Published online Dec 19, 2021. doi: 10.5498/wjp.v11.i12.1274
Peer-review started: April 14, 2021
First decision: June 5, 2021
Revised: June 15, 2021
Accepted: August 31, 2021
Article in press: August 31, 2021
Published online: December 19, 2021
Processing time: 244 Days and 19.4 Hours
Over the past decade, resting-state functional magnetic resonance imaging (rs-fMRI) has concentrated on brain networks such as the default mode network (DMN), the salience network (SN), and the central executive network (CEN), allowing for a better understanding of cognitive deficits observed in mental disorders, as well as other characteristic psychopathological phenomena such as thought and behavior disorganization.
To investigate differential patterns of effective connectivity across distributed brain networks involved in schizophrenia (SCH) and mood disorders.
The sample comprised 58 patients with either paranoid syndrome in the context of SCH (n = 26) or depressive syndrome (Ds) (n = 32), in the context of major depressive disorder or bipolar disorder. The methods used include rs-fMRI and subsequent dynamic causal modeling to determine the direction and strength of connections to and from various nodes in the DMN, SN and CEN.
A significant excitatory connection from the dorsal anterior cingulate cortex to the anterior insula (aI) was observed in the SCH patient group, whereas inhibitory connections from the precuneus to the ventrolateral prefrontal cortex and from the aI to the precuneus were observed in the Ds group.
The results delineate specific patterns associated with SCH and Ds and offer a better explanation of the underlying mechanisms of these disorders, and inform differential diagnosis and precise treatment targeting.
Core Tip: The present study reports a significant excitatory connection from the anterior cingulate cortex to the anterior insula (aI) that was observed in the schizophrenia patient group, whereas inhibitory connections from the precuneus (Pc) to the ventrolateral prefrontal cortex and from aI to Pc were observed in the major depressive episode group. The results delineate specific aberration patterns which correspond to the clinical presentations of the nosological units and can further contribute to a better explanation of the underlying mechanisms of these disorders as well as to inform differential diagnosis and precise treatment targeting.
- Citation: Aryutova K, Paunova R, Kandilarova S, Stoyanova K, Maes MH, Stoyanov D. Differential aberrant connectivity of precuneus and anterior insula may underpin the diagnosis of schizophrenia and mood disorders. World J Psychiatr 2021; 11(12): 1274-1287
- URL: https://www.wjgnet.com/2220-3206/full/v11/i12/1274.htm
- DOI: https://dx.doi.org/10.5498/wjp.v11.i12.1274
Schizophrenia (SCH) is one of the most devastating and socially important diseases, as well as one of the most poorly understood mental conditions that affects people typically in late adolescence/early adulthood and leads to a functional decline in the personal, social and economic aspects of those affected[1]. Although psychotic symptoms, such as delusions and hallucinations, are the most commonly associated behavioral manifestations of SCH, it is primarily a cognitive disorder[2] with an established specific disability profile in terms of cognition and psychosocial dysfun
Unlike other diseases, mental illnesses are classified into diagnostic categories based on top-down, phenomenologically derived criteria. The clinical manifestations of psychiatric disorders are not only multifaceted but also characterized by a high incidence of symptom overlap[10]. As a result of their poor biological validity concerning their etiology due to the limited knowledge about the exact pathological processes, the assessment methods in psychiatry exist outside of the traditional medical framework[11,12]. The contemporary gold standard, represented by the Diagnostic and Statistical Manual of Mental Disorders 5th edition[13] and the International Classification of Diseases X division[14] taxonomies, appears to have a lot of inconsistencies[12]. Thus, the use of those manuals leads to the conclusion that mental illnesses often have high rates of comorbidity, heterogeneity, and presence of intermediate cases[12]. For example, studies show that around half of the SCH cases exhibit symptoms that can be fitted into the category of a Ds[15,16]. In contrast to the presence of a single mental disorder, the presence of two or more mental illnesses is related to increased severity, inadequate pharmacological treatment response, and a substantial suicide risk[17]. This, along with a lack of comprehensive knowledge about the neural and molecular mechanisms that underpin behavioral deviations, explains the therapeutic failures in SCH and mood disorders. All the above-mentioned inconsistencies highlight the imperative need to identify diagnostic biomarkers in psychiatry.
Functional magnetic resonance imaging (fMRI) in psychiatric research is often used to collect signals from brain regions indicating blood oxygen level dependent changes in response to cognitive tasks [task-related fMRI (tr-fMRI)]. Those kinds of studies suggest impaired brain activity during cognitive load involving language, memory, and concentration in patients with mental illness[18-21].
In contrast, resting-state fMRI (rs-fMRI) is a popular instrument for macroscale functional connectomics that can localize low-frequency differences in random brain functions representing interindividual variations in brain activity and mind–brain interactions found in different psychiatric disorders[22]. Over the past decade, rs-fMRI has concentrated on brain networks such as the default mode network (DMN), the salience network (SN), and the central executive network (CEN), allowing for a better understanding of cognitive deficits observed in SCH and mood disorders, as well as other characteristic SCH phenomena such as thought and behavior disorganization[23].
In a previous study[24], we investigated the rs-fMRI effective connectivity in SCH patients with paranoid syndrome and patients with mood disorders with Ds. In addition, in the same sample, we performed tr-fMRI of the von Zerssen’s Paranoid-Depressive Self-Assessment Scale[25], which consists of paranoid-specific, depressive-specific and neutral items. An example of a paranoid-specific item on the scale is “Other people constantly follow and control me”, and an example of a depressive-specific item is “I often feel simply miserable”. The results from tr-fMRI show that the areas that are activated in SCH patients during paranoid-specific items, including the precuneus (Pc) and the posterior cingulate cortex are parts of the DMN, whereas the results for the Ds sample in the same study did not yield any significant clusters of activations. The results from the resting state effective connectivity, however, informed an inhibitory connection from the prefrontal cortex (PFC) to the anterior insula (aI) in the SCH group, which was completely absent in the Ds group. These data led us to assume that disrupted connectivity from the PFC⇒aI at rest may contribute to impaired cognitive functions, behavioral disorganization, and functional disability in people suffering from SCH[26]. Furthermore, the observed DMN activation during the task might be indirect evidence of the inhibitory connection from the PFC⇒aI at rest, which could interfere with the balancing function of the SN as a dynamic switch between the DMN and CEN[24,27].
As the Pc is an integrated element in the DMN and the aI is a central hub in the SN, we hypothesize that functional disruptions in those regions are linked to behavioral abnormalities and cognitive deficits in psychotic disorders. The DMN is characterized by a high baseline firing rate at rest and deactivation at cognitive tasks onset[28]. As the DMN is described as a network that normally activates at rest[29], little is known about the exact mechanisms by which the DMN is activated in SCH patients during the performance of a task. Therefore, our goal was to identify specific patterns in the resting-state effective connectivity of the SN and DMN in the SCH and Ds groups that could explain the behavioral deficits observed in both the paranoid and Ds groups, as well as to test whether these deficits share common or distinct neuropathophysiological patterns.
We decided to test whether at rest, when patients are instructed not to think about anything specific, there was altered connectivity between the components of the SN and DMN, comparing two disease units – SCH (paranoid syndrome) versus mood disorders (Ds).
Hence, the present study was conducted to delineate the effective connectivity patterns at rest with a prior hypothesis that the SN in SCH must have a fundamentally impaired connectivity, which prevents the switching between DMN and CEN, thereby interfering with their basic functions. Our motivation to conduct such a comparative study came from the aforementioned discrepancies, which arise from the lack of biological validity of available diagnostic tools, which ultimately leads to inaccurate diagnosis or high comorbidity in psychotic and affective disorders. By proving neurobiological markers to distinguish the two disorders, we aimed to expand knowledge about their etiology and incorporate it into clinical practice, ultimately optimizing diagnosis and prognosis, and thus choosing the right treatment for these severe mental illnesses.
For the current study, we recruited 58 patients, namely 26 with a paranoid syndrome –in the context of SCH (mean age 39.2 ± 13.2 years, 13 male) and 32 with Ds (mean age 42.9 ± 11.7 years, 10 male) – in the context of major depressive disorder (MDD) (n = 14, mean age 42.4 ± 12 years, 5 male), or bipolar disorder (BD) (n = 18, mean age 43.3 ± 11.8 years, 5 male) – according to the diagnostic criteria of DSM IV Text Revision[30]. The assessment of the participants was performed by experienced psychiatrists (DS, SK and KA) using the general clinical interview[31], the structured Mini-International Neuropsychiatric Interview (M.I.N.I. 6.0)[32], and clinical global impression scale[33]. The positive and negative syndrome scale[34] was chosen for the assessment of the SCH group. A minimum rating of 3 on P1 (delusions) and/or P6 (suspiciousness) was set to secure a reasonable severity of the episode. The severity of the depressive episode was assessed using the Montgomery–Åsberg Depression Rating Scale[35]. A cut-off value of 20 was chosen, which is considered to constitute a DS of moderate severity. Both clinical groups were on stable medication for at least 14 d.
The requirements for participation were the following: age > 18 and < 65 years; lack of metal implants (
Subjects were scanned on a 3Т MRI system (GE Discovery 750w) and the protocol included the following sequences: (1) high-resolution structural scan (Sag 3D T1 FSPGR, slice thickness 1 mm, matrix 256 × 256, relaxation time (TR) 7.2 ms, echo time (TE) 2.3 ms, flip angle 12; and (2) resting-state functional scan-2D echo planar imaging (EPI), with slice thickness 3 mm, matrix 64 × 64, TR 2000 ms, TE 30 ms, 36 slices, flip angle 90, a total of 192 volumes. Before the EPI sequence, subjects were instructed to remain as still as possible with eyes closed and not to think of anything.
The SPM 12 (Statistical Parametric Mapping, http://www.fil.ion.ucl.ac.uk/spm/) software running on MATLAB R2020b for Windows was used to perform data analysis. During the preprocessing of the EP images, they were realigned, co-registered with the structural scans, normalized to Montreal Neurological Institute (MNI) space, and smoothed with a 6-mm full-width-at-half-maximum Gaussian kernel.
First-level resting-state analysis was conducted using a general linear model applied to the time series. Regions of interest (ROIs) were predefined based on their involvement in the SN and the DNM with 6-mm radius spheres. The ROIs with their MNI coordinates are presented in Table 1.
| Region of interest | X | Y | Z | Brodmann area |
| DLPFC (left) | -28 | 46 | 26 | 46 |
| VLPFC–opercular part of the IFG (left) | -41 | 19 | 41 | 44 |
| dACC (left) | 0 | 32 | 26 | 24 |
| aI (left) | -36 | 14 | -4 | 48 |
| Pc (left) | -10 | -54 | 30 | 23 |
These five ROIs were subjected to spectral dynamic causal modeling (spDCM). We used a completely connected model, which meant that each node was linked to the others. A spectral DCM, in contrast to a stochastic DCM, measures effective connec
Statistical analysis of the demographic and clinical characteristics of the participants, as well as of the connectivity strengths of the spDCM model were performed using SPSS for Windows 22.0. The level of significance was set to P < 0.05, two-tailed, for all tests. Student’s t test was used for continuous variables and the
There were no significant differences in age, sex, age of onset, episode duration, and education level between SCH and Ds patients. The clinical characteristics of the whole patient sample are given in detail in Table 2. The two subgroups of Ds (bipolar and unipolar) did not differ significantly in their demographic or clinical variables (Table 3).
We performed the analysis as described above and received various connections between the ROIs. The significant connections are described below within the different groups of patients and between them.
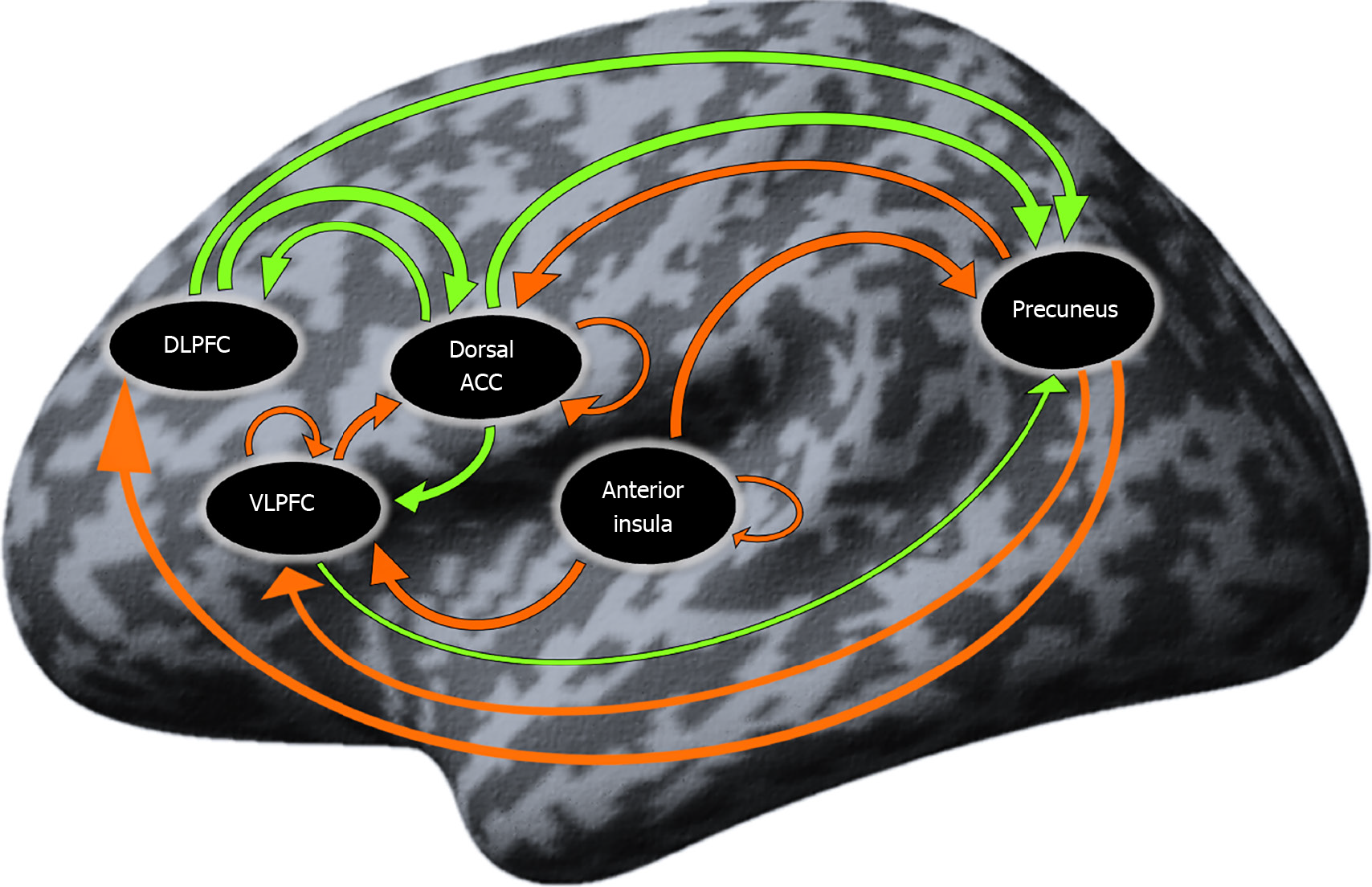
Resting-state effective connectivity in patients with Ds: The coupling connectivity strengths that significantly differed from zero in the group of patients with Ds are presented in Table 4 and Figure 1. There were 15 significant both inhibitory and excitatory connections mainly engaging the prefrontal and parietal regions.
| Connections | Mean | P value |
| dACC→DLPFC | 0.146 | 0.029a |
| Pc→DLPFC | -0.239 | 0.002b |
| VLPFC⊃ | -0.185 | 0.002b |
| dACC→VLPFC | 0.180 | 0c |
| aI→VLPFC | -0.250 | 0.002b |
| Pc→VLPFC | -0.177 | 0.007b |
| DLPFC→dACC | 0.212 | 0.004b |
| VLPFC→dACC | -0.181 | 0.02a |
| dACC⊃ | -0.160 | 0.007b |
| Pc→dACC | -0.228 | 0.006b |
| aI⊃ | -0.216 | 0.003b |
| DLPFC→Pc | 0.090 | 0.047a |
| VLPFC→Pc | 0.133 | 0.029a |
| dACC→Pc | 0.090 | 0.049a |
| aI→Pc | -0.284 | 0c |
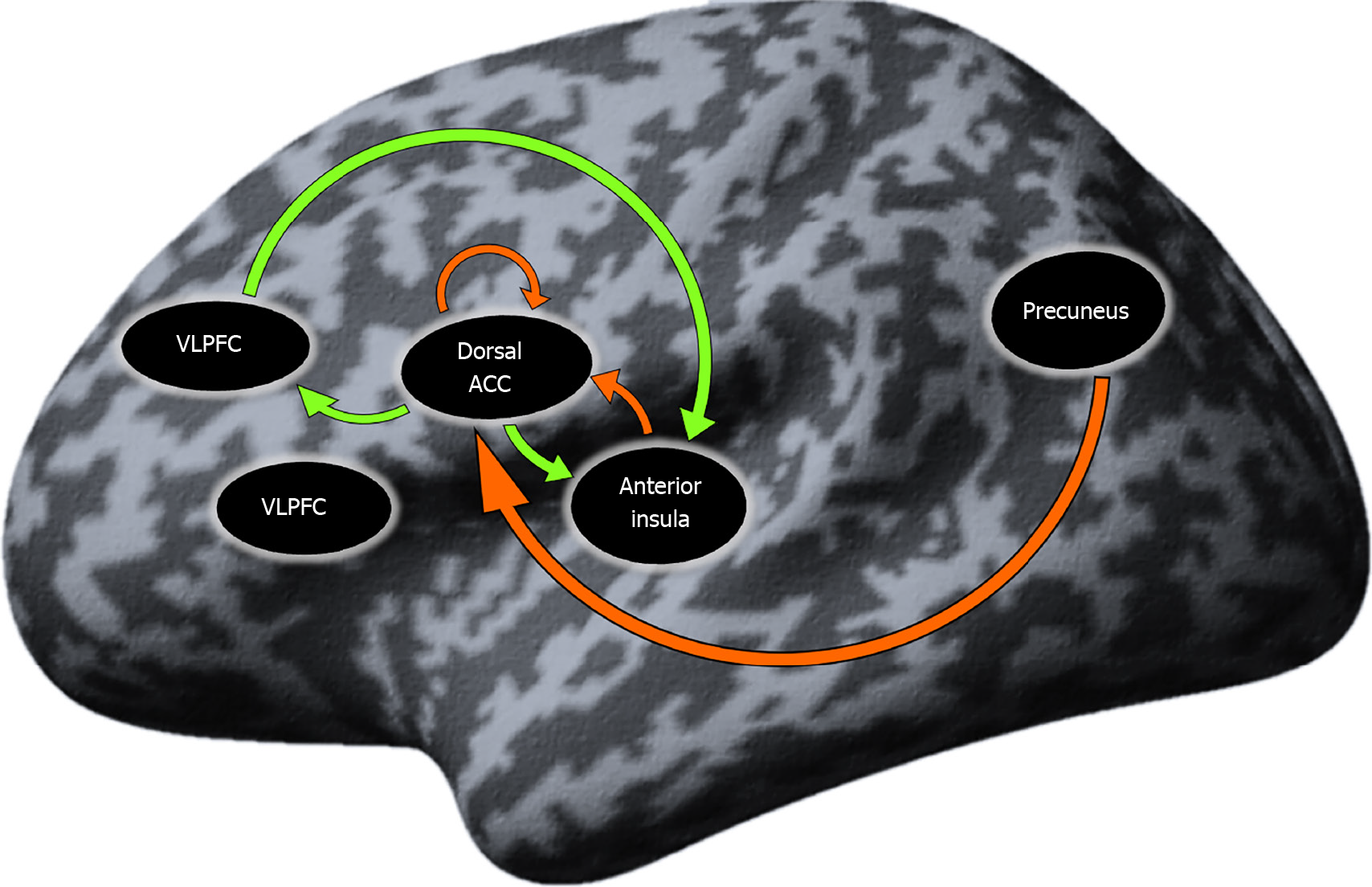
Resting-state effective connectivity in SCH patients: The results from the one-sample t test in the SCH group yielded seven connections that were significantly different from zero. These connections were presented mostly by SN regions and the Pc, as well as self-inhibition of the dorsal anterior cingulate cortex (dACC). The ventrolateral prefrontal cortex (VLPFC) did not present in any of the significant connections. A detailed description of the results is given in Table 5 and Figure 2.
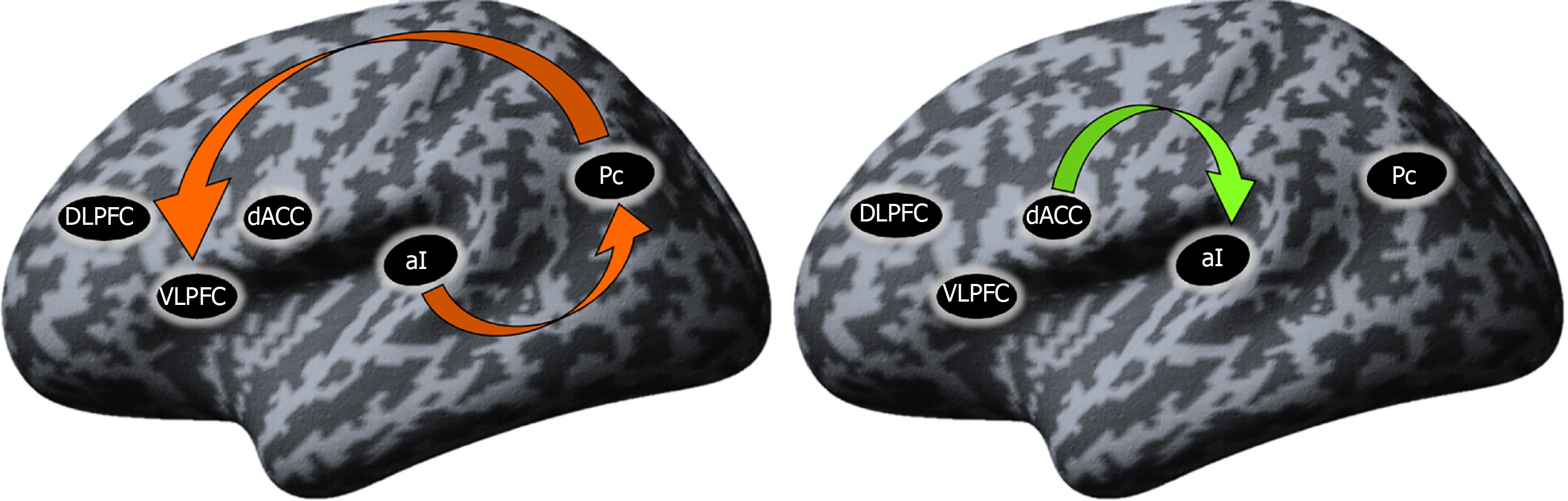
Differences in the resting-state effective connectivity between SCH and Ds patients: To explore the differences between the two groups, independent samples t tests comparing the mean connectivity strengths were performed. The coupling strengths of the connection from the Pc to VLPFC and from the aI to the Pc, both inhibitory connections, were present in the Ds group but absent in the SCH group. In the SCH patients, a significant excitatory connection from the dACC to aI was present that was absent in the Ds group (Figure 3).
The findings of this study suggest that physicians treating patients with SCH and Ds should communicate with their patients that their illness is characterized by alterations in brain connectome data, which in part may explain their symptoms.
Our analysis of the rs-fMRI data demonstrated that, in the patients with Ds, significant excitatory influence was exerted dorsolateral prefrontal cortex (DLPFC)⇒Pc, DLPFC⇒dACC, dACC⇒Pc, dACC⇒DLPFC and VLPFC⇒Pc, while inhibitory influences were exerted Pc⇒dACC, Pc⇒VLPFC, Pc⇒DLPFC, aI⇒Pc, aI⇒VLPFC, and VLPFC⇒dACC and self-inhibition of the VLPFC, dACC and aI.
In the SCH patients, significant effective connectivity, that is, causal interaction in terms of excitatory influence, was exerted dACC⇒aI, dACC⇒DLPFC and DLPFC⇒aI, whereas inhibitory influences were exerted Pc⇒dACC, Pc⇒DLPFC and aI⇒dACC, and self-inhibitory influence of the dACC. The VLPFC in the SCH group did not participate in any interactions with the other ROIs.
However, the main findings from the comparison between the two groups can be summarized as follows: (1) In the Ds group, during rs-fMRI, there was strong involvement of the Pc, which is the central hub of the DMN. There was an inhibitory connection from the aI to the Pc and from the Pc to the PFC; and (2) In the SCH group, during rs-fMRI, there was no DMN activity but instead, there was an excitatory connection from the anterior cingulate cortex to the aI regions that compose the SN.
Concerning the significant deviations observed in the patients with Ds, the following can be discussed. The Pc is a central node of the DMN[37] that becomes more involved during rest when the mind is in a task-free state[38] and its altered function has long been thought to be a neuronal substrate for depression[39]. It plays an important role in the integration of mental processing through its participation in cognitive control functions including visual imagery, episodic memory, and self-directed processes. The observed inhibitory influence from Pc⇒PFC could be related to increased internal ruminating thoughts that are a characteristic trait in patients suffering from Ds[40].
The VLPFC is a component of the PFC that is situated on the inferior frontal gyrus (IFG) that belongs to Brodmann’s field 44, which we included as an ROI in our study. Our results demonstrate that, in the Ds group, the DMN exerted an inhibitory influence upon the PFC (particularly the IFG). Studies on the therapeutic effect of ketamine, which is one of the most effective known treatments in the management of suicidality and self-injurious behavior[41,42], show that ketamine normalizes alterations in this area, namely on its opercular part (which was examined in the current study – Broadman’s field 44)[43].
The rapid effect of ketamine, which is most likely due to its action on the IFG, conjoined with the lack of consistency in the improvement of the condition, indicates that the disturbances in the IFG might be secondary. Our results suggest a model in which the Pc is the primary component responsible for the secondary deficiencies in the IFG function. We hypothesize that the effect of ketamine may be prolonged by combining it with a therapeutic method that acts directly on the Pc, which would ultimately lead to a normalization of the altered effective connectivity (inhibition of Pc⇒IFG) and thus an improvement in IFG function. Such a suitable therapeutic tool could be the repetitive transcranial magnetic stimulation (rTMS)[10], whose effects can be detected not only in the targeted stimulation area but also in other distant areas of the brain that are functionally relevant and occur on existing neural networks[44].
In individuals with Ds, cognitive and emotional aspects linked to the insula are impaired, suggesting that the insula dysfunction may be at the core of the disease’s clinical manifestations[45]. Activity in both the IFG and the aI is often dismissed as being relevant only to motor inhibition or orienting responses and therefore not functionally significant or cognitive. Tops and Boksem[46], however, demonstrated that IFG/aI is involved in dynamic attentional and working memory processing. The IFG/aI is also one of the regions that commonly demonstrates elevated activity related to anxiety and distress[47], which are typical features of the Ds. In addition, antidepressant treatment and sleep deprivation are attributed to a change in activation from the IFG/aI to the DLPFC[48]. This may be due to Ds patients’ difficulty disengaging from issues and rumination, which decreases optimistic prospective and retrospective memory[49].
Analyzing our findings on patients in a depressive episode, we suggest that future research should focus deeper on examining the impaired effective connectivity between important brain regions, most notably the PFC, insula and Pc, relying on the translational approach in neuroscience aimed at incorporating the gained knowledge into clinical practice for more successful treatment options for the Ds. We hypothesize that our findings may serve in this process by combining the psychopharmacological approach, using ketamine, which is known to modulate the function of the IFG, together with a biomedical instrumental method rTMS, targeting the Pc and PFC. Such a translational therapeutic approach could lead to a lasting improvement in the condition and ultimately optimize the prognosis of the disease.
Concerning the significant deviation (excitatory connection from the dACC⇒aI) observed in the SCH group, the following findings can be discussed: The dACC is a key region involved in cognitive and emotional processing[50,51]. Brodmann’s area 24 (dACC, which we examined) was associated in the past with abnormal behavioral manifestations which laid the foundation for bilateral cingulotomy psychosurgery. In such cases, neuropsychological follow-up revealed cognitive deficiency, especially in the executive functions[52]. Heilbronner and Hayden[53] suggested that the dACC is a structure specialized for representing task-state conditions that influence actions and that dACC neurons link context with strategies by combining a variety of task-relevant information to construct a representation of abstract control over decisions and actions.
The aI is an integral center for mediating dynamic interactions between large-scale brain networks engaged in externally oriented attention and self-cognition. This brain structure performs numerous cognitive, affective, and regulatory processes, including interception, emotional reactions, and empathy. The roles delegated to the insula can be conceptualized through a few simple mechanisms: bottom-up identification of salient events; shifting between other large-scale networks to promote access to attention and working memory capacity when a salient event is registered; involvement of the anterior and posterior parts to adjust the autonomic response to salient stimuli; and strong functional connectivity with the ACC[54]. The perception of both visual and auditory emotional information, pain, and subjective projections of the self are all insula-related functions that are disrupted in SCH[55]. The processing of self-representations is important for distinguishing between self-generated and external information, meaning that insula dysfunction could lead to perceptual disturbances, a prevalent symptom of SCH.
The dACC and aI are the two major components constituting the SN. In healthy individuals, the activation of SN is normally observed through a variety of cognitive tasks[54]. Its primary function is to facilitate the switching of brain connectivity between the default mode and the task-related states[56]. Disrupted synchronization between the anti-correlated networks of DMN and CEN that underlie default modes and task-related activity has been postulated as a key pathophysiological characteristic of SCH[57]. Previous research has reported reduced SN connectivity during infor
These assumptions were confirmed in our previous study[24], where we observed altered resting-state connectivity from the PFC⇒aI and task-related activations in the Pc in patients with SCH, indicative of the involvement of neural networks such as the SN and the DMN and their abnormal interactions with each other in SCH etiopathogenesis. Other neuroimaging studies in SCH also reported evidence of reduced inhibition of the DMN during task-related paradigms[60]. We suggested that the inhibitory frontoinsular connection at rest leads to disruption of the salience processing due to the insular dysfunction as a result of its failure as a dynamic switch between the DMN and CEN. As a result, the DMN stays active during tasks and the CEN does not manage to activate at all, which could explain the cognitive deficits observed in SCH individuals reflecting as a serious impact on their functioning in professional and social aspects.
The current data support this hypothesis, as the resting-state excitatory connection observed between SN’s main components – dACC⇒aI – leads to hyperactivity of the SN and ultimately to a salient perception of reality through excessive engagement with indifferent stimuli, which explains the abnormal self-referential thinking that is a characteristic trait of SCH. It appears that inaccurate evaluation of stimuli that would usually be deemed irrelevant seems to be the source of abnormal salience processing in individuals with SCH psychoses. As a result, subthreshold stimuli become excessively attention-getting, which is referred to as aberrant salience by Kapur[61] and proximal salience by Palaniyappan and Liddle[55].
According to Kapur, the central hyperdopaminergic activity causes unwarranted salience to events that would not typically be considered as relevant, resulting in a pathologically exaggerated feeling of the significance of ordinary experiences, perceptual distortions, and delusional causal conclusions. Palaniyappan broadened Kapur's idea of aberrant salience by introducing the concept of proximal salience, with a hypothesis regarding the role of the aI in salience processing to include not just hallucinations and delusions associated with psychosis, but also the debilitating disturbances of cognition and volition associated with chronic SCH. With our current study, we manage to confirm this hypothesis as our finding (excitatory influence of dACC⇒aI) suggests that schizophrenic patients stay in a resting state of aberrant salience. This process may be relevant for the onset of psychotic symptoms, whereby hallucinations are a direct consequence of the aberrant salience[61] of internal representations, and delusions are a cognitive attempt to make sense of these aberrantly salient perceptions and further with the progression of the illness the incorrectly assigned proximal salience[55] produces not only the perceptual and cognitive disturbances of acute psychosis but also the symptoms of behavioral disorganization, via disturbed information processing and goalsetting.
In summary, the results of our study help to understand SCH as a behavioral disorder caused by disintegration across the key brain networks. The abnormal hyperconnectivity of the SN during rest, interfering with its dynamic switch function could explain the inability of the DMN’s components to activate during rest and to deactivate during cognitive load[24]. Our prior hypothesis that the SN in SCH must have a fundamentally impaired connectivity, which prevents the switching between DMN and CEN, thereby interfering with their basic functions, was confirmed by the present study. We suggest that our findings could help both in the biological understanding of the etiology of SCH and in the development and improvement of the therapeutic approach. We visualize a future where translational neuroscience would ultimately integrate psychopathology, psychopharmacology, instrumental methods, and even neurosurgical techniques to restore the brain imbalances by modulating the altered connectivity in the brains of people suffering from SCH[10].
There were several limitations to our study, which need to be considered. First, our study sample was small and there was diagnostic heterogeneity in the Ds group consisting of both patients with MDD and BD, since our comparison is on a syndrome, not a categorical level. Moreover, all the patients were undergoing psychopharmacological treatment, which may be a confounding factor for the determined intraconnectome signatures. An important future aspect in this area may be a study of the longitudinal supervenience between the pretherapeutic and post-therapeutic resting-state signatures across the whole syndromic spectrum including depression– mania–psychosis.
Therefore, even though our data confirm previous studies of dysconnectivity in schizophrenic psychosis, such a model requires additional testing with a larger sample of patients using various imaging tools. Furthermore, the interdisciplinary translational approach in neuroscience can be applied by combining different scientific disciplines such as psychopharmacology, psychopathology, and functional neuroi
We managed to deliver evidence that despite the clinical overlaps, there are objective neuroimaging signatures of disease that can fundamentally distinguish SCH and mood disorders. We propose a model in which the behavioral deviations observed in the Ds group are due to abnormal inhibitory connections in the resting state between nodes of the DMN and SN with the PFC, while in the SCH group there is a hyperconnectivity of the SN, leading to the perception of indifferent internal stimuli as particularly significant. The resting state of aberrant salience observed in the SCH group has the potential to explain the psychotic symptoms, where hallucinations are an attempt to comprehend the abnormal internal salient stimuli, and delusions are a secondary cognitive phenomenon through which the patient explains these abnormal perceptions. In addition, the inclusion of the concept of proximal salience helps to understand the etiology of the persistent and chronic psychoses and the role of the SN and in particular, the insula in the formation of the complex SCH symptoms, such as speech, thought and behavioral disorganization.
The present resting-state functional magnetic resonance imaging (rs-fMRI) study was conducted in two groups of patients – schizophrenia (SCH) and individuals with mood disorders with the depressive syndrome (Ds) – to delineate the effective connectivity patterns at rest with the prior hypothesis that the salience network (SN) in SCH must have a fundamentally impaired connectivity, which prevents the switching between anticorrelated default mode network (DMN) and central executive network (CEN), thereby interfering with their basic functions and that this disruption may serve as neuroimaging biomarker to distinguish between the two groups of patients.
Our motivation to conduct such a comparative study comes from the lack of biological validity of available diagnostic tools, which ultimately leads to inaccurate diagnosis or high rates of comorbidity, and therefore an inadequate choice of treatment for psychotic and affective disorders.
By proving neurobiological markers to distinguish between SCH and mood disorders, we aimed to expand knowledge about their etiology and incorporate it into clinical practice, ultimately optimizing diagnosis and prognosis, and thus choosing the right treatment for these severe mental illnesses.
The methods used include rs-fMRI and subsequent dynamic causal modeling (spDCM) to determine the direction and strength of connections to and from various nodes in the DMN, SN and CEN. The positive and negative syndrome scale was chosen for the assessment of the SCH group, and the severity of the Ds was assessed using the Montgomery–Åsberg Depression Rating Scale. The SPM 12 software running on MATLAB R2020b for Windows was used to perform data analysis. First level resting-state analysis was conducted using a general linear model. Regions of interest were predefined based on their involvement in the SN and the DNM. Furthermore, using the parametric empirical bayes method introduced in SPM12, the individual spDCM models were jointly estimated. Finally, the estimated spDCM models were used to extract connectivity strengths (A-matrix) for further statistical analysis in SPSS.
The coupling strengths of the connection from the precuneus (Pc) to the prefrontal cortex and from the anterior insula (aI) to the Pc, both inhibitory connections were present in the Ds group but absent in the SCH group. In the SCH patients, a significant excitatory connection from the dorsal part of the anterior cingulate cortex to the aI was present which was absent in the Ds study group.
We managed to deliver evidence that despite the clinical overlaps, there are objective neuroimaging signatures of disease that can fundamentally distinguish SCH from mood disorders. The resting state of aberrant salience and proximal salience observed in the schizophrenic group has the potential to explain not only the psychotic symptoms, such as hallucinations and delusions, but also gives insight into the formation of the unique for SCH behavioral and thought disorganization.
We suggest that our findings could help both in the biological understanding of the etiology of SCH and mood disorders in the development and improvement of the therapeutic approach. We visualize a future where translational neuroscience would ultimately integrate psychopathology, psychopharmacology, instrumental methods, and even neurosurgical techniques to restore brain imbalances by modulating the altered connectivity in the brains of people suffering from SCH and mood disorders.
| 1. | Eaton WW, Muntaner C, Sapag JC. Socioeconomic stratification and mental disorder. A handbook for the study of mental health. 1999; 2: 226-255. |
| 2. | Soštarič M, Zalar B. The overlap of cognitive impairment in depression and schizophrenia: a comparative study. Psychiatr Danub. 2011;23:251-256. [PubMed] |
| 3. | Harvey PD. Cognition and the differential diagnosis of schizophrenia. World Psychiatry. 2008;7:30-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Keefe RS. Should cognitive impairment be included in the diagnostic criteria for schizophrenia? World Psychiatry. 2008;7:22-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 109] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 5. | Keefe RS, Fenton WS. How should DSM-V criteria for schizophrenia include cognitive impairment? Schizophr Bull. 2007;33:912-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 275] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 6. | Vanderhasselt MA, De Raedt R. Impairments in cognitive control persist during remission from depression and are related to the number of past episodes: an event related potentials study. Biol Psychol. 2009;81:169-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Chamberlain SR, Sahakian BJ. Cognition in mania and depression: psychological models and clinical implications. Curr Psychiatry Rep. 2004;6:451-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the "right stuff"? Schizophr Bull. 2000;26:119-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2038] [Cited by in RCA: 2072] [Article Influence: 82.9] [Reference Citation Analysis (0)] |
| 9. | Chamberlain SR, Sahakian BJ. The neuropsychology of mood disorders. Curr Psychiatry Rep. 2006;8:458-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 92] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Aryutova K, Paunova R, Kandilarova S, Todeva-Radneva A, Stoyanov D. Implications from translational cross-validation of clinical assessment tools for diagnosis and treatment in psychiatry. World J Psychiatry. 2021;11:169-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 16] [Cited by in RCA: 14] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 11. | Brunoni AR. Beyond the DSM: trends in psychiatry diagnoses. Rev Psiquiatr Clin. 2017;44:154-158. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Lilienfeld SO, Treadway MT. Clashing Diagnostic Approaches: DSM-ICD Versus RDoC. Annu Rev Clin Psychol. 2016;12:435-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 13. | American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®). American Psychiatric Pub. 2013;. |
| 14. | World Health Organization. The ICD-10 Classification of Mental and Behavioural Disorders : Clinical Descriptions and Diagnostic Guidelines. World Health Organization; 1992. [cited 16 March 2021]. Available from: https://apps.who.int/iris/handle/10665/37958. |
| 15. | Pincus HA, Tew JD, First MB. Psychiatric comorbidity: is more less? World Psychiatry. 2004;3:18-23. [PubMed] |
| 16. | Buckley PF, Miller BJ, Lehrer DS, Castle DJ. Psychiatric comorbidities and schizophrenia. Schizophr Bull. 2009;35:383-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 685] [Cited by in RCA: 755] [Article Influence: 44.4] [Reference Citation Analysis (1)] |
| 17. | García-Gutiérrez MS, Navarrete F, Sala F, Gasparyan A, Austrich-Olivares A, Manzanares J. Biomarkers in Psychiatry: Concept, Definition, Types and Relevance to the Clinical Reality. Front Psychiatry. 2020;11:432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 176] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 18. | Eisenberg DP, Berman KF. Executive function, neural circuitry, and genetic mechanisms in schizophrenia. Neuropsychopharmacology. 2010;35:258-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 185] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 19. | Li X, Branch CA, DeLisi LE. Language pathway abnormalities in schizophrenia: a review of fMRI and other imaging studies. Curr Opin Psychiatry. 2009;22:131-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 20. | Iritani S. Neuropathology of schizophrenia: a mini review. Neuropathology. 2007;27:604-608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 21. | Van Snellenberg JX. Working memory and long-term memory deficits in schizophrenia: is there a common substrate? Psychiatry Res. 2009;174:89-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Zuo XN, Xing XX. Test-retest reliabilities of resting-state FMRI measurements in human brain functional connectomics: a systems neuroscience perspective. Neurosci Biobehav Rev. 2014;45:100-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 452] [Cited by in RCA: 540] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 23. | Goulden N, Khusnulina A, Davis NJ, Bracewell RM, Bokde AL, McNulty JP, Mullins PG. The salience network is responsible for switching between the default mode network and the central executive network: replication from DCM. Neuroimage. 2014;99:180-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 551] [Article Influence: 45.9] [Reference Citation Analysis (0)] |
| 24. | Stoyanov D, Aryutova K, Kandilarova S, Paunova R, Arabadzhiev Z, Todeva-Radneva A, Kostianev S, Borgwardt S. Diagnostic Task Specific Activations in Functional MRI and Aberrant Connectivity of Insula with Middle Frontal Gyrus Can Inform the Differential Diagnosis of Psychosis. Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 25. | Zerssen DV, Koeller DM. Paranoid-Depressivitäts-Skala (PD-S)[Paranoia-Depression Scale]. Weinheim (Germany): Beltz, 1976. |
| 26. | Yoon JH, Minzenberg MJ, Ursu S, Ryan Walter BS, Wendelken C, Ragland JD, Carter CS. Association of dorsolateral prefrontal cortex dysfunction with disrupted coordinated brain activity in schizophrenia: relationship with impaired cognition, behavioral disorganization, and global function. Am J Psychiatry. 2008;165:1006-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 204] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 27. | Kandilarova S, Stoyanov D, Kostianev S, Specht K. Altered Resting State Effective Connectivity of Anterior Insula in Depression. Front Psychiatry. 2018;9:83. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 28. | Hayden BY, Smith DV, Platt ML. Electrophysiological correlates of default-mode processing in macaque posterior cingulate cortex. Proc Natl Acad Sci U S A. 2009;106:5948-5953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 133] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 29. | Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci U S A. 2003;100:253-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5006] [Cited by in RCA: 4855] [Article Influence: 211.1] [Reference Citation Analysis (0)] |
| 30. | American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision. American Psychiatric Association; 2000. [cited 16 March 2021]. Available from: https://www.researchgate.net/publication/227942103_Diagnostic_and_Statistical_Manual_of_Mental_Disorders_4th_ed_DSM-IV. |
| 31. | Sullivan HS. The psychiatric interview. Psychiatry. 1951;14:361-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 32. | Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59 Suppl 20:22-33; quiz 34. [PubMed] |
| 33. | Busner J, Targum SD. The clinical global impressions scale: applying a research tool in clinical practice. Psychiatry (Edgmont). 2007;4:28-37. [PubMed] |
| 34. | Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261-276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13937] [Cited by in RCA: 16140] [Article Influence: 413.8] [Reference Citation Analysis (0)] |
| 35. | Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. Br J Psychiatry. 1979;134:382-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9039] [Cited by in RCA: 10133] [Article Influence: 215.6] [Reference Citation Analysis (0)] |
| 36. | Razi A, Kahan J, Rees G, Friston KJ. Construct validation of a DCM for resting state fMRI. Neuroimage. 2015;106:1-14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 217] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 37. | Utevsky AV, Smith DV, Huettel SA. Precuneus is a functional core of the default-mode network. J Neurosci. 2014;34:932-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 517] [Cited by in RCA: 690] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 38. | Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioral correlates. Brain. 2006;129:564-583. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3263] [Cited by in RCA: 3932] [Article Influence: 196.6] [Reference Citation Analysis (0)] |
| 39. | Zhang S, Chen JM, Kuang L, Cao J, Zhang H, Ai M, Wang W, Zhang SD, Wang SY, Liu SJ, Fang WD. Association between abnormal default mode network activity and suicidality in depressed adolescents. BMC Psychiatry. 2016;16:337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 100] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 40. | Cheng W, Rolls ET, Qiu J, Yang D, Ruan H, Wei D, Zhao L, Meng J, Xie P, Feng J. Functional Connectivity of the Precuneus in Unmedicated Patients With Depression. Biol Psychiatry Cogn Neurosci Neuroimaging. 2018;3:1040-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 41. | Zhan Y, Zhang B, Zhou Y, Zheng W, Liu W, Wang C, Li H, Chen L, Yu L, Walter M, Li M, Li MD, Ning Y. A preliminary study of anti-suicidal efficacy of repeated ketamine infusions in depression with suicidal ideation. J Affect Disord. 2019;251:205-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 48] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 42. | Арютова К, Хранов Г. Нови методи за лечение на депресия. Medinfo. 2018;5:10-13 [cited 16 March 2021]. Available from: https://www.medinfo.bg/spisanie/2018/5/statii/novi-metodi-za-lechenie-na-depresija-2687. |
| 43. | Dai D, Lacadie CM, Holmes SE, Cool R, Anticevic A, Averill C, Abdallah C, Esterlis I. Ketamine Normalizes the Structural Alterations of Inferior Frontal Gyrus in Depression. Chronic Stress (Thousand Oaks). 2020;4:2470547020980681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 44. | Vink JJT, Mandija S, Petrov PI, van den Berg CAT, Sommer IEC, Neggers SFW. A novel concurrent TMS-fMRI method to reveal propagation patterns of prefrontal magnetic brain stimulation. Hum Brain Mapp. 2018;39:4580-4592. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 81] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 45. | Sprengelmeyer R, Steele JD, Mwangi B, Kumar P, Christmas D, Milders M, Matthews K. The insular cortex and the neuroanatomy of major depression. J Affect Disord. 2011;133:120-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 46. | Tops M, Boksem MA. A potential role of the inferior frontal gyrus and anterior insula in cognitive control, brain rhythms, and event-related potentials. Front Psychol. 2011;2:330. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 97] [Cited by in RCA: 137] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 47. | Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry. 2007;164:1476-1488. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2627] [Cited by in RCA: 2401] [Article Influence: 126.4] [Reference Citation Analysis (0)] |
| 48. | Wu JC, Gillin JC, Buchsbaum MS, Schachat C, Darnall LA, Keator DB, Fallon JH, Bunney WE. Sleep deprivation PET correlations of Hamilton symptom improvement ratings with changes in relative glucose metabolism in patients with depression. J Affect Disord. 2008;107:181-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 49. | Tops M, Boksem MA, Luu P, Tucker DM. Brain substrates of behavioral programs associated with self-regulation. Front Psychol. 2010;1:152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 50. | Cao W, Luo C, Zhu B, Zhang D, Dong L, Gong J, Gong D, He H, Tu S, Yin W, Li J, Chen H, Yao D. Resting-state functional connectivity in anterior cingulate cortex in normal aging. Front Aging Neurosci. 2014;6:280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 51. | Vytal K, Hamann S. Neuroimaging support for discrete neural correlates of basic emotions: a voxel-based meta-analysis. J Cogn Neurosci. 2010;22:2864-2885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 433] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 52. | Gasquoine PG. Localization of function in anterior cingulate cortex: from psychosurgery to functional neuroimaging. Neurosci Biobehav Rev. 2013;37:340-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 173] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 53. | Heilbronner SR, Hayden BY. Dorsal Anterior Cingulate Cortex: A Bottom-Up View. Annu Rev Neurosci. 2016;39:149-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 326] [Article Influence: 32.6] [Reference Citation Analysis (0)] |
| 54. | Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct. 2010;214:655-667. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4387] [Cited by in RCA: 4196] [Article Influence: 262.3] [Reference Citation Analysis (0)] |
| 55. | Palaniyappan L, Liddle PF. Does the salience network play a cardinal role in psychosis? J Psychiatry Neurosci. 2012;37:17-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 431] [Cited by in RCA: 425] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 56. | Di X, Biswal BB. Identifying the default mode network structure using dynamic causal modeling on resting-state functional magnetic resonance imaging. Neuroimage. 2014;86:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 104] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 57. | Williamson P. Are anti-correlated networks in the brain relevant to schizophrenia? Schizophr Bull. 2007;33:994-1003. [RCA] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 97] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 58. | Schmidt A, Palaniyappan L, Smieskova R, Simon A, Riecher-Rössler A, Lang UE, Fusar-Poli P, McGuire P, Borgwardt SJ. Dysfunctional insular connectivity during reward prediction in patients with first-episode psychosis. J Psychiatry Neurosci. 2016;41:367-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 59. | White TP, Joseph V, Francis ST, Liddle PF. Aberrant salience network (bilateral insula and anterior cingulate cortex) connectivity during information processing in schizophrenia. Schizophr Res. 2010;123:105-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 190] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 60. | Masdeu JC. Neuroimaging in psychiatric disorders. Neurotherapeutics. 2011;8:93-102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 61. | Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160:13-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1765] [Cited by in RCA: 1823] [Article Influence: 79.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed following the Creative Commons Attribution-NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Psychiatry
Country/Territory of origin: Bulgaria
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kaur M, Tung TH, Wang DW S-Editor: Fan JR L-Editor: Kerr C P-Editor: Guo X