Published online Sep 19, 2021. doi: 10.5498/wjp.v11.i9.605
Peer-review started: June 2, 2021
First decision: June 23, 2021
Revised: June 24, 2021
Accepted: August 12, 2021
Article in press: August 12, 2021
Published online: September 19, 2021
Processing time: 104 Days and 19.4 Hours
Chronic gastrointestinal (GI) symptoms and disorders are common in children with autism spectrum disorder and have been shown to be significantly correlated with the degree of behavioral and cognitive impairment. In this unique popula
Core Tip: Children with autism spectrum disorder are at a significantly increased risk for chronic gastrointestinal issues from an early age. Clinicians and caregivers should be made aware of this association and provided with the tools necessary for recognition of gastrointestinal (GI) symptoms in these children. Because the first several years is a critical developmental window in the life of a child, and because of the demonstrated correlation between GI symptom severity and severity of autism spectrum disorder behaviors and cognition, it is plausible that a neurodevelopmental diagnosis may result from an untreated, persistent state of GI/immune dysregulation.
- Citation: Krigsman A, Walker SJ. Gastrointestinal disease in children with autism spectrum disorders: Etiology or consequence? World J Psychiatr 2021; 11(9): 605-618
- URL: https://www.wjgnet.com/2220-3206/full/v11/i9/605.htm
- DOI: https://dx.doi.org/10.5498/wjp.v11.i9.605
Children with autism spectrum disorder (ASD) display gastrointestinal (GI) signs (i.e., abnormalities noted by an observer) more frequently than their neurotypical counterparts[1]. These signs may present as constipation, diarrhea, abdominal pain, vomiting, food refusal, gastroesophageal reflux, malodorous macroscopic malab
Verbally (or non-verbally) communicative children with ASD who can express what they are feeling are known to experience GI symptoms (the self-expressed feeling of an abnormality such as pain) at a greater frequency than their neurotypical counterparts, whereas children with ASD who have an underlying GI disorder, but who are unable to express what they are feeling, are known to manifest GI symptoms as extremes of behaviors, such as aggression, self-injury, and excessive self-repetitive behaviors[2]. It is this group of (treatable) children that is most at risk for not receiving a GI diagnosis and not receiving the proper clinical care.
Finally, core ASD features are more pronounced in GI symptomatic children with ASD, and the severity of GI signs and symptoms have been shown correlate with the severity of ASD features[3]. The constellation of GI sign/symptom presentations in ASD, as well as the GI-behavioral association, has been reviewed elsewhere[4] and will not be the focus of this review. It is worth noting here, however, that the frequent anecdotal reports of improvement (from modest to remarkable) in core ASD features following successful treatment of an underlying GI disorder provides compelling evidence that the two are interrelated.
In this mini review, the fundamental conundrum we wish to explore is: (1) Does chronic gastrointestinal dysfunction, especially at a very young age, predispose a child to developmental delay or disorder (etiology); or (2) Does having a developmental delay or disorder increases the risk of gastrointestinal dysfunction in children (consequence).
The relevance and importance of this information to the mental health professional cannot be overstated. Although it is clear that all core ASD features or their extremes cannot be explained solely by an underlying GI pathology, the documented relationship between these two demands vigilance on the part of those who interact with and care for the child with ASD. If a caregiver or medical professional suspects and/or is made aware that GI signs/symptoms exist, then appropriate pediatric gastroenterology referral is warranted. The logical consequence of acknowledging this relationship between GI signs/symptoms and ASD behaviors (documented[5], though not yet formally addressed in most of the medical literature) is that it will allow for accurate identification and treatment of GI pathology to commence and, when treatment is successful, will ultimately decrease the unfortunate dependence on often unnecessary behavior modifying medications and their associated adverse effect profiles. Most importantly, this strategy directly targets the underlying pathology as opposed to simply modifying the resultant disease related behaviors.
The vast autism-GI literature provides an additional layer of fundamental mechanistic questions regarding the notion that central nervous system (CNS) pathology alone is responsible for ASD behaviors. If GI signs/symptoms correlate with the degree of ASD features in children with ASD, and if they are in some cases the sole presenting indication of an underlying cognitive/behavioral disorder, what might the mechanism be? Although it is tempting to attribute behaviors as directly resulting from unattend
The obligatory first step then in answering this pathophysiologic question lies in the clinical approach taken by the gastroenterologist when confronted with these signs/symptoms in the child with ASD. The standard of care for these children is to address their GI signs/symptoms in identical fashion as one would the child who is neurotypical, while at the same time being mindful of the unique behaviors that may be the only sign of underlying GI pathology. GI evaluation is therefore sign/symptom driven, and when standard non-invasive diagnostic tests (blood, stool, urine, imaging studies) are unrevealing, empiric finite trials of medication (e.g., H2 blockers, laxatives, etc.) may be warranted. If unsuccessful, the standard of care at that point is usually endoscopy with biopsy.
The most important clues as to the mechanism of the unidirectional GI-CNS linkage (beyond the simple behavioral response to pain) would be expected to lie within the GI mucosa, and in our experience treating > 1500 GI-symptomatic children with ASD, the most common pathologic finding at endoscopy in such cases is non-specific inflammation. Therefore, the putative mechanism that will be described in the following sections differs from existing mechanistic hypotheses that have been put forth thus far in the literature in that it is based solely on human GI mucosal pathology, identified specifically (and often uniquely) in children with ASD, that has been published over the past twenty years. A mechanistic theory will then be proposed that incorporates these findings into the published systemic and CNS pathology of ASD.
Kanner[6]’s seminal paper that describes 11 children with autism mentions the finding of GI disturbances in many of them, but it was not until more than 50 years later, in 1998, that the first histologic evaluation and description of GI inflammation of GI symptomatic children with ASD was published. The ongoing and evolving story of ASD related GI inflammation might appear to be better suited for a gastroenterology journal, however since GI disease is being evaluated in the context of ASD, journals focused on autism or psychiatry (ASD is diagnosed as a psychiatric illness, after all) are equally well suited. Regardless of the venue, the story itself is illuminating in so many respects that academic fidelity dictates that if we are to provide meaningful context, any discussion of this topic must necessarily include the chronologic appearances in the literature of evidence of gastrointestinal inflammation.
In 1998, led by world renowned pediatric gastroenterologist John Walker-Smith, the inflammatory bowel disease (IBD) study group at the Royal Free Hospital in London published a case report in the lancet about 12 children with regressive pervasive developmental disorder, characteristic of autism, who underwent diagnostic endos
The following year, in 1999, Horvath et al[9] published a clinical report describing the histologic (but not endoscopic) findings in 36 children with ASD and GI symptoms of diarrhea, flatulence, nighttime awakening, unexplained irritability, and abdominal distention. In that study it was found that 25 (69.4%) of the children had histologic gastroesophageal reflux, almost all of whom had unexplained irritability as a key symptom. Additionally, Paneth cell hyperplasia, a measure of increased crypt mitotic activity secondary to inflammatory mucosal damage, was significantly increased. The authors do not discuss therapeutic interventions.
Before proceeding further through this brief review of the ASD-GI inflammation literature, it is important to note that not all “inflammation” is equal. For example, the cellular infiltrate of acid related disease is composed of different cellular lineages and intensities than, for example, CD. Even within the classification of classic IBD (CD and UC), the types of inflammatory cells differ between the differing IBD diagnoses. Using tissue immunostaining, Furlano et al[10] demonstrated not only a significant non-specific lymphocytic inflammatory plasma cell, CD3, and CD8 T-cell elevation in the colon of GI symptomatic children with ASD compared to healthy controls, but that there was also an excess γδ-T-cell presence that distinguishes autistic colitis from classic IBD.
Attention then turned to the upper gastrointestinal tract where, in 2002, the use of immunohistochemical staining again showed the non-specific increased CD3 and CD8 T-cell inflammatory infiltrate in the mucosa with the additional finding of a thickened basement membrane, a feature often found in the intestine of patients with systemic autoimmune disease such as diabetes, rheumatoid arthritis, and gluten sensitive enteropathy (celiac disease)[11]. However, what distinguished the duodenal inflammation from all previously reported forms of childhood enteropathy was the presence of a unique feature of autoimmunity, namely the presence of IgG colocalizing with complement c1q on the basolateral enterocyte membrane.
Next, in 2003, Ashwood et al[12] reproduced the findings of Furlano and Torrente using the newer technique of flow cytometry (as opposed to immunostaining). This group demonstrated elevated CD3 and CD8 cellular infiltrate in the mucosa (duodenum, terminal ileum, and colon) of 52 GI symptomatic children with ASD when compared to inflamed and non-inflamed mucosal tissues from 54 non ASD controls. They also reported statistically similar degrees of infiltrate compared to inflamed controls having other diagnoses (e.g., IBD, indeterminate colitis, food allergy, celiac disease, etc.). Additionally, intraepithelial lymphocytosis and B-cell infiltration were present in GI symptomatic children with ASD but not in healthy children. The authors noted that this study confirmed and expanded upon the previously described unique nature of the combined inflammatory components in GI symptomatic children with ASD. Importantly, it was the first time that this pathology was shown to exist simultaneously in the small and large intestine.
In 2004, Torrente et al[13] described unique inflammatory pathology in the stomach of 20 (out of 25; 80%) GI symptomatic children with ASD when compared to 30 non-ASD controls. This pathology profile, like the one described by Torrente in the small bowel, was unique and unlike the focal enhanced gastritis seen in CD. Specifically, it consisted of a markedly elevated CD8 T-cell infiltration, marked presence of intraepithelial lymphocytes, and had the same subepithelial basement membrane IgG deposition colocalization with complement c1q seen in the small bowel.
In 2005, Balzola et al[14] reported similar chronic active colitis and gastritis (with gastric pseudopolyps) in a 28-year-old patient with regressive autism. Importantly, capsule endoscopy was also performed and revealed jejunal and ileal erosions and ulcerations. This remains the only published report of capsule endoscopic findings in a patient with autism. The authors suggested that their findings of small bowel disease beyond the limits of the duodenum and terminal ileum “demonstrate the potential for involvement of the entire bowel in this inflammatory disease”.
A 2008 case report of two GI-symptomatic patients (one 18-year-old male; one 19-year-old female) with autism describes the precise histopathologic findings previously reported [i.e., non-specific gastritis, focal active colitis, and lymphonodular hyperplasia (LNH) of the terminal ileum] and reported clinical GI symptom improvement in the male patient following a course of steroids after a failed trial of high dose 5-ASA[15]. In their discussion of the findings, the authors commented that “although the idea of a shared pathophysiology between GI disease and autism remains controversial, the evidence presented so far warrants further exploration at the very least” and they advocated for a heightened awareness and lower threshold for work-up and management on this population.
In 2010, Krigsman et al[16] reported on the retrospective histologic findings (on routine H&E staining) as reported by the hospital pathologists, of 143 consecutive children with ASD and chronic GI symptoms. It remains the largest study of GI symptomatic ASD mucosal biopsy tissue to date. Based on H&E staining, the authors found ileitis in one third (35%), and ileal or colonic chronic active inflammation in nearly three fourths (74%) of the children. LNH, a feature of inflammation that may be observed at any site while not by itself meeting the definition for inflammation, was present in nearly three fourths (73.2%) of the patients, and for this patient series the presence of LNH predicted the presence of inflammation. Regarding LNH in general, and specifically in children with ASD, this finding represented an important observation because although the prominent presence of LNH in GI-symptomatic children with ASD had been noted (and vigorously debated) in the literature, this case series, due to the large size of the cohort, was the first to report a statistically significant link between LNH and actual mucosal inflammation. Regressive or plateau autism was more strongly linked to ileocolitis than early onset autism. This case series did not seek to identify inflammatory characteristics unique to ASD.
In 2013, Walker et al[17] expanded upon the uniqueness of the ASD-associated enterocolitis with characterization of the infiltrates at the molecular level. This case-control study utilized mRNA transcriptome profiling of inflamed ileal and colonic tissue from GI-symptomatic children with ASD and from controls consisting of anatomically matched (ileal and colonic) GI biopsy tissues from GI-symptomatic non-ASD control patients with either: (1) No histologic evidence of pathology; (2) CD; or (3) Ulcerative colitis. Comparison of differentially expressed transcripts between the ASD samples and each of the non-ASD groups showed a clear distinction between the ASD-inflamed mucosa and all other groups. Of particular interest, while there was some degree of overlap between gene expression in inflamed tissue from GI-symptomatic children with ASD and the control samples from non-ASD children with IBD (both CD and UC), no significant overlap existed between ASD and non-diseased controls. The authors noted that this correlates well with the similarities of GI symptom expression and response to therapy as seen in their clinical experience with these patients. Nearly identical molecular profile patterns emerged from both the ileal and colonic mucosal specimens from GI-symptomatic children with ASD which served to confirm the notion of unique ASD inflammatory patterns in both the small and large intestine. The differentially expressed mucosal transcripts unique to ASD that were identified in this study were subsequently evaluated in a follow up 2016 study that compared mucosal gene expression to blood gene expression simultaneously obtained in the same patients, to identify which of these biomarkers might serve as a molecular signature (i.e., a “blood test”) for the presence of ASD-specific inflammatory bowel disease[18].
A subsequent 2016 report by Kushak et al[19] evaluated intestinal function in GI-symptomatic children with autism by measuring duodenal disaccharidase activity (lactase, sucrase, and palmitase), intestinal permeability (endoscopic infusion of rhamnose and lactulose followed by measurements in urine over the next 5 h), and fecal markers of intestinal inflammation (stool calprotectin). Results suggested that intestinal permeability, brush border enzyme activity, and the frequency of inflammation did not differ between children with or without autism. Routine H&E staining showed no significant difference in the frequency of gastric or intestinal inflammation between GI symptomatic ASD and non-ASD children and although 52% of children in the ASD group had some inflammation in the gastrointestinal tract, “it was generally mild and nondiagnostic”. The authors concluded that children with autism who have symptoms of GI disorders exhibit objective findings that are similar to those in children without autism.
Lastly, Alessandria et al[20] investigated the distribution of human leukocyte antigen-DQ2/DQ8 typing in GI-symptomatic patients with ASD, together with its correlation with duodenal histology and response to a gluten free casein-free diet. The study found unexplained duodenal intraepithelial lymphocytosis in 37% (56/151) of GI symptomatic ASD children, none of which were found to have celiac disease serologic markers. The authors concluded that their results suggest that GI-symptomatic children with ASD seem to have a high prevalence of duodenal histologic inflammation that is not linked to celiac disease.
Evaluation and comparison of biopsy obtained mucosal tissue from the terminal ileum, ascending, and descending colon from GI-symptomatic children with pervasive developmental disorder (PDD) with age matched controls was first reported in 2003 by DeFelice et al[21]. Study subjects consisted of 6 children with PDD (3 with a specific diagnosis of autism or ASD) and 9 neurotypical controls. Using an ELISA kit assay of organ culture supernatants, the authors found no statistically significant differences in the concentration of IL-6, IL-8, and IL-1β between PDD and controls. Histologic findings for all patients, based on H&E staining, were nonpathological. The authors concluded that the data failed to support an association between autism and GI inflammation.
In 2004, Ashwood et al[22] prospectively tested the hypothesis that GI-symptomatic children with ASD demonstrated increased numbers of pro-inflammatory cytokine producing CD3+ lymphocytes. As compared to the previously cited 2003 study, this prospective and larger study of 21 children with ASD and 65 neurotypical pediatric controls focused on the intracellular content of pro-inflammatory cytokines as opposed to the cellular supernatant and evaluated the number of pro-inflammatory cytokine containing CD3+ lymphocytes using multicolor flow cytometry. Significant findings included elevated pro-inflammatory cytokine containing lymphocyte counts in children with ASD vs controls, particularly lymphocytes containing TNF-α, IL-2, IL-4, and IFN-γ. Significantly, the counterregulatory IL-10 was reduced in ASD as compared to controls. These trends were found in both the duodenum and colon, as well as in both the epithelium and lamina propria. The authors concluded that that there is a consistent profile of CD3+ lymphocyte cytokines in the small and large intestinal mucosa of GI-symptomatic children with ASD, providing further evidence of a diffuse mucosal immunopathology that may respond to dietary and immunomodulatory therapeutic approaches.
In 2006 Ashwood et al[23] using a different prospective patient cohort consisting of GI-symptomatic children with ASD (n = 18), and typically developing controls (n = 27), including non-inflamed controls (NIC), and inflamed GI control children with CD, further elaborated on their 2004 findings. As in the earlier study, the group used flow cytometry to assess the CD3+ lymphocyte intracellular pro-inflammatory and counterregulatory cytokine profiles, this time in mucosal tissue from the terminal ileum. In this study, the CD3+ lymphocyte cytokine profiles were also measured in peripheral blood and correlated to those from the mucosa. In both the peripheral blood and mucosa, CD3+ TNFα+ and CD3+ IFNγ+ were increased in children with ASD compared to NIC, reaching levels similar to those seen in CD. In contrast, peripheral and mucosal counterregulatory CD3+ IL-10+ were markedly lower in GI-symptomatic children with ASD compared to NIC and CD controls. The authors concluded that there was a unique pattern of peripheral blood and mucosal CD3+ lymphocyte intracellular cytokines, consistent with significant immune dysregulation, in that ASD cohort. This 2006 paper marks the last published investigation of mucosal inflammatory cytokine findings in children with ASD and chronic gastrointestinal disease.
In the years between 2006 and 2018, as the incidence of autism continued to rise (according to official reporting from the CDC) and the interest in identifying biomarkers for ASD was high, there was a renewed focus (and a concomitant rise in peer-reviewed publication activity) on peripheral cytokine activity in children with autism. A 2019 meta-analysis on all circulating (blood, serum, or plasma) pro-inflammatory cytokine related literature in ASD identified 38 studies, with a total of 2487 participants (1393 with ASD and 1094 non-ASD controls) and found evidence for higher concentrations of IFN-γ, IL-1β, IL-6 and TNF-α in participants with autism[24]. A second meta-analysis by the same group, also published in 2019, examined levels of circulating anti-inflammatory cytokines in ASD[25]. This report summarized data from 25 studies with a total of 1754 participants (1022 patients with ASD and 732 controls) and found lower concentrations of IL-10 and IL-1Ra in ASD subjects compared to controls.
Disaccharidases, the enzymes that break down dietary carbohydrates, reside in the brush border lining of the small bowel. Deficiencies in the level of these enzymes and/or their activity can lead to unabsorbed carbohydrates reaching the colon where they are fermented by colonic bacteria. Aside from causing symptoms of diarrhea and gaseousness, these fermentation products have been hypothesized to play a role in exacerbating behavioral symptoms in children with ASD, possibly through abnormal absorption in the permeable GI mucosa. Due to the accessibility of the duodenum via endoscopic biopsy, the levels and activity of these enzymes in the mucosa can be measured relatively easily and as a result, several studies have been able to explore disaccharidase activity deficiencies in children with ASD.
For example, in 1999, Horvath et al[9] evaluated the structure and function of the upper GI tract in a group of 36 GI-symptomatic children with autism and found that 58% (21/36) had deficiencies in at least one brush border disaccharidase enzyme, most often lactase (14/21). Kushak et al[26] measured disaccharidase activity in duodenal biopsies from 199 GI-symptomatic individuals with autism and reported a lactase deficiency in 58% of autistic children < 5 years old and 65% in older patients. Finally, Williams et al[27] in a small case control study that investigated impaired carbohydrate digestion and transport in the GI tract of children with autism and GI disease compared to controls (GI disease alone), found deficient ileal mRNA transcripts encoding intestinal disaccharidases and hexose transporters in the children with ASD. This approach, though novel, did not ultimately measure actual enzyme activity, but the finding of deficient transcript levels provided additional support for the findings reported by Horvath et al[9] and Kushak et al[19].
Of interest is the case-control 2016 study by Kushak et al[19] which again found a similarly high frequency of lactase and other brush border enzyme deficiencies in GI symptomatic children with ASD, but also a similarly high frequency of disaccharidase deficiency in GI symptomatic neurotypical children undergoing diagnostic endoscopy. While disaccharidase deficiency is by no means a unique feature in autism, this finding in persons with ASD suggests it may play an important role in cognitive/be
Intestinal mucosal permeability, or “leaky gut”, has been linked to developmental and behavioral changes in children. For example, a study published in 2010 that evaluated alterations in the intestinal barrier in patients with ASD and their first-degree relatives found a high percentage of abnormal intestinal permeability among patients (36.7%) and their relatives (21.2%) as compared to normal subjects (4.8%)[28]. The authors concluded that their results provided support for the leaky gut hypothesis.
A recent 2019 review of the concept of ‘leaky gut’ discusses how intestinal barrier integrity is commonly measured, what clinical impact a compromised intestinal barrier can have in humans, and sheds light on the nuances of this of this complex topic[29]. Although the review was not specifically about the leaky gut hypothesis in ASD-associated GI disease, the author’s conclusion that “clinicians should be aware of the potential of barrier dysfunction in GI diseases and of the barrier as a target for future therapy” is highly relevant to this discussion.
Between 1996-2016 at least five investigations into the frequency and extent of intestinal permeability in children with ASD were published[19,30-33], but the heterogeneity in study design and the exclusion of GI symptomatic children in some of the studies makes it difficult to draw meaningful conclusions as to the contribution of an excessively permeable membrane to cognitive and behavioral dysfunction in children with ASD and inflammatory GI disease. However, taken together, these studies report an overall greater frequency of increased intestinal permeability in ASD as compared to non-ASD controls, though it appears that this finding does not reach statistical significance in the more rigorous studies.
Lastly, in a study investigating blood-brain barrier and intestinal epithelial barrier function, Fiorentino et al[34] using duodenal mucosal biopsies from GI-symptomatic children with ASD (n = 12) and healthy controls (n = 9), found that 75% of the ASD samples had reduced expression of barrier-forming tight junction proteins, suggesting an impaired gut barrier integrity.
Human health and well-being are inextricably linked to a balanced microbiome and it is well established that intestinal dysbiosis can alter GI physiology, immune function, and even behavior in children with ASD, a population in which microbial gut dysbiosis is common[35]. In the context of the gut-brain-axis in ASD, the influence of the microbiome is an area of intense interest and research activity. Although published studies report imbalances in intestinal microbiome composition in children with ASD[36-38] and have investigated the relationship between dysbiosis and ASD-related cognition, behavior, and GI symptomatology[39], the mechanisms by which the microbiota are thought to alter GI function and behavior are still actively being investigated. A more comprehensive understanding of mechanisms by which dysbiosis occurs, and how it impacts host function, will accelerate the development of clinically relevant mitigation and therapeutic strategies[40].
In clinical gastroenterology, the presence of serological antibodies to normal intestinal flora and their association with IBD has been well established. Though not used clinically as a diagnostic tool, the presence of these antibodies distinguishes one form of IBD from another and emphasizes the prominent role of enteric bacteria and fungi as antigenic stimuli in IBD; only rarely are they present in the general and disease control populations. Their presence does not inform specific treatment strategies, but they do suggest an important microbiome related pathogenic pathway for develop
To date, there have not been published studies that have investigated the frequency of these serologic markers in ASD-associated IBD. It has been the experience of the authors that, although the frequency with which they are present in ASD-IBD is somewhat less than in CD[41,42], they occur with much greater frequency than in the general population. As with CD, there is age-related appearance of these markers such that the anti-flagellan antibodies are prominent in children under 10 years of age, whereas the anti-glycan antibodies (e.g., ASCA) are more dominant in the teenage and young adult patients[43].
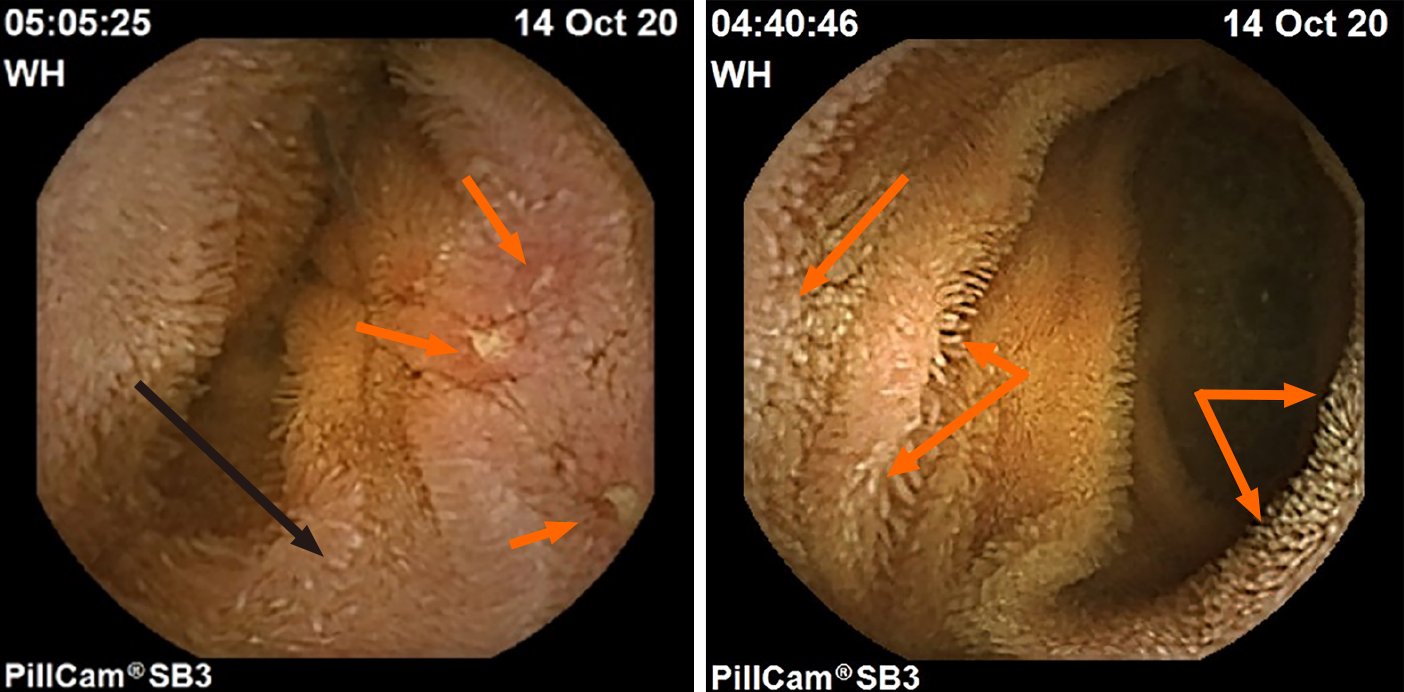
Intestinal lymphangiectasia (the presence of lipid and protein-rich fluid within dilated small intestinal villi) is another endoscopic finding commonly encountered by the authors in teenage and young adult patients with ASD and enteritis who have a long-standing history of untreated gastrointestinal symptoms. Although lymphangiectasia may be found in any number of disease states that prevent the free lymphatic flow away from the intestinal lumen (e.g., elevated central venous pressure secondary to right sided cardiac failure, lymphoma of draining lymph nodes causing distal obstruction, etc.), it has also been noted in intestinal inflammatory states such as CD where the obstruction to outward lymph flow occurs in the intestinal wall layers as it does in ASD-enteritis[44]. Examples of intestinal lymphangiectasia in ASD enteritis co-occurring in the same region as classic IBD aphthous ulcers, and in adjacent mucosa in the same patient is presented in Figure 1. To date, the authors have not encountered protein-losing enteropathy resulting from lymphangiectasia.
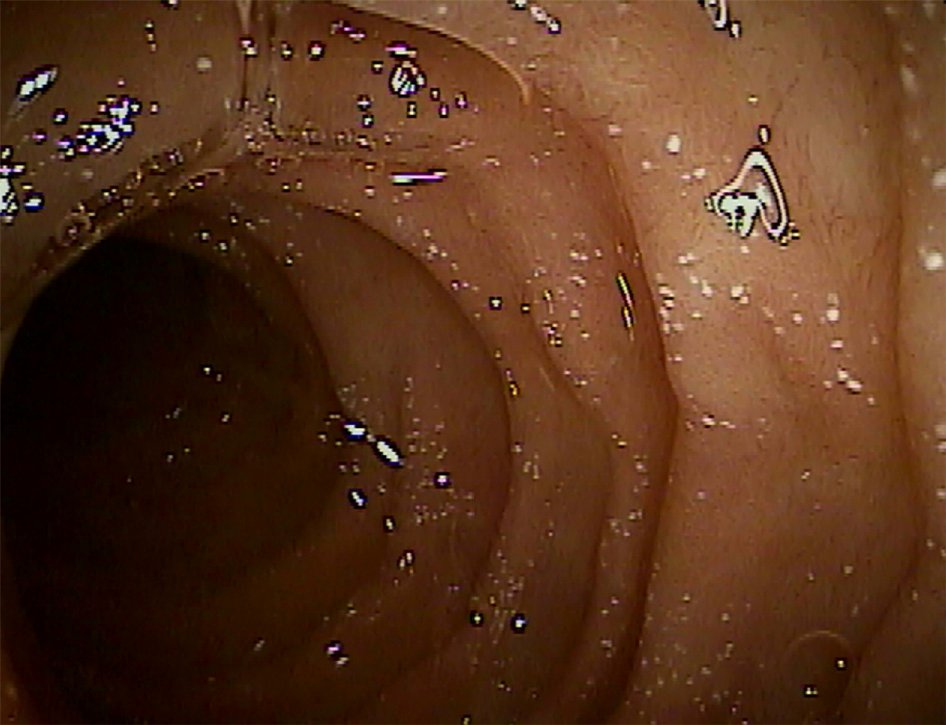
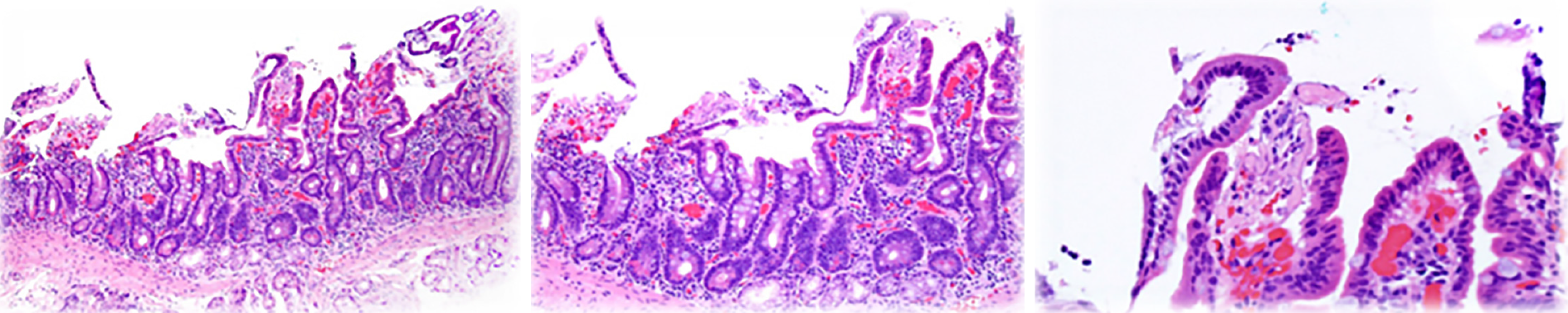
In the experience of the authors, a third type of endoscopic lesion frequently seen in younger ASD-GI children (less than 10 years of age) is that of white spots, either singular or in clusters, that are prominent in the proximal small bowel and present, but much less frequent, in the distal small bowel (Figure 2). They are often, but not always, seen in conjunction with mildly erythematous mucosa, and inflammatory infiltrate is typically not noted on routine H&E staining. Upon histologic multiple sectioning of the biopsy specimen, truncated villi are noted (single or contiguous) that demonstrate a protective fibrous plume emanating from the core of the decapitated villi. Villous decapitation allows luminal contents to evade the highly evolved system of selective absorption and results in increased permeability (Figure 3). In these cases, capsule endoscopy does not reveal the typical lipid filled villi, but rather a white spot on the surface of the mucosa not within a villous structure.
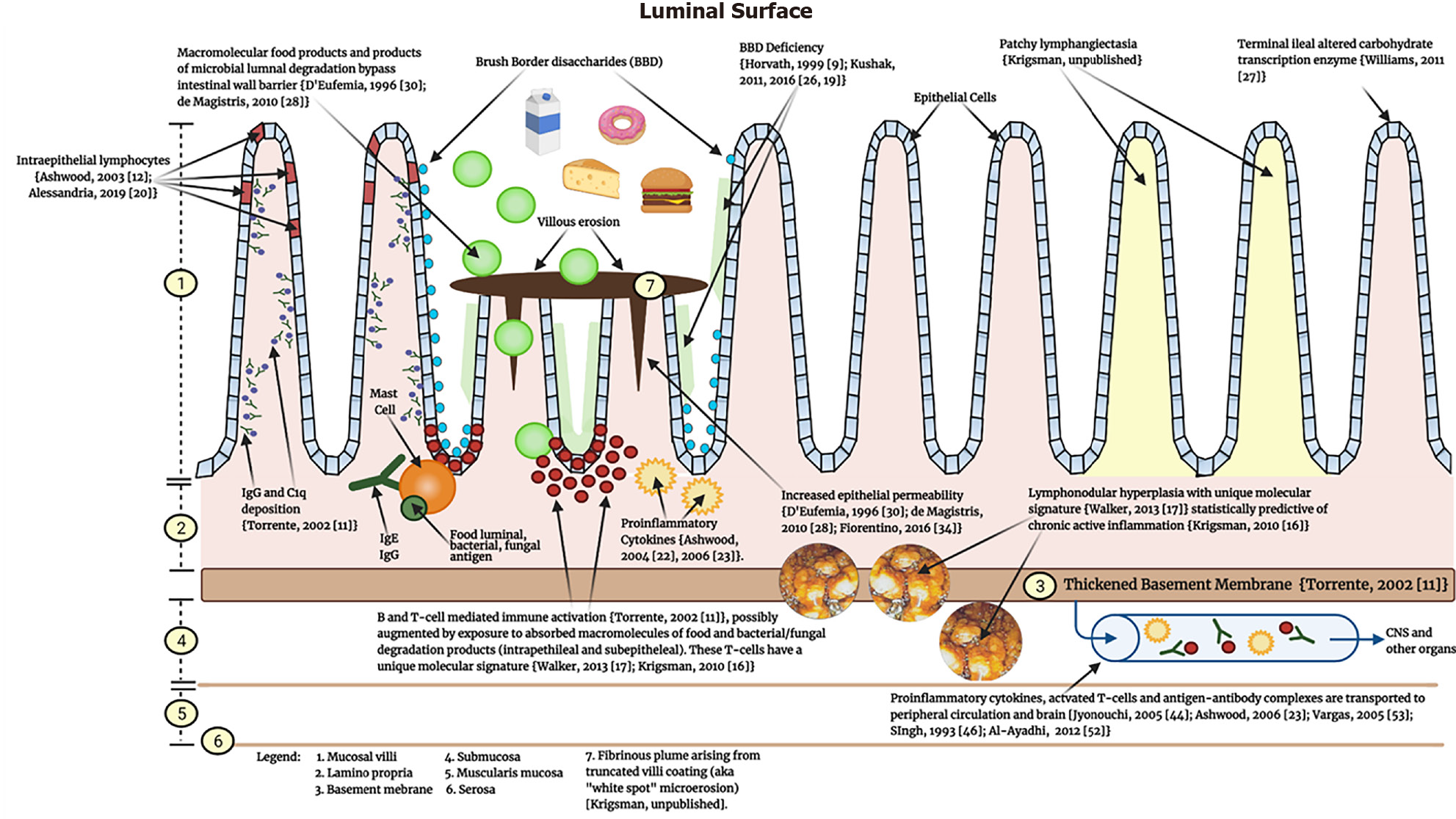
Based on the cumulative published literature discussed to this point detailing what is known about the pathology of the inflamed GI mucosa in GI-symptomatic children with ASD, a unidirectional (bowel to brain) mechanism of enterogenous CNS injury in ASD is proposed (Figure 4).
For reasons that are not yet entirely clear - but that include environmental, epigenetic, maternal-fetal, and perhaps genetic factors - the immune response in ASD is primed towards over-responsiveness. This over-responsiveness manifests as a heavy lamina propria B and T-cell presence, IgG colocalization with complement c1q on the basolateral enterocyte membrane[11], and intraepithelial lymphocytosis[12,20]. Excessive levels of pro-inflammatory cytokines produced by these cell lineages, as well as deficiencies in counter-regulatory IL-10, are consistently present[22,23]. Marked LNH, a persistently occurring feature of GI immunopathology, is typically present and predicts the presence of cellular inflammation[16]. The combination of hypertrophied reactive LNH and the thickened fibrous basement membrane[11] may serve to obstruct, in whole or in part, lymphatic flow away from the mucosa, resulting in lymphangiectasia. The gene transcriptional profile of the inflammatory infiltrate is unique to children with autism and overlaps significantly with CD[17]. Luminal bacteria are antigens known to be targeted by the mucosal antibodies (Krigsman, unpublished observation).
This persistent inflammatory presence in the intestinal mucosa eventually results in mucosal and epithelial architectural damage. Villous destruction occurs (truncated decapitated villi) with attempts at self-reparation by villous production of a fibrinous covering. Inflammatory villous damage and destruction results, as always, in the loss of resident brush border enzymes[9,26] and increased intestinal permeability[28,30,34]. The clinical implications of the latter two findings are that luminal contents (food, degradation products of resident flora, medications) can more easily evade the highly developed mechanisms for selective absorption and enter the mucosa and capillaries of the GI tract as macro, not micro, molecules, thus exposing the local and systemic immune system to unrecognized antigens. The result of this immunologic activity at the level of the intestinal mucosa is the production of activated B and T cells, as well as numerous pro-inflammatory cytokines, antibodies, and antigen-antibody complexes, that may then enter the peripheral circulation. Once in the peripheral circulation, the effects of such a wide array of active inflammatory activity is largely unknown, but would help explain the observation by Jyonouchi[45] who in 2005 described an increase in GI symptoms and systemic pro-inflammatory cytokines after exposure to specific dietary antigens in GI symptomatic ASD children.
The presence of autoantibodies to the GI epithelium, i.e., autoimmunity, often heralds the formation of autoantibodies directed against other organs as well[46] and thus, the inflammatory activity emanating from the bowel may contribute to the development of the numerous CNS autoantibodies described in the literature[47-49]. Additionally, cytokines produced locally in the GI mucosa travel peripherally and can cross the blood brain barrier[50].
Of note, the mechanisms of brain inflammation originating in the bowel may be entirely independent of immune dysregulation of peripheral origin that results in production of anti-CNS antibodies or intracranial cellular and molecular mediators of inflammation. The tendency of autoimmune processes to co-exist in the body and even to augment each other has been discussed elsewhere[46].
Finally, although a number of studies have investigated the role of immunomodulating therapy, targeting inflammatory pathways, as a treatment for autism have appeared over the years (reviewed in 51), the focus of the current review is to understand how the existence of a unique inflammatory bowel disease might impact the brain, and, by extension, how treatment of this IBD might ameliorate core symptoms of ASD. Although “classic” IBD such as CD is not known to affect cognition and behavior in the manner described in ASD, the difference may lie in the unique inflammatory features of ASD-associated enteritis that distinguishes it from CD (as already discussed), and the early age of onset of the ASD enteritis, a time when the brain is most susceptible to toxic insult.
We have presented a mechanistic concept, based on published pathologic immunologic processes occurring in the gastrointestinal tract of GI-symptomatic children with autism, describing how such pathology may be contributing to the cognitive and behavioral deficits seen in ASD. The logical next step is to conduct a prospective treatment study to determine whether standard immunotherapy for IBD results in improvement in GI symptoms and histologic disease, and if this improvement correlates with improvement in the ASD cognitive and behavioral domains as well.
We would like to acknowledge and thank the patients and their families for allowing us the opportunity to work with, study, and observe their children. We also thank Ms. Peyton Lee for her efforts on the creation of Figure 4.
| 1. | McElhanon BO, McCracken C, Karpen S, Sharp WG. Gastrointestinal symptoms in autism spectrum disorder: a meta-analysis. Pediatrics. 2014;133:872-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 451] [Cited by in RCA: 510] [Article Influence: 42.5] [Reference Citation Analysis (0)] |
| 2. | Buie T, Fuchs GJ 3rd, Furuta GT, Kooros K, Levy J, Lewis JD, Wershil BK, Winter H. Recommendations for evaluation and treatment of common gastrointestinal problems in children with ASDs. Pediatrics. 2010;125 Suppl 1:S19-S29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 122] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 3. | Chakraborty P, Carpenter KLH, Major S, Deaver M, Vermeer S, Herold B, Franz L, Howard J, Dawson G. Gastrointestinal problems are associated with increased repetitive behaviors but not social communication difficulties in young children with autism spectrum disorders. Autism. 2021;25:405-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 30] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 4. | Restrepo B, Angkustsiri K, Taylor SL, Rogers SJ, Cabral J, Heath B, Hechtman A, Solomon M, Ashwood P, Amaral DG, Nordahl CW. Developmental-behavioral profiles in children with autism spectrum disorder and co-occurring gastrointestinal symptoms. Autism Res. 2020;13:1778-1789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 77] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 5. | Buie T, Campbell DB, Fuchs GJ 3rd, Furuta GT, Levy J, Vandewater J, Whitaker AH, Atkins D, Bauman ML, Beaudet AL, Carr EG, Gershon MD, Hyman SL, Jirapinyo P, Jyonouchi H, Kooros K, Kushak R, Levitt P, Levy SE, Lewis JD, Murray KF, Natowicz MR, Sabra A, Wershil BK, Weston SC, Zeltzer L, Winter H. Evaluation, diagnosis, and treatment of gastrointestinal disorders in individuals with ASDs: a consensus report. Pediatrics. 2010;125 Suppl 1:S1-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 493] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 6. | Kanner L. Autistic disturbances of affective contact. Acta Paedopsychiatr. 1968;35:100-136. [PubMed] |
| 7. | Wakefield AJ, Murch SH, Anthony A, Linnell J, Casson DM, Malik M, Berelowitz M, Dhillon AP, Thomson MA, Harvey P, Valentine A, Davies SE, Walker-Smith JA. Ileal-lymphoid-nodular hyperplasia, non-specific colitis, and pervasive developmental disorder in children. Lancet. 1998;351:637-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1813] [Cited by in RCA: 1314] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 8. | Wakefield AJ, Harvey P, Linnell J. MMR--responding to retraction. Lancet. 2004;363:1327-8; discussion 1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Horvath K, Papadimitriou JC, Rabsztyn A, Drachenberg C, Tildon JT. Gastrointestinal abnormalities in children with autistic disorder. J Pediatr. 1999;135:559-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 271] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 10. | Furlano RI, Anthony A, Day R, Brown A, McGarvey L, Thomson MA, Davies SE, Berelowitz M, Forbes A, Wakefield AJ, Walker-Smith JA, Murch SH. Colonic CD8 and gamma delta T-cell infiltration with epithelial damage in children with autism. J Pediatr. 2001;138:366-372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 122] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 11. | Torrente F, Ashwood P, Day R, Machado N, Furlano RI, Anthony A, Davies SE, Wakefield AJ, Thomson MA, Walker-Smith JA, Murch SH. Small intestinal enteropathy with epithelial IgG and complement deposition in children with regressive autism. Mol Psychiatry. 2002;7:375-382, 334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 112] [Article Influence: 4.7] [Reference Citation Analysis (1)] |
| 12. | Ashwood P, Anthony A, Pellicer AA, Torrente F, Walker-Smith JA, Wakefield AJ. Intestinal lymphocyte populations in children with regressive autism: evidence for extensive mucosal immunopathology. J Clin Immunol. 2003;23:504-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 122] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 13. | Torrente F, Anthony A, Heuschkel RB, Thomson MA, Ashwood P, Murch SH. Focal-enhanced gastritis in regressive autism with features distinct from Crohn's and Helicobacter pylori gastritis. Am J Gastroenterol. 2004;99:598-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 14. | Balzola F, Barbon V, Repici A, Rizzetto M, Clauser D, Gandione M, Sapino A. Panenteric IBD-like disease in a patient with regressive autism shown for the first time by the wireless capsule enteroscopy: another piece in the jigsaw of this gut-brain syndrome? Am J Gastroenterol. 2005;100:979-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Galiatsatos P, Gologan A, Lamoureux E. Autistic enterocolitis: fact or fiction? Can J Gastroenterol. 2009;23:95-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Krigsman A, Boris M, Goldblatt A, Stott C. Clinical presentation and histologic findings at ileocolonoscopy in children with autistic spectrum disorder and chronic gastrointestinal symptoms. Autism Insights. 2010;2:1-11. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Walker SJ, Fortunato J, Gonzalez LG, Krigsman A. Identification of unique gene expression profile in children with regressive autism spectrum disorder (ASD) and ileocolitis. PLoS One. 2013;8:e58058. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Walker SJ, Beavers DP, Fortunato J, Krigsman A. A Putative Blood-Based Biomarker for Autism Spectrum Disorder-Associated Ileocolitis. Sci Rep. 2016;6:35820. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Kushak RI, Buie TM, Murray KF, Newburg DS, Chen C, Nestoridi E, Winter HS. Evaluation of Intestinal Function in Children With Autism and Gastrointestinal Symptoms. J Pediatr Gastroenterol Nutr. 2016;62:687-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 20. | Alessandria C, Caviglia GP, Campion D, Nalbone F, Sanna C, Musso A, Abate ML, Rizzetto M, Saracco GM, Balzola F. HLA-DQ Genotyping, Duodenal Histology, and Response to Exclusion Diet in Autistic Children With Gastrointestinal Symptoms. J Pediatr Gastroenterol Nutr. 2019;69:39-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | DeFelice ML, Ruchelli ED, Markowitz JE, Strogatz M, Reddy KP, Kadivar K, Mulberg AE, Brown KA. Intestinal cytokines in children with pervasive developmental disorders. Am J Gastroenterol. 2003;98:1777-1782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 22. | Ashwood P, Anthony A, Torrente F, Wakefield AJ. Spontaneous mucosal lymphocyte cytokine profiles in children with autism and gastrointestinal symptoms: mucosal immune activation and reduced counter regulatory interleukin-10. J Clin Immunol. 2004;24:664-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 139] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 23. | Ashwood P, Wakefield AJ. Immune activation of peripheral blood and mucosal CD3+ lymphocyte cytokine profiles in children with autism and gastrointestinal symptoms. J Neuroimmunol. 2006;173:126-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 143] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 24. | Saghazadeh A, Ataeinia B, Keynejad K, Abdolalizadeh A, Hirbod-Mobarakeh A, Rezaei N. A meta-analysis of pro-inflammatory cytokines in autism spectrum disorders: Effects of age, gender, and latitude. J Psychiatr Res. 2019;115:90-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 154] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 25. | Saghazadeh A, Ataeinia B, Keynejad K, Abdolalizadeh A, Hirbod-Mobarakeh A, Rezaei N. Anti-inflammatory cytokines in autism spectrum disorders: A systematic review and meta-analysis. Cytokine. 2019;123:154740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 26. | Kushak RI, Lauwers GY, Winter HS, Buie TM. Intestinal disaccharidase activity in patients with autism: effect of age, gender, and intestinal inflammation. Autism. 2011;15:285-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (1)] |
| 27. | Williams BL, Hornig M, Buie T, Bauman ML, Cho Paik M, Wick I, Bennett A, Jabado O, Hirschberg DL, Lipkin WI. Impaired carbohydrate digestion and transport and mucosal dysbiosis in the intestines of children with autism and gastrointestinal disturbances. PLoS One. 2011;6:e24585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 310] [Cited by in RCA: 367] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 28. | de Magistris L, Familiari V, Pascotto A, Sapone A, Frolli A, Iardino P, Carteni M, De Rosa M, Francavilla R, Riegler G, Militerni R, Bravaccio C. Alterations of the intestinal barrier in patients with autism spectrum disorders and in their first-degree relatives. J Pediatr Gastroenterol Nutr. 2010;51:418-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 399] [Cited by in RCA: 375] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 29. | Camilleri M. Leaky gut: mechanisms, measurement and clinical implications in humans. Gut. 2019;68:1516-1526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 751] [Article Influence: 107.3] [Reference Citation Analysis (0)] |
| 30. | D'Eufemia P, Celli M, Finocchiaro R, Pacifico L, Viozzi L, Zaccagnini M, Cardi E, Giardini O. Abnormal intestinal permeability in children with autism. Acta Paediatr. 1996;85:1076-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 241] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 31. | Robertson MA, Sigalet DL, Holst JJ, Meddings JB, Wood J, Sharkey KA. Intestinal permeability and glucagon-like peptide-2 in children with autism: a controlled pilot study. J Autism Dev Disord. 2008;38:1066-1071. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 32. | Boukthir S, Matoussi N, Belhadj A, Mammou S, Dlala SB, Helayem M, Rocchiccioli F, Bouzaidi S, Abdennebi M. [Abnormal intestinal permeability in children with autism]. Tunis Med. 2010;88:685-686. [PubMed] |
| 33. | Navarro F, Pearson DA, Fatheree N, Mansour R, Hashmi SS, Rhoads JM. Are 'leaky gut' and behavior associated with gluten and dairy containing diet in children with autism spectrum disorders? Nutr Neurosci. 2015;18:177-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 34. | Fiorentino M, Sapone A, Senger S, Camhi SS, Kadzielski SM, Buie TM, Kelly DL, Cascella N, Fasano A. Blood-brain barrier and intestinal epithelial barrier alterations in autism spectrum disorders. Mol Autism. 2016;7:49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 211] [Cited by in RCA: 345] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 35. | Vuong HE, Hsiao EY. Emerging Roles for the Gut Microbiome in Autism Spectrum Disorder. Biol Psychiatry. 2017;81:411-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 333] [Cited by in RCA: 397] [Article Influence: 44.1] [Reference Citation Analysis (0)] |
| 36. | Yang Y, Tian J, Yang B. Targeting gut microbiome: A novel and potential therapy for autism. Life Sci. 2018;194:111-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 85] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 37. | Settanni CR, Bibbò S, Ianiro G, Rinninella E, Cintoni M, Mele MC, Cammarota G, Gasbarrini A. Gastrointestinal involvement of autism spectrum disorder: focus on gut microbiota. Expert Rev Gastroenterol Hepatol. 2021;15:599-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 61] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 38. | Hazan S, Spradling-Reeves KD, Papoutsis A, Walker SJ. Shotgun Metagenomic Sequencing Identifies Dysbiosis in Triplet Sibling with Gastrointestinal Symptoms and ASD. Children (Basel). 2020;7. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 39. | Kang DW, Adams JB, Coleman DM, Pollard EL, Maldonado J, McDonough-Means S, Caporaso JG, Krajmalnik-Brown R. Long-term benefit of Microbiota Transfer Therapy on autism symptoms and gut microbiota. Sci Rep. 2019;9:5821. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 261] [Cited by in RCA: 456] [Article Influence: 65.1] [Reference Citation Analysis (0)] |
| 40. | Eshraghi RS, Davies C, Iyengar R, Perez L, Mittal R, Eshraghi AA. Gut-Induced Inflammation during Development May Compromise the Blood-Brain Barrier and Predispose to Autism Spectrum Disorder. J Clin Med. 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 41. | Lamberts SW, de Herder WW, Kwekkeboom DJ, Bruining HA, Krenning EP. [Insulinoma and octreotide]. Ned Tijdschr Geneeskd. 1992;136:907-910. [PubMed] |
| 42. | Schoepfer AM, Schaffer T, Mueller S, Flogerzi B, Vassella E, Seibold-Schmid B, Seibold F. Phenotypic associations of Crohn's disease with antibodies to flagellins A4-Fla2 and Fla-X, ASCA, p-ANCA, PAB, and NOD2 mutations in a Swiss Cohort. Inflamm Bowel Dis. 2009;15:1358-1367. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 43. | Rieder F, Hahn P, Finsterhoelzl L, Schleder S, Wolf A, Dirmeier A, Lopez R, Shen B, Rogler G, Klebl F, Lang T. Clinical utility of anti-glycan antibodies in pediatric Crohn's disease in comparison with an adult cohort. Inflamm Bowel Dis. 2012;18:1221-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 44. | Cui Y, Lu SY, Xu J, Peng YS, Miao Q, Wang XQ, Chen XY, Ran ZH. Microscopic features of small bowel mucosa of patients with Crohn's disease. BMC Gastroenterol. 2019;19:232. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 45. | Jyonouchi H, Geng L, Ruby A, Reddy C, Zimmerman-Bier B. Evaluation of an association between gastrointestinal symptoms and cytokine production against common dietary proteins in children with autism spectrum disorders. J Pediatr. 2005;146:605-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 69] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 46. | Anaya JM, Corena R, Castiblanco J, Rojas-Villarraga A, Shoenfeld Y. The kaleidoscope of autoimmunity: multiple autoimmune syndromes and familial autoimmunity. Expert Rev Clin Immunol. 2007;3:623-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 47. | Singh VK, Warren RP, Odell JD, Warren WL, Cole P. Antibodies to myelin basic protein in children with autistic behavior. Brain Behav Immun. 1993;7:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 176] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 48. | Singh VK, Lin SX, Yang VC. Serological association of measles virus and human herpesvirus-6 with brain autoantibodies in autism. Clin Immunol Immunopathol. 1998;89:105-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 93] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 49. | Connolly AM, Chez M, Streif EM, Keeling RM, Golumbek PT, Kwon JM, Riviello JJ, Robinson RG, Neuman RJ, Deuel RM. Brain-derived neurotrophic factor and autoantibodies to neural antigens in sera of children with autistic spectrum disorders, Landau-Kleffner syndrome, and epilepsy. Biol Psychiatry. 2006;59:354-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 178] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 50. | Banks WA, Kastin AJ, Broadwell RD. Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation. 1995;2:241-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 515] [Cited by in RCA: 566] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 51. | Hafizi S, Tabatabaei D, Lai MC. Review of Clinical Studies Targeting Inflammatory Pathways for Individuals With Autism. Front Psychiatry. 2019;10:849. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 52. | Al-Ayadhi LY, Mostafa GA. Elevated serum levels of interleukin-17A in children with autism. J Neuroinflammation. 2012;9:158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 144] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 53. | Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA. Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol. 2005;57:67-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1337] [Cited by in RCA: 1522] [Article Influence: 72.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Psychiatry
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Saad K, Siniscalco D S-Editor: Yan JP L-Editor: A P-Editor: Guo X