Published online Sep 19, 2022. doi: 10.5498/wjp.v12.i9.1183
Peer-review started: April 20, 2022
First decision: May 30, 2022
Revised: June 14, 2022
Accepted: August 10, 2022
Article in press: August 10, 2022
Published online: September 19, 2022
Processing time: 152 Days and 14.8 Hours
Evidence suggests that cytokines cause immune disturbances, shape immunological sequelae later in life, and modulate the risk of schizophrenia (SC). Galectin-3 (Gal-3), a multifaceted molecule of the glycan family, is involved in the formation of the immunological synapse and modulates the signalling pathway and effector functions of T lymphocytes, which are major producers of cytokines. We have previously reported elevated serum Gal-3 levels in stable SC patients. However, Gal-3 as a link between cognitive functioning and inflammation has not yet been investigated in SC.
To investigate the relationship between serum Gal-3 levels and cognitive performance, serum cytokines, and white blood cell count in three-month stably treated SC patients.
Twenty-seven patients with SC in remission and 18 healthy volunteers participated in this case-control and correlational study. Clinical assessment was performed using the Positive and Negative Syndrome Scale and the Montreal-Cognitive Assessment. The results of previously measured serum levels of Gal-3, interleukin (IL)-33, soluble suppression of tumorigenicity 2 (sST2), tumor necrosis factor-alpha (TNF-α), IL-6 and IL-17 were used for further statistical analyses, and IL-4, IL-23, IL-1β and transforming growth factor-beta (TGF-β) were now additionally measured with a sensitive enzyme-linked immunosorbent assay. The number of leukocytes in the blood and the percentage of neutrophils, lymphocytes, and monocytes were determined with a standardized routine measurement procedure (Sysmex Technology). Statistical analyses were performed using SPSS 20.0 software.
We found no correlation between serum Gal-3 levels and cognitive functioning in SC patients. A positive correlation was found between the levels of Gal-3 and TNF-α (r = 0.476; P = 0.012), Gal-3 and IL-23 (r = 0.417; P = 0.031), and Gal-3 and sST2 (r = 0.402; P = 0.038). The binary logistic model, which included all nine cytokines measured in this patient sample, indicated the particular role of Gal-3 and TGF-β in the duration of SC. In the stabilization phase of SC, we observed a moderate and negative correlation between serum Gal-3 levels and leukocytes (r = -0.449; P < 0.019). Additional linear regression analysis showed a positive correlation between Gal-3 expression and risperidone dose (F: 4.467; P < 0.045; r2 = 0.396).
The combined activity of Gal-3 and proinflammatory cytokines, TGF-β downregulation and lower counts of leukocytes influence the SC duration. Gal-3 likely manifests indirect immunometabolic regulation of cognition in SC.
Core Tip: In clinical sampling, there is an urge to place the results of biological measurements in a much broader context. Elevated serum galectin-3 (Gal-3) levels in schizophrenia (SC) have not been studied in relation to other peripheral biomarkers and subsequent neuroinflammation. We found that Gal-3 contributes to ongoing peripheral systemic inflammation and disease duration in patients with SC. All of this may be an underlying indirect immunometabolic mechanism for cognitive performance in patients with SC.
- Citation: Minic Janicijevic S, Jovanovic IP, Gajovic NM, Jurisevic MM, Debnath M, Arsenijevic NN, Borovcanin MM. Galectin-3 mediated risk of inflammation in stable schizophrenia, with only possible secondary consequences for cognition. World J Psychiatry 2022; 12(9): 1183-1193
- URL: https://www.wjgnet.com/2220-3206/full/v12/i9/1183.htm
- DOI: https://dx.doi.org/10.5498/wjp.v12.i9.1183
Immune dysregulations during prenatal and postnatal life are increasingly associated with neurodevelopmental disorders and have also recently been shown to be an important etiological construct of schizophrenia (SC)[1,2]. Multiple post-mortem brain and neuroimaging studies have also provided evidence for neuroinflammation in SC[3,4]. One of the best-known hypotheses, proposed by Bechter, links SC to mild and localized encephalitis[5]. There is strong evidence that cytokines cause these immune disturbances, shape immunological sequelae later in life, and modulate SC risk. In particular, T lymphocytes are one of the major producers of cytokines, and it has been reported that blood levels of cytokines derived from various lineages of T lymphocytes such as T helper 1 (Th1), Th2, Th17 and regulatory T cells (Treg) are altered in SC[6-8]. Studies have shown that patients with SC have increased serum concentrations of proinflammatory cytokines, including interleukin (IL)-1β, IL-6, and tumor necrosis factor-alpha (TNF-α)[9,10].
Studies have also shown that Gal-3, a multifaceted molecule in the glycan family, is directly involved in the formation of the immunological synapse and appears to play a pivotal role in modulating the signalling pathway and effector functions of T lymphocytes[11]. It is noteworthy that Gal-3 has both immune and non-immune functions in the brain. Gal-3 appears to play a neuroprotective role in neuronal tissue and is involved in the reparative processes of brain lesions and ischemia. In contrast, Gal-3 may promote microglia-mediated neuroinflammation and contribute to neuroprogression[12]. Gal-3 increases the secretion of proinflammatory cytokines from microglia and astrocytes[13] and is also required for leukocyte recruitment during an acute inflammatory response[14].
Biomarkers that can be conveniently measured in blood may also reflect changes in the central nervous system and dysfunction of the blood-brain barrier (BBB). There is evidence of BBB dysfunction in brain disorders, including SC. Brain microvascular endothelial cells (BMECs) are a key element of the microvasculature that forms the BBB and shields the brain from toxins and reactive immune cells. However, it is not known whether BMECs themselves are functionally compromised and lead to BBB dysfunction in brain disorders[15]. An increased ratio of cerebrospinal fluid to serum albumin in patients with SC suggests increased permeability of the BBB[16]. Given the important role of galectins in cell adhesion, migration, polarity, and chemotaxis, it is likely that modulation of galectin levels in BMECs that form the BBB could compromise BBB integrity and consequently contribute to neuroinflammation[17]. Plasma levels of Gal-3 have been shown to be increased after aneurysmal subarachnoid hemorrhage (SAH), and a Gal-3 inhibitor could potentially prevent post-SAH BBB disruption by inhibiting Gal-3[18].
We have previously reported elevated serum Gal-3 levels in patients with SC who received stable 3-mo antipsychotic therapy[19]. We wanted to go further in exploring Gal-3 interactions and not only measure serum levels during stabilisation of SC. Recently, such an association between Gal-3 and cognition was found in Alzheimer’s disease[20]. In this additional analysis, we tested the hypothesis that serum Gal-3 levels in patients with stable SC might be related to cognitive functioning and different white blood cell counts and types of cytokines in stable SC patients. In this way, we aimed to investigate the possible involvement of this glycan in peripheral systemic inflammation and disease duration, but also its position as a link between cognitive functioning and inflammation, which has not yet been investigated in SC.
Patients with SC in remission (SC in remission) were recruited in 2016 in the Psychiatric Day Hospital of the Kragujevac Clinical Centre. Participants were between 18 and 65 years old. Diagnoses were made using the International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) criteria[21] for SC (F20). The major inclusion criterion was stable mental functioning and adherence to three months of stable antipsychotic depot therapy with risperidone or paliperidone. Add-on therapy for patients included anxiolytics or hypnotics only. A complete medical history was obtained from each patient.
Exclusion criteria were current infections during the three-month remission period, allergies or autoimmune disorders, current anti-inflammatory or antiviral medications, or dual diagnoses of other mental illnesses. Healthy controls (HCs) were recruited during blood donation at the Blood and Blood Products Service of the Kragujevac Clinical Centre, and controls with a family history of psychosis were excluded. All laboratory measurements and immunoassays were performed at the Centre for Molecular Medicine and Stem Cell Research, Faculty of Medical Sciences, University of Kragujevac. The study was conducted after the Ethics Committee of the Kragujevac Clinical Centre gave its approval. Participants were able to give informed consent, and each patient signed the informed consent form before participating in the study.
The study sample was estimated considering the first type error (α) of 0.05 and the power of the study of 0.8 for the two-tailed t-test for two independent samples using the statistical softer G* Power 3.1.9.2. Considering previous studies and similar methods for measuring serum cytokine levels[22], the minimum number of participants required in each group was estimated to be 14.
Psychological assessment was performed by trained raters. Psychopathology was assessed using the Positive and Negative Syndrome Scale (PANSS)[23]. Cognition was assessed using the cognitive factor of the PANSS (consisting of items P2-N5-G11)[24], which primarily refers to sustained attention, and executive functioning such as mental flexibility and problem-solving as components of executive functioning[25]. In addition, cognitive impairment was assessed using the Montreal-Cognitive Assessment (MoCA)[26], a cognitive screening tool for older population with mild cognitive impairment and dementia that has also been shown to be useful in patients with psychosis[27]. The MoCA test assesses multiple cognitive domains including attention, concentration, executive functions, memory, language, visual-constructive skills, conceptualization, and orientation, with a maximum total score of 30 and a lower limit for normal cognition of 26.
Blood samples were taken in the morning (approximately 8 am) after overnight fasting. The blood clot was cut and then centrifuged. After separation, serum samples were stored at -20° until analysis. The results of previously measured serum levels of Gal-3, IL-33, soluble suppression of tumorigenicity 2 (sST2), TNF-α, IL-6 and IL-17[19,28] were used for further statistical analyses, and IL-4, IL-23, IL-1β and transforming growth factor-beta (TGF-β) were now additionally measured using sensitive Enzyme-Linked Immuno-Sorbent Assay kits specific for the human cytokines according to the manufacturer's instructions (R&D System, Minneapolis, MB). The procedure has been described in detail previously[19]. Briefly, 96-well plates coated with capture antibody and incubated overnight were washed with wash buffer and incubated with blocking buffer for 1 h at room temperature. Serum samples or standard recombinant IL-4/IL-23/IL-1β/TGF-β were added to the plates for 2 h before a biotinylated detection antibody and streptavidin peroxidase were applied for 1 h each at room temperature. The plates were developed with substrate reagent for 20 min, and the reaction was stopped by addition of 4 mol/L sulfuric acid. The absorbance was read at 495 nm using a microplate reader. The exact concentration of the above biomarkers was measured by interpolating a standard curve with a series of known concentrations according to the manufacturer's instructions. The values of the measured cytokines are expressed in pg/mL. Blood cell populations were determined using a standardized routine laboratory procedure (Sysmex Technology).
Demographic and clinical data were presented descriptively. Various covariates were included in linear and multiple linear regression models to examine the effects of these variables on the results. Pearson's or Spearman's correlation analysis was used to examine the significance of the correlation between serum Gal-3 levels and blood cell counts, serum cytokine levels, and clinical scores and subscores of PANSS and MoCA. To determine the best prediction of serum cytokine levels for the presence of illness, binary logistic regression analysis was performed. A P-value of ≤ 0.05 was considered statistically significant. Statistical analyses were performed using IBM SPSS Statistics for Windows, version 20.0. Armonk, NY: IBM Corp.
There were no statistically significant differences in age (P = 0.886) and sex (P = 0.851) between patients (n = 27) and HC subjects (n = 18). The demographic and clinical characteristics of the patients were the same as those presented previously[19,28] and are listed in Table 1. Among patients with SC, the duration of illness was 9.95 ± 7.71 years, with 2.18 ± 1.92 years as multiple previous hospitalizations. Most patients were individuals with high school education (n = 22). The mean PANSS total score and subscores, MoCA total score and subscores, and medications taken in the SC group are shown in Table 1.
| Characteristics | SC in remission (n = 27) | Healthy control (n = 18) | P value |
| Age (yr), mean ± SD | 36.18 ± 9.27 | 37.67 ± 9.96 | 0.862 |
| Sex (male/female) | 16/11 | 12/6 | 0.851 |
| Duration of illness (yr), mean ± SD | 9.95 ± 7.71 | - | - |
| Number of previous hospitalizations | 2.18 ± 1.92 | - | - |
| PANSS | |||
| PANSS total score | 99.22 ± 18.2 | - | - |
| Positive syndrome scale | 22.26 ± 5.97 | - | - |
| Negative syndrome scale | 27.52 ± 6.09 | - | - |
| General psychopathology scale | 49.44 ± 7.83 | - | - |
| MoCA | |||
| MoCA total score | 22.74 ± 4.76 | - | - |
| Visuospatial/Executive | 4.11 ± 1.25 | - | - |
| Naming | 2.78 ± 0.69 | - | - |
| Attention | 5.07 ± 1.21 | - | - |
| Language | 1.89 ± 0.69 | - | - |
| Abstraction | 1.41 ± 0.84 | - | - |
| Delayed recall | 1.81 ± 1.62 | - | - |
| Orientation | 5.74 ± 0.81 | - | - |
| Medications | |||
| Long-acting risperidone/paliperidone | 22/5 | - | - |
| Long-acting risperidone dosage 25/37.5/50 mg | 3/9/13 | - | - |
| Cell counts | |||
| Leukocytes (× 109/L) | 6.67 ± 2.06 | - | - |
| Neutrophils (%) | 0.61 ± 0.07 | - | - |
| Lymphocytes (%) | 0.31 ± 0.07 | - | - |
| Monocytes (%) | 0.08 ± 0.02 | - | - |
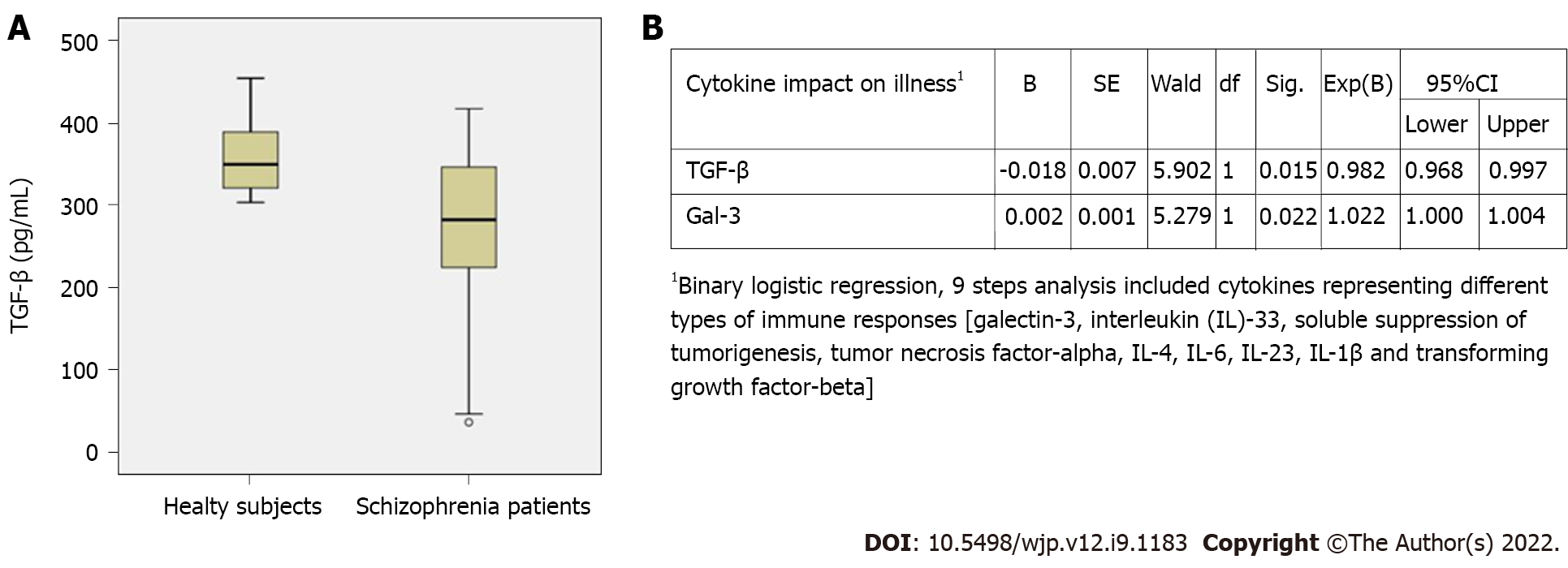
In this study, lower TGF-β levels (272.09 ± 101.59 vs 360.41 ± 45.13, P = 0.003) were observed in patients with SC (Figure 1A), with no difference in serum IL-4, IL-23 and IL-1β levels (data not shown). The binary logistic model, which included the presence of illness as a dependent variable and all measured cytokine serum levels as covariates in a stepwise Backward-Wald method, highlighted the particular role of Gal-3 and TGF-β in SC, both of which have an impact on disease presentation with an odds ratio for Gal-3: 1.002 (95%CI: 1.000-1.004; P = 0.022) and TGF-β: 0.982 (95%CI: 0.9968-0.997; P = 0.015) (Figure 1B), suggesting that higher Gal-3 levels are associated with stabilization in later phases of SC.
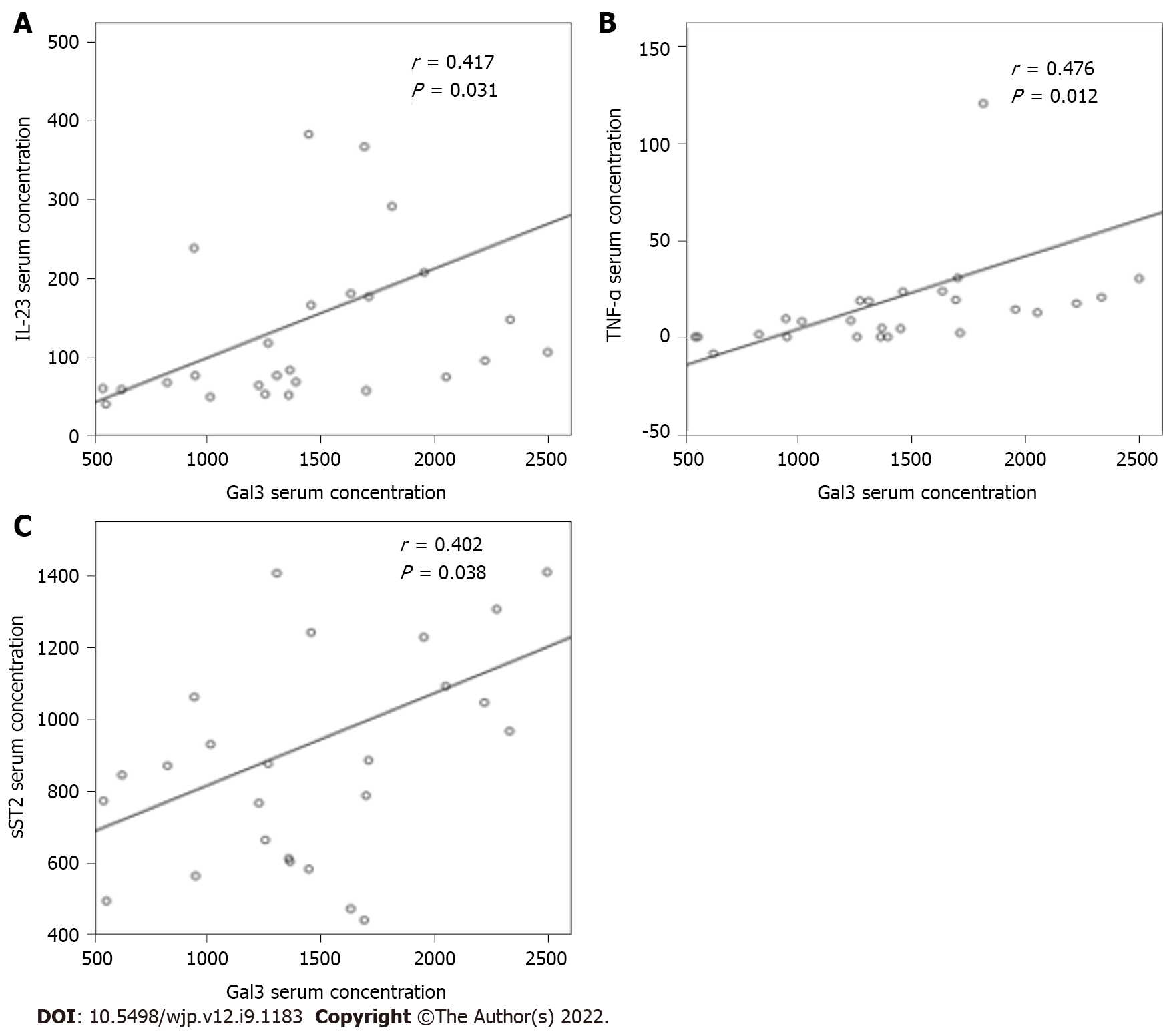
The correlation between Gal-3 serum levels and cognitive functioning considering MoCA total score, subscores, and PANSS Cog was not significant (data not shown). In addition, we now examined the relationship between systemic Gal-3 levels and cytokines with divergent immune properties. A positive and moderate correlation was observed between Gal-3 and TNF-α (r = 0.476; P = 0.012), Gal-3 and IL-23 (r = 0.417; P = 0.031), and Gal-3 and sST2 (r = 0.402; P = 0.038) levels (Figure 2).
Moreover, linear regression analysis revealed a positive correlation between Gal-3 and risperidone dose (F: 4.467; P < 0.045; r2 = 0.396).
We also examined the correlation between Gal-3 and the number of leukocytes (neutrophils, lymphocytes, and monocytes) involved in the immune response. A negative correlation was found between Gal-3 and total leukocyte count (r = -0.449, P < 0.019), with no other significant correlations with the percentages of specific populations.
The current study contains several new and interesting findings. One of the salient findings was a significant correlation between serum Gal-3 levels and levels of proinflammatory cytokines in a stable phase of SC. Serum Gal-3 correlated positively with TNF-α, IL-23, and soluble ST2 in SC in remission (Figure 2) and was associated with downregulation of the counterregulatory cytokine TGF-β and appears to play a role in disrupting leukocyte migration. In addition, the increase in Gal-3 might be influenced by risperidone dosing.
This study was the first to investigate a possible relationship between Gal-3 and cognitive functioning in SC patients. No correlation was found between serum Gal-3 levels and cognitive performance, suggesting a more indirect immunometabolic regulation of cognition in SC, as we have recently discussed[12]. It has been demonstrated that proinflammatory cytokines and mediators of oxidative stress could influence serum Gal-3 levels, and a reciprocal role of Gal-3 in these cascades could not be excluded[29]. Recently, Dal Lin et al[30] (2020) pointed out the close relationship and regulatory effect of cognitive functioning on some molecular processes in the human body, including acute attenuation of oxidative stress and inflammation, which inversely affect Gal-3 levels. Based on these findings, Gal-3 may prove to be a potential therapeutic target in SC.
Currently, there are no studies on the correlation between Gal-3 and proinflammatory cytokine levels in SC patients. In our previous study on the same cohort, we found higher systemic Gal-3 levels[19] and TNF-α[24]. In addition to our study, Kajitani et al[31] (2017) also reported elevated serum Gal-3 levels in a stable phase of SC. In one study, Gal-3 was tested for its capacity to induce proinflammatory cytokines such as TNF-α and IL-6 from plasmacytoid dendritic and form myeloid dendritic cells isolated from blood. This lectin was found to activate both, TNF-α and IL-6[32]. In addition, a pre-clinical model of intracerebral haemorrhage (ICH) also demonstrated increased expression of Gal-3 in perihematomal brain regions after ICH and Gal-3-induced release of IL-6, suggesting a role for Gal-3 in inflammatory responses after ICH[33]. These findings suggest the hypothesis that neuronal damage could be followed by inflammation involving Gal-3. The elevated serum Gal-3 levels observed in SC patients in the current study could lead to BBB disruption and contribute to the persistence of mild chronic neuroinflammation suspected in SC.
In particular, somatic comorbidities common in SC, such as obesity, hyperlipidaemia, dyslipidaemia and type 2 diabetes, could be monitored by measuring Gal-3[34]. Gal-3 correlates positively with obesity and inflammation, as measured by the inflammatory markers IL-6 and C-reactive protein (CRP)[35]. Contrary to this finding, the IL-6 axis was not active in this phase and in the specific subpopulation of patients, but rather overweighted type-1 immune response with representative TNF-α. Taken together, these findings suggest potential systemic inflammatory properties of Gal-3 through its interactions with proinflammatory markers in SC that contribute to immunometabolic processes in SC.
The association of Gal-3 and sST2 and their changes at follow-up with the development of heart failure in patients with ST-segment elevation myocardial infarction showed that the levels of Gal-3 and sST2 were significantly increased at one-year follow-up[36]. Interestingly, the increased serum Gal-3 concentration correlated with the production of IL-17 and exhibited a significant correlation with neutrophil/lymphocyte ratio, white blood cell count, and CRP, but inversely correlated with the production of IL-10 and IL-12 in patients with untreated colorectal cancer[37]. Some findings suggest that Gal-3 is required to efficiently recruit leukocytes during an acute inflammatory response[14]. These findings may indicate the diverse role of Gal-3 in this SC chronic inflammation, as we have previously discussed that Gal-3 plays a predominant role in the resolution of inflammation[12]. In chronic SC, our studies have shown that serum Gal-3 levels are elevated and that Gal-3 is negatively correlated with leukocyte count. This lower leukocyte count may be related to the decline in immunity of patients with SC in later stable phases and their greater susceptibility to infection.
Although the Gal-3 signalling pathway is not well understood, Gal-3 can be secreted into the extracellular space, where it can interact with different structures such as cell surface and extracellular matrix glycoproteins[38]. In autoimmune neuroinflammation, endogenous Gal-3 may potentiate its severity by decreasing the frequency of Treg cells, controlling IL-10 production, and modulating Notch activation[39]. The Notch and TGF-β signalling crosstalk, which plays an important role in regulating endothelial and neural development[40], could also be influenced by Gal-3. Our findings might shed important light on the Notch-TGF-β axis in SC (Figure 1B). As for TGF-β, our previous data indicate that serum levels of TGF-β are significantly increased in patients with SC in relapse and first-episode psychosis compared to healthy subjects[41,42]. However, in the current study, significantly lower TGF-β levels were observed in SC patients in remission compared to a group of HC subjects (Figure 1A), suggesting that TGF-β levels vary during the course of SC.
Regarding the possible influence of antipsychotics, a recent in vitro study reported that the atypical antipsychotic risperidone reduced the production of proinflammatory cytokines by lipopolysaccharide-stimulated glial cells but had no effect on IL-10[43]. However, paliperidone increased TGF-β and IL-10 during acute stress and during prolonged chronic stress[44]. Our recent hypotheses about the involvement of antipsychotics in the processes of glycosylation can be explained by the effects of their higher doses on serum Gal-3 levels. The findings of the current study suggest that higher doses of prescribed risperidone may lead to an increase in Gal-3 levels. Whole-serum proteins show increased glycosylation after antipsychotic use, indicating the usefulness of these processes for understanding the pathogenesis and monitoring the treatment of patients with SC[34,45].
A higher percentage of Gal-3-expressing innate and adaptive immune cells in the lamina propria was observed in patients with comorbid ulcerative colitis and metabolic syndrome[46]; this encouraged us to explore other immune biomarkers in patients with SC. N-acetylcysteine (NAC) has been proposed for the adjunctive treatment of SC and ulcerative colitis[47]. Oral intake of NAC was shown to lower inflammatory biomarkers, CRP and Gal-3 in patients with acute myocardial infarction receiving fibrinolytic therapy[48]. Preliminary results indicated the usefulness of NAC in improving all domains of SC functioning[49].
As a limitation of our study in terms of cognitive assessment, we must consider that only specific domains of cognitive functioning were assessed, using available validated and brief instruments to detect cognitive impairment in SC in our population. Although we tried to exclude all somatic states, we should be aware that comorbidity and psychotropic medication could influence the results of both cognitive functioning and serum measurements. We believe that it is necessary to investigate these issues further in a larger sample with a much more thorough analysis of confounding factors, which has not been done within the scope of this manuscript, but these results are valuable to guide us in the future.
In clinical sampling, there is an urge to place the results of biological measurements into a much wider concept. Higher serum levels of Gal-3 in SC have not been explored in interaction with other peripheral biomarkers reflecting possible inflammatory changes. We observed that Gal-3 contributes to ongoing peripheral systemic inflammation and disease duration in patients with SC. Moreover, its influence on BBB permeability and consequent neuroinflammation should be explored. Our data revealed some new complex roles of Gal-3, such as its possible involvement in neuroinflammation and cognitive processing, contributing to a better understanding of the specific immune profile in patients with SC. Inflammation also appears to be the potential pathway by which Gal-3 may affect cognitive functioning in SC. The efficacy of antipsychotics could be improved and their adverse effects corrected if the role of Gal-3 in glycosylation processes were considered. These findings provide a rationale for further strategies targeting Gal-3 for therapeutic intervention in SC.
Galectin-3 (Gal-3), a multifaceted molecule of the glycan family, modulates T lymphocytes’ signalling pathway and effector functions. We have previously reported elevated serum Gal-3 levels in stable schizophrenia (SC) patients, but Gal-3 as a link between cognitive functioning and inflammation has not yet been investigated in SC.
Elevated serum Gal-3 levels in SC have not been studied in relation to other peripheral biomarkers and subsequent neuroinflammation. All of this may be an underlying indirect immunometabolic mechanism for cognitive performance in patients with SC.
Investigating the relationship between serum Gal-3 levels and cognitive performance, serum cytokines, and white blood cell count in three-month stably treated SC patients could contribute to a better understanding of the specific immune profile in patients with SC.
Twenty-seven patients with SC in remission and 18 healthy volunteers participated in this case-control and correlational study. Clinical assessment was performed using the Positive and Negative Syndrome Scale and the Montreal-Cognitive Assessment. The results of previously measured serum levels of Gal-3, interleukin (IL)-33, soluble suppression of tumorigenicity 2 (sST2), tumor necrosis factor-alpha (TNF-α), IL-6 and IL-17 were used for further statistical analyses, and IL-4, IL-23, IL-1β and transforming growth factor-beta (TGF-β) were now additionally measured with a sensitive enzyme-linked immunosorbent assay. The number of leukocytes in the blood and the percentage of neutrophils, lymphocytes, and monocytes were determined with a standardized routine measurement procedure. Statistical analyses were performed using SPSS 20.0 software.
Serum Gal-3 correlated positively with TNF-α, IL-23, and soluble sST2 in SC in remission and was associated with downregulation of the counterregulatory cytokine TGF-β and appears to play a role in disrupting leukocyte migration. The increase in Gal-3 might be influenced by risperidone dosing.
The combined activity of Gal-3 and proinflammatory cytokines, TGF-β downregulation and lower counts of leukocytes influence the SC duration. Gal-3 likely manifests indirect immunometabolic regulation of cognition in SC.
We observed that Gal-3 contributes to ongoing peripheral systemic inflammation and disease duration in patients with SC. Moreover, its influence on blood-brain barrier permeability and consequent neuroinflammation should be explored. Inflammation also appears to be the potential pathway by which Gal-3 may affect cognitive functioning in SC.
We thank Aleksandar Ilic for excellent technical assistance and Bojana Mircetic for language editing.
| 1. | Debnath M, Venkatasubramanian G, Berk M. Fetal programming of schizophrenia: select mechanisms. Neurosci Biobehav Rev. 2015;49:90-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 2. | Zengeler KE, Lukens JR. Innate immunity at the crossroads of healthy brain maturation and neurodevelopmental disorders. Nat Rev Immunol. 2021;21:454-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 190] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 3. | Trépanier MO, Hopperton KE, Mizrahi R, Mechawar N, Bazinet RP. Postmortem evidence of cerebral inflammation in schizophrenia: a systematic review. Mol Psychiatry. 2016;21:1009-1026. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 312] [Cited by in RCA: 290] [Article Influence: 29.0] [Reference Citation Analysis (0)] |
| 4. | Dong Y, Yong VW. When encephalitogenic T cells collaborate with microglia in multiple sclerosis. Nat Rev Neurol. 2019;15:704-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 123] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 5. | Bechter K. Updating the mild encephalitis hypothesis of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:71-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 6. | Subbanna M, Shivakumar V, Talukdar PM, Narayanaswamy JC, Venugopal D, Berk M, Varambally S, Venkatasubramanian G, Debnath M. Role of IL-6/RORC/IL-22 axis in driving Th17 pathway mediated immunopathogenesis of schizophrenia. Cytokine. 2018;111:112-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 7. | Sahbaz C, Zibandey N, Kurtulmus A, Duran Y, Gokalp M, Kırpınar I, Sahin F, Guloksuz S, Akkoc T. Reduced regulatory T cells with increased proinflammatory response in patients with schizophrenia. Psychopharmacology (Berl). 2020;237:1861-1871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 8. | Kim YK, Myint AM, Lee BH, Han CS, Lee HJ, Kim DJ, Leonard BE. Th1, Th2 and Th3 cytokine alteration in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:1129-1134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 153] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 9. | Upthegrove R, Manzanares-Teson N, Barnes NM. Cytokine function in medication-naive first episode psychosis: a systematic review and meta-analysis. Schizophr Res. 2014;155:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 395] [Article Influence: 32.9] [Reference Citation Analysis (0)] |
| 10. | Dickerson F, Stallings C, Origoni A, Schroeder J, Katsafanas E, Schweinfurth L, Savage C, Khushalani S, Yolken R. Inflammatory Markers in Recent Onset Psychosis and Chronic Schizophrenia. Schizophr Bull. 2016;42:134-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 11. | Chen HY, Fermin A, Vardhana S, Weng IC, Lo KF, Chang EY, Maverakis E, Yang RY, Hsu DK, Dustin ML, Liu FT. Galectin-3 negatively regulates TCR-mediated CD4+ T-cell activation at the immunological synapse. Proc Natl Acad Sci U S A. 2009;106:14496-14501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 162] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 12. | Borovcanin MM, Radosavljevic GD, Pantic J, Milovanovic J, Mijailovic NR, Arsenijevic AN, Arsenijevic NN. Contrasting Roles of the Galectin-3 in the Schizophrenia Onset, Clinical Presentation, and Somatic Comorbidity. Curr Top Med Chem. 2021;21:1471-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Jeon SB, Yoon HJ, Chang CY, Koh HS, Jeon SH, Park EJ. Galectin-3 exerts cytokine-like regulatory actions through the JAK-STAT pathway. J Immunol. 2010;185:7037-7046. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 148] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 14. | Gittens BR, Bodkin JV, Nourshargh S, Perretti M, Cooper D. Galectin-3: A Positive Regulator of Leukocyte Recruitment in the Inflamed Microcirculation. J Immunol. 2017;198:4458-4469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 49] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 15. | Pong S, Karmacharya R, Sofman M, Bishop JR, Lizano P. The Role of Brain Microvascular Endothelial Cell and Blood-Brain Barrier Dysfunction in Schizophrenia. Complex Psychiatry. 2020;6:30-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 16. | Bechter K, Reiber H, Herzog S, Fuchs D, Tumani H, Maxeiner HG. Cerebrospinal fluid analysis in affective and schizophrenic spectrum disorders: identification of subgroups with immune responses and blood-CSF barrier dysfunction. J Psychiatr Res. 2010;44:321-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 161] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 17. | Parikh NU, Aalinkeel R, Reynolds JL, Nair BB, Sykes DE, Mammen MJ, Schwartz SA, Mahajan SD. Galectin-1 suppresses methamphetamine induced neuroinflammation in human brain microvascular endothelial cells: Neuroprotective role in maintaining blood brain barrier integrity. Brain Res. 2015;1624:175-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 18. | Nishikawa H, Liu L, Nakano F, Kawakita F, Kanamaru H, Nakatsuka Y, Okada T, Suzuki H. Modified Citrus Pectin Prevents Blood-Brain Barrier Disruption in Mouse Subarachnoid Hemorrhage by Inhibiting Galectin-3. Stroke. 2018;49:2743-2751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 19. | Borovcanin MM, Janicijevic SM, Jovanovic IP, Gajovic N, Arsenijevic NN, Lukic ML. IL-33/ST2 Pathway and Galectin-3 as a New Analytes in Pathogenesis and Cardiometabolic Risk Evaluation in Psychosis. Front Psychiatry. 2018;9:271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 20. | Ashraf GM, Baeesa SS. Investigation of Gal-3 Expression Pattern in Serum and Cerebrospinal Fluid of Patients Suffering From Neurodegenerative Disorders. Front Neurosci. 2018;12:430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 21. | World Health Organization. International Statistical Classification of Diseases and Related Health Problems Tenth Revision. Geneva: World Health Organization; 1992. |
| 22. | Liu J, Xing Y, Gao Y, Zhou C. Changes in serum interleukin-33 levels in patients with acute cerebral infarction. J Clin Neurosci. 2014;21:298-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Kay SR, Opler LA, Fiszbein A. Positive and Negative Syndrome Scale Manual. North Tonawanda, NY: Multi-Health Systems; 1994. |
| 24. | Rodriguez-Jimenez R, Bagney A, Mezquita L, Martinez-Gras I, Sanchez-Morla EM, Mesa N, Ibañez MI, Diez-Martin J, Jimenez-Arriero MA, Lobo A, Santos JL, Palomo T; PARG. Cognition and the five-factor model of the positive and negative syndrome scale in schizophrenia. Schizophr Res. 2013;143:77-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 25. | Ehmann TS, Khanbhai I, Macewan GW, Smith GN, Honer WG, Flynn S, Altman S. Neuropsychological correlates of the PANSS Cognitive Factor. Psychopathology. 2004;37:253-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Kljajevic V. Montreal Cognitive Assessment: Serb`s Version. Aktuelnosti iz neurologije, psihijatrije i granicnih podrucja, 2009; 17: 31–39. |
| 27. | Gil-Berrozpe GJ, Sánchez-Torres AM, García de Jalón E, Moreno-Izco L, Fañanás L, Peralta V, Cuesta MJ; SEGPEPs group. Utility of the MoCA for cognitive impairment screening in long-term psychosis patients. Schizophr Res. 2020;216:429-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 28. | Borovcanin MM, Minic Janicijevic S, Jovanovic IP, Gajovic NM, Jurisevic MM, Arsenijevic NN. Type 17 Immune Response Facilitates Progression of Inflammation and Correlates with Cognition in Stable Schizophrenia. Diagnostics (Basel). 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 29. | Kumric M, Ticinovic Kurir T, Borovac JA, Bozic J. Role of novel biomarkers in diabetic cardiomyopathy. World J Diabetes. 2021;12:685-705. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 19] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (2)] |
| 30. | Dal Lin C, Brugnolo L, Marinova M, Plebani M, Iliceto S, Tona F, Vitiello G. Toward a Unified View of Cognitive and Biochemical Activity: Meditation and Linguistic Self-Reconstructing May Lead to Inflammation and Oxidative Stress Improvement. Entropy (Basel). 2020;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 31. | Kajitani K, Yanagimoto K, Nakabeppu Y. Serum galectin-3, but not galectin-1, levels are elevated in schizophrenia: implications for the role of inflammation. Psychopharmacology (Berl). 2017;234:2919-2927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 32. | Schroeder JT, Adeosun AA, Bieneman AP. Epithelial Cell-Associated Galectin-3 Activates Human Dendritic Cell Subtypes for Pro-Inflammatory Cytokines. Front Immunol. 2020;11:524826. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 33. | Bonsack F, Sukumari-Ramesh S. Differential Cellular Expression of Galectin-1 and Galectin-3 After Intracerebral Hemorrhage. Front Cell Neurosci. 2019;13:157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 34. | Borovcanin MM, Vesic K, Jovanovic M, Mijailovic NR. Galectin-3 possible involvement in antipsychotic-induced metabolic changes of schizophrenia: A minireview. World J Diabetes. 2021;12:1731-1739. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 35. | Pang J, Nguyen VT, Rhodes DH, Sullivan ME, Braunschweig C, Fantuzzi G. Relationship of galectin-3 with obesity, IL-6, and CRP in women. J Endocrinol Invest. 2016;39:1435-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 36. | Tymińska A, Kapłon-Cieślicka A, Ozierański K, Budnik M, Wancerz A, Sypień P, Peller M, Balsam P, Opolski G, Filipiak KJ. Association of Galectin-3 and Soluble ST2, and Their Changes, with Echocardiographic Parameters and Development of Heart Failure after ST-Segment Elevation Myocardial Infarction. Dis Markers. 2019;2019:9529053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 37. | Shimura T, Shibata M, Gonda K, Nakajima T, Chida S, Noda M, Suzuki S, Nakamura I, Ohki S, Takenoshita S. Association between circulating galectin-3 levels and the immunological, inflammatory and nutritional parameters in patients with colorectal cancer. Biomed Rep. 2016;5:203-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 38. | Le Mercier M, Fortin S, Mathieu V, Kiss R, Lefranc F. Galectins and gliomas. Brain Pathol. 2010;20:17-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 86] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 39. | Fermino ML, Dias FC, Lopes CD, Souza MA, Cruz ÂK, Liu FT, Chammas R, Roque-Barreira MC, Rabinovich GA, Bernardes ES. Galectin-3 negatively regulates the frequency and function of CD4(+) CD25(+) Foxp3(+) regulatory T cells and influences the course of Leishmania major infection. Eur J Immunol. 2013;43:1806-1817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 40. | Blokzijl A, Dahlqvist C, Reissmann E, Falk A, Moliner A, Lendahl U, Ibáñez CF. Cross-talk between the Notch and TGF-beta signaling pathways mediated by interaction of the Notch intracellular domain with Smad3. J Cell Biol. 2003;163:723-728. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 278] [Cited by in RCA: 321] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 41. | Miller BJ, Buckley P, Seabolt W, Mellor A, Kirkpatrick B. Meta-analysis of cytokine alterations in schizophrenia: clinical status and antipsychotic effects. Biol Psychiatry. 2011;70:663-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1188] [Cited by in RCA: 1395] [Article Influence: 93.0] [Reference Citation Analysis (0)] |
| 42. | Meyer U, Schwarz MJ, Müller N. Inflammatory processes in schizophrenia: a promising neuroimmunological target for the treatment of negative/cognitive symptoms and beyond. Pharmacol Ther. 2011;132:96-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 193] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 43. | Obuchowicz E, Bielecka-Wajdman AM, Paul-Samojedny M, Nowacka M. Different influence of antipsychotics on the balance between pro- and anti-inflammatory cytokines depends on glia activation: An in vitro study. Cytokine. 2017;94:37-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 44. | MacDowell KS, Caso JR, Martín-Hernández D, Moreno BM, Madrigal JLM, Micó JA, Leza JC, García-Bueno B. The Atypical Antipsychotic Paliperidone Regulates Endogenous Antioxidant/Anti-Inflammatory Pathways in Rat Models of Acute and Chronic Restraint Stress. Neurotherapeutics. 2016;13:833-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 45. | Telford JE, Bones J, McManus C, Saldova R, Manning G, Doherty M, Leweke FM, Rothermundt M, Guest PC, Rahmoune H, Bahn S, Rudd PM. Antipsychotic treatment of acute paranoid schizophrenia patients with olanzapine results in altered glycosylation of serum glycoproteins. J Proteome Res. 2012;11:3743-3752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 46. | Jovanovic M, Simovic Markovic B, Gajovic N, Jurisevic M, Djukic A, Jovanovic I, Arsenijevic N, Lukic A, Zdravkovic N. Metabolic syndrome attenuates ulcerative colitis: Correlation with interleukin-10 and galectin-3 expression. World J Gastroenterol. 2019;25:6465-6482. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 47. | Rind L, Ahmad M, Khan MI, Badruddeen, Akhtar J, Ahmad U, Yadav C, Owais M. An insight on safety, efficacy, and molecular docking study reports of N-acetylcysteine and its compound formulations. J Basic Clin Physiol Pharmacol. 2021;33:223-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 48. | Wasyanto T, Yasa' A, Jalaludinsyah A. Effect of Oral N-Acetylcysteine Supplementation on the Immunity System in Patients with Acute Myocardial Infarction. Acta Med Indones. 2019;51:311-317. [PubMed] |
| 49. | Pyatoykina AS, Zhilyaeva TV, Semennov IV, Mishanov GA, Blagonravova AS, Mazo GE. [The double-blind randomized placebo-controlled trial of N-acetylcysteine use in schizophrenia: preliminary results]. Zh Nevrol Psikhiatr Im S S Korsakova. 2020;120:66-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
STROBE Statement: The authors have read the STROBE Statement—checklist of items, and the manuscript was prepared and revised according to the STROBE Statement—checklist of items.
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country/Territory of origin: Serbia
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Khosravi M, Iran; Radhakrishnan R, New Zealand; Shu Liu, China S-Editor: Fan JR L-Editor: A P-Editor: Fan JR