Published online Feb 19, 2022. doi: 10.5498/wjp.v12.i2.308
Peer-review started: March 18, 2021
First decision: July 15, 2021
Revised: August 14, 2021
Accepted: January 10, 2022
Article in press: January 10, 2022
Published online: February 19, 2022
Processing time: 335 Days and 24 Hours
Temporal lobe epilepsy (TLE) is the most common focal epilepsy subtype in adults and is frequently accompanied by depression, anxiety and psychosis. Aberrations in total paraoxonase 1 (PON1) status may occur in TLE and these psychiatric conditions.
To examine PON1 status, namely Q192R PON1 genotypes and PON1 enzymatic activities, in TLE.
We recruited 40 normal controls and 104 TLE patients, 27 without comorbidities and 77 with comorbidities including mood disorders (n = 25), anxiety disorders (n = 27) and psychosis (n = 25).
Four-(chloromethyl)phenyl acetate hydrolysis (CMPAase) and arylesterase activities were significantly lower in TLE and mesial temporal sclerosis (MTS) with and without psychiatric comorbidities than those in normal controls. The areas under the receiver operating characteristic curve of CMPAase were 0.893 (0.037) for TLE and 0.895 (± 0.037) for MTS. Partial least squares path analysis showed that there were specific indirect effects of PON1 genotype on TLE severity (P < 0.0001) and psychopathology (P < 0.0001), which were both mediated by lowered CMPAase activity, while arylesterase activity was not significant. The severity of TLE was significantly associated with psychopathology scores. Furthermore, PON1 CMPAase activity was inversely associated with Mini Mental State Examination score.
The severity of TLE and comorbidities are to a large extent explained by reduced PON1 enzyme activities and by effects of the Q192R genotype, which are mediated by reduced CMPAase activity. Total PON1 status plays a key role in the pathophysiology of TLE, MTS and psychiatric comorbidities by increasing the risk of oxidative toxicity. PON1 enzyme activities are new drug targets in TLE to treat seizure frequency and psychiatric comorbidities.
Core Tip: The severity of temporal lobe epilepsy (TLE) and mesial temporal sclerosis and their psychiatric comorbidities including depression, anxiety and psychosis are largely explained by lowered paraoxonase 1 (PON1) enzyme activities, which mediate the effects of the Q192R PON1 genotype on psychopathology and epilepsy severity. It is argued that PON1 status may play a key role in the pathophysiology of TLE, mesial temporal sclerosis and its psychiatric comorbidities by increasing the risk of neuro-oxidative toxicity. It is concluded that PON1 enzyme activities are new drug targets to treat seizure frequency and psychiatric comorbidities in patients with TLE.
- Citation: Michelin AP, Maes MHJ, Supasitthumrong T, Limotai C, Matsumoto AK, de Oliveira Semeão L, de Lima Pedrão JV, Moreira EG, Kanchanatawan B, Barbosa DS. Reduced paraoxonase 1 activities may explain the comorbidities between temporal lobe epilepsy and depression, anxiety and psychosis. World J Psychiatry 2022; 12(2): 308-322
- URL: https://www.wjgnet.com/2220-3206/full/v12/i2/308.htm
- DOI: https://dx.doi.org/10.5498/wjp.v12.i2.308
Patients with epilepsy suffer from recurrent seizures originating from excessive and synchronous firing of groups of neurons in the brain[1,2]. Temporal lobe epilepsy (TLE) is the most common focal epilepsy subtype in adults, with a 40% incidence in relation to all types of epilepsy[3]. Hippocampal sclerosis or mesial temporal sclerosis (MTS), which is associated with neuronal loss and gliosis, is the most common primary pathology, accounting for 36% of all focal pathologies of epilepsy[4,5].
Neuropsychiatric disorders such as mood, anxiety and psychotic disorders are observed in about 30%-70% of TLE patients, and these comorbidities have a significant impact on the patient's quality of life[6-8]. In TLE, comorbid depression has the highest prevalence (42.9%) followed by anxiety disorders (18.4%), especially generalized anxiety disorder (GAD), while psychosis (PSY) shows a lower prevalence (around 5%-7%)[9,10].
In epilepsy, the first seizure may induce reactive oxygen and nitrogen species (ROS/RNS), and when these reactive species are produced in large quantities and exceed the antioxidant defense mechanisms, they may cause oxidative damage to lipids, proteins, DNA and mitochondria, excitotoxicity and neuroinflammation[11,12]. Oxidative neurotoxicity is particularly important in the central nervous system, since the brain is sensitive to oxidative stress due to its high energy and aerobic metabolic demand[13-15]. Mitochondrial dysfunctions arising from ROS/RNS and the consequent oxidative lesions are frequently observed after seizures and during epileptogenesis and, additionally, are associated with neurodegeneration[13]. During seizures, performant antioxidant defenses are extremely important to protect brain tissues against oxidative damage ensuing from lipid peroxidation and aldehyde formation[15]. Experimental studies suggest that these oxidative pathways play an important role in the pathophysiology of TLE and TLE progression[16,17]. In addition, TLE is associated with decreases in antioxidant defenses as indicated by lowered superoxide dismutase[18] and glutathione levels in the hippocampus[19].
The enzyme paraoxonase 1 (PON1) is of particular importance because it is bound to high density lipoprotein (HDL) and has the ability to catalyze the hydrolysis of organic phosphates and lipid peroxides, protecting lipids, HDL and low density lipoprotein (LDL) from oxidation[20]. The PON1 Q192R single nucleotide polymorphism determines in part the catalytic activity and antioxidant properties of PON1 enzymes[21]. The alloenzyme R has a higher efficiency in detoxifying substrates such as paraoxon and 4-(chloromethyl) phenyl acetate (CMPA), and homozygous RR carriers metabolize lipids more efficiently than alloenzyme Q carriers, explaining their stronger protection against lipid peroxidation[22]. Nevertheless, there are only few studies that have examined total PON1 status (that is enzymatic activities and PON1 genotypes) in epilepsy. Dönmezdil et al[23] and Calik et al[24] found significantly lowered serum PON1 and arylesterase activities in patients with epilepsy, although these authors did not measure total PON1 status, which should include total enzyme activities and PON1 genotypes[25]. Moreover, no studies examined the associations between PON1 status and psychiatric comorbidities in TLE, although PON1 status is significantly associated with major depression, anxiety disorders and subtypes of PSY[25].
Hence, the objective of this study was to evaluate PON1 status, namely CMPAase and arylesterase activities as well as PON1 Q192R genotypes, in patients with TLE and MTS with and without comorbid PSY, depression and anxiety.
For this case-control study, 104 patients with TLE and 40 normal controls were recruited. Patients with TLE were admitted to the outpatient clinic of the Comprehensive Epilepsy Unit of King Chulalongkorn Memorial Hospital, Bangkok, Thailand from December 2013 to December 2014. The patients were diagnosed with TLE by a senior neurologist specializing in epilepsy. The latter diagnosis was based on the history of clinical characteristics of seizures, electroencephalography records and magnetic resonance imaging scans performed in all patients. The patients with TLE were subdivided into four subgroups based on the presence of psychiatric comorbidities according to the criteria established in the Diagnostic and Statistical Manual of Mental Disorders, fourth edition, text revision (DSM-IV-TR), namely: (1) Mood disorders due to TLE with depressive characteristics (n = 25); (2) Anxiety Disorder Due to TLE with panic attacks, GAD or obsessive-compulsive symptoms (n = 27); (3) Psychotic disorder due to TLE with delusions or hallucinations (n = 25); and (4) “Pure TLE” when there were no psychiatric comorbidities (n = 27).
Exclusion criteria for healthy controls were a diagnosis of epilepsy, febrile seizures in childhood and any other axis 1 psychiatric disorder and a positive family history of epilepsy, mood disorders or psychotic disorders. Exclusion criteria for TLE patients were: (1) Any other axis 1 disorder, except mood, anxiety and psychotic disorders due to TLE; and (2) Interictive dysphoric disorder. Exclusion criteria for patients with mood disorders due to TLE were anxiety and psychotic disorders. In the patient group with anxiety disorders due to TLE, we excluded patients with mood disorders or PSY, and, in the patient group with psychotic disorder due to TLE, we excluded patients with mood and anxiety disorders. In addition, patients with “pure TLE” did not suffer from any of the above-mentioned psychiatric comorbidities. Exclusion criteria for patients and controls were: (1) (Auto)immune diseases including diabetes, psoriasis, chronic obstructive pulmonary disease, inflammatory bowel disease; (2) Neurodegenerative and neuroinflammatory disorders, such as multiple sclerosis, Parkinson's disease and Alzheimer's disease; (3) Immune, inflammatory or allergic response 3 mo before the start of the study; (4) A lifetime history of treatment with immunomodulatory drugs; (5) Use of therapeutic doses of antioxidants or supplements containing ω3-polyunsaturated fatty acids 3 mo before inclusion in the study; and (6) Pregnant or lactating women.
Prior to participation in the research, all individuals signed a written informed consent form. The Institutional Review Board of the Faculty of Medicine, King Chulalongkorn Memorial Hospital, Bangkok, Thailand, gave their approval to this research (IRB number 305/56) in accordance with the International Guideline for the Protection of Human Research, as established by the Declaration of Helsinki, The Belmont Report, International Conference on Harmonization of Good Clinical Practice and Council for International Organizations of Medical Sciences Guideline.
Semi-structured interviews were conducted by a senior neurologist and a senior psychiatrist specialized in epilepsy. The neurologist collected sociodemographic data and TLE characteristics including family history of epilepsy, age at onset of TLE, type of epilepsy, location of the lesion, seizure frequency, seizure control (seizure free, fairly and poorly controlled seizures); history of post-ictal confusion, type of seizures and use of antiepileptic drugs (AEDs). The diagnosis of TLE was made based on the history of partial seizures and electroencephalography records of epileptiform activities in one or both temporal regions. In addition, the senior neurologist and a radiologist used results of magnetic resonance imaging scans to make the diagnosis of MTS or other types of TLE. Patients and controls were evaluated by the senior psychiatrist to identify psychotic symptoms, anxiety and depression, using DSM-IV-TR criteria. The diagnosis of mood disorders due to TLE comprises major depression in an acute episode or in partial remission and ictus-related depression. The diagnosis of anxiety disorder due to TLE comprises patients with panic, GAD, obsessive-compulsive symptoms and ictus-related anxiety such as fear and horror. Psychotic disorders due to TLE comprise delusions (persecutors, possessed, paranoid and reference ideas), hallucinations (auditory, taste, visual and olfactory) and ictus-related psychoses, as described by Kanchanatawan et al[26]. These psychoses can be ictal, pre-ictal, post-ictal, psychotic aura, peri-ictal, interictal or schizophrenic-like PSY. In this context, fear, horror, forced thoughts, out-of-body experiences and going crazy were not considered to be psychotic.
The senior psychiatrist (BK) also assessed the Brief Psychiatric Rating Scale (BPRS), the Hamilton Depression (HAM-D) and Anxiety (HAM-A) Rating Scale[27-29] and also assessed the Mini Mental State Examination (MMSE)[30] in patients and controls. The body mass index (BMI) was calculated using the ratio between body weight in (kg) and height (m2) and tobacco use disorder was evaluated using the DSM-IV-TR criteria.
Blood samples were collected at 8:00 am, after an overnight fast, and serum was aliquoted and stored at -80 °C until thawed for PON1 status. Total PON1 activity was determined by the formation of phenyl acetate hydrolysis[22]. The rate of phenylacetate hydrolysis was determined on a Perkin Elmer® EnSpire model microplate reader (Waltham, MA, United States) at a wavelength of 270 nm measured over 4 min (16 readings at 15 s between readings) with the temperature maintained at 25 °C. Activity was expressed in U/mL based on the phenyl acetate molar extinction coefficient, which is 1.31 mmol/Lol/Lcm-1. For the stratification of the functional genotypes of the PON1Q192R polymorphism (PON1 192Q/Q, PON1 192Q/R, PON1 192R/R), we used CMPA (Sigma, St. Louis, MO, United States) and phenyl acetate (PA, Sigma). PON1 polymorphism confers differences in hydrolysis capacity, and this allows to stratify the genotypes after phenotypic analysis of enzyme activity. Isoform R has high hydrolysis activity on CMPA, whilst alloenzyme Q has lower hydrolytic activity on CMPA, and both alloforms hydrolyze PA with similar efficacy. Therefore, the reaction with PA is performed with high salt concentrations, which partially inhibits the activity of R allozyme, thereby providing a better distinction between the three functional genotypes. The rate of PA hydrolysis in low salt concentration by arylesterase was also measured.
We used analysis of variance to assess differences in scale variables between diagnostic groups and analysis of contingency tables (χ2-tests) to assess associations among categorical variables. We used multivariate general linear model (GLM) analysis to ascertain the associations between diagnosis and biomarkers while controlling for possible background variables including sex, age, BMI, smoking and the drug state. Consequently, tests for between-subject effects were employed to examine the associations between diagnosis and each of the biomarkers. Model-generated estimated marginal mean values were computed, and protected pair-wise comparisons among treatment means were employed to delineate the differences among the study groups. We used P-corrections for false discovery rate to control for multiple statistical tests[31]. Automatic binary regression analysis was employed to delineate the best biomarker prediction of TLE (controls as reference group). We employed automatic stepwise (step-up) multiple regression analysis to assess the most significant biomarkers predicting the BPRS, HAM-D, HAM-A and MMSE scores. Regression analyses were double-checked for collinearity and bootstrapped using 5000 samples, and the bootstrapped results are shown in case of discrepant results. All tests were two-tailed and a P value of 0.05 was used for statistical significance. IBM SPSS25 (Armonk, NY, United States) (for windows was used to analyze the data. The number of participants was established a priori using GPower: At least 138 people were required to achieve a power of 0.8 (effects size: 0.3; alpha = 0.05; four groups and four covariates) (analysis of covariance).
To examine the causal associations between PON1 genotype and PON1 enzyme activities and TLE characteristics and psychopathology, we performed partial least squares (PLS) path analysis employing SmartPLS[32]. SmartPLS is a structural equation modeling technique that allows to examine causal pathways explaining the effects of input variables (PON1 genotype) on output variables (PON1 activities and clinical aspects of TLS and comorbidities), whereby variables are entered as single indicator variables (PON1 genotype and enzyme activities) or as latent vectors (LV) extracted from TLE features (TLE; aura; postictal confusion; TLE frequency; seizure free, fairly and poorly controlled seizures); and the three psychopathological rating scale scores (BPRS, HAM-A, HAM-D)[32]. We conducted PLS path analysis when the model complied with quality criteria, i.e. model SRMR < 0.080 and when the LVs showed adequate reliability validity as indicated by composite reliability > 0.7, rho_A > 0.8, Cronbach’s alpha > 0.7 and average variance extracted (AVE) > 0.5; while the outer model factor loadings were > 0.6 with P < 0.001[32]. Consequently, we conducted complete and consistent PLS path analysis using 5.000 bootstrap samples to compute path coefficients (with P values) and the significance of total, total indirect and specific indirect effects.
Table 1 shows the socio-demographic data of the participants in this study. There were no significant differences in age, BMI, marital status, or tobacco use disorder between the study groups. There was a trend towards more females in TLE patients with depression and anxiety. Subjects with TLE were somewhat less educated than the healthy controls. Therefore, we have statistically controlled for education in regressions with psychopathology ratings and MMSE as dependent variables. There were no significant differences in seizure frequency, age of onset of TLE, a history of aura, postictal confusion and control of seizures (free of seizures, fair and poor control) between the four TLE subgroups. Patients with psychotic disorder due to TLE showed a higher incidence of status epilepticus as compared with those with “pure TLE”. Table 1 also shows the rating scale scores and MMSE scores in the five subgroups. The BPRS and HAM-A scores were significantly different between the five subgroups, with the lowest levels in controls and highest values in patients with TLE + PSY and TLE + anxiety, respectively. The HAM-D score was significantly higher in patients with TLE + depression than in all other study groups, while the MMSE was significantly lower in TLE patients than in controls, with the lowest scores being established in TLE + PSY.
| Variables | HC1 | TLE2 | TLE + PSY3 | TLE + DEP4 | TLE + ANX5 | F/X2 | df | P value |
| Age (yr) | 37.4 (12.8) | 40.0 (12.8) | 37.9 (10.5) | 39.0 (10.7) | 37.0 (8.2) | 0.34 | 4/141 | 0.849 |
| Sex (♂/♀) | 10/30 | 11/16 | 13/14 | 4/21 | 5/22 | 10.31 | 4 | 0.036 |
| BMI (kg/m2 ) | 24.0 (4.3) | 24.1 (4.0) | 23.5 (3.7) | 23.9 (4.3) | 22.4 (4.3) | 0.79 | 4/140 | 0.535 |
| Married (No/Yes) | 26/14 | 18/9 | 20/7 | 20/5 | 15/11 | 3.58 | 4 | 0.466 |
| Education (yr) | 14.2 (4.9)2,3,4,5 | 11.4 (4.7)1 | 9.4 (4.4)1 | 10.3 (5.4)1 | 10.8 (4.5)1 | 5.14 | 4/141 | 0.001 |
| TUD (No/Yes) | 38/2 | 24/3 | 23/4 | 21/4 | 23/4 | Ψ = 0.136 | - | 0.607 |
| Frequency seizures | - | 29.1 (84.7) | 19.1 (40.7) | 8.0 (17.0) | 9.7 (11.0) | 0.99 | 3/89 | 0.402 |
| Age onset TLE (yr) | - | 17.8 (12.6) | 12.2 (10.1) | 17.6 (8.9) | 16.1 (8.8) | 1.75 | 3/100 | 0.162 |
| Hx Aura (No/Yes) | - | 6/21 | 5/22 | 7/18 | 8/19 | 1.15 | 3 | 0.766 |
| Hx Postictal confusion (No/Yes) | - | 8/19 | 10/16 | 9/16 | 11/15 | 0.97 | 3 | 0.808 |
| Hx Status epilepticus (No/Yes) | - | 24/33 | 14/112 | 21/4 | 13/9 | 10.75 | 3 | 0.013 |
| Seizure control | - | 7/8/8 | 7/8/8 | 5/4/0 | 5/5/10 | Ψ = 0.309 | - | 0.307 |
| BPRS | 18.3 (1.1)2,5 | 23.6 (3.3)1,3,4,5 | 41.3 (5.9)1,2,4,5 | 32.9 (6.7)1,2,3,5 | 29.4 (50.)1,2,3,4 | 115.64 | 4/141 | < 0.001 |
| HAM-D | 0.6 (2.0)2,5 | 4.8 (2.5)1,4,5 | 5.8 (2.9)1,5 | 19.8 (4.9)1,2,3,5 | 10.3 (3.8)1,2,3,4 | 145.21 | 4/140 | < 0.001 |
| HAM-A | 2.6 (5.4)2,5 | 7.8 (3.9)1,3,4,5 | 11.6 (6.7)1,2,3,5 | 18.9 (8.8)1,2,3,5 | 23.8 (5.4)1,2,3,4 | 59.69 | 4/141 | < 0.001 |
| MMSE | 28.3 (2.4)2,5 | 25.1 (4.4)1,3 | 22.4 (5.4)1,2,4,5 | 25.7 (2.4)1,3 | 25.8 (8.9)1,3 | 11.06 | 4/140 | < 0.001 |
The total study group (patients and controls) was at Hardy-Weinberg equilibrium (χ2 = 1.086, df = 1, P = 0.297), while also the control (χ2 = 1.2013, df = 1, P = 0.273) and the TLE (χ2 = 0.530, df = 1, P = 0.467) subgroups were at Hardy-Weinberg equilibrium. There was no significant association between PON1 Q192R genotypes and TLE subgroups (ψ = 0.137, P = 0.251), namely in controls: 1/17/22 vs 10/51/47 in TLE for the QQ, QR and RR genotypes, respectively. There were no significant associations between TLE and different genetic models of the PON1 gene, including allelic, dominant, recessive and overdominant models.
We examined the associations between the activities and diagnosis using multivariate GLM analysis while adjusting for sex, age and BMI. We examined four PON1 activity indices namely PON1 CMPAase and AREase enzyme activities as measured in this study and their residualized values after covarying for PON1 genotypes. The latter explained 70.0% of the variance in PON1 CMPAase and arylesterase activities (F = 173.88, df = 2/145, P < 0.001), with the lowest CMPAase activities and the highest arylesterase activities in QQ carriers.
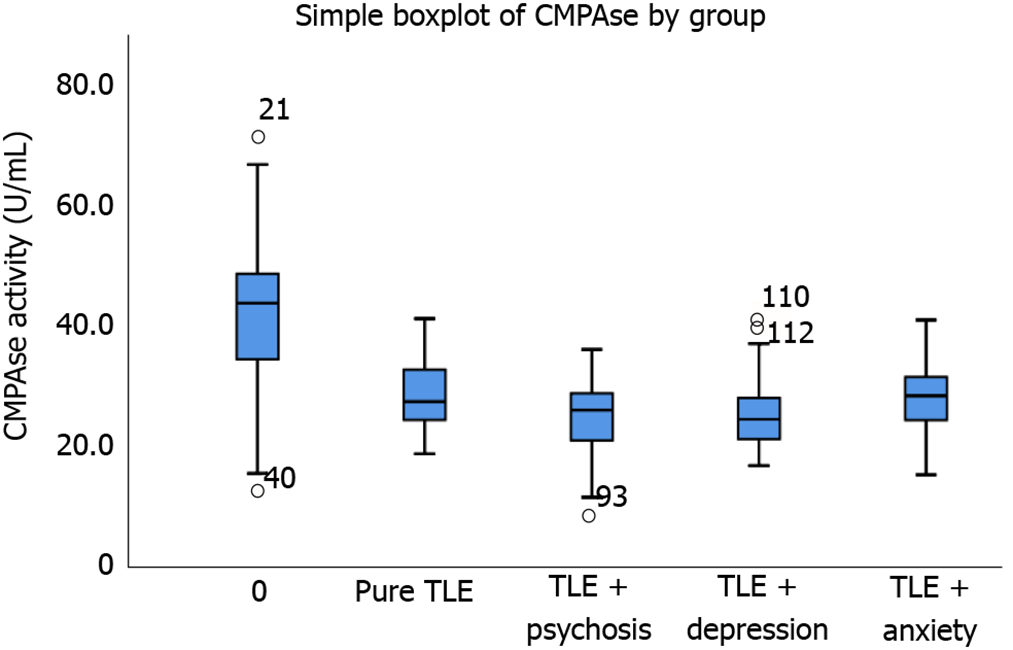
Multivariate GLM analysis showed that there was a significant association between PON1 activity and diagnosis (Table 2). Tests for between-subjects effects showed significant associations between diagnosis and CMPAase and the residualized CMPAase activities with an explained variance of around 43.7%-45.0%. The associations with arylesterase and the residualized arylesterase activity shared around 24.8%-33.0% of the variance. Table 3 shows the model-generated estimated marginal mean values, indicating that all PON1 activities were significantly lowered in TLE patients than in controls. These differences were highly significant, with a distance of around 1.586 standard deviations between controls and patients with TLE + PSY in residualized PON1 CMPAase activity. Figure 1 shows the box-plot of CMPAase activity values in controls, pure TLE and TLE with psychiatric comorbidities.
| Tests | Dependent variables | Exploratory variables | F | df | P value | Partial Eta squared |
| All 4 biomarkers | ||||||
| Multivariate | CMPAase | Diagnosis | 6.49 | 16/410 | < 0.001 | 0.158 |
| Arylesterase | Sex | 1.22 | 4/134 | 0.306 | 0.035 | |
| Res CMPAase | Age | 0.61 | 4/134 | 0.654 | 0.018 | |
| Res Arylesterase | BMI | 1.07 | 4/134 | 0.375 | 0.031 | |
| Between-subject effects | CMPAase | Diagnosis | 28.06 | 4/137 | < 0.001 | 0.450 |
| Res CMPAase | Diagnosis | 26.60 | 4/137 | < 0.001 | 0.437 | |
| Arylesterase | Diagnosis | 11.31 | 4/137 | < 0.001 | 0.248 | |
| Res Arylesterase | Diagnosis | 16.90 | 4/137 | < 0.001 | 0.330 |
| Variables | HC1 | TLE2 | TLE + PSY3 | TLE + DEP4 | TLE + ANX5 |
| CMPAase (U/mL) | 42.1 (1.3)2,3,4,5 | 28.5 (1.6)1 | 24.5 (1.6)1 | 24.8 (1.7)1 | 27.3 (1.7)1 |
| Res CMPAase (z scores) | 1.041 (0.128)2,3,4,5 | -0.196 (0.150)1 | -0.545 (0.163)1 | -0.545 (0.163)1 | -0.375 (0.159)1 |
| Arylesterase (U/mL) | 212.4 (9.0)2,3,4,5 | 156.4 (10.5)1 | 144.2 (10.4)1 | 143.7 (11.4)1 | 137.3 (11.2)1 |
| Res Arylesterase (z scores) | 0.920 (0.140)2,3,4,5 | -0.193 (0.163)1 | -0.425 (0.162)1 | -0.400 (0.177)1 | -0.434 (0.174)1 |
Binary logistic regression analysis with TLE as dependent variable (controls as reference group) showed that the residualized PON1 CMPAase activity was the most significant biomarker discriminating TLE from controls, with a sensitivity of 70.4%, specificity of 90.0% and accuracy of 75.7% (χ2 = 69.74, df = 1, P < 0.001, Nagelkerke = 0.546). The odds ratio was 0.111 (95% confidence interval: 0.053-0.230; Wald = 29.41, P < 0.001; B = 1.515, SE = 0.279).
Table 4 shows that CMPAase and arylesterase activities were significantly lower in MTS (with psychiatric comorbidities), “pure” TLE and “pure” MTS (both without psychiatric comorbidities) than those in controls. The strongest impact was established for CMPAase activity in MTS. The area under the receiver operating characteristic curve using reduced CMPAase activity as discriminatory variable was 0.893 (0.037) for TLE and 0.895 (± 0.037) for MTS.
| PON1 activities | HC | Pure TLE | F | df | P value | Partial eta squared |
| CMPAase (U/mL) | 42.8 (1.7) | 28.8 (1.9) | 30.61 | 1/62 | < 0.001 | 0.331 |
| Arylesterase (U/mL) | 215.4 (10.8) | 156.4 (12.2) | 13.47 | 1/62 | 0.001 | 0.178 |
| MTS | ||||||
| CMPAase (U/mL) | 41.7 (1.5) | 25.6 (1.2) | 81.44 | 1/100 | < 0.001 | 0.449 |
| Arylesterase (U/mL) | 209.8 (9.4) | 139.4 (7.5) | 36.98 | 1/100 | < 0.001 | 0.270 |
| Pure MTS | ||||||
| CMPAase (U/mL) | 42.2 (1.8) | 27.3 (2.6) | 22.61 | 1/52 | < 0.001 | 0.303 |
| Arylesterase (U/mL) | 213.0 (10.8) | 139.5 | 14.65 | 1/52 | < 0.001 | 0.220 |
As shown in Table 2, there were no significant effects of possible confounders including sex, age and BMI. There were also no significant effects of smoking (F = 0.48, df = 4/135, P = 0.748) and the Fagerstrom score (F = 0.16, df = 4/135, P = 0.960). We have also examined the possible effects of treatments with valproate (n = 34), carbamazepine (n = 61), phenytoin (n = 38), levetiracetam (n = 38), lamotrigine (n = 27), phenobarbital (n = 26), clonazepam (n = 10), clobazam (n = 58), topiramate (n = 12), gabapentin (n = 8), antipsychotics (n = 9), antidepressants (n = 16), anxiolytics (n = 10), CaCo3 (n = 13) and folic acid (n = 27). These drug state variables were examined as dummy variables entered altogether in multivariate GLM analysis or one by one in univariate GLM analyses. However, both types of GLM analyses showed no significant effects, even without P-correction for multiple testing. There was no significant association (Spearman rank order correlation) between the number of AEDs and either CMPAase (-0.086, P = 0.398, n = 102) or arylesterase (r = 0.052, P = 0.605, n = 102) activity.
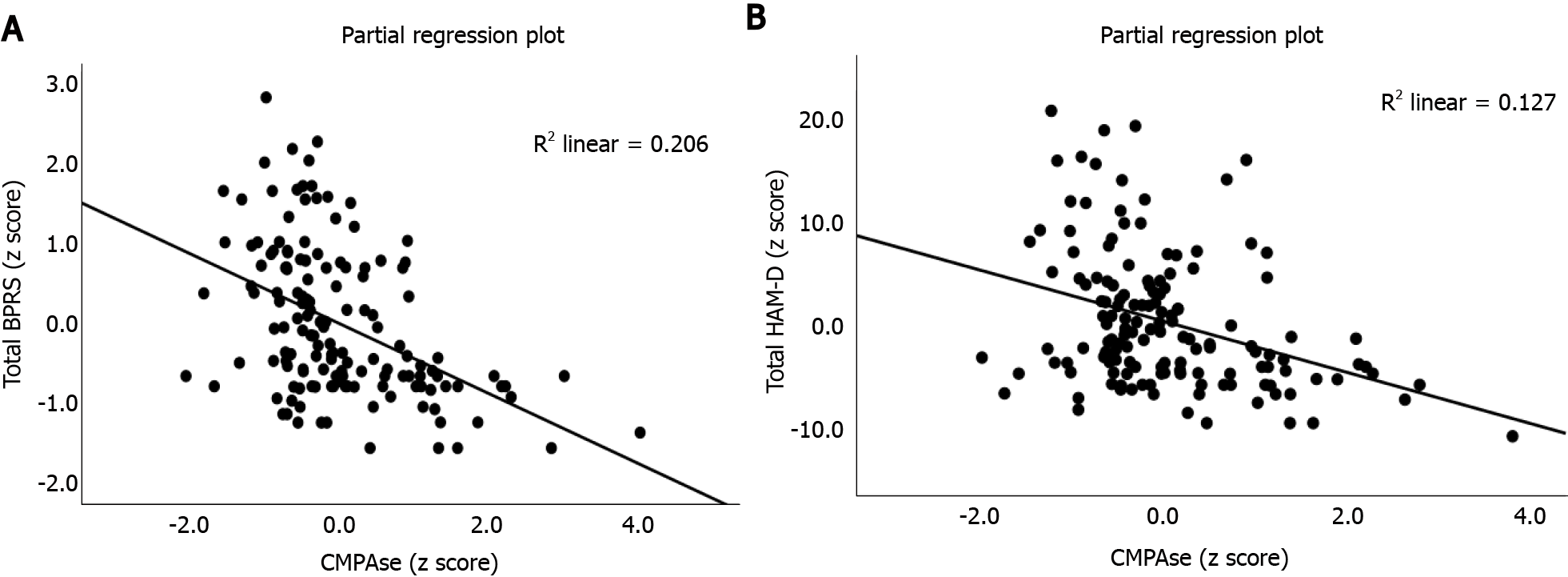
In order to examine the effects of biomarkers on the rating scale scores, we performed automatic multiple regression analysis with the rating scales as dependent variables and the four PON1 measurements (residualized and non-residualized CMPAase and AREase activities), the PON1 genetic models, age, sex as well as education (Table 5). Firstly, we examined associations with the BPRS and two symptoms profiles namely PSY that is sum of BPRS items 4 (conceptual disorganization), 11 (suspiciousness), 12 (hallucinations) and 15 (unusual thought disorders), and negative symptoms namely the sum of BPRS symptoms 3 (emotional withdrawal) and 16 (blunted affect). We found that 29.1% of the variance in the BPRS total score and 11.8% of the variance in PSY was predicted by PON1 CMPAase activity and education (both inversely). Figure 2A shows the inverse association between total BPRS score and CMPAase activity (partial regression based on the first regression in Table 5). The best predictors of negative symptoms were the residualized CMPAase activity, age and education (all inversely correlated) and male sex. We found that 25.4% of the variance in the HAM-D score was predicted by PON1 CMPAase activity, education (both inversely), female sex and being a QQ or RR carrier. Figure 2B shows the partial regression of the total HAM-D score on CMPAase activity. A large part of the variance in suicidal ideation (item 3 of the HAM-D) was explained by QQ genotype and residualized CMPAase activity (inversely associated) combined. We also computed the associations between physiosomatic symptoms, namely the sum of the HAM-A items 11 (anxiety somatic), 12 (somatic symptoms GIS), 13 (somatic symptoms general), 14 (genital symptoms) and 15 (hypochondriasis) and found that 12.2% of its variance was explained by PON1 CMPAase activity (inversely associated). Consequently, we have computed an index of psychomotor retardation (PMR) as z values of item 8 of the HAM-D and item 13 of the BPRS (both PMR) and found that 29.5% of the variance in PMR was explained by residualized CMPAase activity, age and education (all inversely associated) and male sex. CMPAase activity combined with female sex predicted 15.3% of the variance in the total HAM-A score. We have computed an overall psychopathology index as the sum of the z values of the BPRS, HAM-D and HAM-A. This index was best predicted by PON1 CMPAase activity, education (both inversely), female sex and PON1 genotype. We found that 43.4% of the variance in the MMSE score was predicted by education and CMPAase activity (both positively associated).
| Dependent variables | Explanatory variables (model) | β | t value | P value | F model | Df | P value | Partial Eta squared |
| BPRS | CMPAase | -0.444 | -6.09 | < 0.001 | 29.35 | 2/143 | < 0.001 | 0.291 |
| Education | -0.213 | -2.93 | 0.004 | |||||
| Psychosis | CMPAase | -0.260 | -3.20 | 0.002 | 9.56 | 2/143 | < 0.001 | 0.118 |
| Education | -0.167 | -2.06 | 0.041 | |||||
| Negative symptoms | Education | -0.329 | -4.52 | < 0.001 | 12.75 | 4/141 | < 0.001 | 0.226 |
| Sex | -0.329 | -4.29 | < 0.001 | |||||
| Res CMPAase | -0.189 | -2.56 | < 0.011 | |||||
| Age | -0.172 | -2.30 | 0.023 | |||||
| HAM-D | CMPAase | -0.347 | -4.51 | < 0.001 | 11.95 | 4/140 | < 0.001 | 0.254 |
| Education | -0.231 | -3.02 | 0.003 | |||||
| Overdominant model | -0.227 | -3.06 | 0.003 | |||||
| Sex | 0.170 | 2.31 | 0.023 | |||||
| Suicidal ideation | Dominant model | 0.354 | 4.61 | < 0.001 | 13.21 | 2/143 | < 0.001 | 0.156 |
| Res CMPAase | -0.173 | -2.26 | 0.025 | |||||
| Physiosomatic symptoms | CMPAase | -0.349 | -4.47 | < 0.001 | 19.94 | 1/144 | < 0.001 | 0.122 |
| PMR | Education | -0.386 | -5.13 | < 0.001 | 14.75 | 4/141 | < 0.001 | 0.295 |
| Sex | -0.252 | -3.54 | 0.001 | |||||
| Res CMPAase | -0.243 | -3.36 | 0.001 | |||||
| Age | -0.186 | -2.53 | 0.013 | |||||
| HAM-A | CMPAase | -0.350 | -4.80 | < 0.001 | 12.93 | 2/143 | < 0.001 | 0.153 |
| Sex | 0.163 | 2.10 | 0.037 | |||||
| Psychopathology index | CMPAase | -0.430 | -5.83 | < 0.001 | 19.94 | 3/141 | < 0.001 | 0.298 |
| Education | -0.250 | -3.41 | 0.002 | |||||
| Overdominant model | -0.148 | -2.07 | 0.040 | |||||
| MMSE | Education | 0.593 | 9.09 | < 0.001 | 54.48 | 2/142 | < 0.001 | 0.434 |
| CMPAase | 0.175 | 2.69 | 0.008 |
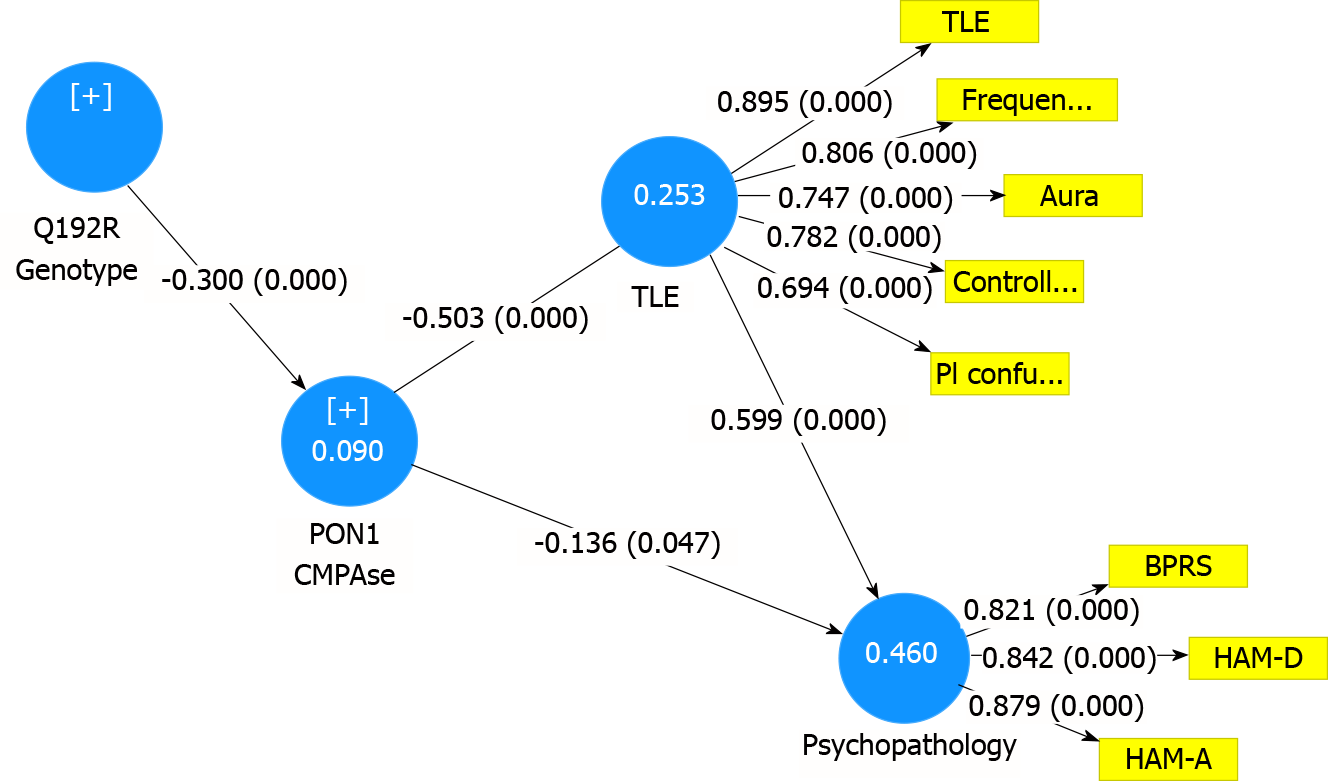
Figure 3 shows the results of a consistent and complete PLS path analysis with the PON1 genotype (additive model) as input variable and a LV extracted from the three rating scale scores (BPRS, HAM-D and HAM-A) as final outcome in a multi-step mediation model with PON1 activities (CMPAase and arylesterase) and a LV extracted from TLE features (frequency, aura, controlled epilepsy and postictal confusion) as mediators. There were no significant effects of arylesterase (after considering the effects of CMPAase), and, therefore, only the latter are shown in this figure. The fit of the model was adequate with SRMR = 0.053, while the construct reliability validity of both LVs was adequate with composite reliability values of 0.890 and 0.855; Cronbach α values of 0.847 and 0.805, rho_A values of 0.898 and 0.810 and AVE values of 0.620 and 0.719 were determined for TLE and psychopathology LVs, respectively. All outer loadings of the indicators of both LVs were > 0.694 (all at P < 0.0001). We found that 46.0% of the variance in the psychopathology index was explained by the TLE LV (positively associated) and PON1 CMPAse activity (inversely associated), while 25.3% of the variance in the TLE LV was explained by CMPAase activity. Finally, the PON1 genotype additive model explained 9% of the variance in CMPAase activity. There were specific indirect effects of PON1 genotype on: (1) The TLE LV, which were mediated by CMPAase activity (t = 4.07, P < 0.0001); and (2) The psychopathology LV mediated by CMPAase activity (t = 1.97, P = 0.048) and the path from PON1 genotype→CMPAase activity→TLE LV→psychopathology LV (t = 3.74, P < 0.0001). Likewise, the PON1 genotype had significant total (indirect) effects on TLE LV (t = 4.07, P < 0.0001) and psychopathology LV (t = 3.87, P < 0.0001). We have also examined the total effects of the QQ, QR and RR genotypes on the TLE and psychopathology LVs and found that QQ (t = 3.39, P = 0.001 and t = 3.20, P = 0.001) and RR (t = -3.35, P = 0.001 and t = -3.26, P = 0.001), but not QR, had significant total effects on the TLE and psychopathology LVs, respectively.
The first major finding of this study is that PON1 CMPAase and arylesterase activities were significantly decreased in patients with TLE, especially MTS, as compared with healthy controls. In our study, reduced levels of CMPAase yielded an area under the receiver operating characteristic curve of around 0.893 for TLE and MTS. These findings extend those of previous publications reporting significantly reduced levels of PON1 and arylesterase in patients with epilepsy when compared to healthy controls[24,33].
The second major finding of this study is that there were no significant differences in PON1 status between TLE without any comorbidities and depression, anxiety or PSY due to TLE, although severity of depression, PSY and anxiety were strongly associated with CMPAase activity. As such, CMPAase and, to a lesser degree, arylesterase activity are important in predicting the severity of psychopathology in TLE. We also observed that the severity of TLE predicts a general index of psychopathology.
Psychiatric comorbidities such as depression and anxiety are prevalent in patients with epilepsy and occur 2 to 3 times more frequently in this group of patients than in people who do not have the disease[34,35]. Some authors found a strong association between low levels of PON1 and CMPAase activities and major depression[36,37], whilst reduced activity of CMPAase was additionally associated with lower quality of life, increased disability and staging of illness[36,38], suggesting that reduced total PON1 and CMPAase activities may play a role in the pathophysiology and progression of mood disorders[36]. In patients with anxiety disorders, decreased levels of PON1 are accompanied by high levels of lipid hydroperoxides as compared with individuals without anxiety[39,40]. CMPAase activity is also inversely associated with symptoms characteristic of (deficit) schizophrenia including PSY, negative symptoms and PMR[37]. The latter authors reported that CMPAase activity was significantly reduced in patients with schizophrenia and that this effect was, to a large extent, determined by increased frequency of the QQ genotype. Noto et al[41] reported a significant decrease in PON1 activity in drug-naïve patients with first-episode PSY.
Our results show that PON1 CMPAase activity is positively associated with the MMSE score, which is significantly reduced in TLE patients, suggesting that PON1 activity protects against cognitive decline in TLE. In this regard, epilepsy per se is accompanied by a neurocognitive decline[42]. In patients with schizophrenia, reduced PON1 activity is strongly associated with neurocognitive deficits[37], whilst in mood disorders, reduced PON1 status is associated with staging of the disorder, which is characterized by increased neurocognitive deficits[43].
The third major finding of this study is the significant association between the PON1 genotype and TLE features including seizure frequency, aura, postictal confusion, uncontrolled seizure type and TLE-associated psychopathology including severity of PSY, depression and anxiety. Thus, PLS path analysis revealed that the PON1 genotype, especially the QQ but also the QR, variants increase risk and severity of TLE and TLE-associated psychopathology and that the RR genotype is protective. Our study indicates that genetically determined decreases in PON1 CMPAase activity as well as reduced PON1 enzyme activities, which occur independently of the PON1 genotype, may be causally related to TLE and its psychiatric comorbidities. As such, alterations in CMPAase and PON1 activities, which are secondary to oxidative stressors[25,43], or environmental factors including nutritional factors and smoking may also be involved[25]. Nutritional factors that may affect PON1 activity include polyphenols, oleic acid, a Mediterranean diet, chokeberry and pomegranate juice, lipids, vitamin C and vitamin A[25]. Interestingly, tobacco use, which lowers PON1 activity, is associated with focal or generalized seizures[44,45], indicating that chemicals in tobacco smoke may have pro-convulsive effects[46]. Harmful and potentially harmful constituents in tobacco that may trigger seizures are carbon monoxide, toluene, cresol, arsenic, acetone, ammonia, lead and hexane[46].
PON1 is a detoxifying enzyme that is associated with HDL[40] and has anti-inflammatory[25] and antioxidant properties, including hydrolyzing lipid peroxides[47]. PON1 activity may protect against lipid, LDL and HDL oxidation and increase HDL's ability to increase cholesterol efflux from macrophages[48]. Furthermore, PON1 protects against macrophages’ pro-oxidative effects, which produce free radicals and myeloperoxidase, resulting in the highly toxic peroxynitrite and hypochlorous acid[40]. Moreover, Borowczyk et al[49] reported that PON1 may hydrolyze homocysteine thiolactone, a toxic metabolite that can induce epileptic seizures in rats and is implicated in neurodegenerative disorders. Importantly, in oxidative stress conditions, PON1 may be damaged by increased myeloperoxidase activity and elevated production of peroxynitrite and hypochlorous acid, leading to reduced antioxidant defenses and thus an increased vulnerability to oxidation of LDL and HDL, which in turn will lower the protective effects of HDL[43]. Therefore, a well-functioning antioxidant system is important to prevent lipid peroxidation and conditions characterized by PON1 gene-associated decreases in PON1 activity (like TLE and MTS) are accompanied by increased risk to develop lipid oxidation.
There is now some evidence of increased oxidation of lipids[50,51] and lipid peroxidation occurring during TLE progression[15]. Moreover, experimental epilepsy/TLE models show increased hydroxyl radicals in the CNS[52], increased lipid peroxidation and reduced antioxidant defenses[15,15,53]. For example, Mojarad and Roghani[18] found increased lipid peroxidation and decreased superoxide dismutase activity in a kainic acid induced TLE model. The latter model is also accompanied by a decrease in the reduced form of glutathione in the hippocampus[19] and increased malondialdehyde, the end product of lipid peroxidation, in the piriform cortex[17]. Moreover, elevated malondialdehyde is associated with a greater vulnerability of the piriform cortex to seizure-induced damage. Lipid peroxidation can alter the permeability of the mitochondrial membrane and enzymes present in the membrane, possibly leading to neurodegenerative processes[12]. Therefore, the results of the current study suggest that reduced PON1 CMPAase activity, which is in part genetically determined, participates in the pathophysiology of TLE and MTS and the onset of comorbid psychopathology through increased oxidative stress. This mechanistic explanation may, at least in part, underpin the strong comorbidity between TLE and psychiatric symptoms. Moreover, the decrease in serum PON1 activity may explain the cognitive impairments in TLE/MTS. Previously, it was reported that reduced PON1 CMPAase activity is associated with cognitive deficits in schizophrenia and dementia[37]. For example, increased ROS levels may cause loss of inhibitory neurons in the hippocampus in patients with epilepsy and induce a hyperexcitability state, which can initiate reactive gliosis and, consequently, mitochondrial dysfunction leading to neurodegeneration[12].
Our results were adjusted for possible effects of sex, age, BMI and smoking, which may affect PON1 enzymatic activity and oxidative biomarkers[25]. All patients in the current study were medicated (AEDs or psychotropic drugs). Some studies, but not all, suggested that AED treatment may influence lipid peroxidation[54]. For example, treatment with levetiracetam may be accompanied by a decrease in serum PON1 and arylesterase activity and a significant increase in oxidized LDL[55]. Nevertheless, in our study, there were no significant effects of AEDs, antipsychotics or mood-stabilizing drugs on PON1 activity, and we found no associations between the number of AEDs patients were taking and PON1 enzymatic activity. Nevertheless, in the present study we did not control for duration of treatment with AEDs or antipsychotics on PON1 activities. Moreover, other studies found no significant differences in oxidative stress indicators between treated and untreated epilepsy patients[56,57]. Arylesterase activity is not significantly different between chronically polymedicated psychiatric patients and controls, suggesting that treatment with psychotropic medications does not induce changes in arylesterase activity[25].
The activities of CMPAse and arylesterase enzymes are significantly decreased in patients with TLE, especially MTS, as compared with healthy controls. The detrimental effects of the PON1 genotype on the severity of TLE, depression, PSY and anxiety are mediated by reduced CMPAase. The aberrations in PON1 status may play a key role in the oxidative pathophysiology of TLE, MTS and psychiatric comorbidities. PON1 enzymatic activity is a new drug target in TLE to treat seizure frequency and psychiatric comorbidities.
Hippocampal sclerosis, also known as mesial temporal sclerosis (MTS), is the most common primary epileptic pathology, accounting for 36% of all focal epileptic pathologies. Depression, anxiety, and psychosis (PSY) affect between 30%–70% of temporal lobe epilepsy (TLE) patients. The pathophysiology of TLE, TLE progression, depression, anxiety and PSY is heavily influenced by oxidative pathways and decreased antioxidant defenses.
The enzyme paraoxonase 1 (PON1) protects against lipid peroxidation, and its activity is influenced in part by a single nucleotide polymorphism in PON1. There has been no research that examined the links between PON1 status and mental comorbidities in TLE.
The goal of this research was to look at PON1 status, namely 4-(chloromethyl) phenyl acetate CMPAase and arylesterase activities, as well as PON1 Q192R genotypes, in patients with TLE and MTS who had comorbid PSY, depression and anxiety.
This is a case-control study that examined 104 patients with TLE and 40 normal controls. TLE patients were divided according to Diagnostic and Statistical Manual of Mental Disorders, fourth edition, text revision criteria into: (1) Mood disorders due to TLE with depressive features (n = 25); (2) Anxiety disorders due to TLE (n = 25); (3) Psychotic disorder due to TLE (n = 25); and (4) "Pure TLE" when there were no psychiatric comorbidities. After an overnight fast, blood samples were obtained at 8:00 a.m., and serum was aliquoted and kept at -80 °C until thawed for total PON1 activity (PON1Q192R polymorphism, and arylesterase and CMPAase activities). Data were analyzed using partial least squares pathway analysis.
PON1 activities were significantly lower in TLE patients than those in controls. The area under the receiver operating characteristic curve using lower CMPAase activity as discriminatory variable was 0.893 (0.037) for TLE and 0.895 (± 0.037) for MTS. We found that 46.0% of the variance in the severity of depressive, anxiety and psychotic symptoms was explained by the severity of TLE features and PON1 CMPAse activity while 25.3% of the variance in TLE severity was explained by CMPAase activity. PON1 QQ and RR, but not QR, had significant effects on severity of TLE and comorbid psychopathology.
In individuals with TLE, particularly MTS, the activity of CMPAse and arylesterase enzymes are much lower than in healthy controls. Reduced CMPAase mediates the negative effects of the PON1 genotype on TLE, depression, PSY and anxiety severity.
Changes in PON1 status play a role in pathophysiology of TLE, MTS and mental comorbidities. PON1 enzymatic activity is a novel therapeutic target in TLE for the treatment of seizure frequency and mental comorbidities.
| 1. | Wickham J, Ledri M, Bengzon J, Jespersen B, Pinborg LH, Englund E, Woldbye DPD, Andersson M, Kokaia M. Inhibition of epileptiform activity by neuropeptide Y in brain tissue from drug-resistant temporal lobe epilepsy patients. Sci Rep. 2019;9:19393. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 2. | Trinka E, Kwan P, Lee B, Dash A. Epilepsy in Asia: Disease burden, management barriers, and challenges. Epilepsia. 2019;60 Suppl 1:7-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 145] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 3. | Engel J Jr; International League Against Epilepsy (ILAE). A proposed diagnostic scheme for people with epileptic seizures and with epilepsy: report of the ILAE Task Force on Classification and Terminology. Epilepsia. 2001;42:796-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1529] [Cited by in RCA: 1409] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 4. | Blümcke I, Thom M, Wiestler OD. Ammon's horn sclerosis: a maldevelopmental disorder associated with temporal lobe epilepsy. Brain Pathol. 2002;12:199-211. [PubMed] |
| 5. | Tai XY, Bernhardt B, Thom M, Thompson P, Baxendale S, Koepp M, Bernasconi N. Review: Neurodegenerative processes in temporal lobe epilepsy with hippocampal sclerosis: Clinical, pathological and neuroimaging evidence. Neuropathol Appl Neurobiol. 2018;44:70-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 86] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 6. | Gilliam FG, Maton BM, Martin RC, Sawrie SM, Faught RE, Hugg JW, Viikinsalo M, Kuzniecky RI. Hippocampal 1H-MRSI correlates with severity of depression symptoms in temporal lobe epilepsy. Neurology. 2007;68:364-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Agrawal N, Mula M. Treatment of psychoses in patients with epilepsy: an update. Ther Adv Psychopharmacol. 2019;9:2045125319862968. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | Vrinda M, Sasidharan A, Aparna S, Srikumar BN, Kutty BM, Shankaranarayana Rao BS. Enriched environment attenuates behavioral seizures and depression in chronic temporal lobe epilepsy. Epilepsia. 2017;58:1148-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 9. | Bragatti JA, Torres CM, Londero RG, Assmann JB, Fontana V, Martin KC, Hidalgo MP, Chaves ML, Bianchin MM. Prevalence of psychiatric comorbidities in temporal lobe epilepsy: the value of structured psychiatric interviews. Epileptic Disord. 2010;12:283-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 10. | Beletsky V, Mirsattari SM. Epilepsy, mental health disorder, or both? Epilepsy Res Treat. 2012;2012:163731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Maes M, Galecki P, Chang YS, Berk M. A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:676-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 786] [Cited by in RCA: 844] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 12. | Puttachary S, Sharma S, Stark S, Thippeswamy T. Seizure-induced oxidative stress in temporal lobe epilepsy. Biomed Res Int. 2015;2015:745613. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 170] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 13. | Patel MN. Oxidative stress, mitochondrial dysfunction, and epilepsy. Free Radic Res. 2002;36:1139-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 96] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Wang X, Wang W, Li L, Perry G, Lee HG, Zhu X. Oxidative stress and mitochondrial dysfunction in Alzheimer's disease. Biochim Biophys Acta. 2014;1842:1240-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 721] [Cited by in RCA: 1002] [Article Influence: 77.1] [Reference Citation Analysis (0)] |
| 15. | Peternel S, Pilipović K, Zupan G. Seizure susceptibility and the brain regional sensitivity to oxidative stress in male and female rats in the lithium-pilocarpine model of temporal lobe epilepsy. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:456-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Gupta YK, Briyal S, Chaudhary G. Protective effect of trans-resveratrol against kainic acid-induced seizures and oxidative stress in rats. Pharmacol Biochem Behav. 2002;71:245-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 118] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Kubera M, Budziszewska B, Jaworska-Feil L, Basta-Kaim A, Leśkiewicz M, Tetich M, Maes M, Kenis G, Marciniak A, Czuczwar SJ, Jagła G, Nowak W, Lasoń W. Effect of topiramate on the kainate-induced status epilepticus, lipid peroxidation and immunoreactivity of rats. Pol J Pharmacol. 2004;56:553-561. [PubMed] |
| 18. | Mojarad TB, Roghani M. The Anticonvulsant and Antioxidant Effects of Berberine in Kainate-induced Temporal Lobe Epilepsy in Rats. Basic Clin Neurosci. 2014;5:124-130. [PubMed] |
| 19. | Shin EJ, Ko KH, Kim WK, Chae JS, Yen TP, Kim HJ, Wie MB, Kim HC. Role of glutathione peroxidase in the ontogeny of hippocampal oxidative stress and kainate seizure sensitivity in the genetically epilepsy-prone rats. Neurochem Int. 2008;52:1134-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 82] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 20. | Asefi M, Vaisi-Raygani A, Bahrehmand F, Kiani A, Rahimi Z, Nomani H, Ebrahimi A, Tavilani H, Pourmotabbed T. Paraoxonase 1 (PON1) 55 polymorphism, lipid profiles and psoriasis. Br J Dermatol. 2012;167:1279-1286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 21. | Aydemir B, Behice Serinkan Cinemre F, Cinemre H, Tüten A, Aytaç Yüksel M, Yılmaz N, Kaya B, Akdemir N, Erdogan E, Madazlı R. Paraoxonase 1 (PON1) Q192R and L55M polymorphisms, lipid profile, lipid peroxidation and lipoprotein-a levels in Turkish patients with pregnancy-related disorders. Gynecol Endocrinol. 2019;35:417-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Richter RJ, Jarvik GP, Furlong CE. Determination of paraoxonase 1 status without the use of toxic organophosphate substrates. Circ Cardiovasc Genet. 2008;1:147-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 93] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 23. | Dönmezdil N, Çevik MU, Özdemir HH, Taşin M. Investigation of PON1 activity and MDA levels in patients with epilepsy not receiving antiepileptic treatment. Neuropsychiatr Dis Treat. 2016;12:1013-1017. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Calik M, Oguz E, Sarikaya S, Kocaturk O, Koca B, Gungor HE, Aksoy N, Yoldas TK, Iscan A. An evaluation of serum paraoxonase together with arylesterase activities and oxidative stress in children with intractable epilepsy: a cross-sectional study. Epilepsy Res. 2014;108:1591-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Moreira EG, Boll KM, Correia DG, Soares JF, Rigobello C, Maes M. Why Should Psychiatrists and Neuroscientists Worry about Paraoxonase 1? Curr Neuropharmacol. 2019;17:1004-1020. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 37] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 26. | Kanchanatawan B, Limothai C, Srikijvilaikul T, Maes M. Clinical predictors of 2-year outcome of resective epilepsy surgery in adults with refractory epilepsy: a cohort study. BMJ Open. 2014;4:e004852. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Hamilton M. The assessment of anxiety states by rating. Br J Med Psychol. 1959;32:50-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6169] [Cited by in RCA: 7048] [Article Influence: 105.2] [Reference Citation Analysis (1)] |
| 28. | Hamilton M. A Rating Scale for depression. J Neurosgery Psychiatr. 1960;23: 56-63. |
| 29. | Overall JE, Gorham DR. The Brief Psychiatric rating scale. Psychol Reports. 1962;10:799-812. |
| 30. | Folstein MF, Folstein SE, McHugh PR. Mini-Mental State. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56757] [Cited by in RCA: 61872] [Article Influence: 1213.2] [Reference Citation Analysis (0)] |
| 31. | Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Test. J Royal Statistical Society. 1997;289-300. [RCA] [DOI] [Full Text] [Cited by in Crossref: 17030] [Cited by in RCA: 26421] [Article Influence: 3302.6] [Reference Citation Analysis (1)] |
| 32. | Ringle CM, Sarstedt M, Straub DW. Editor's comments: a critical look at the use of PLS-SEM in" MIS Quarterly". MIS Quarterly. 2012;36: 3-8. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1064] [Cited by in RCA: 1104] [Article Influence: 78.9] [Reference Citation Analysis (0)] |
| 33. | Işık M, Demir Y, Kırıcı M, Demir R, Şimşek F, Beydemir Ş. Changes in the anti-oxidant system in adult epilepsy patients receiving anti-epileptic drugs. Arch Physiol Biochem. 2015;121:97-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 62] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 34. | Abe C, Denney D, Doyle A, Cullum M, Adams J, Perven G, Dave H, Dieppa M, Hays R, Agostini M, Ding K. Comparison of psychiatric comorbidities and impact on quality of life in patients with epilepsy or psychogenic nonepileptic spells. Epilepsy Behav. 2020;102:106649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 35. | Josephson CB, Jett N. Psychiatric comorbidities in epilepsy. Int Rev Psychiatry. 2017;29:409-424. [RCA] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 142] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 36. | Moreira EG, Correia DG, Bonifácio KL, Moraes JB, Cavicchioli FL, Nunes CS, Nunes SOV, Vargas HO, Barbosa DS, Maes M. Lowered PON1 activities are strongly associated with depression and bipolar disorder, recurrence of (hypo)mania and depression, increased disability and lowered quality of life. World J Biol Psychiatry. 2019;20:368-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 37. | Matsumoto AK, Maes M, Supasitthumrong T, Maes A, Michelin AP, de Oliveira Semeão L, de Lima Pedrão JV, Moreira EG, Kanchanatawan B, Barbosa DS. Deficit schizophrenia and its features are associated with PON1 Q192R genotypes and lowered paraoxonase 1 (PON1) enzymatic activity: effects on bacterial translocation. CNS Spectr. 2021;26:406-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 38. | Maes M, Sirivichayakul S, Matsumoto AK, Maes A, Michelin AP, de Oliveira Semeão L, de Lima Pedrão JV, Moreira EG, Barbosa DS, Geffard M, Carvalho AF, Kanchanatawan B. Increased Levels of Plasma Tumor Necrosis Factor-α Mediate Schizophrenia Symptom Dimensions and Neurocognitive Impairments and Are Inversely Associated with Natural IgM Directed to Malondialdehyde and Paraoxonase 1 Activity. Mol Neurobiol. 2020;57:2333-2345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 39. | Bulut M, Selek S, Bez Y, Karababa IF, Kaya MC, Gunes M, Emhan A, Aksoy N, Sir A. Reduced PON1 enzymatic activity and increased lipid hydroperoxide levels that point out oxidative stress in generalized anxiety disorder. J Affect Disord. 2013;150:829-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 40. | Maes M, Bonifacio KL, Morelli NR, Vargas HO, Moreira EG, St Stoyanov D, Barbosa DS, Carvalho AF, Nunes SOV. Generalized Anxiety Disorder (GAD) and Comorbid Major Depression with GAD Are Characterized by Enhanced Nitro-oxidative Stress, Increased Lipid Peroxidation, and Lowered Lipid-Associated Antioxidant Defenses. Neurotox Res. 2018;34:489-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 41. | Noto C, Ota VK, Gadelha A, Noto MN, Barbosa DS, Bonifácio KL, Nunes SO, Cordeiro Q, Belangero SI, Bressan RA, Maes M, Brietzke E. Oxidative stress in drug naïve first episode psychosis and antioxidant effects of risperidone. J Psychiatr Res. 2015;68:210-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 42. | Veluri N. A Case of Cognitive Decline Resulting from Aging, Temporal Lobe Epilepsy, and Environmental Factors. Case Rep Psychiatry. 2019;2019:9385031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 43. | Maes M, Moraes JB, Congio A, Bonifacio KL, Barbosa DS, Vargas HO, Michelin AP, Carvalho AF, Nunes SOV. Development of a Novel Staging Model for Affective Disorders Using Partial Least Squares Bootstrapping: Effects of Lipid-Associated Antioxidant Defenses and Neuro-Oxidative Stress. Mol Neurobiol. 2019;56:6626-6644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 44] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 44. | Durnin C. Carbon monoxide poisoning presenting with focal epileptiform seizures. Lancet. 1987;1:1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 17] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 45. | Kurt F, Bektaş Ö, Kalkan G, Öncel MY, Yakut HI, Kocabaş CN. Does age affect presenting symptoms in children with carbon monoxide poisoning? Pediatr Emerg Care. 2013;29:916-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 46. | Rong L, Frontera AT Jr, Benbadis SR. Tobacco smoking, epilepsy, and seizures. Epilepsy Behav. 2014;31:210-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 47. | Yildiz A, Gur M, Yilmaz R, Demirbag R, Polat M, Selek S, Celik H, Erel O. Association of paraoxonase activity and coronary blood flow. Atherosclerosis. 2008;197:257-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 48. | Efrat M, Aviram M. Paraoxonases in Inflammation, Infection, and Toxicology. Adv Exp Med Biol. 2010;660:47-60. [RCA] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 49. | Borowczyk K, Shih DM, Jakubowski H. Metabolism and neurotoxicity of homocysteine thiolactone in mice: evidence for a protective role of paraoxonase 1. J Alzheimers Dis. 2012;30:225-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 50. | Infanger DW, Sharma RV, Davisson RL. NADPH oxidases of the brain: distribution, regulation, and function. Antioxid Redox Signal. 2006;8:1583-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 293] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 51. | Roberts LJ, Morrow JD. Measurement of F(2)-isoprostanes as an index of oxidative stress in vivo. Free Radic Biol Med. 2000;28:505-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 758] [Cited by in RCA: 761] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 52. | Arican N, Kaya M, Kalayci R, Uzun H, Ahishali B, Bilgic B, Elmas I, Kucuk M, Gurses C, Uzun M. Effects of lipopolysaccharide on blood-brain barrier permeability during pentylenetetrazole-induced epileptic seizures in rats. Life Sci. 2006;79:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 53. | Mohanan PV, Yamamoto HA. Preventive effect of melatonin against brain mitochondria DNA damage, lipid peroxidation and seizures induced by kainic acid. Toxicol Lett. 2002;129:99-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 56] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 54. | Jeding I, Evans PJ, Akanmu D, Dexter D, Spencer JD, Aruoma OI, Jenner P, Halliwell B. Characterization of the potential antioxidant and pro-oxidant actions of some neuroleptic drugs. Biochem Pharmacol. 1995;49:359-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 79] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 55. | Chen CH, Yang WC, Hsiao YH, Huang SC, Huang YC. High homocysteine, low vitamin B-6, and increased oxidative stress are independently associated with the risk of chronic kidney disease. Nutrition. 2016;32:236-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 56. | Menon B, Ramalingam K, Kumar RV. Oxidative stress in patients with epilepsy is independent of antiepileptic drugs. Seizure. 2012;21:780-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 57. | Levine RL, Williams JA, Stadtman ER, Shacter E. Carbonyl assays for determination of oxidatively modified proteins. Methods Enzymol. 1994;233:346-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1909] [Cited by in RCA: 1929] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country/Territory of origin: Thailand
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Khan MM S-Editor: Fan JR L-Editor: Filipodia P-Editor: Fan JR