. The expression levels of IL-1β, IL-6 and TNF-α in rat serum and the hippocampus were detected by ELISA according to the manufacturer’s protocol (Multisciences, Hangzhou, Zhejiang, China). The absorption was measured at the wavelength of 450 nm.
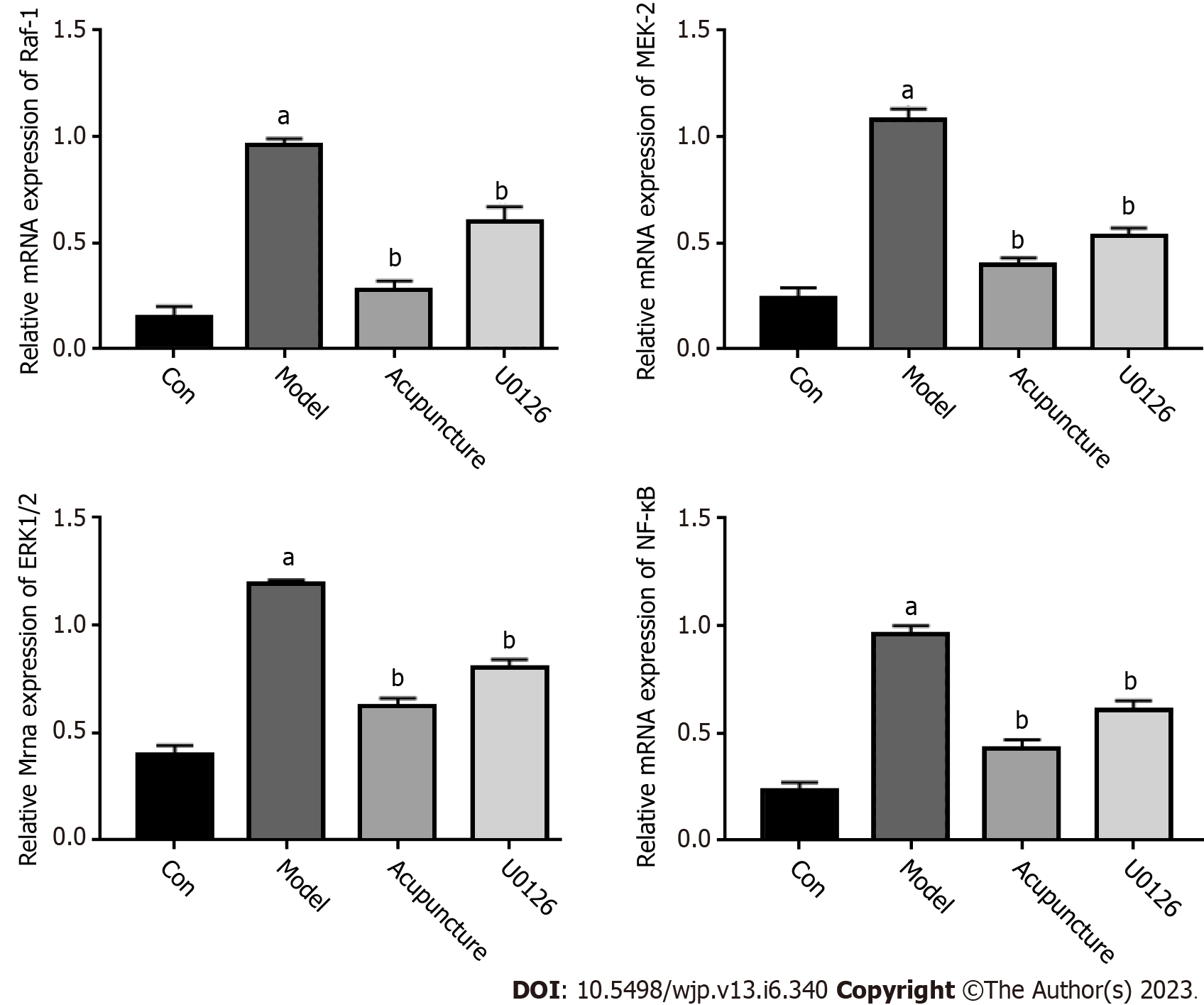
Total RNA in the hippocampus was extracted with Trizol reagent (Servicebio, Wuhan, Hubei, China). RNA concentration and purity were detected using a Nanodrop 2000 spectrophotometer. 1 μg RNA was reverse transcribed into cDNA according to the manufacturer’s protocol (Servicebio, Wuhan, Hubei, China). The obtained cDNA was used as the template in qRT-PCR according to the instructions. The primer sequences were as follows:
GAPDH: F: CTGGAGAAACCTGCCAAGTATG; R: GGTGGAAGAATGGGAGTTGCT; Raf1: F: TGTTTGATGGCTCCAGTTGC; R: AGCGTGCTTTCTTACCTTTGTG; MEK2: F: TGAATGAGCCACCTCCCAAG; R: ATAGCCAGCCAGCGAAGTCC; ERK1/2: F: TTCAGGACCTCATGGAGACGG; R: GCCACATACTCGGTCAGAAAGC; NF-κB: F: GGGACTATGACTTGAATGCGG; R: CAGCCAGGTCCCGTGAAATA.
Total protein in the hippocampus was extracted for Western blot detection. The protein samples were mixed with RAF-1, MEK-2, ERK1/2, NF-κB, and β-actin as an internal reference (all obtained from Servicebio, Wuhan, Hubei, China).
The paraffin sections were deparaffinized for antigen retrieval, washed three times with phosphate buffer saline (PBS), 5 min each time, and then serum-blocked and then added to primary antibody RAF-1 (1:200), primary antibody MEK-2 (1:200), primary antibody ERK1/2 (1:200), primary antibody NF-κB (1:200), and incubated overnight at 4 °C. The sections were washed 3 times with PBS, 5 min each time, diluted secondary antibody (1:1000) was added, incubated at room temperature for 50 min, washed 3 times with PBS, 5 min each time, DAB color developing solution (Servicebio, Wuhan, Hubei, China) was added dropwise, hematoxylin was used as the counterstain and returned to blue, then dehydrated and made transparent. After drying, the sections were mounted and observed under the microscope.
SPSS 24.0 software was used for statistical analysis of the experimental data, measurement data were expressed as mean ± SD, and the t-test (comparison between two groups) or LSD analysis of variance was used when normal distribution and homogeneity of variance were satisfied. For comparisons between multiple groups, the Dunnett's-T3 test was used to compare uneven variance, and P < 0.05 was considered statistically significant.
PCPA was injected intraperitoneally in rats to construct an insomnia model. Compared with the Con group, the sleep latency of rats in the Model group was prolonged and the sleep time was shortened, indicating that the insomnia rat model was successfully established (Figure 1). PCPA can cause general status and behavioral changes in rats[13]. Rats in the Model group showed a poor mental state, fighting and biting each other, with reduced food intake, increased drinking water, dull and damp hair, and poor hair glossiness. After acupuncture treatment, aggressive behavior in rats decreased, food intake increased, and water intake decreased (Table 1). Compared with rats in the Model group, the sleep latency of the Acupuncture group and the U0126 group was shortened and the sleep time was prolonged (Figure 1). In conclusion, acupuncture at Back-Shu acupoints improved cognitive behavioral changes and emotional disturbances caused by insomnia.
| Group | Dietary volume | Drinking volume | Mental state | Hair glossiness | Aggressive behavior | Sensitivity |
| Con | ||||||
| Model | - - - | + + + | - - - | - - - | + + + | - - - |
| Acupuncture | - | + | - | - - | + | - |
| U0126 | - - | + + | - | - - | + | - |
We carried out location experiments to evaluate the memory ability of rats. With the increase in training times, the average escape latency of rats in each group gradually shortened, showing that rats with increased training, remembered the platform. However, the trend in average escape latency in rats was basically the same. After treatment, compared with the Con group, the escape latency of the Model group was prolonged. Compared with the Model group, the escape latency of rats in the Acupuncture group and U0126 group was shortened (Figure 2A). The spatial exploration experiment was used to evaluate the spatial memory ability of rats. The movement trajectories of rats in the Model group showed that the movement trajectories of rats in the four quadrants were basically the same. In contrast, the trajectories of rats in the Con group and the Acupuncture group were primarily concentrated in quadrant Ⅲ (Figure 2B). Compared with the Con group, the time taken to cross the original platform and the quadrants of the original platform were significantly reduced in the Model group. Compared with the Model group, the number of large crossings of the original platform and the quadrant of the original platform increased in the Acupuncture group and the U0126 group (Figure 2C). These results indicated that acupuncture treatment improved the learning and memory ability of rats and promoted the recovery of learning and memory function.
Inflammatory cytokines act as mediating signals between the sleep-wake cycle and the immune system. Abnormal secretion of inflammatory cytokines can cause sleep-wake cycle disorder and lead to insomnia. As shown in Figure 3A-C, compared with the Con group, the secretion of inflammatory cytokines (IL-1β, IL-6 and TNF-α) in serum of the Model group was decreased. After acupuncture and U0126 treatment, the secretion of IL-1β, IL-6, and TNF-α in the serum of rats were significantly increased. In the rat hippocampus, we found the same trend in inflammatory cytokine secretion (Figure 3D-F).
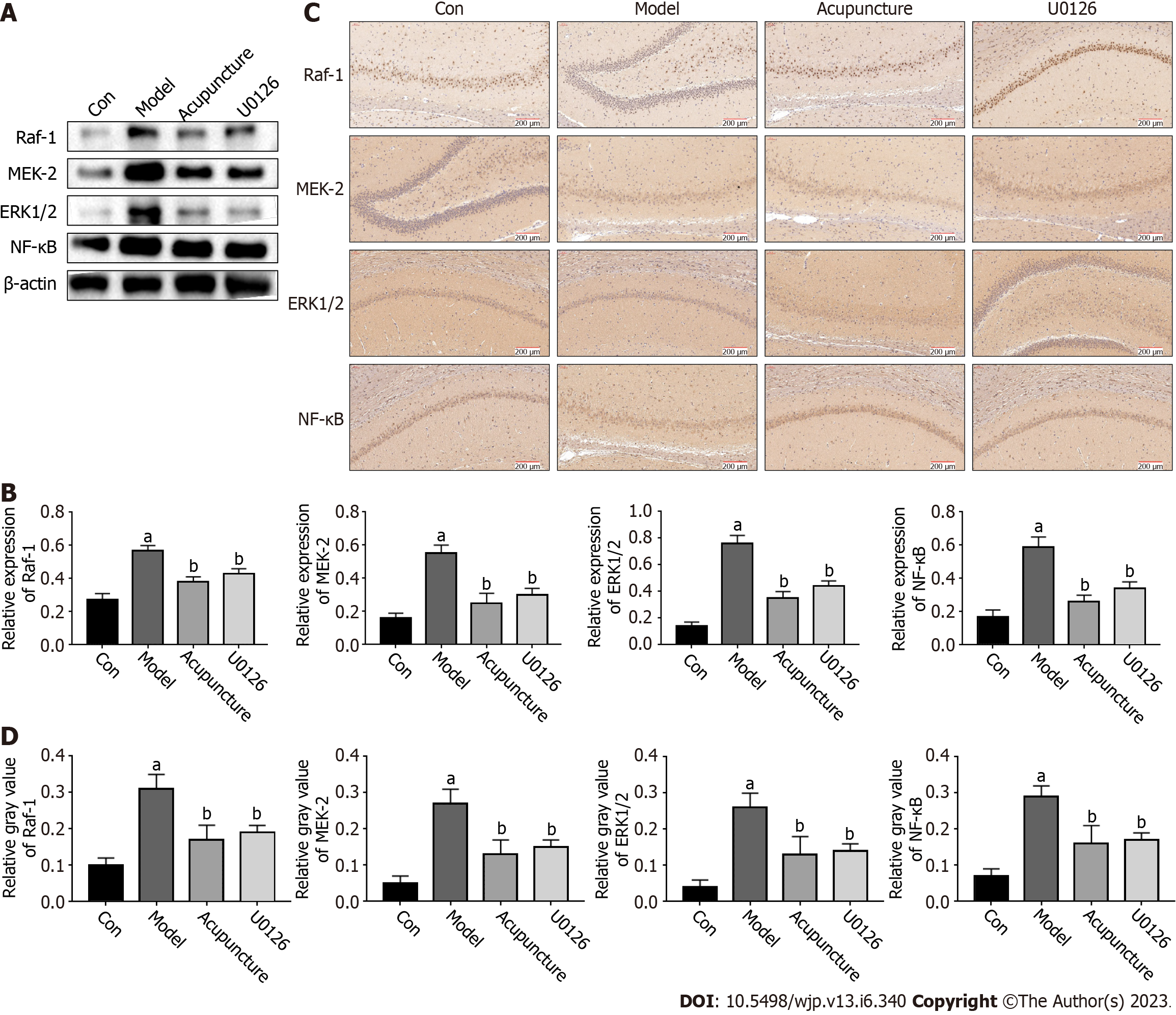
The ERK/NF-κB signaling pathway is closely related to the body's immune system. It plays an important role in the synaptic plasticity of neurons in brain regions, which mainly regulates learning and memory processes. U0126 is a highly potent inhibitor of ERK1/2, MEK-1, and MEK-2. The qRT-PCR results showed that compared with the Con group, the mRNA expression levels of RAF-1, MEK-2, ERK1/2 and NF-κB in the hippocampus of the Model group were increased. In addition, acupuncture and U0126 significantly inhibited the expression of these genes (Figure 4). The protein expression levels of the ERK/NF-κB signaling pathway related genes were detected by Western blot and immunohistochemistry, and the results were consistent with the trend in mRNA expression (Figure 5). In conclusion, we believe that acupuncture at Back-Shu point can improve insomnia in rats by inhibiting the ERK/NF-κB signaling pathway.
Insomnia is a common disease that not only reduces patients' life quality, but also causes other physical diseases, significantly impacting patients' lives[14]. Due to their reproductive ability, easy feeding, easy modeling and experimental operation, and similar sleep-wake cycle and biological regulatory function to humans, rats were selected to construct the insomnia model in this study. PCPA can inhibit sleep and affect the expression of serotonin (5-HT). As an inhibitor of tryptophan hydroxylase, an essential enzyme in the process of 5-HT biosynthesis, PCPA can reduce the concentration of 5-HT in the brain and serum by inhibiting it, thus causing disorder of the sleep-wake cycle[15]. After intraperitoneal injection of PCPA in rats for three consecutive days, the rats showed circadian rhythm disorder, frequent daytime activity, wet and dark hair, increased aggression during stimulation, and mutual fighting and biting behaviors, which lasted for one week, which was consistent with the clinical symptoms caused by insomnia, indicating that the insomnia model was successfully established.
In traditional Chinese medicine, insomnia belongs to the category of "sleepless" and the Back-Shu point is the acupoint on the bladder meridian, which is closely related to the brain. Currently, the five acupoints most commonly used in treating insomnia by acupuncture at Back-Shu point are Xin Yu, Gan Yu, PI Yu, Fei Yu, and Shen Yu[16]. This study found that continuous application of acupuncture at Back-Shu point for seven days can promote appetite, prolong sleep time and improve the mental state, activity, and excessive behavior. The results of the Morris water maze test showed that compared with the Model group, the ability of positioning navigation and space exploration of rats in the Acupuncture group and U0126 group improved to varying degrees, the latency in seeking platform escape was shortened, and the number of swims across the platform was increased. In conclusion, the results show that acupuncture at the Back-Shu point can improve the cognitive behavior changes and emotional disorders caused by insomnia, improve learning and memory ability in rats, and promote learning and memory function recovery.
The sleep-wake cycle is bidirectional in the immune system. Activation of the immune system will change the sleep state, which can lead to increased sleep duration, and lead to the occurrence of insomnia[17]. Cytokines in the sleep-wake cycle and immune system mediate between signals, and through the receptor act on the peripheral nervous system or the central nervous system neurons, astrocytes and microglia, through afferent nerve fibers and blood to the brain signals, cause inflammation and then insomnia[18]. In recent years, the role of inflammatory cytokines in maintaining the NREM sleep cycle has been extensively studied in animal experiments. Anti-inflammatory factors (IL-4, IL-10) can reduce the duration of NREM sleep, and proinflammatory factors (1L-1β, IL-2, IL-6, TNF-α) can promote the duration and depth of NREM sleep[19-21]. The results of the present study showed that compared with the Con group, the proinflammatory cytokines, IL-6, 1L-1β and TNF-α in the Model group rats' serum and hippocampus were significantly decreased (P < 0.01), suggesting that insomnia can result in a proinflammatory cytokine secretion imbalance, affect the body's immune system, resulting in sleep cycle disorder, consistent with previous research results. Compared with the Model group, the secretion of IL-1β, IL-6 and TNF-α in the Acupuncture group and U0126 group were significantly increased, suggesting that acupuncture at Back-Shu point may participate in the regulation of sleep and the immune system, improve the secretion of proinflammatory cytokines, maintain the balance of cytokines, and promote recovery of the body's immune system and the improvement of insomnia.
The MAPK signaling family is divided into the ERK, JNK, ERK5/BMK1 and P38 MAPK signaling pathways, of which the ERK-mediated MAPK signaling pathway is considered a classical MAPK signal transduction pathway[22]. The ERK signaling pathway transfers extracellular signals into cells through the RAF-MEK-ERK cascade effect and then regulates the expression of downstream key target NF-κB, which affects the occurrence of diseases. RAF-1, MEK-2, ERK1/2 and NF-κB are essential targets in the RAF-MEK-ERK-NF-κB pathway, which regulate cell proliferation, apoptosis, differentiation and other physiological activities, and participate in the pathogenesis and clinical symptoms of insomnia[23-25]. This study showed that compared with the Con group, the mRNA and protein expression levels of RAF-1, MEK-2, ERK1/2 and NF-κB in the hippocampus of the Model group were significantly increased (P < 0.01), and the number of positive cells increased, suggesting that the pathogenesis of insomnia may be related to activation of the ERK/NF-κB pathway. To further investigate whether acupuncture at the Back-Shu point improves insomnia by inhibiting the ERK/NF-κB signaling pathway, U0126 was injected into the tail vein of rats. U0126, a highly efficient pathway inhibitor that selects ERK1/2, MEK-1, MEK-2 and other families, can simultaneously inhibit ERK and MEK through a non-competitive pathway, have an impact on the RAF-MEK-ERK cascade reaction, further inhibit cell apoptosis, migration, invasion, and regulate the expression of related proteins[25,26]. Studies have shown that U0126 can effectively inhibit the proliferation, migration and apoptosis of cancer cells in vivo, and antioxidant protection of neuronal cells; thus, it is widely used in the treatment of nerve damage, sleep disorders, decreased learning and memory ability, and cancer caused by hypoxia and ischemia[27-29]. Compared with the Model group, the mRNA and protein expression levels of RAF-1, MEK-2, ERK1/2 and NF-κB in the Acupuncture group and U0126 group were significantly down-regulated (P < 0.01), suggesting that acupuncture at Back-Shu point and injection of U0126 inhibited activation of the ERK/NF-κB signaling pathway.
In summary, acupuncture at Back-Shu point can promote appetite, prolong sleep time, improve learning and memory ability, maintain the balance of proinflammatory cytokine secretion, and improve rats' mental state, activity, and excessive behavior. We believe that the pathogenesis of insomnia may be related to activation of the ERK/NF-κB signaling pathway. Acupuncture at Back-Shu point may inhibit conduction of the ERK/NF-κB signaling pathway, down-regulate the mRNA and protein expression of RAF-1, MEK-2, ERK1/2 and NF-κB in the pathway, increase the secretion levels of proinflammatory cytokines IL-1β, IL-6 and TNF-α, maintain the balance of cytokines, and protect nerve cells. Thus, acupuncture may promote the body's immune system recovery and play a role in the treatment of insomnia.
The characteristics of insomnia are dissatisfaction with sleep, including difficulty beginning or maintaining sleep, waking up with/or early morning, accompanied by related daytime damage, such as fatigue and emotional disorders. Western medicine mainly uses sedatives and hypnotic drugs to treat insomnia, and long-term use is prone to drug resistance and other adverse reactions. Acupuncture has a good curative effect and unique advantages in the treatment of insomnia.
To explore the molecular mechanism of acupuncture at Back-Shu point for insomnia.
To provides a new insight into the treatment of insomnia by acupuncture at Back-Shu point from the perspective of traditional Chinese medicine.
We first prepared a rat model of insomnia, and then carried out acupuncture for 7 consecutive days to explored the effect of acupuncture. The Morris water maze test was used to assess behavioral change. The detailed mechanism research was detected by RT-qPCR, ELISA, and Western blot.
Some Western drugs can improve insomnia symptoms, but their high drug dependence and adverse reactions limit their clinical application. Our study provides new insights into the treatment of insomnia symptoms from the perspective of traditional Chinese medicine. Our study fills a gap in the treatment of insomnia with acupuncture at Back-Shu point and provides a new treatment for insomnia.
This study suggest that acupuncture at the Back-Shu point can improve the insomnia by inhibiting the ERK/NF-κB signaling pathway and increasing the release of inflammatory cytokines in the hippo-campus. Acupuncture at the Back-Shu point has promoted the treatment of insomnia and benefited the public's sleep health.
This paper provides new ideas for the treatment of insomnia from the perspective of acupuncture.
| 1. | Li O, Wang F. [Acupuncture at back-shu points of five zang, Geshu (BL 17) and Shenmen (HT 7) for the treatment of menopausal insomnia]. Zhongguo Zhen Jiu. 2018;38:4693-4672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 2. | Riemann D, Baglioni C, Bassetti C, Bjorvatn B, Dolenc Groselj L, Ellis JG, Espie CA, Garcia-Borreguero D, Gjerstad M, Gonçalves M, Hertenstein E, Jansson-Fröjmark M, Jennum PJ, Leger D, Nissen C, Parrino L, Paunio T, Pevernagie D, Verbraecken J, Weeß HG, Wichniak A, Zavalko I, Arnardottir ES, Deleanu OC, Strazisar B, Zoetmulder M, Spiegelhalder K. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. 2017;26:675-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 823] [Cited by in RCA: 1290] [Article Influence: 143.3] [Reference Citation Analysis (0)] |
| 3. | Tang Y, Li Z, Yang D, Fang Y, Gao S, Liang S, Liu T. Research of insomnia on traditional Chinese medicine diagnosis and treatment based on machine learning. Chin Med. 2021;16:2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Acuff NV, Li X, Elmore J, Rada B, Watford WT. Tpl2 promotes neutrophil trafficking, oxidative burst, and bacterial killing. J Leukoc Biol. 2017;101:1325-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Du R, Wang JL, Wang YL. Role of RhoA/MERK1/ERK1/2/iNOS signaling in ocular ischemic syndrome. Graefes Arch Clin Exp Ophthalmol. 2016;254:2217-2226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Zhang Q, Liu L, Hu Y, Shen L, Li L, Wang Y. Kv1.3 Channel Is Involved In Ox-LDL-induced Macrophage Inflammation Via ERK/NF-κB signaling pathway. Arch Biochem Biophys. 2022;730:109394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (1)] |
| 7. | Kim SY, Han SD, Kim M, Mony TJ, Lee ES, Kim KM, Choi SH, Hong SH, Choi JW, Park SJ. Mentha arvensis Essential Oil Exerts Anti-Inflammatory in LPS-Stimulated Inflammatory Responses via Inhibition of ERK/NF-κB Signaling Pathway and Anti-Atopic Dermatitis-like Effects in 2,4-Dinitrochlorobezene-Induced BALB/c Mice. Antioxidants (Basel). 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 8. | Kim SO, Park JY, Jeon SY, Yang CH, Kim MR. Saikosaponin a, an active compound of Radix Bupleuri, attenuates inflammation in hypertrophied 3T3-L1 adipocytes via ERK/NF-κB signaling pathways. Int J Mol Med. 2015;35:1126-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Yan HJ, Qi GQ, Ma Y. Effect of propofol on myocardial ischemia-reperfusion injury through MAPK/ERK pathway. Eur Rev Med Pharmacol Sci. 2019;23:11051-11061. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 10. | Ye HK, Zhang HH, Tan ZM. MiR-328 inhibits cell apoptosis and improves cardiac function in rats with myocardial ischemia-reperfusion injury through MEK-ERK signaling pathway. Eur Rev Med Pharmacol Sci. 2020;24:3315-3321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 11. | Li JJ, Xie C, Zhao N, Wang C, Chen YF. [Regularity of Acupoint Selection in Treatment of Insomnia by Catgut Embedding Therapy]. Zhen Ci Yan Jiu. 2018;43:670-673. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 12. | Oh HK, Park SJ, Bae SG, Kim MJ, Jang JH, Ahn YJ, Woo H, Kwon G, Ryu JH. Kami-ondam-tang, a traditional herbal prescription, attenuates the prepulse inhibition deficits and cognitive impairments induced by MK-801 in mice. J Ethnopharmacol. 2013;146:600-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Lv YB, Zhou Q, Yan JX, Luo LS, Zhang JL. Enzymolysis peptides from Mauremys mutica plastron improve the disorder of neurotransmitter system and facilitate sleep-promoting in the PCPA-induced insomnia mice. J Ethnopharmacol. 2021;274:114047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 14. | Dopheide JA. Insomnia overview: epidemiology, pathophysiology, diagnosis and monitoring, and nonpharmacologic therapy. Am J Manag Care. 2020;26:S76-S84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 107] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 15. | Hong J, Chen J, Kan J, Liu M, Yang D. Effects of Acupuncture Treatment in Reducing Sleep Disorder and Gut Microbiota Alterations in PCPA-Induced Insomnia Mice. Evid Based Complement Alternat Med. 2020;2020:3626120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 16. | Chen Y, Zhao Y, Tan RY, Zhang PY, Long T, Shi Y, Zheng HB. The Influence of Stomach Back-Shu and Front-Mu Points on Insular Functional Connectivity in Functional Dyspepsia Rat Models. Evid Based Complement Alternat Med. 2021;2021:2771094. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Sgro M, Kodila ZN, Brady RD, Reichelt AC, Mychaisuk R, Yamakawa GR. Synchronizing our clocks as we age: the influence of the brain-gut-immune axis on the sleep-wake cycle across the lifespan. Sleep. 2022;45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | He S, Chen XX, Ge W, Yang S, Chen JT, Niu JW, Xia L, Chen GH. Are Anti-Inflammatory Cytokines Associated with Cognitive Impairment in Patients with Insomnia Comorbid with Depression? A Pilot Study. Nat Sci Sleep. 2021;13:989-1000. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Brager AJ, Ehlen JC, Castanon-Cervantes O, Natarajan D, Delisser P, Davidson AJ, Paul KN. Sleep loss and the inflammatory response in mice under chronic environmental circadian disruption. PLoS One. 2013;8:e63752. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Ballesteros-Zebadua P, Custodio V, Franco-Perez J, Rubio C, González E, Trejo C, Celis MA, Paz C. Whole-brain irradiation increases NREM sleep and hypothalamic expression of IL-1β in rats. Int J Radiat Biol. 2014;90:142-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Zielinski MR, Gerashchenko D, Karpova SA, Konanki V, McCarley RW, Sutterwala FS, Strecker RE, Basheer R. The NLRP3 inflammasome modulates sleep and NREM sleep delta power induced by spontaneous wakefulness, sleep deprivation and lipopolysaccharide. Brain Behav Immun. 2017;62:137-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 22. | Lee S, Rauch J, Kolch W. Targeting MAPK Signaling in Cancer: Mechanisms of Drug Resistance and Sensitivity. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 184] [Cited by in RCA: 567] [Article Influence: 94.5] [Reference Citation Analysis (0)] |
| 23. | Sun Y, Liu WZ, Liu T, Feng X, Yang N, Zhou HF. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J Recept Signal Transduct Res. 2015;35:600-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 836] [Cited by in RCA: 1368] [Article Influence: 124.4] [Reference Citation Analysis (0)] |
| 24. | Rai SN, Dilnashin H, Birla H, Singh SS, Zahra W, Rathore AS, Singh BK, Singh SP. The Role of PI3K/Akt and ERK in Neurodegenerative Disorders. Neurotox Res. 2019;35:775-795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 349] [Article Influence: 49.9] [Reference Citation Analysis (0)] |
| 25. | Lavoie H, Gagnon J, Therrien M. ERK signalling: a master regulator of cell behaviour, life and fate. Nat Rev Mol Cell Biol. 2020;21:607-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 797] [Article Influence: 132.8] [Reference Citation Analysis (0)] |
| 26. | Wang T, Wu J, Dong W, Wang M, Zhong X, Zhang W, Dai L, Xie Y, Liu Y, He X, Liu W, Madhusudhan T, Zeng H, Wang H. The MEK inhibitor U0126 ameliorates diabetic cardiomyopathy by restricting XBP1's phosphorylation dependent SUMOylation. Int J Biol Sci. 2021;17:2984-2999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 23] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 27. | Wang A, Zhang H, Liang Z, Xu K, Qiu W, Tian Y, Guo H, Jia J, Xing E, Chen R, Xiang Z, Liu J. U0126 attenuates ischemia/reperfusion-induced apoptosis and autophagy in myocardium through MEK/ERK/EGR-1 pathway. Eur J Pharmacol. 2016;788:280-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 57] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 28. | Ye H, Zhang Y, Wang Y, Xia J, Mao X, Yu X. The restraining effect of baicalein and U0126 on human cervical cancer cell line HeLa. Mol Med Rep. 2017;16:957-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 29. | Dumoulin MC, Aton SJ, Watson AJ, Renouard L, Coleman T, Frank MG. Extracellular signal-regulated kinase (ERK) activity during sleep consolidates cortical plasticity in vivo. Cereb Cortex. 2015;25:507-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Jing Shao National Famous, CZ0250-04.
Specialty type: Psychiatry
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Griffiths MD, United Kingdom; Hossain MS, United Kingdom S-Editor: Li L L-Editor: A P-Editor: Zhao S