Published online Apr 19, 2022. doi: 10.5498/wjp.v12.i4.615
Peer-review started: March 19, 2021
First decision: May 5, 2021
Revised: May 15, 2021
Accepted: February 23, 2022
Article in press: February 23, 2022
Published online: April 19, 2022
Processing time: 390 Days and 6.9 Hours
Fibromyalgia (FM) patients are treated with antidepressants, and in most cases, these drugs lose efficacy or present side effects. Intravenous lidocaine (IL) is an anesthetic drug used in some FM trials.
To systematically review the safety and efficacy of IL in FM patients.
To systematically search PubMed for articles in English, Spanish, and Japanese with English Abstracts on FM and lidocaine between 1966 and February 2021. This study was registered at PROSPERO.
We found only ten articles published in this field, with a total of 461 patients. Females predominated varying from 95% to 100% in the studies. Age varied from 40.9 to 55 years old. Disease duration varied from 1 mo to 6.4 years. Lidocaine dose varied from 2 to 7.5 mg/kg via intravenous infusion. Follow-up period varied from 65.7 to 90 days. Regarding outcomes, most studies used the visual analogue scale (VAS) for pain; before short-term lidocaine administration, VAS was between 6.1 and 8.1 and after treatment was between 1.7 and 4.5 mm. Concerning long term lidocaine, VAS varied from 30% to 35.4% after lidocaine infusion. Side effects were observed in 0% to 39.6% of cases, they were usually mild or moderate.
This study demonstrates the short-term effectiveness and safety of intravenous lidocaine in FM patients. However, more studies, including long-term follow-up, are still needed.
Core Tip: This is the first systematic review on lidocaine studies in fibromyalgia patients.
- Citation: de Carvalho JF, Skare TL. Lidocaine in fibromyalgia: A systematic review. World J Psychiatry 2022; 12(4): 615-622
- URL: https://www.wjgnet.com/2220-3206/full/v12/i4/615.htm
- DOI: https://dx.doi.org/10.5498/wjp.v12.i4.615
Fibromyalgia is a painful chronic disease characterized by diffuse pain for over three months with associated co-morbidities including headaches, irritable bowel syndrome, anxiety, depression, and others[1]. FM is the third most common musculoskeletal condition and may affect 0.4% (in Greece) to 8.8% (in Turkey) of a population and has a global prevalence of 2.7%[1].
Standard treatments for FM include physical exercise, psychological intervention, and medication. Regarding pharmacological treatment, antidepressants are the leading choice for this condition. However, adverse effects can lead to dropouts, which range from 9% to 23% in short-term studies and from 11.4% to 27.2% in long-term studies[2]. Lack of efficacy is also observed during FM treatment, which can reach between 50 to 60% of cases[2]. Thus, different treatment modalities are desired for unresponsive patients or who present side effects with drugs.
Lidocaine is a topical anesthetic drug used worldwide to treat specific clinical situations such as systemic sclerosis. It is used intravenously in chronic pain and arrhythmia cases[3]. Intravenous lidocaine has been shown to control the symptoms of diabetic neuropathy[4]; there are some studies on intravenous lidocaine use in FM patients with controversial results[5-14].
In light of this, the objective of this article is to perform a systematic review of the safety and efficacy of lidocaine in FM patients.
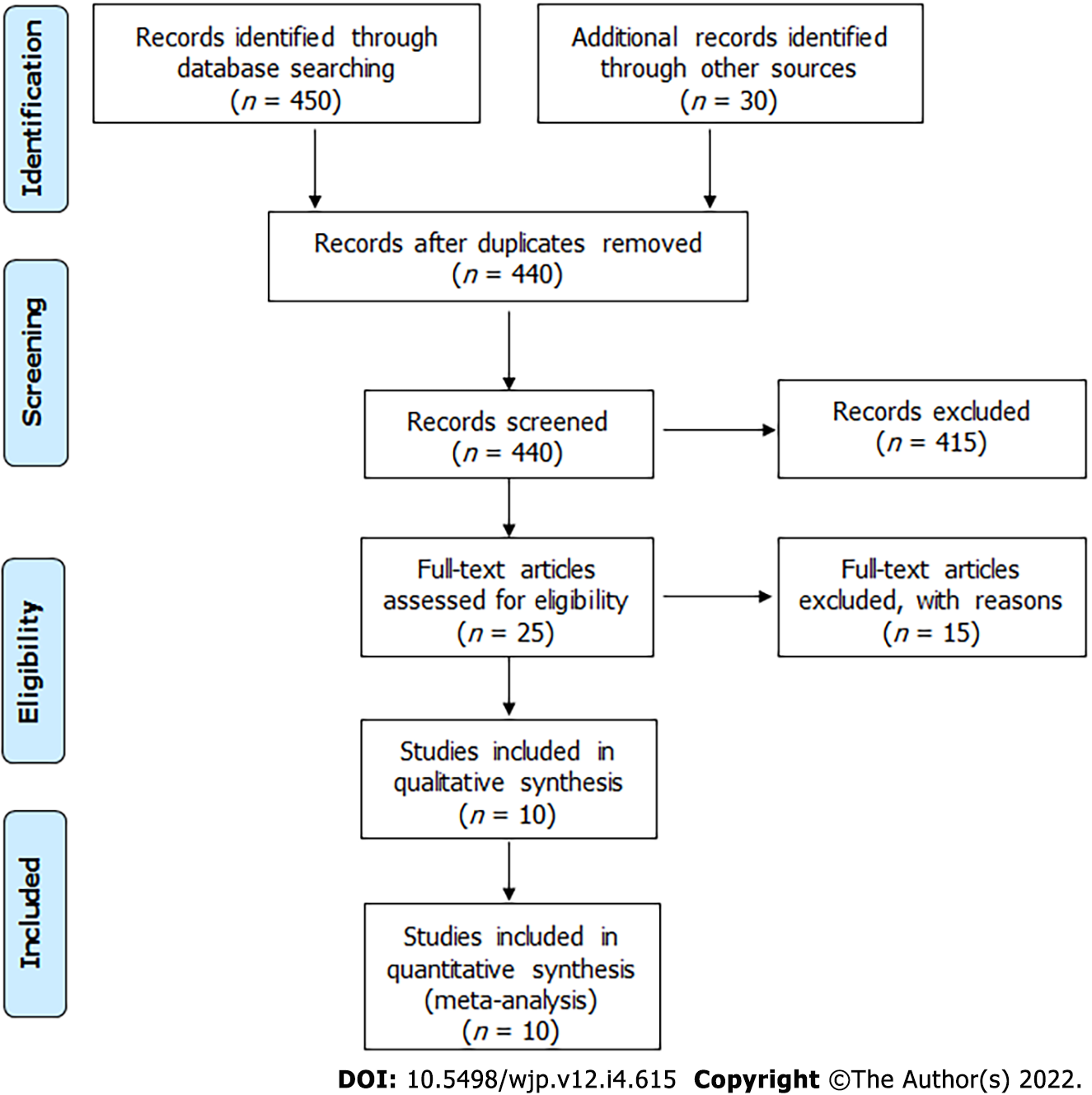
We performed a systematic search of articles published in PubMed/MEDLINE, Web of Sciences, LILACS, and Scielo from 1966 to November 2020 using the following MeSH entry terms: "lidocaine" and "fibromyalgia." We used equivalent strategies in other databases. All related articles are based on "lidocaine" and "fibromyalgia" without language restriction. The reference lists in the selected articles were analyzed to identify other publications. Initially, two authors (JFC and TLS) performed the literature search and independently selected the study abstracts. In the second stage, the same reviewers independently read the full-text articles selected by abstracts. Disagreements arising in consensus meetings were resolved by a third reviewer. The authors followed PRISMA guidelines[15]. We designed a standardized form to extract the following information from relevant articles regarding authors, year of publication, number of patients studied, demographic data, disease duration, study follow-up, pre- and post-intervention VAS, lidocaine posology, and outcomes (Figure 1).
This study was registered at PROSPERO under number CRD42021227210.
Demographic and clinical data and pre- and post-lidocaine treatment VAS scores for FM patients are shown in Table 1.
| Ref. | Study design | N, female sex | Age, yr | Disease duration | Follow-up | Lidocaine prescription | Concomitant treatment | Short-term VAS, | Long-term VAS | Other outcomes | Adverse effects | ||
| Pre and post lidocaine | Pre and post, placebo | Pre and post lidocaine | Pre and post placebo | ||||||||||
| Verd et al[5] | Prospective | 48, 95.8% | Median age-55 | 90 d | Escalating dose from 2 mg/kg to 5 mg/kg per day, IV during 10 d | - | Pain measured by BPI 29.5→26.5 | - | In 90 d BPI = 30.0 | - | Improved in MOS and EXPEC; Short-lived improvement in BPI, BFI and depression | Nausea (n = 8); Worsening pain (n = 1) | |
| Wilderman et al[6] | Retrospective | 74, 9.7% | 51.3 | NA | 5 mg/kg→65.7 d; 7.5 mg/kg→86.3 d; 7.5 mg/kg→90.9 d | Escalating doses: 5 mg/kg, 7.5 mg/kg and 7.5 mg/kg + magnesium 2.5 g IV | None | ∆ VAS in 5 mg/kg = 2.41; ∆ VAS in 7.5 mg/kg = 3.15; ∆ VAS in 7.5 mg/kg + Mg = 3.62 | NA | Pain relief:In 30.2% of 5 mg/kg- median time 62 d; In 39.1% in 7.5 mg/kg; median time 62.5 d; 40.6% in 7.5 mg/kg + Mg; Median time 64 d | NA | - | 24/222 infusions (10.8%)-dizziness, nausea, hyperglycemia, headache, lip numbness and mild dyspnea |
| Kim et al[7] | Retrospective | 55, 94.5% | NA | NA | After 1 infusion | 5 mg/kg (maximum of 500 mg), IV | 7.6 ± 1.6→5.8 ± 2.2 | - | - | - | Caucasians and non-smokers had better results | NA | |
| Albertoni Giraldes et al[8] | RCT | 42, 95% | 42.4 ± 9.4 | 6.0 ± 5.05 | 8 wk | 250 mg/wk – for 4 wk IV; vs saline | Amitriptyline 25 mg, paracetamol if needed. | 6 ± 1.3 3.9 ± 2.8 | 7.2 ± 1.3→2.7 ± 2.9 | - | - | IL-1, IL-6 and IL-8 values did not change | Placebo equal to lidocaine: nausea, vomiting, drowsiness, paresthesia, constipation and dry mouth |
| Staud et al[9] | Prospective | 62, 100% | 45.8 ± 14.8 | NA | Data collection just after injections | Group 1 (n = 20)- 4 injections of 50 mg lidocaine, IM; Group 2 (n = 21)- 2 injections 50 mg lidocaine + 2 saline, IM; Group 3 (n = 21)- four injections saline, IM | Muscle relaxing drugs and/or tricyclics were allowed | VAS declined 38% | - | - | - | Mechanical and heat hyperalgesia decreased significantly | NA |
| Vlainich et al[10] | RCT, | 30, 100% | Group 1-40.9 ± 11.6; Group 2-44.7 ± 10.5 | NA | 4 wk | Group 1- (n = 15) lidocaine 240 mg/wk for 4 wk, IV; Group 2- (n = 15) Saline | Amitriptyline 25 mg | 7.6 ± 0.8→4.1 ± 2.3 | 7.0 ± 1.2→4.0 ± 2.1 | - | - | norepinephrine and serotonin levels unchanged dopamine levels ↑ week 4 in the placebo group. | No |
| Schafranski et al[11] | Prospective | 23, 95.6% | NA | NA | 4 wk | Sequential lidocaine infusions from 2-5 mg/kg for 5 d, IV | None | 8.1 ± 1.7→6.8 ± 2.4 | - | Mean VAS of pain = 7.1 ± 2.3 in 30 d | - | FIQ, HAQ improved significantly | No |
| Raphael et al[12] | Prospective and retrospective | 106, 92% prospective arm (to see side effects); 50, 82%retrospective arm (to see efficacy) | 51.4 prospective arm; 50.2 retrospective arm | Prospective arm- NA; 6.6 ± 4.5 yr in retrospective arm | N/A | Started at 5 mg/kg-100 mg and increased to 5 mg/kg+150 mg (maximum 550 mg) IV; For 6 consecutive days | None | Only in the retrospective arm 9→5; Mean duration pain relief 11.5 ± 6.5 wk | - | - | No improvement in work status; improvement in several sociological and psychological dimensions | Only in the prospective arm; 2 major effects: (pulmonary edema and supra ventricular tachycardia); 42/106 minor effects: Hypotension (n = 17); Headache (n = 8), hypertension (n = 5), tachycardia (n = 1), arrhythmia (n = 1), pulmonary edema (n = 1) | |
| Bennett et al[13] | Prospective | 10, 100% | 44.2 | 16 (1-192) mo | 4 wk | Started at 250 mg/d and increased by 50 mg/d to 500 mg/dfor 6 d, IV | Haloperidol 0.5 mg/d + clomipramine 10 mg/d or Amitriptyline 10 mg/d | 8 4.1 | - | Mean VAS of pain = 5.4 in 30 d | - | Stopped analgesics. Mood improved but not statistically significant | None |
| Sörensen et al[14] | Double blind, placebo-controlled | 11, 100% | 41, (range 21-59) | 5 yr (range 2-11) | 1 wk after 2nd injection | 2 injections, IV; 5 mg/kg vs saline | Paracetamol or dextropropoxyphene | (VAS from 0-100); 6.1→4.5 | (VAS from 0-100); 51→51 | - | - | Tender points, muscle endurance and muscle strength (except dorsiflexors of wrist) unchanged | NA |
There were only ten articles published in this field, with a total of 461 patients. Females predominated varying from 95% to 100% in the studies. Age varied from 40.9 to 55 years old. Disease duration varied from 1 month to 6.4 years.
Lidocaine IV dosage varied from 2 to 7.5 mg/kg. Follow-up was from 65.7 to 90 d.
Regarding outcome, most studies evaluated VAS. Before lidocaine, VAS ranged from 6.1 to 8.1 and after treatment, from 1.7 to 4.5 mm in the short term. Concerning long term after lidocaine infusion, VAS varied 30% to 35.4%.
Side effects were observed in 0% to 39.6% of cases, usually with mild or moderate repercussions. These effects were dizziness, nausea, vomiting, hyperglycemia, headache, lip numbness, mild dyspnea, paresthesia, dry mouth, and increasing pain. The significant effects were pulmonary edema and supraventricular tachycardia.
This is the first study to systematically review the therapeutic effects of intravenous lidocaine in FM patients.
The study strengths are: (1) The inclusion of studies with patients with international criteria for FM; and (2) The exclusion of case reports, case series, and observational studies. Prospective studies present a higher degree of evidence.
The analgesic properties of intravenous lidocaine were first observed in 1962 when used to treat postoperative pain[16]. Thirty-six years later, a study demonstrated that lidocaine might be used to treat postoperative pain, reducing hospital stay in patients who had undergone radical prostatectomy[17]. Lidocaine acts by blocking sodium channels on the neuronal membrane that may play a role in the pathogenesis of inflammatory and neuropathic pain[6].
Previous studies have demonstrated the efficacy of intravenous lidocaine in FM patients. Bennett and Tai[13] described improvement in pain scores were maintained even 30 d after lidocaine infusion. Furthermore, Sörensen et al[14] evaluating 12 fibromyalgia patients showed improvements in VAS pain scores during and 15 min after a 30 min infusion of lidocaine in a double-blind placebo-controlled crossover study. Three of the 12 patients who responded to lidocaine had their pain reduced. The authors reported no statistically significant differences between FM and placebo groups in tender points, muscle strength (hip flexors and handgrip), and muscle endurance. However, the lidocaine group exhibited a significant improvement in wrist dorsiflexion muscle strength[14].
Raphael et al[12] conducted a prospective study of the adverse effects of lidocaine in 106 patients with FM and a retrospective questionnaire study of the efficacy of this drug in 50 FM patients. Serial infusions of IV lidocaine were administered for six consecutive days at 5 mg/kg minus 100 mg and increased by 50 mg/d to 5 mg/kg plus 150 mg over 6 h, with the maximum allowable dose being 550 mg. Pain was measured using an 11-point VAS, in a 4-point verbal scale of pain severity (none, mild, moderate, severe), and according to the average number of hours per day in pain. Pain relief was also measured on the 11-point VAS along with pain relief duration. The psychological and social impact of the pain were evaluated by measuring depression, coping ability, dependency, and several other items using the 11-point scales. Pain score and relief interruption, pain mean duration, and verbal assessment were significantly reduced following lidocaine treatment. Mean pain relief duration was 11.5 ± 6.5 wk, ranging from 0 to 36 wk. Psychosocial measurements significantly improved after lidocaine treatment in all parameters except work status.
Schafranski et al[11], in an open trial, showed similar results after five sequential lidocaine infusions with rising dosages (2-5 mg/kg, days 1-5). The Fibromyalgia Impact Questionnaire (FIQ) and a VAS for pain were applied before lidocaine infusion and immediately, and 30 d after the 5th infusion. They observed significant reductions in FIQ and VAS after the fifth infusion which were maintained after 30 d[11].
Finally, some limitations were observed in our study. For instance, no comparison between lidocaine and classical antidepressants used in FM were available in literature. The number of participants was low and future studies should include large patient samples with more long-term follow-up; this would enable a better understanding of the course of this therapeutic modality in FM.
The present study was a systematic review of all prospective studies that evaluated the role of lidocaine in FM patients and found excellent short-term efficacy. Future studies using larger FM patient samples and long-term follow-up which address the safety and efficacy of lidocaine are needed.
Lidocaine is used to treat fibromyalgia patients.
As there are some articles that evaluated the role of lidocaine as therapy of fibromyalgia patients, the authors thought it is important to systematically review this literature.
The authors had the objective to perform the first systematic review on lidocaine in the treatment of fibromyalgia.
Systematic review based on PRISMA guidelines and PROSPERO register.
Most studies showed reduction of pains measured by visual analogic scale after lidocaine infusion.
This systematic review showed that lidocaine is effective and safe for fibromyalgia treatment, mainly in short-term.
Future studies with large number of participants to evaluate the safety and efficacy of lidocaine for fibromyalgia is needed, as short and long-term studies.
| 1. | Sarzi-Puttini P, Giorgi V, Marotto D, Atzeni F. Fibromyalgia: an update on clinical characteristics, aetiopathogenesis and treatment. Nat Rev Rheumatol. 2020;16:645-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 460] [Article Influence: 76.7] [Reference Citation Analysis (0)] |
| 2. | Calandre EP, Rico-Villademoros F, Slim M. An update on pharmacotherapy for the treatment of fibromyalgia. Expert Opin Pharmacother. 2015;16:1347-1368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 3. | Niesters M, Martini C, Dahan A. Ketamine for chronic pain: risks and benefits. Br J Clin Pharmacol. 2014;77:357-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 330] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 4. | Kastrup J, Petersen P, Dejgård A, Angelo HR, Hilsted J. Intravenous lidocaine infusion--a new treatment of chronic painful diabetic neuropathy? Pain. 1987;28:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 199] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 5. | Verd M, Ribera H, Sansaloni C, de Vicente MJ, M. Truyols M. Efficacy of lidocaine infusions in fibromyalgia. Rev Soc Esp del Dolor. 2020;27:287-291. [DOI] [Full Text] |
| 6. | Wilderman I, Pugacheva O, Perelman VS, Wansbrough MCT, Voznyak Y, Zolnierczyk L. Repeated Intravenous Lidocaine Infusions for Patients with Fibromyalgia: Higher Doses of Lidocaine Have a Stronger and Longer-Lasting Effect on Pain Reduction. Pain Med. 2020;21:1230-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Kim YH, Moyse D, Horazeck C, Hsia HL, Roldan CJ, Huh B, Roy L. Lidocaine infusion decreases pain scores in a fibromyalgia pain population with significant differential pain relief secondary to smoking status. Glob J Anesth. 2017;4:16-22. [DOI] [Full Text] |
| 8. | Albertoni Giraldes AL, Salomão R, Leal PD, Brunialti MK, Sakata RK. Effect of intravenous lidocaine combined with amitriptyline on pain intensity, clinical manifestations and the concentrations of IL-1, IL-6 and IL-8 in patients with fibromyalgia: A randomized double-blind study. Int J Rheum Dis. 2016;19:946-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Staud R, Weyl EE, Bartley E, Price DD, Robinson ME. Analgesic and anti-hyperalgesic effects of muscle injections with lidocaine or saline in patients with fibromyalgia syndrome. Eur J Pain. 2014;18:803-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Vlainich R, Issy AM, Sakata RK. Effect of intravenous lidocaine associated with amitriptyline on pain relief and plasma serotonin, norepinephrine, and dopamine concentrations in fibromyalgia. Clin J Pain. 2011;27:285-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Schafranski MD, Malucelli T, Machado F, Takeshi H, Kaiber F, Schmidt C, Harth F. Intravenous lidocaine for fibromyalgia syndrome: an open trial. Clin Rheumatol. 2009;28:853-855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Raphael JH, Southall JL, Treharne GJ, Kitas GD. Efficacy and adverse effects of intravenous lignocaine therapy in fibromyalgia syndrome. BMC Musculoskelet Disord. 2002;3:21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Bennett MI, Tai YM. Intravenous lignocaine in the management of primary fibromyalgia syndrome. Int J Clin Pharmacol Res. 1995;15:115-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 14. | Sörensen J, Bengtsson A, Bäckman E, Henriksson KG, Bengtsson M. Pain analysis in patients with fibromyalgia. Effects of intravenous morphine, lidocaine, and ketamine. Scand J Rheumatol. 1995;24:360-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 161] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 15. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18665] [Cited by in RCA: 18038] [Article Influence: 1061.1] [Reference Citation Analysis (1)] |
| 16. | Bartlett EE, Hutaserani Q. Lidocaine (xylocaine) for the relief of postoperative pain. J Am Med Womens Assoc. 1962;17:809-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 17. | Groudine SB, Fisher HA, Kaufman RP Jr, Patel MK, Wilkins LJ, Mehta SA, Lumb PD. Intravenous lidocaine speeds the return of bowel function, decreases postoperative pain, and shortens hospital stay in patients undergoing radical retropubic prostatectomy. Anesth Analg. 1998;86:235-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 57] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country/Territory of origin: Brazil
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gicchino MF S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ