Published online Aug 19, 2021. doi: 10.5498/wjp.v11.i8.463
Peer-review started: February 24, 2021
First decision: July 4, 2021
Revised: July 12, 2021
Accepted: July 29, 2021
Article in press: July 29, 2021
Published online: August 19, 2021
Processing time: 169 Days and 3.8 Hours
Sleep dysfunction is a common problem in people with schizophrenia, and side effects of treatment often exacerbate metabolic and cardiovascular risk and may induce extrapyramidal side effects. Melatonin (N-acetyl-5-methoxytryptamine) is an endogenously produced hormone which has demonstrated direct and indirect antioxidant and neuroprotective effects. Previous studies have explored the use of exogenous melatonin in improving sleep outcomes in the general population, yet indications for use in schizophrenia are unclear.
To synthesize the evidence from clinical trials investigating prescribed melatonin as an adjunctive therapy in patients with schizophrenia.
A systematic literature review of MEDLINE (Ovid), Embase, PsychINFO, and PubMed on the 27/08/20; and CINAHL and Cochrane Library databases, was conducted. Inclusion criteria were: a peer-reviewed clinical trial published in English; included a group of patients with schizophrenia; used melatonin as an adjunctive therapy; and reported any outcome of any duration. Exclusion criteria were: neurodegenerative diseases, primary sleep disorders, co-morbid substance use or animal studies.
Fifteen studies were included in the current review with the following primary outcomes: sleep (n = 6), metabolic profile (n = 3), tardive dyskinesia (n = 3), cognitive function (n = 2) and benzodiazepine discontinuation (n = 1).
Adjunctive melatonin therapy has some positive outcomes for sleep, metabolic profile and tardive dyskinesia in patients with schizophrenia. No beneficial effect of melatonin was observed on outcomes of cognition or benzodiazepine discontinuation. Future studies utilizing larger samples and investigations specifically comparing the effect of melatonin as adjunctive therapy with different antipsychotics in patients with schizophrenia are required.
Core Tip: This systematic review synthesized the results of clinical trials that have investigated the effect of exogenous melatonin as adjunctive therapy for patients with schizophrenia. Some positive outcomes were demonstrated for sleep improvement and attenuating antipsychotic-induced metabolic side effects. Future investigations are required to determine differential effects of melatonin when used in conjunction with a range of antipsychotic medications.
- Citation: Duan C, Jenkins ZM, Castle D. Therapeutic use of melatonin in schizophrenia: A systematic review. World J Psychiatr 2021; 11(8): 463-476
- URL: https://www.wjgnet.com/2220-3206/full/v11/i8/463.htm
- DOI: https://dx.doi.org/10.5498/wjp.v11.i8.463
Schizophrenia is a chronic psychiatric disorder characterized by a combination of positive (hallucinations and delusions) and negative symptoms affecting thought, perception, cognition and behaviour through complex mechanisms, often resulting in significant deterioration of function[1]. Along with the cardinal features of psychosis, associated side effects of medications and sleep dysfunction can cause lifelong impact on patients’ quality of life[2].
Second-generation antipsychotics (SGAs) are the current standard for treating schizophrenia and related illnesses due to the comparatively lower rates of extrapyramidal side effects (EPS), as compared with first generation antipsychotics[3]. However, it has been demonstrated that EPS remain a potential side effect of SGAs[4]; tardive dyskinesia (TD) remains a risk even with these newer agents[5], with limited understanding of both the mechanisms and treatment options[6]. Furthermore, significant weight gain and an increased incidence of metabolic syndrome (MetS) and cardiovascular disease risk have also been established in association with certain SGA medications[7].
Sleep dysfunction is also common in people with schizophrenia, as demonstrated by poor sleep efficiency and disrupted circadian rhythms[8,9]. Studies have shown that there is decreased endogenous secretion of melatonin in patients with schizophrenia, and this pattern can persist despite improvement of sleep quantity and quality with antipsychotic agents[10]. Benzodiazepines (BZDs) are widely used to ameliorate sleep disruption in schizophrenia. While only recommended for short-term use, many patients remain on BZDs long-term, suffering additional effects including sedation, increased risk of falls, and cognitive impairment[11,12].
In an effort to address sleep dysfunction in individuals with schizophrenia, various studies have explored the use of exogenous melatonin. Melatonin (N-acetyl-5-methoxytryptamine) is an endogenously produced hormone naturally secreted at night from the pineal gland in a circadian rhythm to promote sleep. Adjunctive administration of exogenous melatonin has been recognized to have therapeutic benefit in sleep disorders in the general population[13,14]; however, it remains relatively less explored in people with schizophrenia. Furthermore, melatonin has also demonstrated direct and indirect antioxidant and neuroprotective effects, suggesting various potential clinical uses for treatment in schizophrenia, notably EPS and MetS[15].
The current review aims to synthesize the evidence from clinical trials investigating the effect of adjunctive use of melatonin on any outcome in individuals with schizophrenia.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were adhered to throughout the review.
The literature search was conducted online for papers in the English language with no restriction of publication date, using MEDLINE (Ovid), Embase, PsychINFO, and PubMed on 27/08/20; and CINAHL and the Cochrane Library on 28/08/20. Syntax was translated using Polyglot to search the databases appropriately. Search terms included the keywords of “schizophrenia”, “melatonin” and with the following trade names — circadin, regulin, benedorm, melaxen or melovine, and “clinical trials” (of any type). Boolean operators OR was used to combine synonyms of the keywords, and AND was used to combine search terms. MeSH headings were specifically used for “schizophrenia”, “melatonin” and “clinical trials” as well as searching for these terms in the title and abstract. Additional studies were identified from a manual search of the reference lists of included articles and registered clinical trials.
The inclusion criteria for papers in this systematic review were: (1) A peer-reviewed research article published in English; (2) Included a group of participants with a diagnosis of schizophrenia (including its subtypes), paraphrenia, delusional psychoses, paranoid psychosis, psychosis not otherwise specified, schizophreniform disorder, schizotypal disorder or schizoaffective disorder; (3) A clinical trial using melatonin as adjunctive therapy; (4) Collected an outcome measure of any duration; and (5) Included original data in the paper. Exclusion criteria included review papers, meta-analyses, case reports, animal studies or studies with patient populations that included neurodegenerative diseases, organic causes of disease, and primary sleep disorders.
Search results were exported to Endnote bibliographic management software, duplicates removed, and the remainder uploaded to Covidence systematic review software (www.covidence.org) by Duan C Two authors (Duan C, Castle D) independently screened records on title and abstract and then full text against the exclusion criteria and disagreements were resolved by discussion.
Two reviewers (Jenkins ZM and Duan C) independently extracted data and consensus was confirmed by a third reviewer (Castle D). Extracted data included information on study characteristics, objectives and outcomes. A meta-analysis was not performed as there were too few similarities across studies in terms of study methods and outcome measures. The risk of bias among included studies was assessed independently by two authors (Duan C and Jenkins ZM) using the Cochrane risk-of-bias tool for randomised trials[16] and consensus was confirmed by a third reviewer (Castle D). Studies were classified into three classes of quality, viz.: low, moderate or high risk of bias. The risk of bias was not used as an exclusion criterion in the selection of studies, to provide a complete overview of available data.
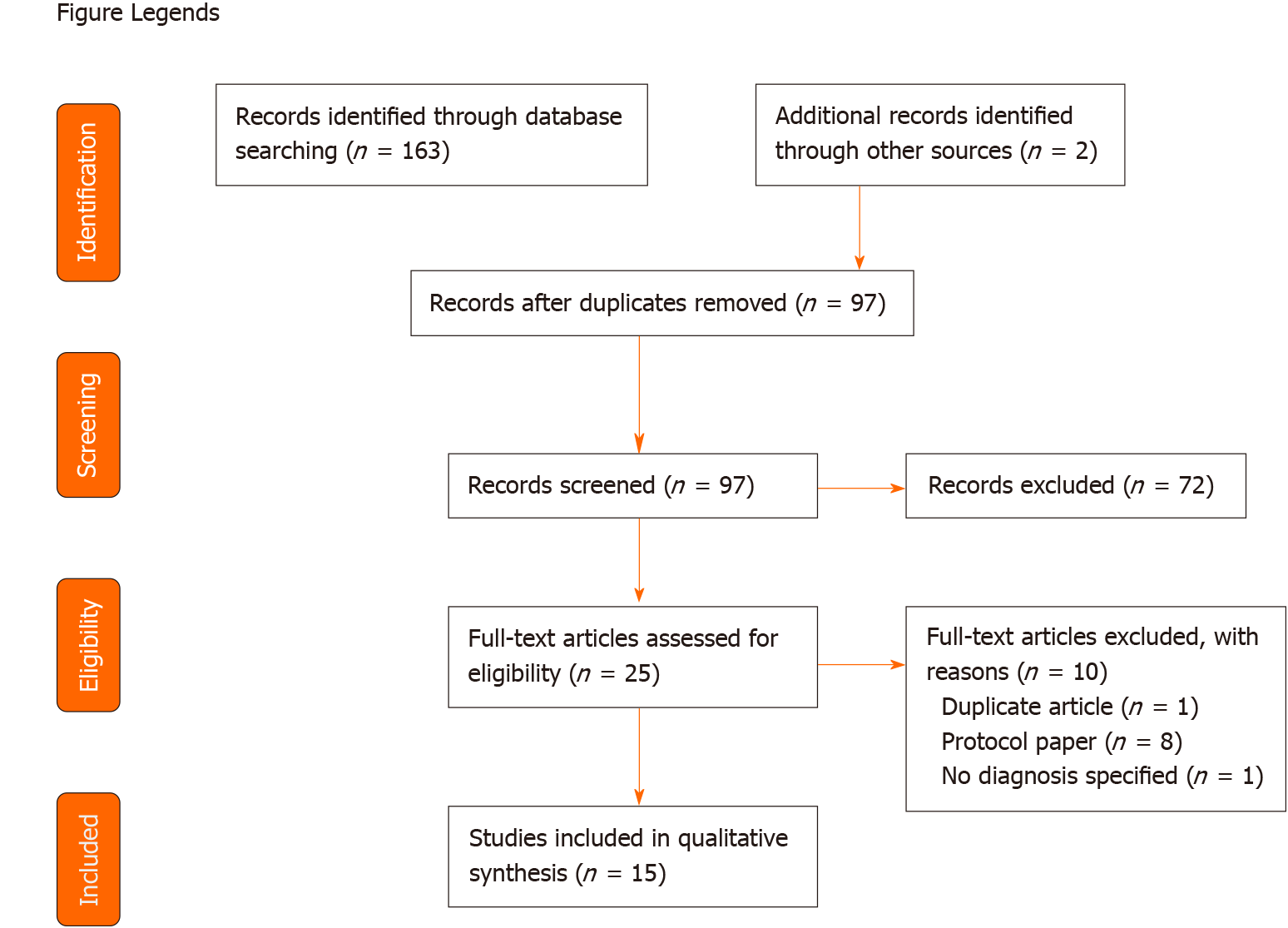
A total of 163 papers were identified from the search and an additional two papers were found from the reference list of the identified papers. Sixty eight duplicate records were excluded and the titles and abstracts of the remaining 97 papers were screened independently by two authors (Duan C and Castle D). After title and abstract screening, 25 papers were read in full and assessed for eligibility. A further 10 papers were excluded, leaving 15 papers included for qualitative synthesis (see Figure 1 for an overview of the study selection process).
Characteristics of the 15 included studies for qualitative synthesis are shown in Tables 1-5. Four trials had a crossover design, two were open-label and nine utilized a randomized double-blind parallel design. The 15 trials included a total of 626 participants (351 male, 252 female, 23 not specified) with sample sizes ranging from 10-120. Diagnoses in the studies included 269 participants with schizophrenia, 268 with paranoid schizophrenia, 7 with disorganized schizophrenia, 27 with schizoaffective disorder and 55 with bipolar disorder (the bipolar disorder patients were part of a cohort including people with schizophrenia, hence their inclusion).
| Ref. | Sample size (sex) melatonin; control | Age (yr) melatonin; control | Dose, duration | Diagnosis | Inclusion criteria | Study design | Outcomes | Significant findings related to melatonin | Risk of bias |
| Shamir et al[18], 2000 | 14 (11 M, 3 F), 14 (11 M, 3 F) | Overall: 42.3 ± 13.1 | 2 mg CR melatonin or placebo/day for 3 wk | SZA (n = 2); Paranoid SCZ (n = 10); Disorganised SCZ (n = 2) | Diagnosis of chronic SCZ (as per DSM-IV criteria); poor sleep quality | Randomized, double-blind, crossover trial (1 wk washout) | Sleep latency (min), REM sleep (%), REM sleep latency (min), total sleep time (min), sleep efficiency (%), duration of wakefulness (min), stage 1 sleep (%), slow wave sleep (%) | REM Sleep latency (min): Melatonin: 1st night > 2nd night | High |
| Sleep efficiency (%): Melatonin: 1st night < 2nd night | |||||||||
| Duration of wakefulness (min): Melatonin: 1st night > 2nd night | |||||||||
| Stage 1 sleep (%): Placebo: 1st night < 2nd night | |||||||||
| Shamir et al[17], 2000 | 19 (12 M, 7 F); 19 (12 M, 7 F) | Overall: 42 ± 5 | 2 mg CR melatonin or placebo/day for 3 wk | SZA (n = 5); Paranoid SCZ (n = 9); Disorganised SCZ (n = 5) | Diagnosis of chronic SCZ (as per DSM-IV criteria); poor sleep quality | Randomized, double-blind, crossover trial (1 wk washout) | Urinary 6-SMT excretion, sleep efficiency (%), sleep latency (min), total sleep time (min), wake after sleep onset duration (min), fragmentation index (%), number of awakenings (N) | Sleep efficiency (%): Melatonin > Placebo | Some concerns |
| Suresh Kumar et al[19], 2007 | 20 (13 M, 7 F); 20 (14 M, 6 F) | 38.4 ± 14.4; 36.0 ± 13.4 | Patient determined dosage of melatonin or placebo for 15 d | Paranoid SCZ (n = 40) | Diagnosis of paranoid SCZ (as per DSM-IV criteria); illness duration < 1 yr; clinically stable; receiving same dose of haloperidol for the past month, insomnia present for past 2 wk | Double-blind, placebo-controlled study | Time taken to fall asleep (min), number of awakenings (n), duration of sleep (min), self-report sleep questionnaire | Number of awakenings (N): Melatonin < Placebo | High |
| Duration of sleep (min): Melatonin > Placebo | |||||||||
| Self-report sleep questionnaire: Time to fall asleep, quality of sleep, depth of sleep, freshness on awakening, morning headache, morning mental dullness, mood, overall functioning were superior in Melatonin group vs Placebo | |||||||||
| Mishra et al[20], 2020 | PPS: 30 (15 M, 15 F); 30 (21 M, 9 F). PNS: 30 (21 M, 9 F); 30 (14 M, 16 F) | PPS: 38.6 ± 10.68; 34.0 ± 8.38. PNS: 34.97 ± 12.35; 37.87 ± 3.84 | 8 mg/d Ramelteon + monotherapy vs monotherapy alone for 4 wk | SCZ (n = 120). Patients were categorized into PPS (n = 60) and PNS (n = 60) groups based on PANSS scoring | Diagnosis of SCZ (as per DSM-5 criteria); aged between 18-65 yr; treatment naïve or had not taken treatment for 4 wk | Randomized, open-label, rater-blinded, parallel design clinical trial | Quality of sleep (PSQI), melatonin excretion (urinary melatonin 6aMTs), serum AANAT, symptom severity (PANSS) | Change in serum melatonin at 14:00 h: PPS and PNS: Melatonin > Control | High |
| Change in serum melatonin 2 h after add on therapy: PPS and PNS: Melatonin > Control | |||||||||
| Change in urinary melatonin: PPS and PNS: Melatonin > Control | |||||||||
| Change in serum AANAT: PPS and PNS: Melatonin > Control | |||||||||
| PSQI: PPS and PNS: Melatonin > Control | |||||||||
| Change in PANSS total score: PPS and PNS: Total score improved Melatonin > Control; PPS: Decreased positive symptoms in Melatonin > Control; PNS: Decrease negative symptoms in Melatonin > Control | |||||||||
| Baandrup et al[22], 2016 | 20 (11 M, 9 F); 28 (18 M, 10 F) | 47.7 ± 8.2; 45.9 ± 10.3 | 2 mg/d PR melatonin or placebo for 24 wk | Paranoid SCZ (n = 38), Non-paranoid SCZ (n = 2), SZA (n = 2), BP (n = 6) | Diagnosis of SCZ, SZA or BP (as per ICD-10 criteria); treated with 1 antipsychotic and 1 BZD for 3 m | Randomized, double-blind clinical trial | Actigraphy (sleep and 24 h rhythm activity variables) | - | Some concerns |
| Baandrup et al[21], 2016 | 28 (14 M, 14 F); 27 (15 M, 12 F) | 48.8 ± 7.1; 49.1 ± 12.2 | 2 mg/d PR melatonin or placebo for 24 wk | Paranoid SCZ (n = 42), non-paranoid SCZ (n = 2), SZA (n = 3), BP (n = 8) | Diagnosis of SCZ, SZA or BP (as per ICD-10 criteria); treated with 1 antipsychotic and 1 BZD for 3 mo | Randomized, double-blind clinical trial | PSQI, polysomnography (n = 23; total sleep time, sleep latency, REM latency, time awake after sleep onset, number of awakenings, sleep architecture) | PSQI sleep quality: Melatonin > Placebo | Some concerns |
| Ref. | Sample size (sex) melatonin; control | Age (yr) melatonin; control | Dose, duration | Diagnosis | Inclusion criteria | Study design | Outcomes | Significant findings related to melatonin | Risk of bias |
| Borba et al[23], 2011 | 14 (8 M, 6 F); 6 (5 M, 1 F) | 49 ± 7; 56 ± 9 | 8 mg/d Ramelton or placebo for 8 wk | SCZ (n = 11), SZA (n = 9) | Diagnosis of SCZ (as per DSM-IV criteria); aged between 18-65 yr; BMI > 27 kg/m2, insulin resistance or any component of metabolic syndrome or a BMI of > 30 kg/m2 | Double-blind, placebo-controlled pilot trial | Waist circumference (cm), abdominal fat as measured by DEXA, glucose metabolism, C-reactive protin, lipids, psychopathology (PANSS; HDRS, HCQoL), sleep quality (SSS, FSI, MOSSS), adverse effects (SATEE) | Total cholesterol; cholesterol-to-HDL ratio; LDL particle number: Melatonin < Placebo | High |
| Adverse effects: Melatonin vs control group: drowsiness (57% vs 33%), heart burn (21% vs 0%), cough (21% vs 0%), akathisia (21% vs 0%), increased urinary frequency (14% vs 0%), problems with memory or concentration (21% vs 0%) | |||||||||
| Arthralgia/myalgia and anxiety: Placebo > Melatonin | |||||||||
| Modabbernia et al[24], 2014 | 18 (13 M, 5 F); 18 (12 M, 6 F) | 32.7 ± 7.3; 32.8 ± 8.2 | 3 mg/d melatonin + Olanzapine or placebo + Olanzapine for 8 wk | SCZ (n = 36) | Diagnosis of SCZ (as per DSM-IV criteria); aged between 18-65 yr; in their first-episode eligible for starting olanzapine | Randomized, double- blind, placebo-controlled, and parallel-group study | Anthropometric measures, BP, FBS, fasting plasma insulin, Psychopathology (PANSS) | Weight, BMI and Waist circumference: Melatonin < Placebo | Some concerns |
| PANSS total score: Melatonin < Placebo | |||||||||
| Romo-Nava et al[25], 2014 | 20 (10 M, 10 F); 24 (12 M, 12F) | Overall: 29.5 ± 8.3 | 5 mg/d CR melatonin or placebo for 8 wk | SCZ (n = 24); BP (n = 20) | Diagnosis of SCZ or BP type I (as per DSM-IV criteria); aged between 18-45 yr, initiated treatment with SGAs < 3 mo | Double-blind, placebo-controlled study | Anthropometric measures, body composition, BP, Lipids, Glucose, PANSS, CGI-S. BPD only: HDRS, YMRS. Schizophrenia only: CDS | Weight gain, waist circumference, DBP, fat mass, triglycerides: Melatonin (BP only) < Placebo | Some concerns |
| Ref. | Sample size (sex) melatonin; control | Age (yr) melatonin; control | Dose, duration | Diagnosis | Inclusion criteria | Study design | Outcomes | Significant findings related to melatonin | Risk of bias |
| Shamir et al[26], 2000 | 19 (8 M, 11 F); 19 (8 M, 11 F) | Overall: 74.0 ± 9.5 | 2 mg/d CR melatonin or placebo for 4 wk | SCZ (n = 19) | Diagnosis of SCZ of > 20 yr (as per DSM-IV criteria), TD > 5 yr, antipsychotic treatment > 10 yr | Double-blind, placebo-controlled, crossover trial (2 wk washout) | AIMS | - | Some concerns |
| Shamir et al[27], 2001 | 22 (11 F, 11 M); 22 (11 F, 11 M) | Overall: 64.2 ± 14.3 | 10 mg/d CR melatonin or placebo for 6 wk | SCZ (n = 22) | Diagnosis of SCZ and anti-psychotic-induced TD (as per DSM-IV criteria) | Double-blind, placebo-controlled, crossover trial (4 wk washout) | AIMS | AIMS: Melatonin < Placebo | Some concerns |
| Castro et al[28], 2011 | 7; 6 (sex NR) | Overall: 59.9 ± 2.7 | 20 mg/d melatonin or placebo for 12 wk | SCZ (n = 11); BP (n = 2) | Diagnosis of neuroleptic-induced TD (as per DSM-IV criteria) | Randomized, double blind, placebo-controlled pilot study | AIMS; BPRS | - | High |
| Ref. | Sample size (sex) melatonin; control | Age (yr) melatonin; control | Dose, duration | Diagnosis | Inclusion criteria | Study design | Outcomes | Significant findings related to melatonin | Risk of bias |
| Shirayama et al[29], 2014 | 10 (NR); - | 42.5 ± 7.3 | 8 mg/d melatonin for 6 mo | SCZ (n = 10) | Diagnosis of SCZ (as per DSM-IV criteria); symptoms stable for 3 mo | Open-label study | TMT (A and B), WCST, VFT, Stroop Test, DSPDT, IGT, RAVLT | RAVLT (total, delayed recall and recognition): Improved at 6-mo compared to baseline | High |
| Baandrup et al[30], 2017 | 40 (21 M, 19 F); 40 (24 M, 16 F) | 47.4 ± 8.6; 49.0 ± 12.1 | 2 mg/d CR melatonin or placebo for 24 wk | Paranoid SCZ (n = 62), non-paranoid SCZ (n = 6), SZA (n = 3), BP (n = 9) | Diagnosis of SCZ, SZA or BP (as per ICD-10 criteria); treated with 1 antipsychotic and 1 BZD for 3 mo | Randomized, double-blind clinical trial | BACS (domains: verbal memory, working memory, motor speed, verbal fluency, letter fluency, attention and processing speed, executive function), WHO-Five WBI, SWN, PSP, UKU, PANSS | - | Low |
| Ref. | Sample size (sex) melatonin; control | Age (yr) melatonin; control | Dose, duration | Diagnosis | Inclusion criteria | Study design | Outcomes | Significant findings | Risk of bias |
| Baandrup et al[12], 2016 | 42 (23 M, 19 F); 44 (25 M, 19 F) | 47.9 ± 8.7; 49.4 ± 12.3 | 2 mg/d PR melatonin or placebo for 24 wk | Paranoid SCZ (n = 67), Non-paranoid SCZ (n = 6), SZA (n = 3), BP (n = 10) | Diagnosis of SCZ, SZA or BP (as per ICD-10 criteria); treated with 1 antipsychotic drug and 1 BZD drug for 3 mo; able to understand Danish | Randomized, double-blind clinical trial | Mean daily dosage of BZD, pattern of BZD dosage, BZD cessation proportion, BWSQ-2 | - | Some concerns |
One study was classified as at low risk of bias, nine were classified as having a moderate risk of bias and six were classified as being at high risk of bias (see outcome Tables for overall classification).
Studies were grouped according to the following primary outcome measures: sleep (n = 6; see Table 1), metabolic profile (n = 3; see Table 2), TD (n = 3; see Table 3), cognitive function (n = 2; see Table 4) and BZD tapering (n = 1; see Table 5).
Six studies primarily assessed the impact of melatonin therapy on sleep parameters in individuals with schizophrenia[17-22], see Table 1.
Two studies assessed the effect of controlled release melatonin formulation on sleep parameters over a three week period[17,18]; one demonstrated a significant improvement in sleep efficiency over three weeks of melatonin treatment, as compared to placebo[17] while the other reported worsened sleep efficiency, prolonged REM sleep latency and increased duration of wakefulness on the first night, as compared to the second night in participants who received melatonin[18]. Another investigation employed a patient-determined dosage of melatonin over a 15 d period; those who received melatonin had a decreased number of awakenings, increased sleep duration and superior self-report sleep parameters than controls[19].
The impact of melatonin on circadian rhythms was assessed by Mishra et al[20] according to change in serum and urinary melatonin levels, which increased over the trial period in participants who received melatonin therapy, compared with controls. Moreover, significant improvements in sleep (as measured by the PSQI) and symptoms of schizophrenia (as measured by the PANSS) were demonstrated by patients who received ramelteon as an add-on to antipsychotic therapy, compared to antipsychotic therapy alone[20].
Two publications reported different sleep outcomes from the same study whose primary aim was to observe the effect of melatonin on BZD discontinuation/reduction in patients with a diagnosis of schizophrenia or bipolar disorder. One reported no difference between add-on melatonin therapy on circadian rest-activity rhythms[22], compared to placebo. However, the other publication highlighted that participants who received melatonin reported superior subjective sleep quality than the placebo group[21].
Three studies assessed whether melatonin attenuates antipsychotic-induced metabolic side effects[23-25], see Table 2.
Modabbernia et al[24] investigated the impact of melatonin on patients with first episode schizophrenia. They reported significantly less increase in weight gain, body mass index (BMI) and total cholesterol over an eight week trial as well as significantly greater reductions in psychiatric symptoms (as assessed by the PANSS) in patients who received melatonin when initiating olanzapine, compared to placebo. Another investigation assessed the impact of melatonin on patients with schizophrenia or bipolar disorder taking SGAs[25]. For the group as a whole, melatonin attenuated weight gain, waist circumference increase and was associated with decreased diastolic blood pressure. However, subgroup analysis of participants with schizophrenia alone demonstrated no significant changes as a result of melatonin therapy[25].
Borba et al[23] investigated whether melatonin could attenuate the metabolic side-effects of antipsychotics in patients with a pre-existing BMI of above 27 kg/m2 and at least one component of MetS. They demonstrated no change in anthropometric outcomes but did observe decreased total cholesterol, cholesterol-to-high-density lipoprotein ratio and low-density lipoprotein (LDL) particle number in those who received melatonin, compared to placebo[23]. Moreover, they reported significantly lower levels of arthralgia/myalgia and anxiety, yet significantly more side effects of drowsiness (57% vs 33%), heart burn (21% vs 0%), cough (21% vs 0%), akathisia (21% vs 0%), increased urinary frequency (14% vs 0%), and problems with memory or concentration (21% vs 0%) in those who received melatonin in comparison with those on placebo[23].
Three studies investigated the impact of melatonin therapy on symptoms of TD[26-28], see Table 3.
The use of 2 mg/d adjunctive melatonin therapy over a four week period did not affect severity of TD in an initial study by Shamir et al [26]. However, a follow-on study from the same investigators found that 10 mg/d of melatonin over a six week period was associated with a significant decrease in TD severity (as measured by the Abnormal Involuntary Movement Scale; AIMS) in patients with chronic schizophrenia, as compared to placebo[27]. In a more recent study, however, Castro et al[28] used 20 mg/d of melatonin over a 12 wk period, yet did not observe any difference from placebo in terms of TD symptoms.
Two studies investigated the impact of melatonin on cognitive function in people with schizophrenia, over a period of 24 wk[29,30], see Table 4.
The only significant change in cognitive function associated with melatonin treatment was improved memory on a verbal learning task post-treatment (8 mg/d for six months), as compared to baseline levels in one of these studies[29]. In contrast, Baandrup et al[30] did not report any change in any domain of cognitive functioning — as assessed by the Brief Assessment of Cognition in Schizophrenia (BACS) cognitive battery — in patients receiving 2 mg/d prolonged-release melatonin for 24 wk.
Baandrup et al[21,22,30] investigated the impact of melatonin during discontinuation or reduction of BZDs and reported various outcomes in four papers, three of which are reported above. Overall, they found that add-on melatonin did not impact average BZD dosage, dosage pattern, cessation proportions or BZD withdrawal symptoms over their 24-wk trial, see Table 5[12].
The current review provides a synthesis of investigations using melatonin for individuals with schizophrenia, across a diverse range of outcomes. The various outcomes identified are discussed below.
The impact of melatonin on sleep function was the most commonly reported outcome in the current review, with some positive outcomes. Two studies assessed the efficacy of melatonin in improving sleep efficiency (defined as proportion of time asleep over total time in bed). One demonstrated improved efficiency[17] while one reported worsened sleep efficiency[18]. However, the worsened sleep efficiency (and accompanying prolonged REM sleep latency and increased duration of wakefulness) was a comparison between sleep parameters on the first night to the second night of the study. The study objective was to investigate the impact of melatonin on ‘the first night effect (FNE)’, whereby individuals have a tendency to experience poorer sleep quality on the first night of a sleep evaluation study[18]. Therefore, while this study demonstrated that melatonin does not ameliorate the FNE, conclusions cannot be drawn about extended sleep efficiency.
The only study in the current review that permitted a patient-determined dosage of melatonin revealed a significantly lower number of night-time awakenings, a longer duration of sleep and improved subjective sleep quality in patients with schizophrenia and comorbid insomnia, as compared to placebo[19]. While the modal dose of melatonin over the trial was 3 mg/d, the authors conceded that conclusions could not be made regarding the optimal dose of melatonin for improved sleep outcomes. Moreover, they did not employ an objective measure of sleep, such as polysomnographic or actigraphic assessments, recommending that further investigation is required[19].
The impact of melatonin on circadian rhythm was assessed in two studies; increased objective measures of serum and urinary melatonin were observed in patients who received melatonin[20], yet actigraphic observations of circadian rhythm revealed no change with melatonin treatment[22]. However, the actigraphic assessments were taken in patients who were concurrently discontinuing BZD medication, limiting generalisation. The same investigation reported improved subjective sleep quality in patients tapering off BZDs[21], suggestive of some beneficial impacts of melatonin.
Overall, melatonin’s main indication has been to treat disorders of sleep based on its physiological regulatory effects. In mental health populations, there has been increasing use in treatment of disorders that have a sleep dysfunction component[31]. In patients with schizophrenia, exogenous melatonin induced secretion of endogenous melatonin in a single study and improved sleep efficiency in another. Future replications utilizing a controlled melatonin dosage, free from the impact of BZD discontinuation, are required in order to clarify the indications for melatonin on sleep in people with schizophrenia.
The efficacy of melatonin in ameliorating metabolic side effects of antipsychotics among patients with schizophrenia was explored in three studies; two of which reported some benefits[23,24].
Adjunctive treatment of melatonin was effective in attenuating weight gain in first episode schizophrenia patients who were initiating treatment with olanzapine[24]. However, no significant benefit of melatonin on body weight were observed in two separate studies of patients with an established history of antipsychotic treatment[23,25]. A tentative interpretation of these findings is that the use of melatonin in conjunction with the initiation of antipsychotic treatment may assist in the initial weight gain associated with first episode psychosis and initiation of antipsychotics[32,33], but further studies are required.
The only other beneficial metabolic outcome was an improvement in total cholesterol and LDL-particle number in participants who received ramelteon in a pilot study[23]. However, the inclusion criteria for that study specified patients with a pre-existing BMI of above 27 and a component of the MetS, therefore it may be that melatonin is more effective for those with poorer initial metabolic health. There is also the possibility of the finding simply reflecting regression to the mean. While no significant beneficial impact on lipid profile was seen in the larger studies included in this review, there was a trend towards improvement in triglyceride levels in first episode patients[24]; further studies are required.
Given that some metabolic outcomes were improved in patients with schizophrenia[23,24] and bipolar disorder[25] taking antipsychotic medication, and a recent animal study demonstrated efficacy of melatonin in attenuating antipsychotic-induced weight gain[34], there are positive indications for the adjunctive use of melatonin. It was also suggested that the variation in efficacy may be partly explained by the relative metabolic risk associated with different antipsychotics[25]. Melatonin reduced weight gain in patients receiving medium risk antipsychotics (quetiapine and risperidone), an effect that was not seen in patients receiving high risk antipsychotics (clozapine and olanzapine)[25]. Therefore, future studies should also investigate the differential impact of adjunctive melatonin therapy on individual antipsychotic medications.
Three studies in the current review assessed the impact of melatonin on TD severity using the AIMS, with one group suggesting that both duration and dosage of melatonin therapy are pertinent to alleviating symptoms of TD. Shamir et al[27] initially found no beneficial impact of a low dose of melatonin on TD, yet their later investigation using a higher dose of melatonin for a prolonged period suggested clinical efficacy for symptoms of TD[27]. However, these results were not replicated in a further study[28]. While melatonin has demonstrated efficacy in attenuating symptoms of TD in animal models, it has been suggested that this may be due to anti-dopaminergic activity masking the movements, as opposed to treating TD per se[35].
It is well established that schizophrenia is associated with impairments across a wide range of cognitive domains[36]. The current review identified only one study whose primary objective was to observe the effect of add-on melatonin on cognitive function in patients with schizophrenia[29]. While a test of memory function (RAVLT) improved over six months in patients with schizophrenia, this improvement was in comparison to baseline assessments rather than controls[29]. Moreover, given the study design was open-label and conducted in a small sample size, this result requires further replication. Cognitive outcomes were assessed as a secondary outcome in a separate study whose primary objective was to observe the efficacy of melatonin as facilitator of reduction or discontinuation of chronic use of BZDs[30]. No cognitive improvements were observed in the cognitive battery (BACS), consistent with the literature suggesting that pharmacological adjuncts are yet to prove effectiveness in significantly enhancing cognition in patients with schizophrenia[37].
One study in the current review investigated the effects of melatonin in facilitating reduction or discontinuation of chronic use of BZDs in patients with schizophrenia[12], concluding that adjunctive melatonin had no significant effect on BZD dosage or cessation over 24 wk. Moreover, any interpretation of secondary outcomes from this investigation would need to be considered within the context of BZD withdrawal.
The interpretations from the current review are limited by the small sample sizes of included studies. Melatonin formulations and dosages differed across studies. Moreover, risk of bias assessment indicated that only one study was deemed at low risk of bias, with the remainder carrying moderate or high risk of bias. Furthermore, some samples included patients with diagnoses of schizophrenia and bipolar disorder, making it difficult to delineate the effect of melatonin for schizophrenia alone. It remains an open question whether efficacy of melatonin for sleep in people with schizophrenia endures over the longer term: future studies should address this important topic.
The current review synthesized the results of clinical trials investigating the effect of adjunctive melatonin therapy on any outcome in patients with schizophrenia. To date, investigated outcomes include sleep function, metabolic benefits, TD attenuation, cognitive function and as an adjunct to BZD discontinuation. Positive outcomes were demonstrated for the use of melatonin in improving sleep efficiency and circadian rhythm as well as certain metabolic outcomes, specifically in first-episode patients initiating antipsychotic treatment. One study reported benefit for the use of melatonin in attenuating TD, but this was not found in other studies. There was no observed benefit for the use of melatonin in improving cognitive function or facilitating BZD discontinuation in individuals with schizophrenia. Given that the pharmacokinetics of melatonin and interactions with other drugs are unclear, future studies investigating these in relation to specific antipsychotic medications are required. Moreover, the range of melatonin dosage used and the duration of the studies in the current review highlights that there is currently no standardized melatonin recommendation and the effects of contamination and different formulations are unknown.
Schizophrenia is a chronic psychiatric condition consisting of positive and negative symptoms causing significant impacts on life. Current treatment includes second-generation antipsychotics (SGAs), the use of which is associated with side effects including: increased metabolic risk, sleep dysfunction and extrapyramidal side effects (EPS). Exogenous melatonin has been demonstrated to attenuate sleep dysfunction in the general population, however its indication in schizophrenia has been relatively unexplored. The proven antioxidant and neuroprotective effects of melatonin suggest potential for therapeutic benefit in adjunctive treatment of schizophrenia, especially in attenuating side effects associated with SGAs.
Current therapeutic treatment for schizophrenia often causes side effects including increased cardiovascular and metabolic risk, sleep dysfunction and EPS. Adjunctive use of melatonin has been suggested to benefit sleep disturbances, however its indication in schizophrenia has remained unclear. Therefore, we synthesized the current evidence for the effect of adjunctive use of melatonin on any outcome in individuals with schizophrenia.
To synthesize clinical trials conducted to date that have investigated the use of melatonin as an adjunctive therapy for individuals with schizophrenia in improving any therapeutic outcome.
A systematic literature search was conducted on MEDLINE (Ovid), Embase, PsychINFO, PubMed, CINAHL and Cochrane Library for clinical trials using melatonin as an adjunctive therapy that included a group of patients with schizophrenia. PRISMA guidelines were adhered to and the Cochrane risk-of-bias tool for randomized controlled trials was used by two authors to assess the trials independently, with consensus confirmed by a third reviewer.
A total of 15 trials were included for qualitative synthesis after assessing for eligibility and removing duplicates. The trials assessed the following primary outcomes: sleep (n = 6), metabolic profile (n = 3), tardive dyskinesia (n = 3), cognitive function (n = 2) and benzodiazepine discontinuation (n = 1).
Positive outcomes were demonstrated for the use of melatonin in improving sleep efficiency and certain metabolic outcomes, specifically in first-episode patients initiating antipsychotic treatment. Currently, there is limited therapeutic indication for the use of melatonin in treatment of tardive dyskinesia, cognitive function or facilitating benzodiazepine discontinuation. Limitations included small sample sizes and no standardization of the duration and/or dosage of adjunctive melatonin used.
Future studies are required to confirm these improvements, determine the pharmacokinetic interactions of melatonin with specific antipsychotic medications and develop a standardized duration and dosage of adjunctive melatonin treatment. Moreover, a long-term safety and efficacy profile remains to be determined.
| 1. | Freedman R. Schizophrenia. N Engl J Med. 2003;349:1738-1749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 509] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 2. | Benson KL. Sleep in schizophrenia: impairments, correlates, and treatment. Psychiatr Clin North Am. 2006;29:1033-45; abstract ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 74] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 3. | Leucht S, Pitschel-Walz G, Abraham D, Kissling W. Efficacy and extrapyramidal side-effects of the new antipsychotics olanzapine, quetiapine, risperidone, and sertindole compared to conventional antipsychotics and placebo. A meta-analysis of randomized controlled trials. Schizophr Res. 1999;35:51-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 447] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 4. | Divac N, Prostran M, Jakovcevski I, Cerovac N. Second-generation antipsychotics and extrapyramidal adverse effects. Biomed Res Int. 2014;2014:656370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 128] [Cited by in RCA: 206] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 5. | Carbon M, Kane JM, Leucht S, Correll CU. Tardive dyskinesia risk with first- and second-generation antipsychotics in comparative randomized controlled trials: a meta-analysis. World Psychiatry. 2018;17:330-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 126] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 6. | Lerner PP, Miodownik C, Lerner V. Tardive dyskinesia (syndrome): Current concept and modern approaches to its management. Psychiatry Clin Neurosci. 2015;69:321-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 68] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 7. | Pillinger T, McCutcheon RA, Vano L, Mizuno Y, Arumuham A, Hindley G, Beck K, Natesan S, Efthimiou O, Cipriani A, Howes OD. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis. Lancet Psychiatry. 2020;7:64-77. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 500] [Cited by in RCA: 671] [Article Influence: 111.8] [Reference Citation Analysis (0)] |
| 8. | Rao ML, Gross G, Strebel B, Halaris A, Huber G, Bräunig P, Marler M. Circadian rhythm of tryptophan, serotonin, melatonin, and pituitary hormones in schizophrenia. Biol Psychiatry. 1994;35:151-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 115] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 9. | Monti JM, Monti D. Sleep in schizophrenia patients and the effects of antipsychotic drugs. Sleep Med Rev. 2004;8:133-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 141] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 10. | Monteleone P, Natale M, La Rocca A, Maj M. Decreased nocturnal secretion of melatonin in drug-free schizophrenics: no change after subchronic treatment with antipsychotics. Neuropsychobiology. 1997;36:159-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Petursson H, Lader MH. Withdrawal from long-term benzodiazepine treatment. Br Med J (Clin Res Ed). 1981;283:643-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 232] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 12. | Baandrup L, Lindschou J, Winkel P, Gluud C, Glenthoj BY. Prolonged-release melatonin vs placebo for benzodiazepine discontinuation in patients with schizophrenia or bipolar disorder: A randomised, placebo-controlled, blinded trial. World J Biol Psychiatry. 2016;17:514-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Ferracioli-Oda E, Qawasmi A, Bloch MH. Meta-analysis: melatonin for the treatment of primary sleep disorders. PLoS One. 2013;8:e63773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 250] [Cited by in RCA: 292] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 14. | Auld F, Maschauer EL, Morrison I, Skene DJ, Riha RL. Evidence for the efficacy of melatonin in the treatment of primary adult sleep disorders. Sleep Med Rev. 2017;34:10-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 216] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 15. | Morera-Fumero AL, Abreu-Gonzalez P. Role of melatonin in schizophrenia. Int J Mol Sci. 2013;14:9037-9050. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 16. | Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Assessing risk of bias in a randomized trial. Cochrane Handbook Syst Rev Intervent. 2019;205-228. |
| 17. | Shamir E, Laudon M, Barak Y, Anis Y, Rotenberg V, Elizur A, Zisapel N. Melatonin improves sleep quality of patients with chronic schizophrenia. J Clin Psychiatry. 2000;61:373-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 82] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Shamir E, Rotenberg VS, Laudon M, Zisapel N, Elizur A. First-night effect of melatonin treatment in patients with chronic schizophrenia. J Clin Psychopharmacol. 2000;20:691-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 19. | Suresh Kumar PN, Andrade C, Bhakta SG, Singh NM. Melatonin in schizophrenic outpatients with insomnia: a double-blind, placebo-controlled study. J Clin Psychiatry. 2007;68:237-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Mishra A, Maiti R, Mishra BR, Jena M, Nath S, Sahu P. Effect of add-on ramelteon therapy on sleep and circadian rhythm disruption in patients with schizophrenia: A randomized controlled trial. Eur Neuropsychopharmacol. 2020;31:109-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Baandrup L, Glenthøj BY, Jennum PJ. Objective and subjective sleep quality: Melatonin vs placebo add-on treatment in patients with schizophrenia or bipolar disorder withdrawing from long-term benzodiazepine use. Psychiatry Res. 2016;240:163-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 23] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 22. | Baandrup L, Fasmer OB, Glenthøj BY, Jennum PJ. Circadian rest-activity rhythms during benzodiazepine tapering covered by melatonin vs placebo add-on: data derived from a randomized clinical trial. BMC Psychiatry. 2016;16:348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Borba CP, Fan X, Copeland PM, Paiva A, Freudenreich O, Henderson DC. Placebo-controlled pilot study of ramelteon for adiposity and lipids in patients with schizophrenia. J Clin Psychopharmacol. 2011;31:653-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 24. | Modabbernia A, Heidari P, Soleimani R, Sobhani A, Roshan ZA, Taslimi S, Ashrafi M, Modabbernia MJ. Melatonin for prevention of metabolic side-effects of olanzapine in patients with first-episode schizophrenia: randomized double-blind placebo-controlled study. J Psychiatr Res. 2014;53:133-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 25. | Romo-Nava F, Alvarez-Icaza González D, Fresán-Orellana A, Saracco Alvarez R, Becerra-Palars C, Moreno J, Ontiveros Uribe MP, Berlanga C, Heinze G, Buijs RM. Melatonin attenuates antipsychotic metabolic effects: an eight-week randomized, double-blind, parallel-group, placebo-controlled clinical trial. Bipolar Disord. 2014;16:410-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 26. | Shamir E, Barak Y, Plopsky I, Zisapel N, Elizur A, Weizman A. Is melatonin treatment effective for tardive dyskinesia? J Clin Psychiatry. 2000;61:556-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Shamir E, Barak Y, Shalman I, Laudon M, Zisapel N, Tarrasch R, Elizur A, Weizman R. Melatonin treatment for tardive dyskinesia: a double-blind, placebo-controlled, crossover study. Arch Gen Psychiatry. 2001;58:1049-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 100] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Castro F, Carrizo E, Prieto de Rincón D, Rincón CA, Asián T, Medina-Leendertz S, Bonilla E. Effectiveness of melatonin in tardive dyskinesia. Invest Clin. 2011;52:252-260. [PubMed] |
| 29. | Shirayama Y, Takahashi M, Suzuki M, Tsuruoka Y, Sato K. Effects of Add-on Ramelteon on Cognitive Impairment in Patients with Schizophrenia: An Open-label Pilot Trial. Clin Psychopharmacol Neurosci. 2014;12:215-217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 30. | Baandrup L, Fagerlund B, Glenthoj B. Neurocognitive performance, subjective well-being, and psychosocial functioning after benzodiazepine withdrawal in patients with schizophrenia or bipolar disorder: a randomized clinical trial of add-on melatonin vs placebo. Eur Arch Psychiatry Clin Neurosci. 2017;267:163-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 31. | Janicak PG, Kledzik AM, Thorne MC. The role of melatonin in psychiatric disorders. Psychopharm Rev. 2011;46:49-55. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (1)] |
| 32. | Addington J, Mansley C, Addington D. Weight gain in first-episode psychosis. Can J Psychiatry. 2003;48:272-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 33. | Strassnig M, Miewald J, Keshavan M, Ganguli R. Weight gain in newly diagnosed first-episode psychosis patients and healthy comparisons: one-year analysis. Schizophr Res. 2007;93:90-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 34. | Miron IC, Popescu F, Enăchescu V, Cristea OM, Stoicănescu EC, Amzoiu E, Amzoiu M, Popescu FD. Combination of Olanzapine Pamoate with Melatonin and Metformin: Quantitative Changes in Rat Adipose Tissue. Curr Health Sci J. 2019;45:372-382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 35. | Nelson LA, McGuire JM, Hausafus SN. Melatonin for the treatment of tardive dyskinesia. Ann Pharmacother. 2003;37:1128-1131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 36. | Schaefer J, Giangrande E, Weinberger DR, Dickinson D. The global cognitive impairment in schizophrenia: consistent over decades and around the world. Schizophr Res. 2013;150:42-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 435] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 37. | Harvey PD, Bowie CR. Cognitive enhancement in schizophrenia: pharmacological and cognitive remediation approaches. Psychiatr Clin North Am. 2012;35:683-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Psychiatry
Country/Territory of origin: Australia
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hosak L S-Editor: Gao CC L-Editor: A P-Editor: Ma YJ