Published online Aug 19, 2021. doi: 10.5498/wjp.v11.i8.491
Peer-review started: November 29, 2020
First decision: March 16, 2021
Revised: March 29, 2021
Accepted: July 5, 2021
Article in press: July 5, 2021
Published online: August 19, 2021
Processing time: 256 Days and 6.7 Hours
Visual hallucination (VH) refers to a spontaneous visual perception without corresponding external stimuli and often occurs in ophthalmological and neuropsychiatric disorders. It is associated with poor quality of life, and increased patient hospitalization and nursing home admission. To date, a scientometric analysis of research on VH is lacking.
To objectively summarize the features of VH research and gain insights into the emerging trends in research on VH.
CiteSpace V was used in this article. Publication outputs, document types, geographic distributions, co-authorship status, research hotspots, and co-citation status were analyzed. A total of 2176 original articles and 465 reviews were included in the database downloaded from the Web of Science Core Collection. We selected the top 50 most cited or occurring articles or items to create a visualized network with a 1-year interval. In the document co-citation analysis stage, we performed clustering analysis on co-cited references, and log likelihood tests were used to name the clusters.
The results showed that most publications can be classified into neurology, sports, and ophthalmology studies. In addition, North America, Europe, Asia and Australia published the most documents. Some well-known authors have always had a leading role in this field; meanwhile, new authors keep emerging. A relatively stable cooperation has been formed among many authors. Furthermore, neuropsychiatric symptom and functional connectivity are the top hotspots. Research on VH in dementia with Lewy bodies and Parkinson’s disease (PD) have received much attention. Studies on VH in PD are likely to be the new emerging trends in the future, especially the mechanisms of VH.
Research on VH has formed a complete system. More large-scale clinical and in-depth basic research are required to better understand the mechanisms underlying VH, which will contribute to our understanding of the patho
Core Tip: Visual hallucination (VH) is very common and research on VH keeps emerging. In this review, CiteSpace V was used to objectively summarize the features of VH research and gain insights into the emerging trends for research on VH. Publication outputs, document types, geographic distributions, co-authorship status, research hotspots, and co-citation status were analyzed.
- Citation: Zhong M, Wu Z, Jiang X, Shen B, Zhu J, Zhang L. Knowledge domain and emerging trends in visual hallucination research: A scientometric analysis. World J Psychiatr 2021; 11(8): 491-506
- URL: https://www.wjgnet.com/2220-3206/full/v11/i8/491.htm
- DOI: https://dx.doi.org/10.5498/wjp.v11.i8.491
Visual hallucination (VH) is a spontaneous visual perception in the absence of corresponding external stimuli; it has been aptly described by Collerton et al[1] as seeing things that are not there. It is a common symptom often associated with eye diseases (e.g., Charles Bonnet syndrome [CBS])[2,3] and neuropsychiatric conditions (e.g., Parkinson’s disease [PD][4-6], dementia with Lewy bodies [DLB][7-9], epilepsy[10], schizophrenia[11,12], occipital stroke[13]). In some cases, it can be a side effect of medications, such as anticholinergics, dopamine agonists, and a wide range of medications modulating diverse neurochemical pathways[14]. VH is related to the poisoning and withdrawal of alcohol, cannabis, and cocaine and other physical conditions such as physical illness and stress[15-17]. A small percentage of healthy individuals have reported experienced VH in their life[18,19]. Although VH occurs in a significant proportion of cases, it mostly occurs sporadically in healthy people. The frequent occurrence of VH tends to be a signal of pathology[20].
The prevalence of VH varies widely in different diseases. Almost 40% of people with eye or visual pathway disease, typically macular degeneration, develop VH known as CBS[3]. VH is also a core feature for DLB diagnosis and has a 54%-70% prevalence in DLB[21,22]. Furthermore, 22%-78% of patients with PD suffer from VH[23]. Hallucinations have been described as a hallmark of schizophrenia, showing a prevalence of 36.5%[24]. The lifetime prevalence of VH in healthy subjects is 3.4%[25]. Progressive and recurrent VH is often associated with a likelihood of poor life quality, increased patient hospitalization, and nursing home admission[26,27]. In addition, it is a risk factor for dementia and is associated with the high mortality rate of patients with dementia[28,29].
Extensive research on VH has been widely conducted worldwide, and a large number of papers have been published. However, to the best of our knowledge, VH has not been systematically reviewed by scientometric analysis. The knowledge domain and emerging trends of existing research have not been fully understood. Therefore, objectively summarizing the features of VH and gaining insights into the new emerging trends for research on VH are crucial. This work conducted a systematic and scientific analysis of the research on VH by using CiteSpace, a powerful tool for data analysis and visualization[30]. The findings elaborate on annual publications, document type, co-country, co-authorship, burst keywords, and document co-citation.
Our data were retrieved from the Web of Science Core Collection (WOSCC), which is the specified article data source for CiteSpace as it contains citation information. An initial topic search for ‘visual hallucination’ resulted in 3178 records published between 1985 and 2020. We filtered out conference abstracts and proceedings and corrigendum documents, which were less representative[31]. We believe that original research papers can better represent the state of the research field compared with other types of documents. Review papers can attach additional importance to the representative papers selected by domain experts[32]. A total of 2641 publications which consist of 2176 articles and 465 reviews, were selected as a database to be used in subsequent analysis (537 were excluded). Then we downloaded raw data, which included full records and cited references, from WOSCC in the form of plain text files.
CiteSpace V based on Java was utilized for information visualization analysis, which provides insights into VH research and makes it easy to effectively follow the progress of information[30,32,33]. In this study, we selected the top 50 most cited or occurring articles or items to create a visualized network with a 1-year interval. In the document co-citation analysis stage, we performed clustering analysis on co-cited references, in which similar references were combined to determine related research fields. Moreover, log likelihood tests (LLRs) typically provide the unique and best results that consider all of the contents of a cluster; thus, we extracted noun phrases from the keywords of articles that cited a cluster on the basis of LLR to characterize the nature of the cluster[34]. Office Excel 2019 was also applied to our study.
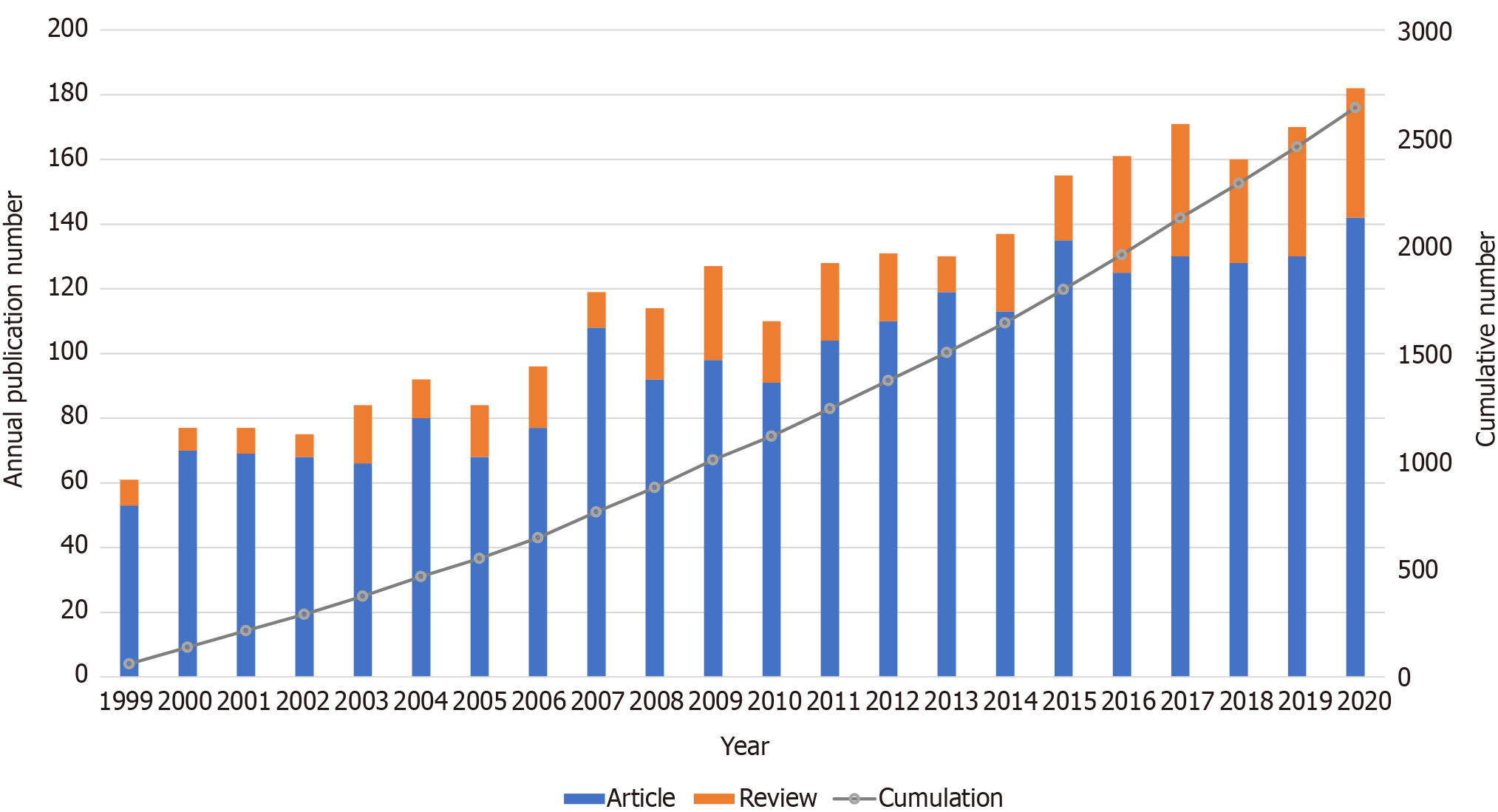
To determine the general trend of VH research, we summarized the publications of original articles and reviews over the years. The earliest record we found in WOSCC was published in 1999. The results in Figure 1 show that the publication outputs are mainly in a fluctuating growth trend, with an increase from 61 in 1999 to 182 in 2020.
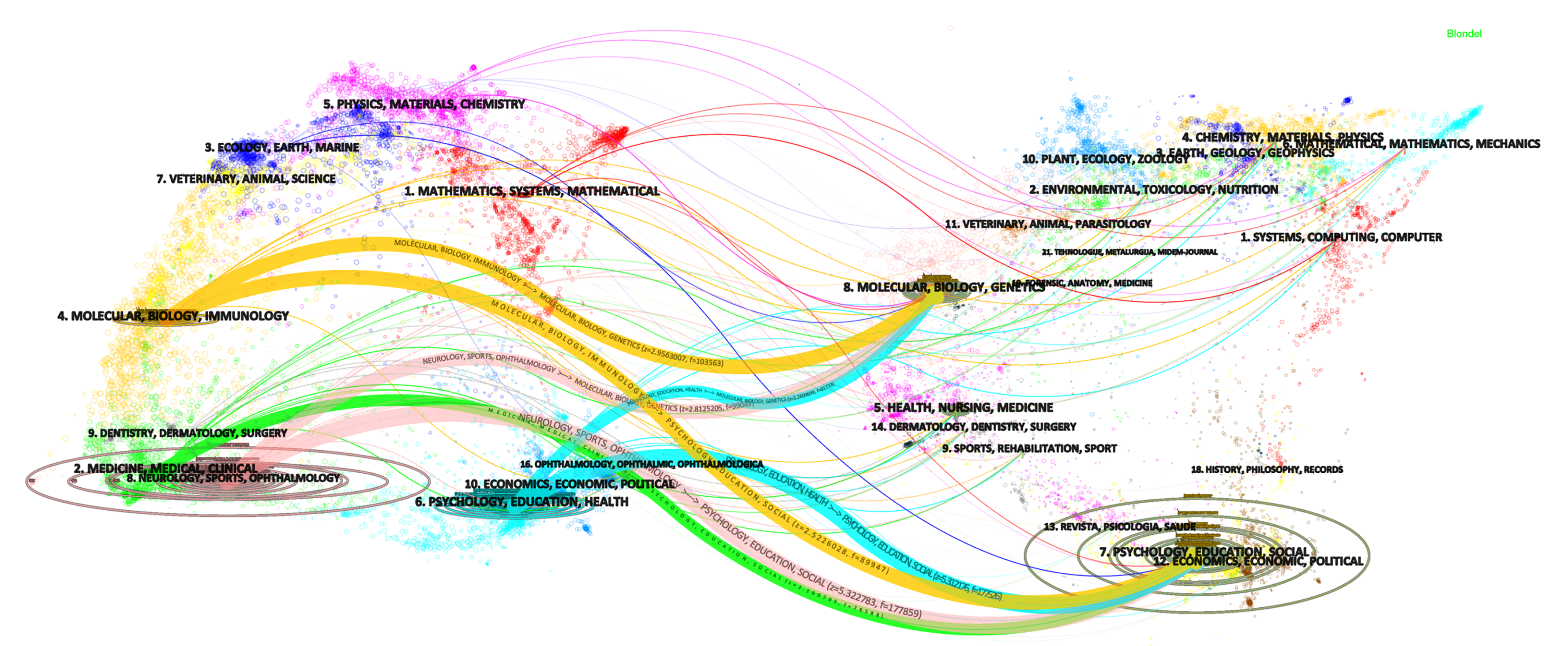
Dual-map overlays in CiteSpace can help reveal the trends of the scientific portfolio under a background of a global map of scientific literature. The background has two base maps, the left part shows a base map of citing journals and the right part shows the cited journals, each containing a network of over 10000 journals. Similar journals form a cluster, which is labeled on the basis of the terms in the journal titles of the cluster. The reference relationships between the left and right parts are connected by colored curves that indicate how a current research obtains inspiration from previous works[33]. The vertical and horizontal axes of the ellipse in the left part respectively indicate the number of articles and authors published in journals. The number of citations determines the size of the ellipse in the right part[34]. Figure 2 shows a dual-map overlay visualization of the citing and cited papers with regard to the topic search on VH. Four threads of citations stand out. They originate from four clusters in the citing base map: the orange threads from the cluster of molecular, biology and immunology, the pink threads from the cluster of neurology sports and ophthalmology, the blue threads from the cluster of psychology, education and health, and the green threads from the cluster of medicine, medical and clinical. These threads generally point to two clusters in the cited base map. One is the cluster of molecular, biology and genetics; the other is the cluster of psychology, education and social. New developments are highlighted in red from the publication point of view. The new progression of VH is in the field of mathematics, systems and mathematical, which is worth further research.
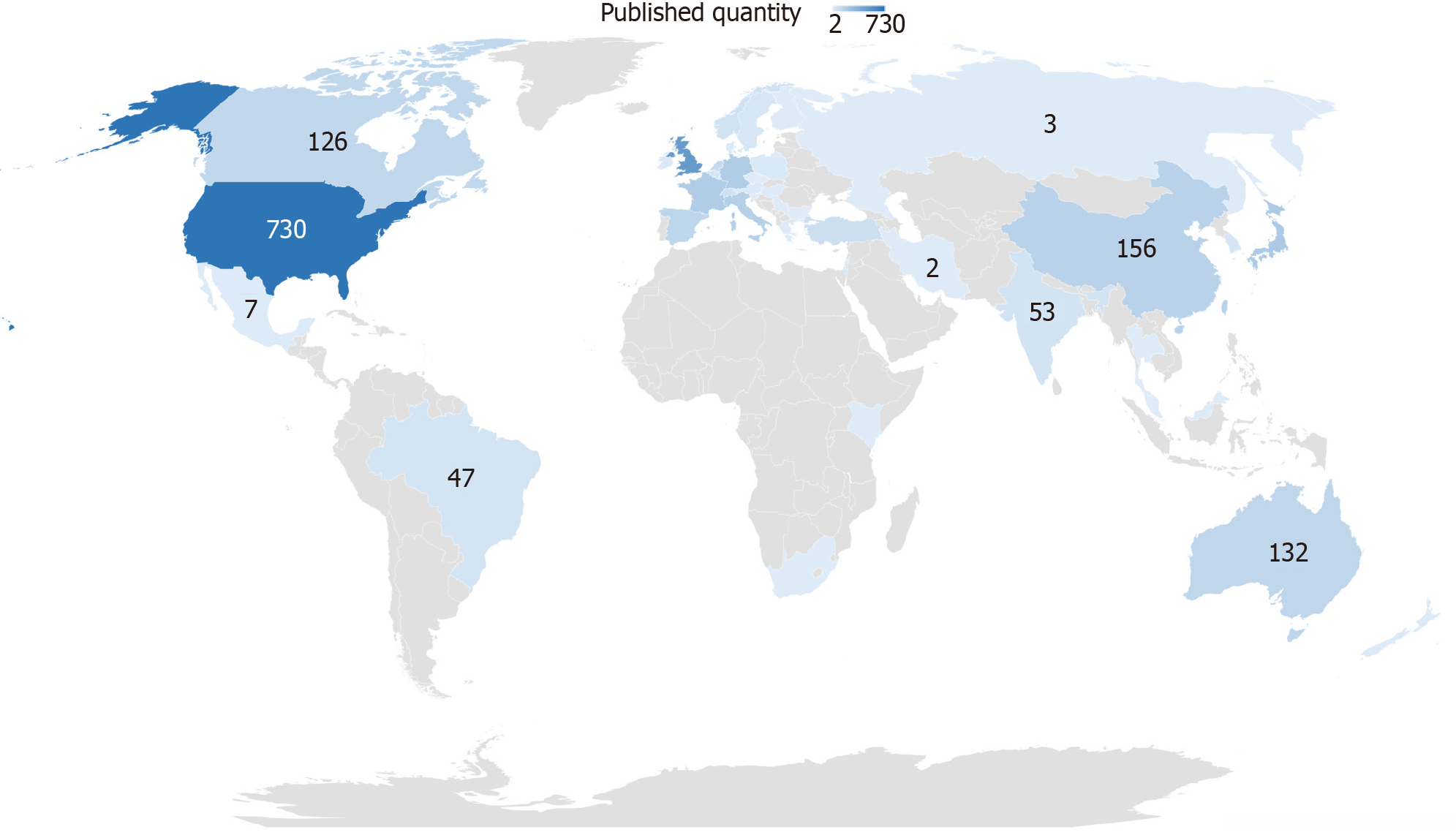
The total number of publications by country was analyzed to understand their geographic distribution. We checked the names of all countries and merged some regions into corresponding countries. The analysis is presented in Figure 3. The larger the published quantity, the deeper the color. The United States, England, and Japan were the top three countries.
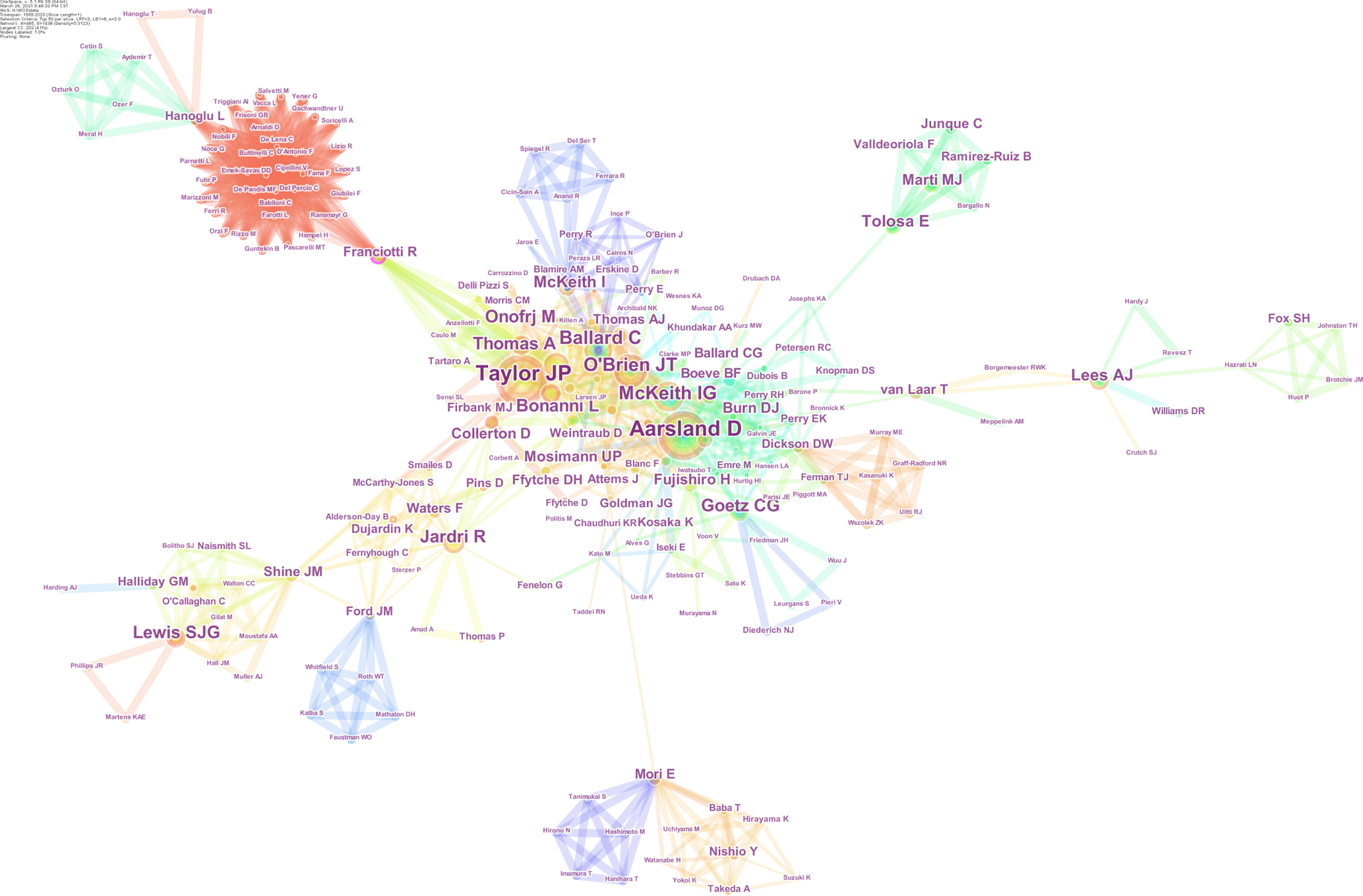
Co-authorship was analyzed to detect active authors and their cooperation in the field. For accuracy and objectivity, we reviewed all of the authors’ names to reduce misidentification. The result is displayed in Figure 4. The font size of each author’s name corresponds to the number of articles by each author, which represents the contribution of the author to this field. The color of the tree ring stands for the year in which the author published his or her articles. The thickness of the tree ring represents the number of his or her articles in a particular year. Collaborative intensity between authors is indicated by the thickness of a connecting line. Chronological order information is included in the color of the lines that appear together between nodes: blue represents the oldest, green the middle, and red the newest. In summary, top-productivity authors have greatly contributed to this field, and a relatively stable cooperation has formed among many authors. More than 485 authors have made contributions to the research on VH. Among these authors, Taylor JP (40 articles) ranked first, followed by Aarsland D (38 articles), O'Brien JT (25 articles) and McKeith IG (24 articles).
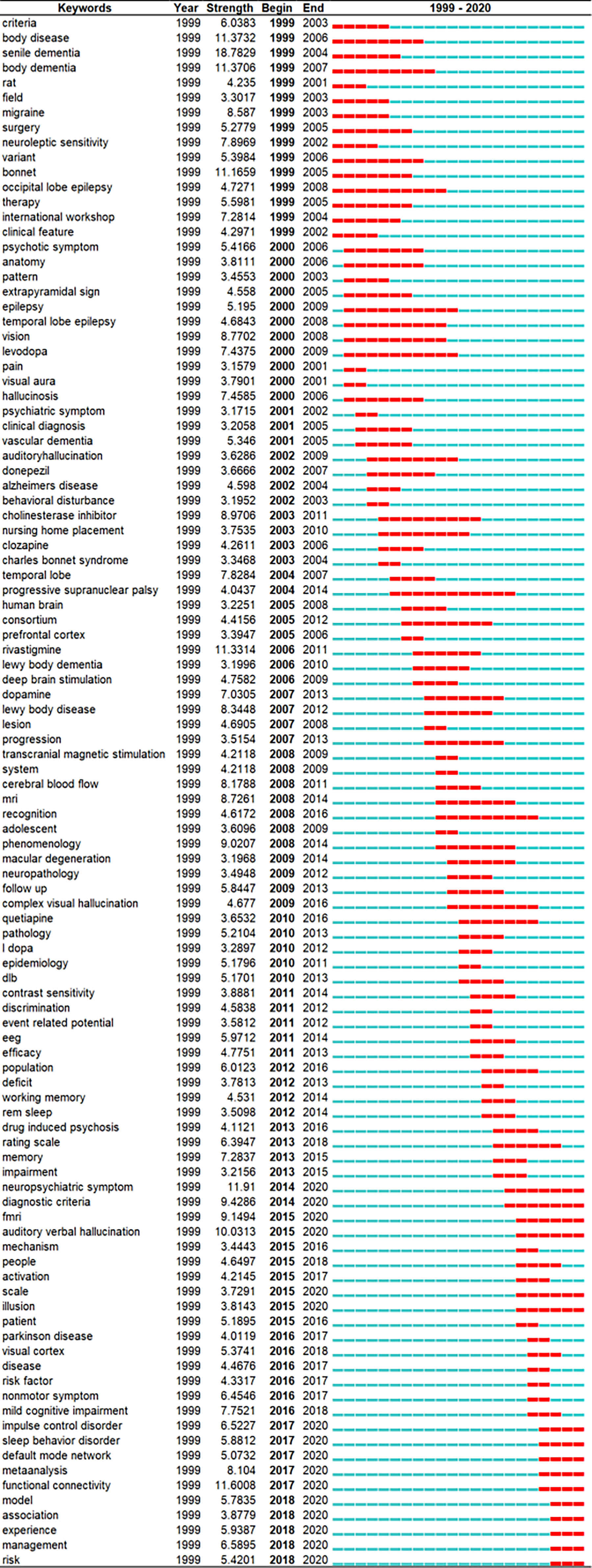
The topics involved in VH can be described by the keywords extracted from each article in the dataset[31]; however, new insights into this field should be determined. The burst patterns of keywords reveal research hotspots in the field of VH because a burst of a keyword is a sharp increase of the keyword that is likely to have a great influence[32]. Among the 340 selected keywords, 104 have the strongest strength of burst during 1999-2020. Figure 5 lists the top 104 keywords with strongest burst. The blue line represents the timeline from 1999 to 2020, and the red line stands for the years when a keyword has burst. Among the top 104 keywords, particular attention was paid to those keywords that remain to have a burst until 2020, such as ‘neuropsychiatric symptom’ and ‘functional connectivity’. Other burst keywords included ‘auditory verbal hallucination, diagnostic criteria, functional magnetic resonance imaging (fMRI), meta-analysis, management, impulse control disorder, sleep behavior disorder, and default mode network’.
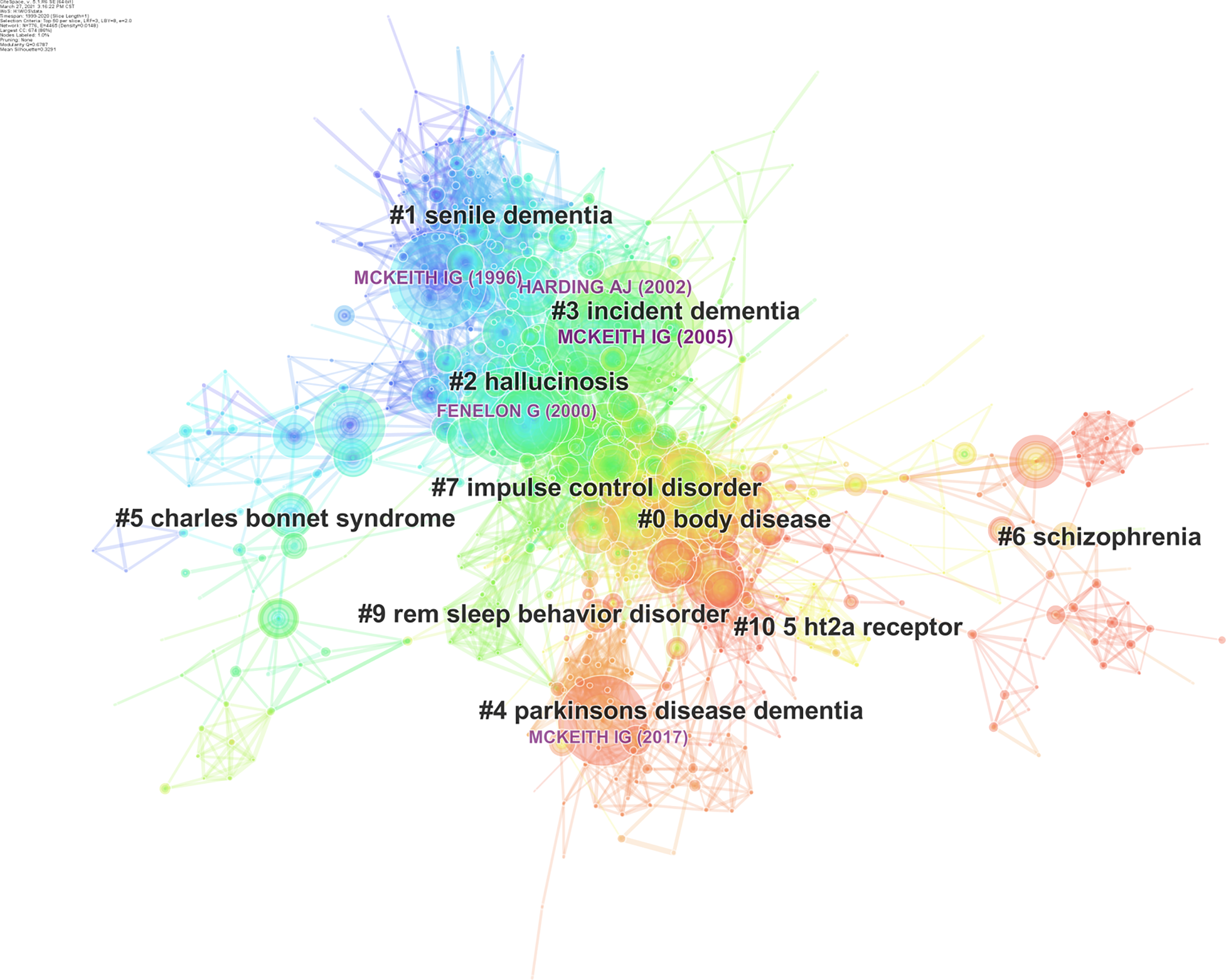
The most outstanding function in CiteSpace is co-citation analysis, which was used to analyze all references of the 2641 documents downloaded from WOSCC, and the top 50 most-cited references each year was select. As a result, 776 nodes of references were generated and automatically linked in the visual interface. Only if two references were cited by the same document could they be connected. If two references were often cited by documents together, they tended to have a close relationship, so they were classified to the same cluster. In our analysis, the smallest clusters were filed out, and 69 clusters were left. The information on all references formed the intellectual base of this field, and 776 highly cited references were classic documents[32]. By mining the most classic references in each cluster, we can understand the general development process and research frontiers of the VH research field. An overview of a co-cited reference network is shown in Figure 6. The overall structure can be divided into three major parts: the upper left part of the nodes and links, which represent the co-citation of the first 7 years from 1999 to 2005, is essentially in blue. The central part of the network is mainly in green and yellow, which indicates that the relationship was probably constructed between 2006 and 2012. The bottom right part is predominantly in red, and connections are formed credibly in the most recent 8 years.
The quantity of the clusters can be measured via two indexes: Modularity Q value and Silhouettre value. Modularity Q value is a network modularization index with a value range of 0-1. Q > 0.3 indicates that the structure of a certain cluster is significant. Silhouettre value indicates the homogeneity or consistency of the cluster. The closer the S value is to 1, the better the homogeneity of the cluster is. When S > 0.5, the clustering result can be considered reasonable; when S > 0.7, the clustering result is efficient. The number of references constituting the cluster must be greater than 10[35]. Table 1 lists the major clusters of co-cited references selected from 69 clusters. In general, the 10 clusters in the table represent 10 research directions in the field.
| Cluster | Size | Silhouette | Mean (year) | Label (LLR) |
| 0 | 154 | 0.727 | 2011 | Body disease |
| 1 | 110 | 0.883 | 1997 | Senile dementia |
| 2 | 82 | 0.715 | 2001 | Hallucinosis |
| 3 | 71 | 0.77 | 2005 | Incident dementia |
| 4 | 70 | 0.903 | 2014 | Parkinson's disease dementia |
| 5 | 57 | 0.957 | 2001 | Charles bonnet syndrome |
| 6 | 52 | 0.968 | 2014 | Schizophrenia |
| 7 | 47 | 0.822 | 2008 | Impulse control disorder |
| 9 | 11 | 0.975 | 2006 | Rem sleep behavior disorder |
| 10 | 11 | 0.99 | 2010 | 5 HT2a receptor |
The earliest formed cluster is Cluster #1 senile dementia, whose average publication year is 1997. It has more than 100 references as its members. A common theme of this cluster is identifying DLB from Alzheimer's disease, which are both belong to senile dementia[36-38]. The 82 members of Cluster #2 are evenly published in 2001, mainly involve PD with hallucination and focus on phenomenology[39]. Cluster #5 Charles bonnet syndrome, #3 incident dementia, and #6 schizophrenia introduce CBS, DLB and schizophrenia, respectively. These diseases all have VH as a hallmark. The research foundation in the three fields was mostly established in 2001, 2005, and 2014. Their high silhouette values indicate a high homogeneity of the clusters. Importantly, Cluster #2 and #3 are very close to each other in Figure 6; this may be partly because both PD and DLB are Lewy bodies (LB) diseases[40], and persistent VH is related to the spread of LBs[29].
The largest cluster is Cluster #0 labeled body disease, which includes 154 complete references. These references’ average publication year is 2011. Cluster #4 Parkinson’s disease dementia (PDD), which has 70 group members with an average publication year of 2014. These two clusters constitute the main body of VH research in the last decade and are closely connected in Figure 6 because they include research that introduces PD with VH. Cluster #0 focuses on the evidence of changes in brain structure and function in PD with VH[41,42], while Cluster #4 is mainly about the comparison and management of VH in PDD and DLB[43,44].
Other clusters, such as Cluster #7 impulse control disorder and Cluster #9 REM sleep behavior disorder (RBD) are formed more recently. Impulse control disorder and RBD are both included in the non-motor symptoms of PD. The co-cited references in these two clusters demonstrate the relationship between these two symptoms and VH respectively. Cluster #10 5-HT2A involves the investigation of serotonin 2A receptor in PD with psychosis (PDP)[45,46]. It includes publications averagely in 2010.
To detect emerging trends in the field, we further investigated the top high-quality co-cited references. In this part, we used the first author’s name plus the publication year as a notion to refer to the articles extracted from 776 co-cited articles. Top three references are shown in Table 2 as ranked by citation counts, centrality, burst and sigma respectively.
| Values | Ref. | Journal, volume, start page | Cluster # | |
| Counts | 124 | Mckeith et al[69], 2005 | Neurology, 65, 1863 | 3 |
| 93 | Harding et al[70], 2002 | Brain, 125, 391 | 3 | |
| 91 | Mckeith et al[36], 1996 | Neurology, 47, 1113 | 1 | |
| Centrality | 0.09 | Aarsland et al[71], 2002 | J Neurol Neurosur Ps, 72, 708 | 1 |
| 0.08 | Goetz et al[72], 2011 | Movement Disord, 26, 2196 | 0 | |
| 0.07 | Cummings et al[46], 2014 | Lancet, 383, 533 | 0 | |
| Bursts | 44.12 | Mckeith et al[74], 2017 | Neurology, 89, 88 | 4 |
| 43.96 | Mckeith et al[36], 1996 | Neurology, 47, 1113 | 1 | |
| 43.86 | Mckeith et al[69], 2005 | Neurology, 65, 1863 | 3 | |
| Sigma | 4.49 | Teunisse et al[79], 1996, | Lancet, 347, 794 | 5 |
| 4.36 | Cummings et al[46], 2014 | Lancet, 383, 533 | 0 | |
| 4.04 | Mckeith et al[69], 2005 | Neurology, 65, 1863 | 3 | |
Research on VH has formed a complete system. A total of 2641 articles were published between 1999 and 2020. Apparently, more original articles than reviews were published every year. In terms of the cumulative number of publications, the cumulation in 2020 (2641) is approximately twice that in 2011 (1244). Following the analysis of the chart, we can predict that this study period will further attract attention and continually grow in the next few years.
On a global scale, many countries conduct numerous studies on VH. This field has attracted wide attention worldwide. However, research imbalance between countries exists. To date, studies on VH have been predominantly conducted in North America (e.g., United States and Canada), Europe (e.g., United Kingdom, France, Germany), Asia (e.g., Japan and China), and Australia. The United States published the largest number of studies between 1999 and 2020. England was the second country that stands out in studies involving VH.
Dr. Taylor JP and Dr. Aarsland D are pioneers in the field. Dr. Taylor JP pushes back the frontiers of DLB[47] and plays an important role in the studies on the visual cortical excitability in the VH of DLB[7,48-50]. Dr. Aarsland D opens doors to reveal the psychiatric symptoms of PD[51-54]; his outstanding contribution to this field is clarifying that the presence of hallucination in PD is an important contributor to institutionalization and caregiver distress[55,56]. In addition, a group of new experts has emerged in this field in 2020, and their cooperation is very close. The team is mainly engaged in the research of electroencephalogram and evoked potentials of VHs, which is a new research breakthrough[57,58].
The most active topics of this field are ‘neuropsychiatric symptom’ and ‘functional connectivity’. For example, the burst period of keywords ‘neuropsychiatric symptom’ is from 2014 to 2020, and the burst intensity is 11.91. Cross-diseases (mainly psychiatric and neurological diseases) VH research started in 2014[59]. This article sparked a heated discussion on the relationship between VH in psychiatric and neurological diseases. Many scholars tend to believe that VH in different neuropsychiatry disorders have the same mechanism. Some experts have proposed that impairments in attentional network activity can explain all hallucinations in various diseases[60]. Yao et al[61] proposed that apart from schizophrenia, aberrant default mode network (DMN) was also found to contribute to the VH in PD. Meanwhile, the dorsal attention network (DAN) and the ventral attention network (VAN) also play important roles in the occurrence of VH[62]. DMN involves the function of sensory information perception and processing, while VAN engages attention to salient stimuli and DAN generates selective attention. The underactivation of VAN and overactivation of DAN and DMN lead to the recall of previously stored perception information, resulting in VH[63]. In the cohort of patients with VH, thinner retinal nerve fiber layer thickness was found by using spectral domain optical coherence tomography[64], and grey matter atrophy in visual perception region was shown in structural magnetic resonance imaging. These all provide evidence to support the attention deficit network model.
The keyword ‘functional connectivity’ burst from 2017 to 2020 with a burst intensity of 11.6008. Although current research has produced deep insights into the symptoms and nature of VH, the pathophysiology and etiology of VH remain unclear. Therefore, understanding neural mechanisms has considerable scientific and clinical significance. Evidence from the autopsy can only reveal changes in VH and not explain the cause of VH[65]. Functional neuroimaging studies are beneficial at capturing spontaneous VH in the neuroimaging scanner and the examination is noninvasive[66]. fMRI can investigate changes in specific parts of the brain rather than gross brain abnormalities[67]. It can reveal alterations in brain connectivity even before structural deficits occur. Resting state and task state are two main types of functional neuroimaging in the studies on VH[66]. The most recent research into the neural underpinnings of VH in schizophrenia concluded that the lateral occipital cortex (LOC) of patients showed increased connection with the frontoparietal task-control network and thalamus in the resting state; however, during task switching, LOC has an increase in interaction with the DMN[68].
Emerging trends of VH were identified based on structural and temporal properties derived from the relevant publications.
Given the groundbreaking contributions, the most cited articles in the research field are often considered the landmarks[31]. Mckeith IG (2005) and Harding AJ (2002) are at the top of the list. Both of them are in Cluster #3. Mckeith IG (1996) is the third most cited article and belongs to Cluster #1. Notably, Mckeith IG is a pioneer in the field of DLB and has published a series of DLB clinical guidelines in Neurology. Mckeith IG (1996) proposed the first consensus guideline for the clinicopathological diagnosis of DLB[36]. In 2005, Mckeith et al[69] published the third revised clinical diagnostic and treatment criteria for DLB, which included management in the criteria for the first time. These two guidelines authoritatively summarized the clinicopathological diagnosis of DLB and showed the direction of DLB treatment at that time. The article by Harding et al[70] entitled Visual hallucinations in Lewy body disease relate to Lewy bodies in the temporal lobe advanced the finding that temporal lobe LB is strikingly associated with VH given the distribution of LB in the brain. This result is a remarkable finding that links the clinical and pathological features of DLB together[70].
Pivot articles often refer to gateway articles between two densely connected subfields with a unique position. This type of articles can provide insights into emerging trends. The top ranked article by centrality is Aarsland D (2002) in Cluster #1, which has a centrality of 0.09. This double-blind, randomized, placebo-controlled study included 14 PDD patients to study the safety and effectiveness of the cholinesterase inhibitor donepezil in the treatment of PDD. This study lasted for 20 wk and finally they found that donepezil is safe and effective, also it does not worsen motor symptoms of PD[71]. This outstanding discovery has important therapeutic implications for PDD. The second one is Goetz CG (2011) in Cluster #0, with centrality of 0.08. Goetz et al[72] followed up 60 patients with PD but without hallucinations at baseline for more than 10 years. VH was found to dominate in early hallucination profile. This discovery revealed the outstanding position of VH in PDP. The third is Cummings J (2014) in Cluster #0, with centrality of 0.07. Cummings et al[46] conducted a randomized controlled double-blind trial on patients with PDP and concluded that this population can achieve benefit from Pimavanserin, a selective serotonin 5-HT2A inverse agonist, which is the only U.S. Food and Drug Administration-approved medication for PDP[23]. This study was a phase 3 trial that marks a critical breakthrough in the treatment of PDP, in which VH is a common symptom[73].
The importance of burst cannot be overemphasized. Through burst testing of all cited articles, we easily found that Mckeith IG (2017), Mckeith IG (1996), and Mckeith IG (2005) are on the top of the diagram. They are the milestones in relation to DLB. We discussed the last two articles in front part. The top one was Mckeith IG (2017) in Cluster #4. Mckeith et al[74] renewed the consensus report of DLB in 2017. Compared with the 2005 edition, the 2017 edition clearly distinguished clinical features and biological markers. According to different clinical features and biological markers, the diagnosis was divided into probable and possible DLB. Consistency of the diagnostic criteria for DLB will be more conducive to further research on DLB.
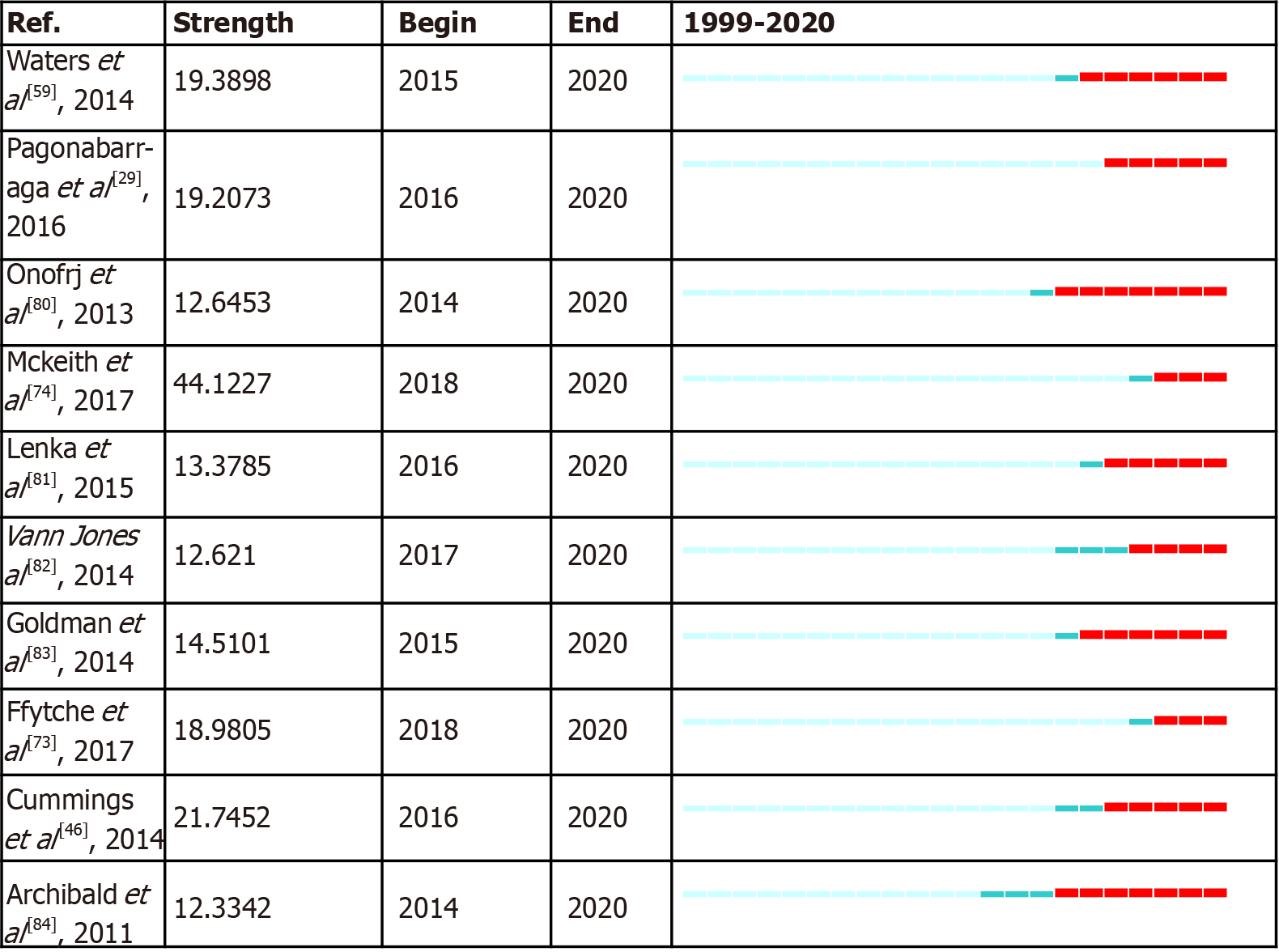
It is valuable to examine the citation burst in more recent articles while eliminating the overshadow burst of those landmark articles. The top 10 references that retained citation burst until 2020 are shown in Figure 7, which anticipates emerging trends in the future. In general, a new emerging trend involves PD with VH. Waters F (2014) is a relatively early article comparing VH in different diseases[59]. This article opened up a new dimension in VH research. Although many hypotheses have been proposed to explain the mechanism of VH, and many imaging and electrophysiological studies have partly proved these hypotheses; it is still unclear whether VH in different diseases has the same mechanism, and further research is needed. Pagonabarraga J (2016) is the only article that had citation burst as soon as its publication. PD minor hallucination is not a newly found phenomenon in PD but is underestimated because previous research on PDP focused on the study of well-structured VH. Pagonabarraga et al[29] reported that minor hallucination is the most frequent symptom in PDP and may even occur before the onset of parkinsonism. The third paper was Onofrj M (2013). This paper reviews the hypothetical mechanisms of VH in PD and DLB. To date, three predominant mechanistic models have been presented: a disturbance between top-down and bottom-up aspects of visual perception[1,75]; chronic deafferentation causing hyperexcitability to cortical structures involved in vision[3,76]; and the misattribution of internal imagery[59,77,78]. The authors tend to identify with the attention deficit network model as mentioned before.
Sigma is defined as: (centrality + 1)burstness, which can simultaneously measure a cited reference’s structural centrality and citation burstness. Teunisse RJ (1996) is at the top of the list, which objectively describes the characteristics of CBS by using a semi-structured interview[79]. The advanced nature of this article lies not only in the formation of the prototype semi-structured interview of VH but also in the occurrence of CBS, which is partly due to sensory deprivation and low arousal. The second was Cummings J (2014) in Cluster #4, Mckeith IG (2005) ranked third in this part and their importance is self-evident.
Through systematic analysis of the literature of VH over the past 22 years, we found that with the yearly progress of research on VH, its mystery has been gradually unveiled. Current research mainly focuses on neuropsychiatry. Countless countries, institutions, and authors have collaborated together and contributed to this field. North America, Europe, Asia and Australia showed outstanding contributions, and Dr. Taylor JP and Dr. Aarsland D are the most active contributors. Several research hotspots in the field of VH were detected in the recent research. The neuropsychiatry symptom and MRI function connectivity have been paid much attention. In the field of VH, neurodegenerative diseases, especially PD and DLB, were found to be widely studied. We believe that research on these two diseases will continue to advance the field of VH.
Although VH is of clinical importance, and its pathophysiology or treatment is mainly focused on single diseases and its mechanisms remain unclear. Additional clinical studies are required to provide higher evidence-based support for the diagnosis and treatment of VH. We investigated the basic literature related to VH and concluded that the small number of such studies is partly caused by difficulty in inducing and testing VH in animal models. Further efforts are required in this direction to obtain profound insights into the mechanisms that underlie VH. This issue will have important pathophysiologic and possible therapeutic implications in the future.
Visual hallucination (VH) refers to a spontaneous visual perception without corresponding external stimuli and often occurs in ophthalmological and neuropsychiatric disorders. It is associated with poor life quality, increased patient hospitalization, and nursing home admission.
To date, there is a lack of scientometric analysis of the research on VH.
To objectively summarize the features of VH research and gain insights into the emerging trends for research on VH.
CiteSpace V was used in this article. Publication outputs, document types, geographic distributions, co-authorship status, research hotspots, and co-citation status were analyzed. A total of 2176 original articles and 465 reviews were included in the database downloaded from the Web of Science Core Collection.
The results showed that most publications can be classified into neurology, sports and ophthalmology studies. In addition, North America, Europe, Asia, and Australia published the most documents. Some well-known authors have always had a leading role in this field; meanwhile, new authors keep emerging. A relatively stable cooperation has been formed among many authors. Furthermore, neuropsychiatric symptom and functional connectivity are the top hotspots. Research on VH in dementia with Lewy bodies and Parkinson’s disease (PD) have received much attention.
Studies on VH in PD are likely to be the new emerging trends in the future, especially the mechanism of VH.
More large-scale clinical and in-depth basic research studies are required to better understand the mechanisms underlie VH, which will contribute to our understanding of pathophysiology and therapy in VH.
| 1. | Collerton D, Perry E, McKeith I. Why people see things that are not there: a novel Perception and Attention Deficit model for recurrent complex visual hallucinations. Behav Brain Sci. 2005;28:737-757; discussion 757. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 288] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 2. | Minakaran N, Soorma T, Bronstein AM, Plant GT. Charles Bonnet syndrome and periodic alternating nystagmus: Moving visual hallucinations. Neurology. 2019;92:e1072-e1075. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Coltheart M. Charles Bonnet Syndrome: Cortical Hyperexcitability and Visual Hallucination. Curr Biol. 2018;28:R1253-R1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Wood RA, Hopkins SA, Moodley KK, Chan D. Fifty Percent Prevalence of Extracampine Hallucinations in Parkinson's Disease Patients. Front Neurol. 2015;6:263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 5. | Clegg BJ, Duncan GW, Khoo TK, Barker RA, Burn DJ, Yarnall AJ, Lawson RA. Categorising Visual Hallucinations in Early Parkinson's Disease. J Parkinsons Dis. 2018;8:447-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Firbank MJ, Parikh J, Murphy N, Killen A, Allan CL, Collerton D, Blamire AM, Taylor JP. Reduced occipital GABA in Parkinson disease with visual hallucinations. Neurology. 2018;91:e675-e685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 75] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 7. | Taylor JP, Firbank M, O'Brien JT. Visual cortical excitability in dementia with Lewy bodies. Br J Psychiatry. 2016;208:497-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | López-Mora DA, Camacho V, Lleó A, Fernández A, Carrió I. The Added Value of Dynamic 18F-Florbetapir PET in the Assessment of Dementia With Lewy Bodies. Clin Nucl Med. 2018;43:e85-e86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Heitz C, Noblet V, Cretin B, Philippi N, Kremer L, Stackfleth M, Hubele F, Armspach JP, Namer I, Blanc F. Neural correlates of visual hallucinations in dementia with Lewy bodies. Alzheimers Res Ther. 2015;7:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Doud A, Julius A, Ransom CB. Visual Phenomena in Occipital Lobe Epilepsy: "It's Beautiful! JAMA Neurol. 2018;75:1146-1147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Cachia A, Amad A, Brunelin J, Krebs MO, Plaze M, Thomas P, Jardri R. Deviations in cortex sulcation associated with visual hallucinations in schizophrenia. Mol Psychiatry. 2015;20:1101-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 12. | Rolland B, Amad A, Poulet E, Bordet R, Vignaud A, Bation R, Delmaire C, Thomas P, Cottencin O, Jardri R. Resting-state functional connectivity of the nucleus accumbens in auditory and visual hallucinations in schizophrenia. Schizophr Bull. 2015;41:291-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 13. | Rafique SA, Richards JR, Steeves JK. rTMS reduces cortical imbalance associated with visual hallucinations after occipital stroke. Neurology. 2016;87:1493-1500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Beaulieu-Boire I, Lang AE. Behavioral effects of levodopa. Mov Disord. 2015;30:90-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 15. | Núñez LA, Gurpegui M. Cannabis-induced psychosis: a cross-sectional comparison with acute schizophrenia. Acta Psychiatr Scand. 2002;105:173-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Mitchell J, Vierkant AD. Delusions and hallucinations of cocaine abusers and paranoid schizophrenics: a comparative study. J Psychol. 1991;125:301-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 38] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Hielscher E, Connell M, Lawrence D, Zubrick SR, Hafekost J, Scott JG. Prevalence and correlates of psychotic experiences in a nationally representative sample of Australian adolescents. Aust N Z J Psychiatry. 2018;52:768-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 18. | Pignon B, Geoffroy PA, Gharib A, Thomas P, Moutot D, Brabant W, Weens B, Dupond MP, Caron A, Falissard B, Medjkane F, Jardri R. Very early hallucinatory experiences: a school-based study. J Child Psychol Psychiatry. 2018;59:68-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 19. | Pearson J, Chiou R, Rogers S, Wicken M, Heitmann S, Ermentrout B. Sensory dynamics of visual hallucinations in the normal population. Elife. 2016;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 20. | Aynsworth C, Collerton D, Dudley R. Measures of visual hallucinations: Review and recommendations. Clin Psychol Rev. 2017;57:164-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Eversfield CL, Orton LD. Auditory and visual hallucination prevalence in Parkinson's disease and dementia with Lewy bodies: a systematic review and meta-analysis. Psychol Med. 2019;49:2342-2353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 22. | Jellinger KA. Dementia with Lewy bodies and Parkinson's disease-dementia: current concepts and controversies. J Neural Transm (Vienna). 2018;125:615-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 201] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 23. | Armstrong MJ, Okun MS. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA. 2020;323:548-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 845] [Cited by in RCA: 1840] [Article Influence: 306.7] [Reference Citation Analysis (0)] |
| 24. | Chyzhyk D, Graña M, Öngür D, Shinn AK. Discrimination of schizophrenia auditory hallucinators by machine learning of resting-state functional MRI. Int J Neural Syst. 2015;25:1550007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 25. | van Ommen MM, van Beilen M, Cornelissen FW, Smid HG, Knegtering H, Aleman A, van Laar T; GROUP Investigators. The prevalence of visual hallucinations in non-affective psychosis, and the role of perception and attention. Psychol Med. 2016;46:1735-1747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Goetz CG, Stebbins GT. Mortality and hallucinations in nursing home patients with advanced Parkinson's disease. Neurology. 1995;45:669-671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 189] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 27. | Mosimann UP, Rowan EN, Partington CE, Collerton D, Littlewood E, O'Brien JT, Burn DJ, McKeith IG. Characteristics of visual hallucinations in Parkinson disease dementia and dementia with lewy bodies. Am J Geriatr Psychiatry. 2006;14:153-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 110] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 28. | Goetz CG, Fan W, Leurgans S, Bernard B, Stebbins GT. The malignant course of "benign hallucinations" in Parkinson disease. Arch Neurol. 2006;63:713-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 107] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 29. | Pagonabarraga J, Martinez-Horta S, Fernández de Bobadilla R, Pérez J, Ribosa-Nogué R, Marín J, Pascual-Sedano B, García C, Gironell A, Kulisevsky J. Minor hallucinations occur in drug-naive Parkinson's disease patients, even from the premotor phase. Mov Disord. 2016;31:45-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 171] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 30. | Chen C. Searching for intellectual turning points: progressive knowledge domain visualization. Proc Natl Acad Sci U S A. 2004;101 Suppl 1:5303-5310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1484] [Cited by in RCA: 1412] [Article Influence: 64.2] [Reference Citation Analysis (1)] |
| 31. | Chen C, Hu Z, Liu S, Tseng H. Emerging trends in regenerative medicine: a scientometric analysis in CiteSpace. Expert Opin Biol Ther. 2012;12:593-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 499] [Cited by in RCA: 686] [Article Influence: 49.0] [Reference Citation Analysis (0)] |
| 32. | Chen C, Dubin R, Kim MC. Emerging trends and new developments in regenerative medicine: a scientometric update (2000 - 2014). Expert Opin Biol Ther. 2014;14:1295-1317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 449] [Article Influence: 37.4] [Reference Citation Analysis (1)] |
| 33. | Chen C, Leydesdorff L. Patterns of connections and movements in dual-map overlays: A new method of publication portfolio analysis. J Assoc Inf Sci Technol. 2014;65:334-351. [RCA] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 180] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 34. | Yao L, Hui L, Yang Z, Chen X, Xiao A. Freshwater microplastics pollution: Detecting and visualizing emerging trends based on Citespace II. Chemosphere. 2020;245:125627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 109] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 35. | Chen C, Ibekwe-SanJuan F, Hou J. The structure and dynamics of cocitation clusters: A multiple-perspective cocitation analysis. J Am Soc Inf Sci Technol. 2010;61 ):1386-1409. [RCA] [DOI] [Full Text] [Cited by in Crossref: 684] [Cited by in RCA: 601] [Article Influence: 37.6] [Reference Citation Analysis (0)] |
| 36. | McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, Salmon DP, Lowe J, Mirra SS, Byrne EJ, Lennox G, Quinn NP, Edwardson JA, Ince PG, Bergeron C, Burns A, Miller BL, Lovestone S, Collerton D, Jansen EN, Ballard C, de Vos RA, Wilcock GK, Jellinger KA, Perry RH. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47:1113-1124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2500] [Cited by in RCA: 2455] [Article Influence: 81.8] [Reference Citation Analysis (0)] |
| 37. | Lobotesis K, Fenwick JD, Phipps A, Ryman A, Swann A, Ballard C, McKeith IG, O'Brien JT. Occipital hypoperfusion on SPECT in dementia with Lewy bodies but not AD. Neurology. 2001;56:643-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 253] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 38. | Ballard C, Holmes C, McKeith I, Neill D, O'Brien J, Cairns N, Lantos P, Perry E, Ince P, Perry R. Psychiatric morbidity in dementia with Lewy bodies: a prospective clinical and neuropathological comparative study with Alzheimer's disease. Am J Psychiatry. 1999;156:1039-1045. [PubMed] |
| 39. | Fénelon G, Mahieux F, Huon R, Ziégler M. Hallucinations in Parkinson's disease: prevalence, phenomenology and risk factors. Brain. 2000;123 ( Pt 4):733-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 697] [Cited by in RCA: 647] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 40. | Schumacher J, Thomas AJ, Taylor JP. Dynamic functional connectivity changes in Lewy body disease. Brain. 2019;142:e68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 41. | Knolle F, Garofalo S, Viviani R, Justicia A, Ermakova AO, Blank H, Williams GB, Arrondo G, Ramachandra P, Tudor-Sfetea C, Bunzeck N, Duezel E, Robbins TW, Barker RA, Murray GK. Altered subcortical emotional salience processing differentiates Parkinson's patients with and without psychotic symptoms. Neuroimage Clin. 2020;27:102277. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 42. | Meppelink AM, de Jong BM, Teune LK, van Laar T. Regional cortical grey matter loss in Parkinson's disease without dementia is independent from visual hallucinations. Mov Disord. 2011;26:142-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 43. | Taylor JP, McKeith IG, Burn DJ, Boeve BF, Weintraub D, Bamford C, Allan LM, Thomas AJ, O'Brien JT. New evidence on the management of Lewy body dementia. Lancet Neurol. 2020;19:157-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 225] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 44. | Onofrj M, Espay AJ, Bonanni L, Delli Pizzi S, Sensi SL. Hallucinations, somatic-functional disorders of PD-DLB as expressions of thalamic dysfunction. Mov Disord. 2019;34:1100-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 45. | Ballanger B, Strafella AP, van Eimeren T, Zurowski M, Rusjan PM, Houle S, Fox SH. Serotonin 2A receptors and visual hallucinations in Parkinson disease. Arch Neurol. 2010;67:416-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 181] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 46. | Cummings J, Isaacson S, Mills R, Williams H, Chi-Burris K, Corbett A, Dhall R, Ballard C. Pimavanserin for patients with Parkinson's disease psychosis: a randomised, placebo-controlled phase 3 trial. Lancet. 2014;383:533-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 493] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 47. | McKeith IG, Ferman TJ, Thomas AJ, Blanc F, Boeve BF, Fujishiro H, Kantarci K, Muscio C, O'Brien JT, Postuma RB, Aarsland D, Ballard C, Bonanni L, Donaghy P, Emre M, Galvin JE, Galasko D, Goldman JG, Gomperts SN, Honig LS, Ikeda M, Leverenz JB, Lewis SJG, Marder KS, Masellis M, Salmon DP, Taylor JP, Tsuang DW, Walker Z, Tiraboschi P; prodromal DLB Diagnostic Study Group. Research criteria for the diagnosis of prodromal dementia with Lewy bodies. Neurology. 2020;94:743-755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 380] [Cited by in RCA: 538] [Article Influence: 89.7] [Reference Citation Analysis (0)] |
| 48. | Erskine D, Taylor JP, Thomas A, Collerton D, McKeith I, Khundakar A, Attems J, Morris C. Pathological Changes to the Subcortical Visual System and its Relationship to Visual Hallucinations in Dementia with Lewy Bodies. Neurosci Bull. 2019;35:295-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 49. | Taylor JP, Firbank MJ, He J, Barnett N, Pearce S, Livingstone A, Vuong Q, McKeith IG, O'Brien JT. Visual cortex in dementia with Lewy bodies: magnetic resonance imaging study. Br J Psychiatry. 2012;200:491-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 50. | Taylor JP, Firbank M, Barnett N, Pearce S, Livingstone A, Mosimann U, Eyre J, McKeith IG, O'Brien JT. Visual hallucinations in dementia with Lewy bodies: transcranial magnetic stimulation study. Br J Psychiatry. 2011;199:492-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 51] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 51. | Aarsland D, Marsh L, Schrag A. Neuropsychiatric symptoms in Parkinson's disease. Mov Disord. 2009;24:2175-2186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 350] [Article Influence: 21.9] [Reference Citation Analysis (1)] |
| 52. | Aarsland D, Larsen JP, Cummins JL, Laake K. Prevalence and clinical correlates of psychotic symptoms in Parkinson disease: a community-based study. Arch Neurol. 1999;56:595-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 246] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 53. | Aarsland D, Larsen JP, Lim NG, Janvin C, Karlsen K, Tandberg E, Cummings JL. Range of neuropsychiatric disturbances in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry. 1999;67:492-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 379] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 54. | Aarsland D, Brønnick K, Alves G, Tysnes OB, Pedersen KF, Ehrt U, Larsen JP. The spectrum of neuropsychiatric symptoms in patients with early untreated Parkinson's disease. J Neurol Neurosurg Psychiatry. 2009;80:928-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 183] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 55. | Aarsland D, Larsen JP, Karlsen K, Lim NG, Tandberg E. Mental symptoms in Parkinson's disease are important contributors to caregiver distress. Int J Geriatr Psychiatry. 1999;14:866-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 56. | Aarsland D, Larsen JP, Tandberg E, Laake K. Predictors of nursing home placement in Parkinson's disease: a population-based, prospective study. J Am Geriatr Soc. 2000;48:938-942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 516] [Cited by in RCA: 540] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 57. | Franciotti R, Pilotto A, Moretti DV, Falasca NW, Arnaldi D, Taylor JP, Nobili F, Kramberger M, Ptacek SG, Padovani A, Aarlsand D, Onofrj M, Bonanni L; E-DLB consortium. Anterior EEG slowing in dementia with Lewy bodies: a multicenter European cohort study. Neurobiol Aging. 2020;93:55-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 58. | Pascarelli MT, Del Percio C, De Pandis MF, Ferri R, Lizio R, Noce G, Lopez S, Rizzo M, Soricelli A, Nobili F, Arnaldi D, Famà F, Orzi F, Buttinelli C, Giubilei F, Salvetti M, Cipollini V, Franciotti R, Onofri M, Fuhr P, Gschwandtner U, Ransmayr G, Aarsland D, Parnetti L, Farotti L, Marizzoni M, D'Antonio F, De Lena C, Güntekin B, Hanoğlu L, Yener G, Emek-Savaş DD, Triggiani AI, Paul Taylor J, McKeith I, Stocchi F, Vacca L, Hampel H, Frisoni GB, Bonanni L, Babiloni C. Abnormalities of resting-state EEG in patients with prodromal and overt dementia with Lewy bodies: Relation to clinical symptoms. Clin Neurophysiol. 2020;131:2716-2731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 59. | Waters F, Collerton D, Ffytche DH, Jardri R, Pins D, Dudley R, Blom JD, Mosimann UP, Eperjesi F, Ford S, Larøi F. Visual hallucinations in the psychosis spectrum and comparative information from neurodegenerative disorders and eye disease. Schizophr Bull. 2014;40 Suppl 4:S233-S245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 224] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 60. | Shine JM, O'Callaghan C, Halliday GM, Lewis SJ. Tricks of the mind: Visual hallucinations as disorders of attention. Prog Neurobiol. 2014;116:58-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 61. | Yao N, Shek-Kwan Chang R, Cheung C, Pang S, Lau KK, Suckling J, Rowe JB, Yu K, Ka-Fung Mak H, Chua SE, Ho SL, McAlonan GM. The default mode network is disrupted in Parkinson's disease with visual hallucinations. Hum Brain Mapp. 2014;35:5658-5666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 108] [Cited by in RCA: 123] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 62. | Muller AJ, Shine JM, Halliday GM, Lewis SJ. Visual hallucinations in Parkinson's disease: theoretical models. Mov Disord. 2014;29:1591-1598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 63. | Shine JM, Halliday GM, Naismith SL, Lewis SJ. Visual misperceptions and hallucinations in Parkinson's disease: dysfunction of attentional control networks? Mov Disord. 2011;26:2154-2159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 156] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 64. | Lee JY, Kim JM, Ahn J, Kim HJ, Jeon BS, Kim TW. Retinal nerve fiber layer thickness and visual hallucinations in Parkinson's Disease. Mov Disord. 2014;29:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 65. | O'Brien J, Taylor JP, Ballard C, Barker RA, Bradley C, Burns A, Collerton D, Dave S, Dudley R, Francis P, Gibbons A, Harris K, Lawrence V, Leroi I, McKeith I, Michaelides M, Naik C, O'Callaghan C, Olsen K, Onofrj M, Pinto R, Russell G, Swann P, Thomas A, Urwyler P, Weil RS, Ffytche D. Visual hallucinations in neurological and ophthalmological disease: pathophysiology and management. J Neurol Neurosurg Psychiatry. 2020;91:512-519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 83] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 66. | Zmigrod L, Garrison JR, Carr J, Simons JS. The neural mechanisms of hallucinations: A quantitative meta-analysis of neuroimaging studies. Neurosci Biobehav Rev. 2016;69:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 156] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 67. | Schultz SK, Andreasen NC. Schizophrenia. Lancet. 1999;353:1425-1430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 174] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 68. | Li K, Sweeney JA, Hu XP. Context-dependent dynamic functional connectivity alteration of lateral occipital cortex in schizophrenia. Schizophr Res. 2020;220:201-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 69. | McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del Ser T, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VM, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M; Consortium on DLB. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65:1863-1872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4043] [Cited by in RCA: 3682] [Article Influence: 175.3] [Reference Citation Analysis (0)] |
| 70. | Harding AJ, Broe GA, Halliday GM. Visual hallucinations in Lewy body disease relate to Lewy bodies in the temporal lobe. Brain. 2002;125:391-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 381] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 71. | Aarsland D, Laake K, Larsen JP, Janvin C. Donepezil for cognitive impairment in Parkinson's disease: a randomised controlled study. J Neurol Neurosurg Psychiatry. 2002;72:708-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 210] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 72. | Goetz CG, Stebbins GT, Ouyang B. Visual plus nonvisual hallucinations in Parkinson's disease: development and evolution over 10 years. Mov Disord. 2011;26:2196-2200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 73. | Ffytche DH, Creese B, Politis M, Chaudhuri KR, Weintraub D, Ballard C, Aarsland D. The psychosis spectrum in Parkinson disease. Nat Rev Neurol. 2017;13:81-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 251] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 74. | McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, Aarsland D, Galvin J, Attems J, Ballard CG, Bayston A, Beach TG, Blanc F, Bohnen N, Bonanni L, Bras J, Brundin P, Burn D, Chen-Plotkin A, Duda JE, El-Agnaf O, Feldman H, Ferman TJ, Ffytche D, Fujishiro H, Galasko D, Goldman JG, Gomperts SN, Graff-Radford NR, Honig LS, Iranzo A, Kantarci K, Kaufer D, Kukull W, Lee VMY, Leverenz JB, Lewis S, Lippa C, Lunde A, Masellis M, Masliah E, McLean P, Mollenhauer B, Montine TJ, Moreno E, Mori E, Murray M, O'Brien JT, Orimo S, Postuma RB, Ramaswamy S, Ross OA, Salmon DP, Singleton A, Taylor A, Thomas A, Tiraboschi P, Toledo JB, Trojanowski JQ, Tsuang D, Walker Z, Yamada M, Kosaka K. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology. 2017;89:88-100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3653] [Cited by in RCA: 3146] [Article Influence: 349.6] [Reference Citation Analysis (0)] |
| 75. | Lefebvre S, Baille G, Jardri R, Plomhause L, Szaffarczyk S, Defebvre L, Thomas P, Delmaire C, Pins D, Dujardin K. Hallucinations and conscious access to visual inputs in Parkinson's disease. Sci Rep. 2016;6:36284. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 76. | Manford M, Andermann F. Complex visual hallucinations. Clinical and neurobiological insights. Brain. 1998;121 ( Pt 10):1819-1840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 300] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 77. | Shine JM, Keogh R, O'Callaghan C, Muller AJ, Lewis SJ, Pearson J. Imagine that: elevated sensory strength of mental imagery in individuals with Parkinson's disease and visual hallucinations. Proc Biol Sci. 2015;282:20142047. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 61] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 78. | Fernyhough C. Modality-general and modality-specific processes in hallucinations. Psychol Med. 2019;49:2639-2645. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 79. | Teunisse RJ, Cruysberg JR, Hoefnagels WH, Verbeek AL, Zitman FG. Visual hallucinations in psychologically normal people: Charles Bonnet's syndrome. Lancet. 1996;347:794-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 233] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 80. | Onofrj M, Taylor JP, Monaco D, Franciotti R, Anzellotti F, Bonanni L, Onofrj V, Thomas A. Visual hallucinations in PD and Lewy body dementias: old and new hypotheses. Behav Neurol. 2013;27:479-493. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 37] [Reference Citation Analysis (0)] |
| 81. | Lenka A, Jhunjhunwala KR, Saini J, Pal PK. Structural and functional neuroimaging in patients with Parkinson's disease and visual hallucinations: A critical review. Relat Disord. 2015;21:683-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 82. | Vann Jones SA, O'Brien JT. The prevalence and incidence of dementia with Lewy bodies: a systematic review of population and clinical studies. Psychol Med. 2014;44:673-683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 361] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 83. | Goldman JG, Stebbins GT, Dinh V, Bernard B, Merkitch D, deToledo-Morrell L, Goetz CG. Visuoperceptive region atrophy independent of cognitive status in patients with Parkinson's disease with hallucinations. Brain. 2014;137:849-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 84. | Archibald NK, Clarke MP, Mosimann UP, Burn DJ. Visual symptoms in Parkinson's disease and Parkinson's disease dementia. Mov Disord. 2011;26:2387-2395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 154] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Psychiatry
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Shiina A S-Editor: Gao CC L-Editor: Filipodia P-Editor: Wang LYT