Published online Oct 19, 2021. doi: 10.5498/wjp.v11.i10.830
Peer-review started: February 28, 2021
First decision: March 30, 2021
Revised: April 11, 2021
Accepted: August 3, 2021
Article in press: August 3, 2021
Published online: October 19, 2021
Processing time: 228 Days and 17.4 Hours
Dopaminergic neurotoxicity is characterized by damage and death of dopaminergic neurons. Parkinson's disease (PD) is a neurodegenerative disorder that primarily involves the loss of dopaminergic neurons in the substantia nigra. Therefore, the study of the mechanisms, as well as the search for new targets for the prevention and treatment of neurodegenerative diseases, is an important focus of modern neuroscience. PD is primarily caused by dysfunction of dopaminergic neurons; however, other neurotransmitter systems are also involved. Research reports have indicated that the glutamatergic system is involved in different pathological conditions, including dopaminergic neurotoxicity. Over the last two decades, the important functional interplay between dopaminergic and glutamatergic systems has stimulated interest in the possible role of metabotropic glutamate receptors (mGluRs) in the development of extrapyramidal disorders. However, the specific mechanisms driving these processes are presently unclear. The participation of the universal neuronal messenger nitric oxide (NO) in the mechanisms of dopaminergic neurotoxicity has attracted increased attention. The current paper aims to review the involvement of mGluRs and the contribution of NO to dopaminergic neurotoxicity. More precisely, we focused on studies conducted on the rotenone-induced PD model. This review is also an outline of our own results obtained using the method of electron paramagnetic resonance, which allows quantitation of NO radicals in brain structures.
Core Tip: Dopaminergic neurotoxicity is characterized by damage and death of dopaminergic neurons. Chronic systemic exposure to rotenone (an inhibitor of mitochondrial complex I and a commonly used pesticide) induced dopaminergic degeneration and reproduced many features of human Parkinson's disease in rats. The current paper aims to review the involvement of metabotropic glutamate receptors and the contribution nitric oxide to dopaminergic neurotoxicity.
- Citation: Bashkatova V. Metabotropic glutamate receptors and nitric oxide in dopaminergic neurotoxicity. World J Psychiatr 2021; 11(10): 830-840
- URL: https://www.wjgnet.com/2220-3206/full/v11/i10/830.htm
- DOI: https://dx.doi.org/10.5498/wjp.v11.i10.830
The dopaminergic system is a part of the brain that plays a key role in the control of locomotor activity, stress reactions, aggressive behavior, and mechanisms of the formation of dependence in humans and animals[1,2]. Dopaminergic neurotoxicity is characterized by damage and death of dopaminergic neurons. The contribution of dopaminergic neurotoxicity to the pathogenesis of several disorders of the central nervous system (CNS), such as Parkinson's disease (PD)[3,4], Tourette syndrome[5], drug abuse[6,7], and schizophrenia[8,9], has been postulated. Currently, neurodegenerative diseases are a major cause of disability around the world. PD is the second-leading cause of neurodegenerative disorder after Alzheimer's disease (AD)[4]. PD is manifested primarily by movement disturbances. Mental health disorders are also a serious nonmotor feature of PD[10,11]. Thus, psychotic symptoms are not uncommon among individuals with PD, with a prevalence rate of approximately 25%-30%[12,13]. In this regard, the study of mechanisms, as well as the search for ways to prevent and treat PD, is not only an important medical problem but also a social problem[14].
A growing body of evidence has demonstrated that glutamatergic neurotransmission plays an important role in the mechanisms of dopaminergic brain damage[15,16]. Previous studies have shown that modulation of metabotropic glutamate receptors (mGluRs) may be considered a more promising way to alter the activity of the brain glutamatergic system than direct action on ionotropic glutamate receptors of the N-methyl-D-aspartate and amino-methyl-phosphonic acid subtypes[17]. However, the neurochemical and neuropsychological effects of mGluRs on dopaminergic neurotoxicity remain poorly understood.
The association between the neurotransmitter function of glutamate and the formation of neuronal messenger nitric oxide (NO) has received increased attention in recent years. NO is considered to be the first representative of a novel family of signaling molecules with neurotransmitter properties[18,19]. NO is a labile free radical that is involved in many physiological processes[20]. It is assumed that together with some important physiological functions in the CNS, NO can have either neuroprotective or neurotoxic actions, depending on its redox state[21]. There are growing numbers of studies concerning the involvement of NO in the mechanisms of dopaminergic neurotoxicity[22-24]. Measurement of NO is technically difficult due to its rapid chemical reactions with a wide range of molecules, such as free radicals, metals, thiols, etc[25]. Thus, accurate detection and quantification are critical to understanding health and disease[26]. Most of the NO measurement techniques in the literature are indirect[27,28]. In our work, we used the direct electron paramagnetic resonance (EPR) method, which allows us to estimate the generation of NO radicals directly in brain tissue[29-31]. The current paper aims to review the involvement of mGluRs and the contribution of NO to dopaminergic neurotoxicity. More precisely, we focused on studies conducted on the rotenone-induced Parkinson's disease model. This review is also an outline of our own results obtained using the EPR method, which allows quantitation of NO radicals in brain structures.
The use of animal models to study neurological diseases associated with dopaminergic neurotoxicity allows for in-depth study of their neuropathophysiology[32,33]. Pathologically, the hallmark of idiopathic PD is the loss of dopaminergic neurons in the substantia nigra (SN). However, in the absence of nigral involvement, noncatecholaminergic neurons are also affected[34]. Agents that selectively damage or disrupt catecholaminergic systems, such as reserpine, methamphetamine (METH), 6-hydroxydopamine (6-OHDA), and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), have been used to develop PD models of PD[35,36]. Based on experimental and clinical evidence, PD was the first neurological disease to be modeled and subsequently treated with neurotransmitter replacement therapy[35]. Recent studies have shown that nigrostriatal dopamine degeneration can be induced via overexpression of α-synuclein using viral vectors or transgenic techniques. In addition, protein aggregation pathology can be triggered by inoculating preformed fibrils of α-synuclein in the SN or the striatum[37,38]. Transgenic animals that overexpress α-synuclein were used to study the role of this protein in dopaminergic degeneration[39,40]. Nevertheless, although transgenic models offer insight into the causes of the pathogenesis of PD or Lewy body (LB)-like formation, the lack of sequential loss of neurons in the SNc remains a major limitation for these models[40]. Conversely, toxin-based models recreate selective nigrostriatal cell death and show extensive motor dysfunction. However, these toxin models do not reproduce the extranigral degeneration that also occurs as part of the disease and lack the pathological hallmark of LB inclusions[39,41]. Betarbet et al[42] reported that chronic, systemic introduction of rotenone, an inhibitor of mitochondrial complex I, accurately replicates many aspects of the pathology of PD. Additionally, in rotenone-treated animals, α-synuclein-and polyubiquitin-positive aggregates were observed in dopamine neurons of the SN[43,44]. The advantages of this model include a slow and specific loss of DA neurons. The disadvantages of the model include the duration of drug administration, as well as sometimes high animal mortality[36]. Currently, several modifications of rotenone-induced Parkinson's disease models have been created. These modifications are different in the mode of administration as well as in the dosage and duration of treatment with rotenone[44-46]. The fact that rotenone is still widely used in agriculture as a pesticide increases the relevance of studying this model[47-49]. However, while the behavioral effects of rotenone administration are well characterized, the mechanisms of rotenone action are still poorly understood.
A significant number of studies have revealed that the excitotoxicity of glutamate contributes to the development of dopaminergic neurotoxicity[50-52]. It has been reported that various neurotoxic agents, including rotenone and METH, can severely damage both ionotropic and metabotropic glutamate receptors, which leads to the progression of toxic effects[53]. An important functional interplay between the dopaminergic and glutamatergic systems has stimulated the consideration of mGluRs as potential therapeutic targets in PD[54-56]. Eight mGluRs (GRM1 to GRM8) have been identified and divided into three groups based on their sequence similarity and pharmacology[57,58]. All mGluRs are family C G-protein-coupled receptors that participate in the modulation of synaptic transmission and neuronal excitability throughout the CNS[59]. Studies have shown that mGluR-mediated mechanisms have been implicated in both neuroprotection and neurotoxicity. The involvement of mGluRs in the control of movement, spatial and olfactory memory and nociception has been demonstrated[57,60,61]. Studies have shown the antiparkinsonian potential of mGluR modulation in groups I, II and III in experimental MPTP and 6-OHDA models of PD[56,62]. It has been reported that group I mGluR antagonism and groups II and III mGluR activation improve some motor symptoms of PD by regulating excitatory and inhibitory transmission in the basal ganglia[55]. However, the mechanism by which these mGluR ligands may alleviate the symptoms of parkinsonism in animal models is largely unknown.
Because the rotenone model of PD has attracted much attention, we searched the PubMed and Google Scholar databases for articles concerning the effect of mGluR on the rotenone model of neurotoxicity. In the end, by consensus, primary articles were selected as relevant to our goals (Table 1). Thus, it has been reported that the application of a group III mGluR agonist (L-AP-4) significantly reduced the toxicity of rotenone in a culture of TH+ midbrain neurons[63]. The authors hypothesized that activation of group III mGluR decreases the selective toxicity of rotenone to dopamine neurons by activating the MAP kinase pathway to stabilize microtubules[63]. In contrast, activation of group I mGluRs enhances rotenone-induced toxicity in MN9D cells[64]. The modulation of the mGluR5 type in rotenone-induced PD models has attracted much attention from researchers. Pharmacological inhibition of mGluR5 has beneficial anti-akinetic effects in animal models of PD; however, the mechanism by which these antagonists alleviate PD symptoms is largely unknown[65]. Previous studies have shown that downregulation of mGluR5 promotes cell apoptosis in a model of rotenone-induced cellular PD. Moreover, conditioned media derived from rotenone-treated dopaminergic MN9D neuronal cells have been found to enhance the production of reactive oxygen species (ROS), which can be further attenuated by an mGlu5 agonist[66]. The selective mGluR5 antagonist 2-methyl-6-(phenylethynyl) pyridine (MPEP) prevented rotenone-induced DNA damage in MN9D dopaminergic neurons through a mechanism involving ROS-related mitochondrial dysfunction[65]. It has been demonstrated that mGluR5 expression is decreased in a time- and dose-dependent manner in rotenone-treated MN9D cells[67]. It has been reported that oxidized extracellular cysteine/cystine redox potential plays a role in mGluR5 activity in the rotenone rat model of PD[68]. In our studies, MPEP (3 mg/kg) reduced the intensity of catalepsy in rats after long-lasting administration of rotenone at a dose of 1.5 mg/kg[69]. We observed that the mGluR5 antagonist partially prevented the increase in NO generation evoked by rotenone[69]. In another study, it was shown that the behavioral effects of MPEP (2.5 mg/kg) were less pronounced in rats receiving a higher dose of rotenone (2.5 mg/kg) following the same duration of neurotoxin administration[70]. It has been reported that coadministration of MPEP with rotenone reduces the descent latency in the grid test at day 60 but does not block the decrease in DA and serotonin levels induced by treatment with this neurotoxin[70]. Thus, the behavioral effects of mGluR5 were notably dependent on the dose of rotenone administered. The above findings indicate that mGluR5 inhibition produces an inhibitory effect on ROS and NO activity[65,66,69]. In summary, an analysis of the literature data supports the notion that antagonists of mGluR5 are considered promising targets for the treatment of pathological conditions induced by dopaminergic neurotoxicity.
| Ref. | Object of study | mGluR type | Ligand |
| Luo et al[67], 2019 | Cell culture; rats | mGluR5 | Antagonist MPEP |
| Bai et al[66], 2018 | Cell culture | mGluR5 | Agonist CHPG; antagonist MPEP |
| Xia et al[65], 2015 | Cell culture, rats | mGluR5 | Antagonist MPEP |
| Bashkatova et al[69], 2012 | Rats | mGluR5 | Antagonist MPEP |
| Sun et al[64], 2012 | Cell culture, rats | Group I mGluR | Agonist DHPG |
| Zhu et al[68], 2012 | Cell culture, rats | mGluR5 | Antagonist MPEP |
| Alam et al[70], 2009 | Rats | mGluR5 | Antagonist MPEP |
| Jiang et al[63], 2006 | Cell culture | Group III mGluR | Agonist L-AP-4 |
Currently, mitochondrial dysfunction is thought to be associated with NO pathways in glutamate neurotoxicity[71]. NO is a gaseous chemical messenger that modulates many functions of the nervous system, including the release of neurotransmitters, interneuronal communication, synaptic plasticity, receptor state, and intracellular signal transduction[20,72]. The role of NO as a biological mediator is primarily determined by its physical and chemical properties. NO is generated by the enzyme NO synthase (NOS), which is widely distributed in the brain[19,73]. One of the possible mechanisms of the neurotoxic effect of NO may be the reaction of NO with ROS, leading to the formation of a highly toxic product peroxynitrite[24,74,75]. The short half-life of the NO radical is 2-5 s[20]. There are several indirect methods for determining NO and its products/metabolites in biological fluids and tissues. One of the most frequently used indirect methods for the determination of NO is the measurement of nitrite and nitrate by spectroscopy using the Griess reagent[27]. Another well-known method for indirect measurement of NO is the quantitative determination of 3-nitrotyrosine (a product of tyrosine nitration)[76]. The applicability of these indirect methods seems to be problematic. Quantitative determination of nitrites by the Griess method is falsified in the presence of reducing agents as well as thiol groups[77]. These data are consistent with our opinion that only direct methods may be used for reliable determination of NO levels. One of the most accurate and correct ways to measure these data is through use of the EPR method[26,31,78,79].
Recent reports claim that NO is involved in neurotoxicity elicited by dopaminergic neurotoxins[24,74,79]. Over the past two decades, significant advances have been made in improving knowledge about the role of NO in the mechanisms of PD pathogenesis[21,80,81]. Thus, in brains from victims of PD, a nitrosyl species, identified as nitrosyl hemoglobin, has been observed in the SN[82]. Previous studies have shown that neurotoxic agents such as MPTP[83], 6-OHDA[84] or METH[74] induce a significant increase in the production of 3-nitrotyrosine in the striatum. The protective effect of neuronal NOS inhibitors has been demonstrated in the MPTP neurotoxicity model in mice[85].
NO is cytotoxic, partly due to its effects on mitochondria[86]. Research reports have shown that NO is involved in rotenone-induced neurotoxicity (Table 2). However, the detailed mechanisms of this process are not well understood. Our data indicate that following a single injection of rotenone (1.5 mg/kg), the levels of NO in all studied brain areas were indistinguishable from those in control animals[78]. The data obtained correspond with the results of other authors. Thus, acute administration of rotenone at a significantly higher dose (15 mg/kg) did not affect the level of hydroxyl radical generation[87]. The NO level reached its maximum in dopaminergic structures, the prefrontal cortex, and the NAc 60 d after administration of rotenone[78]. We observed a more than 2-fold elevation in NO generation in all studied brain structures of rats only after repeated injections of rotenone[78]. These results are consistent with other studies investigating NO metabolites/products in the brains of rats treated with rotenone (Table 2). Thus, a significant increase (by 200.0%) in the concentration of nitrite determined by the spectrophotometric method was observed[88]. Authors suggest that overproduction of NO may be associated with Snitrosylation or nitration of certain important proteins[88]. It was reported that the production of 3-nitrotyrosine in the brains of rats treated with rotenone for 40 d increased significantly[76]. In recent years, several studies have investigated the potential neuroprotective properties of various plant extracts[89-91], polyphenolic agents[92] and flavonoids[93] in a rotenone model of PD. The authors measured the level of nitrites as a marker of neurotoxicity in the dopaminergic brain structures of animals after long-lasting administration of rotenone (Table 2). The above studies indicate that the content of NO and its metabolites in the brain is currently considered one of the markers of rotenone-induced neurotoxicity.
| Ref. | Dose of rotenone | Duration | NO or its products/metabolites | Method of NO determination |
| Kumar et al[91], 2021 | 2.0 mg/kg | 28 | Nitrite | Spectrophotometry |
| Parkhe et al[92], 2020 | 2.0 mg/kg | 21 | Nitrite | Spectrophotometry |
| Sharma et al[93], 2020 | 2.0 mg/kg | 28 | Nitrite | Spectrophotometry |
| Sun et al[80], 2019 | 1.5 mg/kg | 28 | Nitrate/nitrite | NO assay kit |
| Jayaraj et al[90], 2019 | 1.5 mg/kg | 28 | Nitrite | Spectrophotometry |
| Abdel-Salam et al[89], 2017 | 1.5 mg/kg × 3 | 7 | Nitrite | Spectrophotometry |
| Javed et al[86], 2016 | 2.5 mg/kg | 28 | Nitrite | Spectrophotometry |
| Xiong et al[88], 2015 | 1.5 mg/kg | 6 | Nitrite | Spectrophotometry |
| Tapias et al[23], 2014 | 3.0 mg/kg | Individually1 | 3-NT (3-nitrotyrosine) | Immunofluorescence |
| Bashkatova et al[69], 2012 | 1.5 mg/kg | 60 | NO (Nitroxyl radical) | EPR |
| Bashkatova et al[78], 2004 | 1.5 mg/kg | 60 | NO (Nitroxyl radical) | EPR |
| He et al[76], 2003 | 2 mg/kg × 3 | 40 | 3-NT (3-nitrotyrosine) | HPLC |
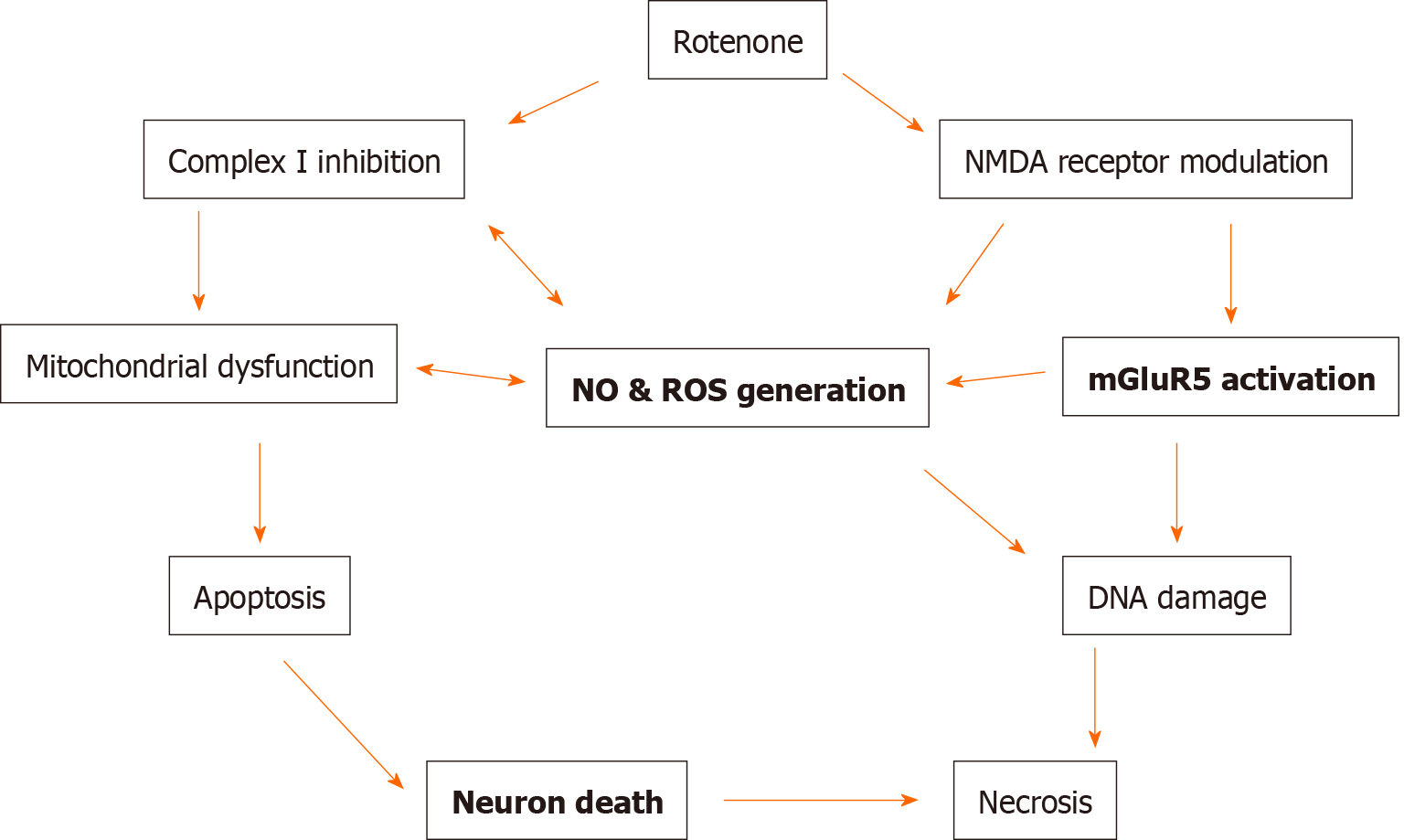
In our opinion, it seems to be critical to study the dynamics of NO changes in various brain structures during long-term administration of rotenone. This issue is practically not studied. Our results demonstrated a significant enhancement of NO generation in the NAc after 20 d of treatment with rotenone, while the NO level was not elevated yet in the frontal cortex[78]. These data may indicate that dopaminergic neurons in the NAc may be intrinsically susceptible to oxidative damage compared to other neurons. Taken together, our results, as well as literature data, allow us to conclude that rotenone can produce a neurotoxic effect and cause an increased production of free radicals, including NO, only with long-lasting chronic administration. This, in turn, confirms the assumption that the cascade of biochemical reactions causing the development of neurotoxic processes can be triggered only after repeated injections of rotenone[35,78,87,94]. In summary, the data obtained indicate prospects for further research on the interaction of dopaminergic, mGluR and NO systems in rotenone models of PD to search for and study the mechanism of action of substances with neuroprotective properties (Figure 1).
This review summarizes several newly discovered mechanisms of dopaminergic neurotoxicity (Figure 1). Current treatments for PD are mainly the administration of dopaminergic drugs. However, dopaminergic drugs are only symptomatic treatments and are limited by several side effects. Understanding the pathogenetic mechanisms of the onset and development of PD is of great clinical importance. Recent studies on drug development have focused on emerging new molecular mechanisms, including modulation of mGluRs and NO formation. Despite the growing number of studies demonstrating the positive effect of some mGluR ligands on motor symptomatology in PD models, there are still no drugs in clinical practice targeting mGluRs to restore neurological disorders of PD. Treatment with NO scavengers/NOS inhibitors may be another potential neuroprotective strategy for diseases associated with dopaminergic neurotoxicity. In addition, our preliminary results and literature data suggest that an increase in the formation of NO radicals in some brain structures may precede the onset of behavioral disorders in rats treated with rotenone. Therefore, finding a possible correlation between the generation of NO radicals and the onset of neurological disturbances during long-term application of rotenone can be an important step in understanding the pathogenesis of rotenone-induced neurotoxicity We can assume that in the future, the determination of NO generation may become a test for the early diagnosis of PD in patients who do not yet have specific symptoms of the disease.
Additionally, long-term application and widespread use of synthetic insecticides have resulted in the accumulation of their residues in food, milk, water, and soil and have adverse health effects for humans[95]. Although all natural insecticides are not completely safe, it seems necessary to phase out the use of rotenone pesticides in agriculture and replace them with natural (“organic”) pesticides with maximum safety.
In summary, alternative treatment strategies beyond dopaminergic drugs might be a major topic of future PD therapy. In conclusion, the findings demonstrate that modulation of mGluR and NO formation suggests the possibility of developing new treatment strategies for PD.
| 1. | Girault JA, Greengard P. The neurobiology of dopamine signaling. Arch Neurol. 2004;61:641-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 296] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 2. | Klein MO, Battagello DS, Cardoso AR, Hauser DN, Bittencourt JC, Correa RG. Dopamine: Functions, Signaling, and Association with Neurological Diseases. Cell Mol Neurobiol. 2019;39:31-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 704] [Cited by in RCA: 649] [Article Influence: 92.7] [Reference Citation Analysis (1)] |
| 3. | Strange PG. Dopamine receptors in the basal ganglia: relevance to Parkinson's disease. Mov Disord. 1993;8:263-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Marino BLB, de Souza LR, Sousa KPA, Ferreira JV, Padilha EC, da Silva CHTP, Taft CA, Hage-Melim LIS. Parkinson's Disease: A Review from Pathophysiology to Treatment. Mini Rev Med Chem. 2020;20:754-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 139] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 5. | Jimenez-Shahed J. Medical and Surgical Treatments of Tourette Syndrome. Neurol Clin. 2020;38:349-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 6. | Volkow ND, Morales M. The Brain on Drugs: From Reward to Addiction. Cell. 2015;162:712-725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 743] [Cited by in RCA: 894] [Article Influence: 89.4] [Reference Citation Analysis (0)] |
| 7. | Barenys M, Reverte I, Masjosthusmann S, Gómez-Catalán J, Fritsche E. Developmental neurotoxicity of MDMA. A systematic literature review summarized in a putative adverse outcome pathway. Neurotoxicology. 2020;78:209-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Remington G, Foussias G, Agid O, Fervaha G, Takeuchi H, Hahn M. The neurobiology of relapse in schizophrenia. Schizophr Res. 2014;152:381-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 9. | Kostrzewa RM, Wydra K, Filip M, Crawford CA, McDougall SA, Brown RW, Borroto-Escuela DO, Fuxe K, Gainetdinov RR. Dopamine D2 Receptor Supersensitivity as a Spectrum of Neurotoxicity and Status in Psychiatric Disorders. J Pharmacol Exp Ther. 2018;366:519-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Fénelon G, Alves G. Epidemiology of psychosis in Parkinson's disease. J Neurol Sci. 2010;289:12-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 206] [Cited by in RCA: 220] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 11. | Naasan G, Shdo SM, Rodriguez EM, Spina S, Grinberg L, Lopez L, Karydas A, Seeley WW, Miller BL, Rankin KP. Psychosis in neurodegenerative disease: differential patterns of hallucination and delusion symptoms. Brain. 2021;144:999-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 89] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 12. | Ffytche DH, Creese B, Politis M, Chaudhuri KR, Weintraub D, Ballard C, Aarsland D. The psychosis spectrum in Parkinson disease. Nat Rev Neurol. 2017;13:81-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 251] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 13. | Tampi RR, Tampi DJ, Young JJ, Balachandran S, Hoq RA, Manikkara G. Evidence for using pimavanserin for the treatment of Parkinson's disease psychosis. World J Psychiatry. 2019;9:47-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Prenger MTM, Madray R, Van Hedger K, Anello M, MacDonald PA. Social Symptoms of Parkinson's Disease. Parkinsons Dis. 2020;2020:8846544. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 15. | Finlay C, Duty S. Therapeutic potential of targeting glutamate receptors in Parkinson's disease. J Neural Transm (Vienna). 2014;121:861-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 16. | Piccirillo S, Magi S, Preziuso A, Castaldo P, Amoroso S, Lariccia V. Gateways for Glutamate Neuroprotection in Parkinson's Disease (PD): Essential Role of EAAT3 and NCX1 Revealed in an In Vitro Model of PD. Cells. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Parsons CG, Danysz W, Zieglgänsberger W. Excitatory amino acid neurotransmission. Handb Exp Pharmacol. 2005;249-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 18. | Ignarro LJ. Nitric oxide. A novel signal transduction mechanism for transcellular communication. Hypertension. 1990;16:477-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 347] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 19. | Garthwaite J. NO as a multimodal transmitter in the brain: discovery and current status. Br J Pharmacol. 2019;176:197-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 20. | Dawson TM, Snyder SH. Gases as biological messengers: nitric oxide and carbon monoxide in the brain. J Neurosci. 1994;14:5147-5159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 786] [Cited by in RCA: 751] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 21. | Jiménez-Jiménez FJ, Alonso-Navarro H, Herrero MT, García-Martín E, Agúndez JA. An Update on the Role of Nitric Oxide in the Neurodegenerative Processes of Parkinson's Disease. Curr Med Chem. 2016;23:2666-2679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 22. | Pierucci M, Galati S, Valentino M, Di Matteo V, Benigno A, Pitruzzella A, Muscat R, Di Giovanni G. Nitric oxide modulation of the basal ganglia circuitry: therapeutic implication for Parkinson's disease and other motor disorders. CNS Neurol Disord Drug Targets. 2011;10:777-791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Tapias V, Cannon JR, Greenamyre JT. Pomegranate juice exacerbates oxidative stress and nigrostriatal degeneration in Parkinson's disease. Neurobiol Aging. 2014;35:1162-1176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 24. | Tewari D, Sah AN, Bawari S, Nabavi SF, Dehpour AR, Shirooie S, Braidy N, Fiebich BL, Vacca RA, Nabavi SM. Role of Nitric Oxide in Neurodegeneration: Function, Regulation, and Inhibition. Curr Neuropharmacol. 2021;19:114-126. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 25. | Csonka C, Páli T, Bencsik P, Görbe A, Ferdinandy P, Csont T. Measurement of NO in biological samples. Br J Pharmacol. 2015;172:1620-1632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 26. | Bryan NS, Grisham MB. Methods to detect nitric oxide and its metabolites in biological samples. Free Radic Biol Med. 2007;43:645-657. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 765] [Cited by in RCA: 684] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 27. | Tsikas D. Methods of quantitative analysis of the nitric oxide metabolites nitrite and nitrate in human biological fluids. Free Radic Res. 2005;39:797-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 281] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 28. | Li CZ, Alwarappan S, Zhang W, Scafa N, Zhang X. Metallo Protoporphyrin Functionalized Microelectrodes for Electrocatalytic Sensing of Nitric Oxide. Am J Biomed Sci. 2009;1:274-282. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 29. | Olesen SP, Møller A, Mordvintcev PI, Busse R, Mülsch A. Regional measurements of NO formed in vivo during brain ischemia. Acta Neurol Scand. 1997;95:219-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Bashkatova V, Kraus M, Prast H, Vanin A, Rayevsky K, Philippu A. Influence of NOS inhibitors on changes in ACH release and NO level in the brain elicited by amphetamine neurotoxicity. Neuroreport. 1999;10:3155-3158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Hogg N. Detection of nitric oxide by electron paramagnetic resonance spectroscopy. Free Radic Biol Med. 2010;49:122-129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 78] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 32. | Carvey PM, Punati A, Newman MB. Progressive dopamine neuron loss in Parkinson's disease: the multiple hit hypothesis. Cell Transplant. 2006;15:239-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 120] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 33. | Duda J, Pötschke C, Liss B. Converging roles of ion channels, calcium, metabolic stress, and activity pattern of Substantia nigra dopaminergic neurons in health and Parkinson's disease. J Neurochem. 2016;139 Suppl 1:156-178. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 120] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 34. | Del Tredici K, Rüb U, De Vos RA, Bohl JR, Braak H. Where does parkinson disease pathology begin in the brain? J Neuropathol Exp Neurol. 2002;61:413-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 538] [Cited by in RCA: 535] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 35. | Betarbet R, Sherer TB, Greenamyre JT. Animal models of Parkinson's disease. Bioessays. 2002;24:308-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 387] [Cited by in RCA: 393] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 36. | Blesa J, Przedborski S. Parkinson's disease: animal models and dopaminergic cell vulnerability. Front Neuroanat. 2014;8:155. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 346] [Cited by in RCA: 375] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 37. | Chesselet MF. In vivo alpha-synuclein overexpression in rodents: a useful model of Parkinson's disease? Exp Neurol. 2008;209:22-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 152] [Cited by in RCA: 140] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 38. | Cenci MA, Björklund A. Animal models for preclinical Parkinson's research: An update and critical appraisal. Prog Brain Res. 2020;252:27-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 39. | Bezard E, Yue Z, Kirik D, Spillantini MG. Animal models of Parkinson's disease: limits and relevance to neuroprotection studies. Mov Disord. 2013;28:61-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 148] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 40. | Visanji NP, Brotchie JM, Kalia LV, Koprich JB, Tandon A, Watts JC, Lang AE. α-Synuclein-Based Animal Models of Parkinson's Disease: Challenges and Opportunities in a New Era. Trends Neurosci. 2016;39:750-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 129] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 41. | Lane E, Dunnett S. Animal models of Parkinson's disease and L-dopa induced dyskinesia: how close are we to the clinic? Psychopharmacology (Berl). 2008;199:303-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 42. | Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci. 2000;3:1301-1306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2576] [Cited by in RCA: 2620] [Article Influence: 100.8] [Reference Citation Analysis (0)] |
| 43. | Sherer TB, Kim JH, Betarbet R, Greenamyre JT. Subcutaneous rotenone exposure causes highly selective dopaminergic degeneration and alpha-synuclein aggregation. Exp Neurol. 2003;179:9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 508] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 44. | Cannon JR, Tapias V, Na HM, Honick AS, Drolet RE, Greenamyre JT. A highly reproducible rotenone model of Parkinson's disease. Neurobiol Dis. 2009;34:279-290. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 622] [Cited by in RCA: 591] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 45. | Alam M, Mayerhofer A, Schmidt WJ. The neurobehavioral changes induced by bilateral rotenone lesion in medial forebrain bundle of rats are reversed by L-DOPA. Behav Brain Res. 2004;151:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 52] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 46. | Fleming SM, Zhu C, Fernagut PO, Mehta A, DiCarlo CD, Seaman RL, Chesselet MF. Behavioral and immunohistochemical effects of chronic intravenous and subcutaneous infusions of varying doses of rotenone. Exp Neurol. 2004;187:418-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 166] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 47. | Tanner CM, Kamel F, Ross GW, Hoppin JA, Goldman SM, Korell M, Marras C, Bhudhikanok GS, Kasten M, Chade AR, Comyns K, Richards MB, Meng C, Priestley B, Fernandez HH, Cambi F, Umbach DM, Blair A, Sandler DP, Langston JW. Rotenone, paraquat, and Parkinson's disease. Environ Health Perspect. 2011;119:866-872. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1148] [Cited by in RCA: 1003] [Article Influence: 66.9] [Reference Citation Analysis (7)] |
| 48. | Pouchieu C, Piel C, Carles C, Gruber A, Helmer C, Tual S, Marcotullio E, Lebailly P, Baldi I. Pesticide use in agriculture and Parkinson's disease in the AGRICAN cohort study. Int J Epidemiol. 2018;47:299-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 49. | Wu AP, He Y, Ye SY, Qi LY, Liu L, Zhong W, Wang YH, Fu H. Negative effects of a piscicide, rotenone, on the growth and metabolism of three submerged macophytes. Chemosphere. 2020;250:126246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 50. | Difazio MC, Hollingsworth Z, Young AB, Penney JB Jr. Glutamate receptors in the substantia nigra of Parkinson's disease brains. Neurology. 1992;42:402-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 51. | Iovino L, Tremblay ME, Civiero L. Glutamate-induced excitotoxicity in Parkinson's disease: The role of glial cells. J Pharmacol Sci. 2020;144:151-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 252] [Article Influence: 42.0] [Reference Citation Analysis (0)] |
| 52. | Wang J, Wang F, Mai D, Qu S. Molecular Mechanisms of Glutamate Toxicity in Parkinson's Disease. Front Neurosci. 2020;14:585584. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 155] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 53. | Jakaria M, Park SY, Haque ME, Karthivashan G, Kim IS, Ganesan P, Choi DK. Neurotoxic Agent-Induced Injury in Neurodegenerative Disease Model: Focus on Involvement of Glutamate Receptors. Front Mol Neurosci. 2018;11:307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 54. | Dekundy A, Pietraszek M, Schaefer D, Cenci MA, Danysz W. Effects of group I metabotropic glutamate receptors blockade in experimental models of Parkinson's disease. Brain Res Bull. 2006;69:318-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 107] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 55. | Amalric M. Targeting metabotropic glutamate receptors (mGluRs) in Parkinson's disease. Curr Opin Pharmacol. 2015;20:29-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 56. | Sebastianutto I, Cenci MA. mGlu receptors in the treatment of Parkinson's disease and L-DOPA-induced dyskinesia. Curr Opin Pharmacol. 2018;38:81-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 59] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 57. | Pin JP, Bockaert J. Get receptive to metabotropic glutamate receptors. Curr Opin Neurobiol. 1995;5:342-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 100] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 58. | Nicoletti F, Bockaert J, Collingridge GL, Conn PJ, Ferraguti F, Schoepp DD, Wroblewski JT, Pin JP. Metabotropic glutamate receptors: from the workbench to the bedside. Neuropharmacology. 2011;60:1017-1041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 549] [Cited by in RCA: 513] [Article Influence: 34.2] [Reference Citation Analysis (0)] |
| 59. | Endoh T. Characterization of modulatory effects of postsynaptic metabotropic glutamate receptors on calcium currents in rat nucleus tractus solitarius. Brain Res. 2004;1024:212-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 60. | Rouse ST, Marino MJ, Bradley SR, Awad H, Wittmann M, Conn PJ. Distribution and roles of metabotropic glutamate receptors in the basal ganglia motor circuit: implications for treatment of Parkinson's disease and related disorders. Pharmacol Ther. 2000;88:427-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 114] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 61. | Boccella S, Marabese I, Guida F, Luongo L, Maione S, Palazzo E. The Modulation of Pain by Metabotropic Glutamate Receptors 7 and 8 in the Dorsal Striatum. Curr Neuropharmacol. 2020;18:34-50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 62. | Matarredona ER, Santiago M, Venero JL, Cano J, Machado A. Group II metabotropic glutamate receptor activation protects striatal dopaminergic nerve terminals against MPP+-induced neurotoxicity along with brain-derived neurotrophic factor induction. J Neurochem. 2001;76:351-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 63. | Jiang Q, Yan Z, Feng J. Activation of group III metabotropic glutamate receptors attenuates rotenone toxicity on dopaminergic neurons through a microtubule-dependent mechanism. J Neurosci. 2006;26:4318-4328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 64. | Sun L, Gu L, Wang S, Yuan J, Yang H, Zhu J, Zhang H. N-acetylcysteine protects against apoptosis through modulation of group I metabotropic glutamate receptor activity. PLoS One. 2012;7:e32503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 65. | Xia N, Zhang Q, Wang ST, Gu L, Yang HM, Liu L, Bakshi R, Yang H, Zhang H. Blockade of metabotropic glutamate receptor 5 protects against DNA damage in a rotenone-induced Parkinson's disease model. Free Radic Biol Med. 2015;89:567-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 66. | Bai XX, Gu L, Yang HM, Xi SS, Xia N, Zhang S, Zhang H. Downregulation of metabotropic glutamate receptor 5 inhibits hepatoma development in a neurotoxin rotenone-induced Parkinson's disease model. Toxicol Lett. 2018;288:71-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 67. | Luo WY, Xing SQ, Zhu P, Zhang CG, Yang HM, Van Halm-Lutterodt N, Gu L, Zhang H. PDZ Scaffold Protein CAL Couples with Metabotropic Glutamate Receptor 5 to Protect Against Cell Apoptosis and Is a Potential Target in the Treatment of Parkinson's Disease. Neurotherapeutics. 2019;16:761-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 68. | Zhu JW, Yuan JF, Yang HM, Wang ST, Zhang CG, Sun LL, Yang H, Zhang H. Extracellular cysteine (Cys)/cystine (CySS) redox regulates metabotropic glutamate receptor 5 activity. Biochimie. 2012;94:617-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 69. | Bashkatova VG, Sudakov SK. Role of metabotropic glutamate receptors in the mechanisms of experimental parkinsonism development. Bull Exp Biol Med. 2012;153:655-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 70. | Alam M, Danysz W, Schmidt WJ, Dekundy A. Effects of glutamate and alpha2-noradrenergic receptor antagonists on the development of neurotoxicity produced by chronic rotenone in rats. Toxicol Appl Pharmacol. 2009;240:198-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 71. | Manucha W. Mitochondrial dysfunction associated with nitric oxide pathways in glutamate neurotoxicity. Clin Investig Arterioscler. 2017;29:92-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 72. | Moncada S, Bolaños JP. Nitric oxide, cell bioenergetics and neurodegeneration. J Neurochem. 2006;97:1676-1689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 412] [Cited by in RCA: 412] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 73. | Dawson VL. Nitric oxide: role in neurotoxicity. Clin Exp Pharmacol Physiol. 1995;22:305-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 89] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 74. | Imam SZ, Newport GD, Itzhak Y, Cadet JL, Islam F, Slikker W Jr, Ali SF. Peroxynitrite plays a role in methamphetamine-induced dopaminergic neurotoxicity: evidence from mice lacking neuronal nitric oxide synthase gene or overexpressing copper-zinc superoxide dismutase. J Neurochem. 2001;76:745-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 94] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 75. | Ramdial K, Franco MC, Estevez AG. Cellular mechanisms of peroxynitrite-induced neuronal death. Brain Res Bull. 2017;133:4-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 76. | He Y, Imam SZ, Dong Z, Jankovic J, Ali SF, Appel SH, Le W. Role of nitric oxide in rotenone-induced nigro-striatal injury. J Neurochem. 2003;86:1338-1345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 93] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 77. | Tsikas D. Analysis of nitrite and nitrate in biological fluids by assays based on the Griess reaction: appraisal of the Griess reaction in the L-arginine/nitric oxide area of research. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;851:51-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 444] [Cited by in RCA: 578] [Article Influence: 28.9] [Reference Citation Analysis (0)] |
| 78. | Bashkatova V, Alam M, Vanin A, Schmidt WJ. Chronic administration of rotenone increases levels of nitric oxide and lipid peroxidation products in rat brain. Exp Neurol. 2004;186:235-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 78] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 79. | Bashkatova V, Philippu A. Role of nitric oxide in psychostimulant-induced neurotoxicity. AIMS Neurosci. 2019;6:191-203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 80. | Sun C, Wang Y, Mo M, Song C, Wang X, Chen S, Liu Y. Minocycline Protects against Rotenone-Induced Neurotoxicity Correlating with Upregulation of Nurr1 in a Parkinson's Disease Rat Model. Biomed Res Int. 2019;2019:6843265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 81. | Ardah MT, Bharathan G, Kitada T, Haque ME. Ellagic Acid Prevents Dopamine Neuron Degeneration from Oxidative Stress and Neuroinflammation in MPTP Model of Parkinson's Disease. Biomolecules. 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 82. | Cammack R, Shergill JK, Ananda Inalsingh V, Hughes MN. Applications of electron paramagnetic resonance spectroscopy to study interactions of iron proteins in cells with nitric oxide. Spectrochim Acta A Mol Biomol Spectrosc. 1998;54A:2393-2402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 83. | Pennathur S, Jackson-Lewis V, Przedborski S, Heinecke JW. Mass spectrometric quantification of 3-nitrotyrosine, ortho-tyrosine, and o,o'-dityrosine in brain tissue of 1-methyl-4-phenyl-1,2,3, 6-tetrahydropyridine-treated mice, a model of oxidative stress in Parkinson's disease. J Biol Chem. 1999;274:34621-34628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 210] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 84. | Ferger B, Themann C, Rose S, Halliwell B, Jenner P. 6-hydroxydopamine increases the hydroxylation and nitration of phenylalanine in vivo: implication of peroxynitrite formation. J Neurochem. 2001;78:509-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 28] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 85. | Aras S, Tanriover G, Aslan M, Yargicoglu P, Agar A. The role of nitric oxide on visual-evoked potentials in MPTP-induced Parkinsonism in mice. Neurochem Int. 2014;72:48-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 86. | Prentice H, Modi JP, Wu JY. Mechanisms of Neuronal Protection against Excitotoxicity, Endoplasmic Reticulum Stress, and Mitochondrial Dysfunction in Stroke and Neurodegenerative Diseases. Oxid Med Cell Longev. 2015;2015:964518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 163] [Cited by in RCA: 198] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 87. | Leng A, Feldon J, Ferger B. Rotenone increases glutamate-induced dopamine release but does not affect hydroxyl-free radical formation in rat striatum. Synapse. 2003;50:240-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 88. | Xiong ZK, Lang J, Xu G, Li HY, Zhang Y, Wang L, Su Y, Sun AJ. Excessive levels of nitric oxide in rat model of Parkinson's disease induced by rotenone. Exp Ther Med. 2015;9:553-558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 89. | Abdel-Salam OME, Youness ER, Ahmed NA, El-Toumy SA, Souleman AMA, Shaffie N, Abouelfadl DM. Bougainvillea spectabilis flowers extract protects against the rotenone-induced toxicity. Asian Pac J Trop Med. 2017;10:478-490. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 90. | Jayaraj RL, Beiram R, Azimullah S, Meeran MFN, Ojha SK, Adem A, Jalal FY. Lycopodium Attenuates Loss of Dopaminergic Neurons by Suppressing Oxidative Stress and Neuroinflammation in a Rat Model of Parkinson's Disease. Molecules. 2019;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 91. | Kumar S, Kumar P. The Beneficial effect of rice bran extract against rotenone-induced experimental Parkinson's disease in rats. Curr Mol Pharmacol. 2021;14:428-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 92. | Parkhe A, Parekh P, Nalla LV, Sharma N, Sharma M, Gadepalli A, Kate A, Khairnar A. Protective effect of alpha mangostin on rotenone induced toxicity in rat model of Parkinson's disease. Neurosci Lett. 2020;716:134652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 36] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 93. | Sharma S, Raj K, Singh S. Neuroprotective Effect of Quercetin in Combination with Piperine Against Rotenone- and Iron Supplement-Induced Parkinson's Disease in Experimental Rats. Neurotox Res. 2020;37:198-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 75] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 94. | Javed H, Azimullah S, Haque ME, Ojha SK. Cannabinoid Type 2 (CB2) Receptors Activation Protects against Oxidative Stress and Neuroinflammation Associated Dopaminergic Neurodegeneration in Rotenone Model of Parkinson's Disease. Front Neurosci. 2016;10:321. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 144] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 95. | Mossa AH, Mohafrash SMM, Chandrasekaran N. Safety of Natural Insecticides: Toxic Effects on Experimental Animals. Biomed Res Int. 2018;2018:4308054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Neurosciences
Country/Territory of origin: Russia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Xu WX S-Editor: Zhang H L-Editor: A P-Editor: Wang LYT