Published online Dec 19, 2023. doi: 10.5498/wjp.v13.i12.1027
Peer-review started: September 12, 2023
First decision: September 25, 2023
Revised: October 20, 2023
Accepted: November 8, 2023
Article in press: November 8, 2023
Published online: December 19, 2023
Processing time: 98 Days and 4.2 Hours
Cerebral apoplexy patients are prone to cognitive impairment, and it is very important to choose appropriate treatment methods to improve their cognitive impairment after stroke.
To evaluate the effects of enhanced external counterpulsation (EECP) in con
In this retrospective study, data from 60 patients with poststroke cognitive impairment due to stroke who were treated in our hospital from February 2021 to July 2022 were analyzed and divided into a treatment group (n = 30) and a control group (n = 30) according to the different nursing methods applied. Patients in the treatment group received EECP in addition to atorvastatin, while those in the control group received atorvastatin alone. Mini-Mental State Examination (MMSE), Montreal Cognitive Assessment (MoCA) and activities of daily living (ADL) scale scores were compared between the two groups. Additionally, the two groups were compared in terms of serum acetylcholine (ACh), acetylcholinesterase (AChE), nitric oxide (NO), endothelin-1 (ET-1), β2-microglobulin (β2-MG), glial fibrillary acidic protein (GFAP), and visinin-like protein 1 (VILIP-1) in the serum. Blood flow measurements from the anterior cerebral artery (ACA), middle cerebral artery (MCA) and posterior cerebral artery (PCA) were compared between the two groups before and after treatment, and the pulsatility index (PI) and resistance index (RI) of each artery were determined.
MMSE, MoCA, and ADL scores all improved in both groups following treatment, with the study group showing more improvement than the control group (P < 0.05). After treatment, there were statistically significant increases in both ACh and NO levels, whereas decreases occurred in AChE, ET-1, β2-MG, VILIP-1, and GFAP, levels and the PI and RI of the left-ACA, right-ACA, left-MCA, right-MCA, left-PCA, and right-PCA. The study group showed greater gains in all metrics than the control group (P < 0.05).
EECP combined with atorvastatin is effective in the treatment of cognitive impairment after stroke and can effectively improve the cognitive function, neurotransmitter levels, and brain tissue damage status of patients.
Core Tip: Enhanced extracorporeal counterpulsation and atorvastatin are widely used in the treatment of stroke patients with cognitive impairment, but the effect of enhanced counterpulsation combined with atorvastatin on cognitive function of stroke patients with cognitive impairment has not been discussed. The objective of this study was to compare the efficacy of enhanced external counterpulsation combined with atorvastatin vs atorvastatin alone in the treatment of post-stroke cognitive impairment. Combined therapy is better than atorvastatin therapy alone.
- Citation: Duan Y, Tang HX. Efficacy of enhanced extracorporeal counterpulsation combined with atorvastatin in the treatment of cognitive impairment after stroke. World J Psychiatry 2023; 13(12): 1027-1036
- URL: https://www.wjgnet.com/2220-3206/full/v13/i12/1027.htm
- DOI: https://dx.doi.org/10.5498/wjp.v13.i12.1027
A patient’s quality of life is drastically altered after suffering a stroke, which is an acute cerebrovascular event that can result in brain ischemia, hypoxic damage, and neurological abnormalities as well as sequelae such as language dysfunction, limb malfunction, and cognitive impairment. Cognitive impairment after stroke is caused by degenerative diseases resulting from neural tissue ischemia. Therefore, patients with cognitive impairment following a stroke may achieve some improvement in their clinical symptoms with clinical therapy for nutritional neuropathy, improvement of microcirculation, and hemorheology, along with physical exercise[1,2]. Safe, effective, and noninvasive, enhanced external counterpulsation (EECP) is a mechanical circulatory aid method commonly employed in the treatment of coronary heart disease and angina pectoris. Multiple recent studies have demonstrated the efficacy of EECP in treating ischemic cerebrovascular disease, sleep disturbances, and psychological and psychiatric diseases[3,4]. The mechanism of EECP is to increase both the arterial and venous return of both lower limbs, increase coronary blood flow, and improve the perfusion of the heart, brain, kidneys, and other organs[5]. Statins, which are hydroxymethylglutaryl coenzyme A reductase inhibitors, can significantly boost patients’ cognitive performance and postpone disease progression[6] through their anti-inflammatory, antithrombotic, endothelium-protective, and antioxidant properties. The purpose of this research was to examine the effects of EECP in conjunction with atorvastatin on cognitive performance, neurotransmitter levels, and recovery from brain tissue damage in patients with poststroke cognitive impairment. The findings are detailed below.
Data from 60 patients with poststroke cognitive impairment due to stroke who were treated in our hospital from February 2021 to July 2022 were analyzed, and the patients were divided into a treatment group (n = 30) and a control group (n = 30) according to the different nursing methods applied. There were 21 men and 9 women in the study group, with ages ranging from 49 to 74 (mean = 61.40, SD = 5.59) years; the average duration since stroke onset was 6.30 ± 1.62 mo, and the average duration since cognitive impairment onset was 5.57 ± 1.14 mo. In the control group, the age ranged from 49 to 73 years, with a mean of 60.30 ± 5.84 years. There were 19 men and 11 women in the control group; the mean duration since stroke onset was 6.50 ± 1.28 mo, and the mean duration since the onset of cognitive impairment was 3.75 ± 0.78 mo. Overall, there was little to no difference in these data between the two groups (P > 0.05). All protocols in this study were approved by the ethics committee of the Shengjing Hospital of China Medical University and abided by the ethical guidelines of the Declaration of Helsinki. The ethics committee waived the requirement for informed consent.
All patients were diagnosed with stroke by imaging examination, and color ultrasound examination showed that there was a mural thrombus in the bilateral carotid arteries. The patients were diagnosed with mild cognitive impairment as described in the study of Ismail et al[7]: (1) All imaging examinations showed findings in accordance with the diagnostic criteria for stroke; (2) Progressive impairment of cognitive function; (3) Mild memory impairment; (4) Ability to continue daily life; and (5) Cognitive impairment less severe than the threshold for a diagnosis in the Diagnostic and Statistical Manual of Mental Disorders[8]. Reduced capacity to orient oneself, recognize objects, and express oneself in language served as the primary clinical indications.
(1) Imaging findings, physical examination findings, and a thorough review of the patient’s medical history all corroborated the diagnosis, which matched all of the criteria laid forth in the Diagnostic Essentials of Various Cerebrovascular Diseases[9]; (2) Absence of any life-threatening organ malfunction; and (3) Voluntary participation in the research.
(1) Neurological disease, such as Alzheimer’s disease, Parkinson’s disease, or epilepsy; (2) History of brain trauma; (3) Previous stroke or stroke-like event; (4) Existing mental disability before the stroke; and (5) Severe anxiety, depression, or other mental illness.
Atorvastatin was used in conjunction with EECP to treat patients in the research group (Pfizer Pharmaceutical Co., Ltd., National Drug Approval No.: H20051408). Patients in the control group were given only atorvastatin. Oxygen saturation was tracked using an EECP instrument (a PECP/TM) to measure EECP. The machine was first warmed for approximately 10-15 min. After the patient was positioned in an appropriate posture for treatment, sandpaper was used to smooth the skin around the electrode connection site, and alcohol was used to disinfect the immediate area. The white, red, and black electrodes were fixed under the left clavicle or near the manubrium, the apex of the heart, the upper right abdomen, or the lower right rib. Then, a finger pulse oximeter was placed on a finger of the right hand of the patient, ensuring that the electrode was in a position free from or minimally affected by vibration, the red and white electrodes were not too close, and the electrode position did not affect the inflatable cuff. The standard inflation pressure ranged from 0.025 to 0.045 MPa, with adjustments made during the operation based on the patient’s response to the pressure. At the end of treatment, the finger pulse oximeter was removed, the inflatable cuff was unfastened, the electrode leads and electrodes were removed, and the patient was helped in tidying up his or her clothes and leaving the counterpulsation bed. The patient was required to remain under observation for 10 min without any discomfort before leaving. The treatment was performed once a day, 1 h/session, 6 d a week for 6 consecutive weeks, for a total of 36 sessions. Atorvastatin (20 mg) was given once daily for 24 wk.
Clinical data of patients in the two groups were collected, and the Mini-Mental State Examination (MMSE)[10], Montreal Cognitive Assessment (MoCA)[11] and activities of daily living (ADL)[12] scores were compared between the two groups. Serum acetylcholine (ACh), acetylcholinesterase (AChE), nitric oxide (NO), endothelin-1 (ET-1), β2-microglobulin (β2-MG), visinin-like protein-1 (VILIP-1) and glial fibrillary acidic protein (GFAP) levels were compared between the two groups. The blood flow conditions of the bilateral anterior cerebral artery (ACA), middle cerebral artery (MCA) and posterior cerebral artery (PCA) were compared between the two groups before and after treatment. The pulsatility index (PI) and resistance index (RI) of each artery were calculated according to the blood flow velocity of the left and right arteries during the peak systolic period (Vs), end-diastolic blood velocity (Vd) and average blood flow velocity (Vm), with PI = (Vs-VD)/Vm, and RI = (Vs-VD)/Vs.
The MMSE includes five different elements, with a total of 30 items and a total score of 30 points. The better the patient’s mental condition, the higher the score. The total possible score on the MoCA is 30 points, and a MoCA score < 26 points indicates that the patient has cognitive impairment. The total possible score on the ADL scale is 100 points, and the higher the score, the better the patient’s daily living ability. The abbreviations for the cerebral arteries are left ACA (LACA), right ACA (RACA), left MCA (LMCA), right MCA (RMCA), left PCA (LPCA), and right PCA (RPCA).
SPSS 20.0 was employed for processing and analyzing the data. The values for the measurements are presented as “mean ± SD”. The t test for independent samples was used to evaluate differences between the groups. Within-group comparisons of pre- and posttreatment values were performed using the paired t test. The χ2 test was used to make comparisons, with count data being reported as frequencies and category ratios. P < 0.05 was considered to indicate a statistically significant difference.
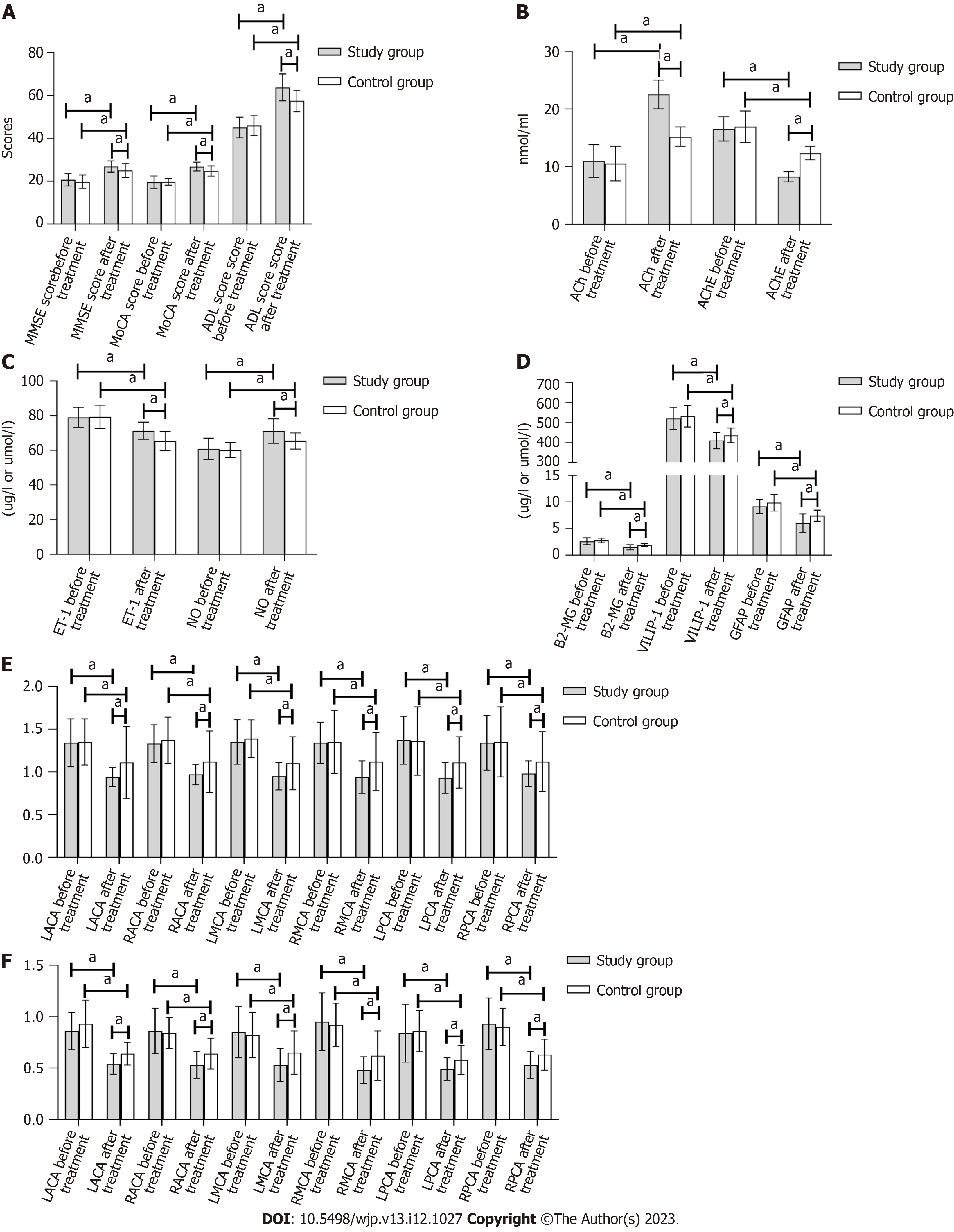
There were no statistically significant differences between the groups in the pretreatment MMSE, MoCA, or ADL scores (P > 0.05). The treatment group showed statistically significant (P < 0.05) improvements in the MMSE, MoCA, and ADL scores after treatment compared to the control group, as shown in Table 1 and Figure 1A.
| Group | MMSE score | MoCA score | ADL score | |||
| Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | |
| Study group (n = 30) | 20.63 ± 2.94 | 26.80 ± 2.59a | 19.50 ± 2.84 | 26.77 ± 2.08a | 45.00 ± 4.78 | 63.70 ± 6.25a |
| Control group (n = 30) | 19.73 ± 3.10 | 24.97 ± 3.29a | 19.70 ± 1.60 | 24.70 ± 2.42a | 45.97 ± 4.62 | 57.40 ± 4.99a |
| P value | 0.253 | 0.020 | 0.738 | 0.001 | 0.429 | < 0.001 |
| t value | 1.154 | 2.400 | 0.336 | 3.545 | 0.797 | 4.314 |
There were no statistically significant differences in the pretreatment ACh or AChE level between the two groups. In both groups, the ACh level increased after therapy, while the AChE level decreased (P < 0.05). There were statistically significant (P < 0.05) improvements in all indices in the experimental group that were not present in the control group, as shown in Table 2 and Figure 1B.
There was no significant change in the ET-1 or NO level between the two groups before treatment (P > 0.05). After therapy, the ET-1 level in both groups decreased, and the NO level increased. The study group showed significantly (P < 0.05) greater improvement than the control group in all areas, as shown in Table 3 and Figure 1C.
There were no significant variations in the β2-MG, VILIP-1, or GFAP levels between the two groups prior to therapy (P > 0.05). After treatment, the β2-MG, VILIP-1, and GFAP levels decreased in both groups, with significantly lower levels in the study group (P < 0.05), as shown in Table 4 and Figure 1D.
| Group | Β2-MG (mg/L) | VILIP-1 (ng/L) | GFAP (mg/L) | |||
| Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | |
| Study group (n = 30) | 2.64 ± 0.65 | 1.49 ± 0.48a | 520.59 ± 55.39 | 409.67 ± 40.77a | 9.17 ± 1.32 | 6.04 ± 1.70a |
| Control group (n = 30) | 2.79 ± 0.42 | 1.93 ± 0.28a | 531.47 ± 54.03 | 435.83 ± 37.09a | 9.87 ± 1.56 | 7.44 ± 1.06a |
| P value | 0.294 | < 0.001 | 0.444 | 0.012 | 0.064 | < 0.001 |
| t value | 1.061 | 4.339 | 0.771 | 2.599 | 1.888 | 3.817 |
The PI values of the LACA, RACA, LMCA, RMCA, LPCA, and RPCA were not significantly different between the two groups before treatment (P > 0.05). As expected, therapy resulted in a reduction of pretreatment PI values in all examined vessels (LACA, RACA, LMCA, RMCA, LPCA, and RPCA) in both groups, with the study group showing significantly (P < 0.05) lower PI levels than the control group, as presented in Table 5 and Figure 1E.
| Group | LACA | RACA | LMCA | RMCA | LPCA | RPCA | ||||||
| Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | |
| Study group | 1.34 ± 0.28 | 0.94 ± 0.11a | 1.33 ± 0.22 | 0.97 ± 0.12a | 1.35 ± 0.26 | 0.95 ± 0.16a | 1.34 ± 0.24 | 0.94 ± 0.19a | 1.37 ± 0.28 | 0.93 ± 0.18a | 1.34 ± 0.32 | 0.98 ± 0.15a |
| Control group | 1.35 ± 0.27 | 1.11 ± 0.42a | 1.37 ± 0.27 | 1.12 ± 0.36a | 1.39 ± 0.22 | 1.10 ± 0.31a | 1.35 ± 0.37 | 1.12 ± 0.34a | 1.36 ± 0.40 | 1.11 ± 0.30a | 1.35 ± 0.41 | 1.12 ± 0.35a |
| P value | 0.832 | 0.040 | 0.536 | 0.049 | 0.490 | 0.024 | 0.905 | 0.013 | 0.857 | 0.007 | 0.983 | 0.047 |
| t value | 0.213 | 2.139 | 0.622 | 2.043 | 0.695 | 2.339 | 0.120 | 2.589 | 0.180 | 2.805 | 0.021 | 2.052 |
The RI values of the LACA, RACA, LMCA, RMCA, LPCA, and RPCA were similar in the two groups before treatment (P > 0.05). Treatment resulted in a substantial (P < 0.05) reduction in the RI values of the LACA, RACA, LMCA, RMCA, LPCA, and RPCA in both groups, as shown in Table 6 and Figure 1F.
| Group | LACA | RACA | LMCA | RMCA | LPCA | RPCA | ||||||
| Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | Before treatment | After treatment | |
| Study group | 0.86 ± 0.18 | 0.54 ± 0.10a | 0.86 ± 0.22 | 0.53 ± 0.13a | 0.85 ± 0.25 | 0.53 ± 0.16a | 0.95 ± 0.28 | 0.48 ± 0.13a | 0.84 ± 0.28 | 0.49 ± 0.11a | 0.93 ± 0.25 | 0.53 ± 0.13a |
| Control group | 0.93 ± 0.23 | 0.64 ± 0.11a | 0.84 ± 0.15 | 0.64 ± 0.15a | 0.82 ± 0.22 | 0.65 ± 0.21a | 0.92 ± 0.21 | 0.62 ± 0.24a | 0.86 ± 0.20 | 0.58 ± 0.14a | 0.90 ± 0.18 | 0.63 ± 0.15a |
| P value | 0.225 | 0.001 | 0.729 | 0.004 | 0.720 | 0.014 | 0.582 | 0.007 | 0.701 | 0.006 | 0.566 | 0.008 |
| t value | 1.226 | 3.661 | 0.349 | 3.033 | 0.360 | 2.547 | 0.553 | 2.847 | 0.386 | 2.864 | 0.578 | 2.750 |
The degree of cognitive impairment in stroke patients is strongly correlated with their prognosis, and elderly individuals are especially prone to cognitive impairment as a sequela of cerebral infarction. Therefore, it is crucial to find efficient ways to reduce stroke patients’ cognitive impairment. According to relevant studies, atorvastatin serves antioxidant and antithrombotic functions and protects vascular endothelial function. It can play a direct role in protecting nerves, reducing the abundance of macrophages in atherosclerotic plaques, enhancing the integrity of fibrous plaque caps, inhibiting vascular inflammation, protecting the vascular endothelium, and inhibiting cognitive impairment after a stroke caused by vascular factors. EECP is a safe and cost-effective treatment that is mainly suitable for the treatment of coronary heart disease and angina pectoris. Numerous investigations conducted recently have demonstrated that EECP significantly affects ischemic cerebrovascular disorders and heart failure[13,14]. According to a prior study, EECP can reduce the clinical symptoms of coronary artery disease by enhancing vascular endothelial function[15]. EECP is an important means of cardiovascular auxiliary circulation that can simultaneously increase the arterial and venous return of both lower limbs and improve coronary blood flow. Patients’ diastolic blood pressure and cardiac output as well as blood perfusion to the heart, brain, kidneys, and other organs can all be improved by wrapping balloon sleeves around the thighs, calves, and buttocks and then inflating and deflating the balloons using an air supply system. EECP has been shown to enhance arterial blood flow, increase blood perfusion in brain tissue, improve brain cell metabolism, and facilitate neurological function recovery[16]. Numerous clinical trials have demonstrated that EECP is able to successfully increase blood flow to ischemic areas, restore nerve cell activity, and facilitate the opening of collateral circulation in the ischemic penumbra[17,18].
This research compared the effects of EECP combined with atorvastatin to those of atorvastatin alone between two groups. The results indicated that after therapy, the MMSE, MoCA, and ADL scores in the study group improved more than those in the control group (P < 0.05). These findings demonstrate that the combined application of EECP and atorvastatin might be more effective than atorvastatin alone in enhancing patients’ cognitive function and daily living abilities and that such enhancements could serve as direct indicators of the efficacy of therapy. The endothelium lining the blood vessels is vulnerable to oxidative damage, which can be caused by atherosclerosis. Atorvastatin is a commonly used lipid-lowering drug that stabilizes atherosclerotic plaques. EECP can play a role in increasing cerebral blood flow and perfusion and improving neural function, such that the combined application of these two treatments is more effective. Previous studies have also shown that EECP combined with conventional drugs can be used to treat poststroke cognitive impairment more effectively than the same drugs alone[19]. Learning and memory are two physiological processes that benefit greatly from the functioning of the central cholinergic system. AChE catalyzes the decomposition reaction that produces ACh, which binds to ACh receptors. Degeneration of cholinergic neurons is common in patients with cognitive impairment after stroke[20]. ACh can participate in neuronal activity and regulate synaptic plasticity. In this investigation, the improvement in ACh and AChE levels following therapy was larger in the study group than in the control group (P < 0.05). The treatment of cognitive impairment following a stroke, which may be connected to the management of AChE and ACh levels, is said to benefit significantly from the combination of EECP and atorvastatin. The vascular endothelium serves both barrier and endocrine functions. Dysfunction of vascular endothelial cells can lead to damage to the blood-brain barrier, causing toxic substances to accumulate around nerve cells; triggering inflammatory reactions; damaging brain white matter, neuronal axons and synapses; and leading to cognitive impairment. Vascular endothelial damage, atherosclerosis, brain tissue damage, increased ET-1 expression, and decreased NO expression result in spasms of small cerebral vessels and damage to the nerve fiber network. It has been reported that the serum ET-1 and NO levels in patients with dementia are closely related to endothelial dysfunction of small cerebral vessels and can be used as a marker of endothelial dysfunction[21]. Patients with cognitive impairment following stroke showed significant improvement in ET-1 and NO levels in the treatment group compared to the control group, suggesting that combination therapy can increase the degree of vascular endothelial function more effectively than pharmacotherapy alone. Atorvastatin’s lipid-lowering action, ability to mitigate atherosclerosis and protective effect on vascular endothelial function are all well documented. EECP has been shown to ameliorate cognitive impairment by normalizing systolic and diastolic vascular function, controlling vascular tension, and boosting endothelial function in blood vessels. Thus, when applied in combination, they could play synergistic roles, resulting in an enhanced therapeutic effect.
All nucleated cells contain β2-MG, which might increase the risk of an inflammatory response and subsequent brain injury. With a negative effect on cognition, β2-MG has been linked to a host of serious health problems[22]. According to relevant studies, serum β2-MG levels are high in patients with cognitive impairment after stroke[23]. It has been shown that the expression level of VILIP-1, a small-molecule cytosolic protein typically dispersed in nerve cells, is positively linked with the presence of brain damage[24]. Serum levels of GFAP, an intermediate filament protein found in glial cells, are significantly elevated in those who have had a stroke and are experiencing cognitive impairment[25]. The fact that the levels of β2-MG, VILIP-1, and GFAP improved more in the study group than in the control group suggests that the combination therapy may promote the healing of damaged brain tissue in patients, which merits additional investigation into the specific mechanism of action. Patients with poststroke cognitive impairment who received combination therapy showed greater improvements in the PI and RI values of the LACA, RACA, LMCA, RMCA, LPCA, and RPCA than those who received atorvastatin therapy alone. This is because of the synergistic impact of atorvastatin’s lipid-lowering and antithrombotic effects and the capacity of EECP to increase cerebral blood flow perfusion.
In conclusion, EECP combined with atorvastatin can effectively improve cognitive function, daily living ability, vascular endothelial function, neural function, and cerebral blood flow in patients with poststroke cognitive dysfunction, indicating the clinical value of this combination. A limitation of this study is that it was a retrospective study, and the number of cases that could be included in the observation was small, which may have led to bias in the results. In the future, a prospective multicenter study with a larger sample should be conducted to further verify the results of this study.
The research background is the discussion on the treatment of patients with cognitive dysfunction after stroke. The current research status is that atorvastatin is widely used to treat cognitive impairment after stroke, and the research significance is to provide a new treatment plan for cognitive impairment after stroke and improve clinical efficacy.
With the treatment of stroke patients with cognitive dysfunction as the research topic, more effective treatment plans need to be explored to improve the prognosis of stroke patients with cognitive dysfunction. The significance of this study is to affirm the better treatment of cerebral stroke patients with cognitive dysfunction, and promote the innovation of clinical treatment of cerebral stroke cognitive endometrial methods.
The objective of this study was to compare the therapeutic effect of different treatment methods and to observe the advantages of enhanced external counterpulsation (EECP) combined with atorvastatin over atorvastatin alone. In this study, enhanced in vitro rebuttal combined with atorvastatin was effective in the treatment of cognitive dysfunction in stroke patients, including cognitive function, ability of daily living, vascular endothelial function, nerve function and cerebral blood flow, confirming that the combined treatment has good clinical effects and providing a new reference for the treatment of cognitive dysfunction in stroke patients in the future.
The clinical data of the patients were analyzed retrospectively and grouped according to different treatment methods. Then, independent sample t test, paired sample t test and χ2 test were used to statistically analyze the general data of the two groups, including mental state, cognitive function, daily living ability score, neurotransmitter level, vascular endothelial function index level, cognitive-related index and cerebral blood flow index before and after treatment. The feature of retrospective study is to explore the cause through the results, and it is easier to obtain the case data.
EECP combined with atorvastatin can significantly improve cognitive function, mental state, ability of daily living, vascular endothelial function, nerve function and cerebral blood flow in the treatment of stroke patients with cognitive dysfunction, which provides a new treatment method for the treatment of stroke patients with cognitive dysfunction and requires further prospective exploration. To further verify the effectiveness of this treatment method.
Vascular endothelial dysfunction can lead to impairment of the blood-brain barrier, leading to cognitive dysfunction. Therefore, attention should be paid to the effect of treatment on vascular endothelial function. Based on the better treatment effect of EECP combined with atorvastatin, the more effective treatment scheme should be selected in clinic.
Biological indicators can effectively reflect the severity of the patient’s disease, and the effect of treatment regimen on the level of novel biomarkers in patients with post-stroke cognitive dysfunction needs to be further explored.
| 1. | Rost NS, Brodtmann A, Pase MP, van Veluw SJ, Biffi A, Duering M, Hinman JD, Dichgans M. Post-Stroke Cognitive Impairment and Dementia. Circ Res. 2022;130:1252-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 502] [Article Influence: 125.5] [Reference Citation Analysis (0)] |
| 2. | Zhang X, Bi X. Post-Stroke Cognitive Impairment: A Review Focusing on Molecular Biomarkers. J Mol Neurosci. 2020;70:1244-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 90] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 3. | Caceres J, Atal P, Arora R, Yee D. Enhanced external counterpulsation: A unique treatment for the "No-Option" refractory angina patient. J Clin Pharm Ther. 2021;46:295-303. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 4. | Bai J, Wu D, Zhang J. A simulation study of external counterpulsation. Comput Biol Med. 1994;24:145-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Raza A, Steinberg K, Tartaglia J, Frishman WH, Gupta T. Enhanced External Counterpulsation Therapy: Past, Present, and Future. Cardiol Rev. 2017;25:59-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 44] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
| 6. | Hou Chenchen, Liu Dan. [Meta analysis of the efficacy of atorvastatin calcium in the treatment of cognitive impairment caused by cerebrovascular disease]. South China Journal of National Defense Medicine. 2021;35:651-656+677. |
| 7. | Ismail Z, Black SE, Camicioli R, Chertkow H, Herrmann N, Laforce R Jr, Montero-Odasso M, Rockwood K, Rosa-Neto P, Seitz D, Sivananthan S, Smith EE, Soucy JP, Vedel I, Gauthier S; CCCDTD5 participants. Recommendations of the 5th Canadian Consensus Conference on the diagnosis and treatment of dementia. Alzheimers Dement. 2020;16:1182-1195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 176] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 8. | Peng D, Zhu R, Xu X. [Diagnostic and Statistical Manual of Mental Disorders-5 Diagnostic Criteria for Neurocognitive Disorders (draft)]. Chinese Journal of Geriatrics. 2011;30:6. |
| 9. | Wang X. [Diagnostic key points of various cerebrovascular diseases]. China Rural Medicine. 1996;2:2. |
| 10. | Zwecker M, Levenkrohn S, Fleisig Y, Zeilig G, Ohry A, Adunsky A. Mini-Mental State Examination, cognitive FIM instrument, and the Loewenstein Occupational Therapy Cognitive Assessment: relation to functional outcome of stroke patients. Arch Phys Med Rehabil. 2002;83:342-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 94] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | You JS, Chen RZ, Zhang FM, Zhou ZY, Cai YF, Li GF. The chinese (cantonese) montreal cognitive assessment in patients with subcortical ischemic vascular dementia. Dement Geriatr Cogn Dis Extra. 2011;1:276-282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 12. | Mlinac ME, Feng MC. Assessment of Activities of Daily Living, Self-Care, and Independence. Arch Clin Neuropsychol. 2016;31:506-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 488] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 13. | Valenzuela PL, Montalvo Z, Torrontegi E, Sánchez-Martínez G, Lucia A, de la Villa P. Enhanced External Counterpulsation and Recovery From a Plyometric Exercise Bout. Clin J Sport Med. 2020;30:416-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Rayegani SM, Heidari S, Maleki M, Seyed-Nezhad M, Heidari M, Parhizgar SE, Moradi-Joo M. Safety and effectiveness of enhanced external counterpulsation (EECP) in refractory angina patients: A systematic reviews and meta-analysis. J Cardiovasc Thorac Res. 2021;13:265-276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Valenzuela PL, Sánchez-Martínez G, Torrontegi E, Montalvo Z, Lucia A, de la Villa P. Enhanced External Counterpulsation and Short-Term Recovery From High-Intensity Interval Training. Int J Sports Physiol Perform. 2018;13:1100-1106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Soran O. Alternative Therapy for Medically Refractory Angina: Enhanced External Counterpulsation and Transmyocardial Laser Revascularization. Heart Fail Clin. 2016;12:107-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Lin L, Zhu W, Ma N, Lin X, Yang H. Evaluation of enhanced external counterpulsation therapy for nonarteritic anterior ischemic optic neuropathy. BMC Ophthalmol. 2020;20:236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Li B, Wang W, Mao B, Zhang Y, Chen S, Yang H, Niu H, Du J, Li X, Liu Y. Hemodynamic effects of enhanced external counterpulsation on cerebral arteries: a multiscale study. Biomed Eng Online. 2019;18:91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Chen Q, Xu X, Zhao X, Li H. [Analysis of the effect of external counterpulsation combined with drug therapy on cognitive dysfunction after stroke]. China Practical Medicine. 2018;13:110-111. |
| 20. | Ballinger EC, Ananth M, Talmage DA, Role LW. Basal Forebrain Cholinergic Circuits and Signaling in Cognition and Cognitive Decline. Neuron. 2016;91:1199-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 560] [Article Influence: 62.2] [Reference Citation Analysis (0)] |
| 21. | Xu ZP, Yang SL, Zhao S, Zheng CH, Li HH, Zhang Y, Huang RX, Li MZ, Gao Y, Zhang SJ, Zhan PY, Zhang LF, Deng L, Wei S, Liu YC, Ye JW, Ren HJ, Li N, Kong CX, Wang X, Fang L, Zhou QZ, Jiang HW, Li JR, Wang Q, Ke D, Liu GP, Wang JZ. Biomarkers for Early Diagnostic of Mild Cognitive Impairment in Type-2 Diabetes Patients: A Multicentre, Retrospective, Nested Case-Control Study. EBioMedicine. 2016;5:105-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 22. | Chen F, Liu J, Li FQ, Wang SS, Zhang YY, Lu YY, Hu FF, Yao RQ. β2-Microglobulin exacerbates neuroinflammation, brain damage, and cognitive impairment after stroke in rats. Neural Regen Res. 2023;18:603-608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 23. | Zhang M, Li M, Xue J, Yang Q, Wang T. Serum β Study on the correlation between 2-microglobulin combined with lipoprotein associated phospholipase A2 and cognitive impairment after stroke. Practical Geriatrics. 2022;36:23-26. [DOI] [Full Text] |
| 24. | Bradley-Whitman MA, Roberts KN, Abner EL, Scheff SW, Lynn BC, Lovell MA. A novel method for the rapid detection of post-translationally modified visinin-like protein 1 in rat models of brain injury. Brain Inj. 2018;32:363-380. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 25. | Huss A, Abdelhak A, Mayer B, Tumani H, Müller HP, Althaus K, Kassubek J, Otto M, Ludolph AC, Yilmazer-Hanke D, Neugebauer H. Association of Serum GFAP with Functional and Neurocognitive Outcome in Sporadic Small Vessel Disease. Biomedicines. 2022;10:1869. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Asiamah N, Ghana; Meaney MJ, Canada S-Editor: Wang JJ L-Editor: A P-Editor: Xu ZH