Published online Sep 19, 2020. doi: 10.5498/wjp.v10.i9.212
Peer-review started: December 27, 2019
First decision: April 2, 2020
Revised: June 16, 2020
Accepted: August 24, 2020
Article in press: August 24, 2020
Published online: September 19, 2020
Processing time: 259 Days and 15.6 Hours
Delirium is a common disorder in elderly medical inpatients with serious adverse outcomes and is characterized by sudden onset, disturbance in attention, awareness, consciousness and cognition, and often with behavioural disturbances. Central to understanding delirium, is understanding mechanisms by which body and brain wellbeing are linked and in particular how brain responses to bodily homeostatic stress is mediated. A number of studies have investigated the relationship between insulin-like growth factor I (IGF-I) and delirium in medically ill hospitalised patients with conflicting results. However, none have investigated growth hormone (GH) which is related to IGF-I via negative feedback.
To investigate the relationship between serum levels of IGF-I and GH, and the occurrence of delirium.
Prospective, longitudinal, observational study. Consecutive elderly inpatients (aged 70+), were assessed twice weekly with Montreal cognitive assessment (MoCA), Confusion assessment method (CAM), Acute Physiology and Chronic Health Evaluation II. Delirium was defined using CAM. Previous history of dementia was evaluated with the Informant Questionnaire on Cognitive Decline in the Elderly. IGF-I and GH levels were estimated with the ELISA method. Generalized estimating equations (GEE) model was applied for the first five assessments to analyze those longitudinal data.
The sample consisted of 198 participants (mean age 80.63 ± 6.81; range 70-97). Of these 92 (46.5%) were females. Eighty six (43.4%) were identified with a history of dementia. Incident or prevalent delirium during hospitalisation was identified with CAM in 40 participants (20.2%). Evaluation of missing values with Little's MCAR test indicated that they were missing completely at random (MCAR χ2 = 12.24, u: 9, P = 0.20). Using GEE for the analysis we found that low MoCA scores, low levels of IGF-I and high levels of GH were significantly associated with any delirium (prevalence, incident, or fluctuating , during the study period (Wald χ2 = 12.231; u: 1, P < 0.001, Wald χ2 = 7.196, u: 1, P = 0.007, Wald χ2 = 6.210; : u: 1, P = 0.013 respectively).
The results show that low levels of IGF-I, high levels of GH and low scores in cognition are independently associated with the occurrence of any delirium during the hospitalisation of medically ill older people. The results of the study supports the hypothesis that deficits in the immunoreactivity of the brain (low cerebral reserve) may be associated with delirium.
Core Tip: The present work investigates the association of serum levels of insulin-like growth factor I (IGF-I) and growth hormone (GH) with delirium presence in older medically ill hospitalised people. We found, in accordance with previous studies, that low levels of serum IGF-I and high levels of GH together with cognitive deficits are associated with the occurrence of delirium.
- Citation: Adamis D, Coada I, Eikelenboom P, Chu CS, Finn K, Melvin V, Williams J, Meagher DJ, McCarthy G. Delirium, insulin-like growth factor I, growth hormone in older inpatients. World J Psychiatr 2020; 10(9): 212-222
- URL: https://www.wjgnet.com/2220-3206/full/v10/i9/212.htm
- DOI: https://dx.doi.org/10.5498/wjp.v10.i9.212
Delirium is a syndrome which often presented with sudden onset, and disorders of the cognitive, consciousness, motor, affective, and perceptual domains. Those disturbances often are fluctuated[1]. It also manifested with different motor subtypes like hyperactive, hypoactive or mixed states[2].
Delirium is a common disorder in elderly medical inpatients. In Irish hospitals the point prevalence of delirium is estimated around 20%[3]. Higher rates are reported among patients in palliative and intensive care settings[3,4]. Delirium has severe bad consequences like, delays in hospital discharges increased hospital budgets[5], higher rates of institutionalisation[6], and perhaps increased mortality[7]. The old-fashioned concept of delirium as a brief, transient, and highly reversible condition is no longer supported by longitudinal studies. Accumulating evidence support that delirium is associated with persistent cognitive and functional problems[8,9] and delirium may be an accelerating and possibly causal factor in the development of dementia[10]. Notably, these adverse outcomes are independent of the severity of physical illness that cause delirium. Despite the understanding that delirium is caused by physical illness, it is not clear yet how those physical causes without direct connection with brain, can produce such a consistent complex neuropsychiatric picture as found in delirium. Thus, the pathophysiology of delirium remains unclear and many pathophysiological mechanisms have been hypothesised and suggested but none yet proved[5,11].
Delirium often occurs in the context of infectious illness and the external administration of cytokines in a number of medical illness for therapeutic reasons, can results to delirium. Thus, in an effort to clarify the pathophysiology of delirium a number of studies have investigated cytokine levels, but the results are conflicted and inconclusive[12]. However, from our previous work[13] we found that low circulating levels of neuroprotective factors of IGF-1 and interleukin-1 receptor antagonist (IL-1RA) were connected with delirium. Although there are few studies with firm conclusions about the role of cytokines in delirium, a hypothesis in which we work is that cerebral deficits may be the reason for the occurrence of delirium. This has led our research group to work on the general hypothesis that delirium is associated with already existed deficits in the brain regarding the immunoreactivity and the readiness to respond to the external “insult” (e.g., physical illness). Low levels of neuroprotective factors may possibly explain the onset of delirium rather than the actual trigger or “insult” factor. This can explain the observations that the severity of physical illness is not a risk factor for delirium at least in older populations[14]. One of those neuroprotective factors which has been investigated in delirium is the IGF-I.
IGF-I is regulate the body growth and metabolism but involves also in different brain functions. Circulating IGF-I is produced mainly from the liver but it can be produced by any cell type. The receptors of IGF-I are on almost all different cells including brain and have neuroprotective effects[15,16]. In addition IGF-I receptors play significant role in the integrity and regulation of blood-brain barrier, they have high expression in the cells that constitute it and they facilitate the access of serum IGF-I to entry into the brain[17].
In the Central Nervous System the IGF-I is produced by neurons and glial cells[18] and plays an extensive role in the development, plasticity and survival of neurons. IGF-I involves in the production of neurotransmitters, blocks apoptosis in damaged neurons, and thus has effects on cognition and cognitive decline during ageing or in other neurocognitive disorders like dementia and delirium[16,19-21].
However, IGF-I is not independent. The secretion of IGF-I is under the control of growth hormone (GH) which is called the GH/IGF-1 axis. GH is pleiotropic hormone which regulates many functions like feeding, growth, metabolism, reproduction, and immune system function. The secretion of GH is stimulated by the GHRH from the hypothalamus. GH secretion is also influenced by IGF-I, which involves a negative feedback mechanism[22,23].
Higher levels of GH are associated with risen levels IGF-I until a certain point where a plateau is reached[24]. It has been reported[25] that cerebrospinal levels of somatostatin (Growth hormone Inhibiting hormone) are significantly lower during delirium but and later at follow-up. This does not indicate a directly link between delirium and GH, but indicates a disturbed GH/IGF-1 axis in delirium. In the same line, in patients admitted to the intensive care unit it was found that low levels of IGF-I were correlated with high levels of GH[26]. In addition, a study[27] reported that administration of human GH in aged women following hip operation had increased IGF-I levels. Therefore, until now we do not know the way of the interaction of GH and IGF-I during delirium. In our previous review we have identified a lack of research work in relation to GH in studies which investigated IGF-I and delirium[12].
Nevertheless, a number of studies have investigated the relationship of IGF-I with delirium in medically ill hospitalised patients as well as in patients undergoing surgery with conflicting results. A resent meta-analysis[28] showed that there are indications of an association of IGF-1 and delirium but the authors also calling for further research into this area.
Given the previous studies and the new updates we carried out a new study in older medically ill hospitalised patients with the aim to find out the relationship of the circulating levels of IGF-I and GH to the delirium (in both prevalent and incident).
The present study was designed as a pragmatic prospective, longitudinal study. The study was carried out in a University Hospital in Sligo in the North-West of Ireland. The inclusion criteria were: (1) Consecutive admitted patients in the elderly medical wards; and (2) To be 70 years old and above. Exclusion criteria were (1) Patients who were readmitted and had already participate in the study; (2) Patients intubated or with aphasia; (3) Patients in a terminal stage of illness; and (4) Patients unable to speak English. A time frame of 72 h since admission was in place for the assessment of the eligibility for recruitment and the recruitment of participants.
Those patients who fulfilled the inclusion criteria and consented had an assessment at first day. Then seven more assessments were followed in a regular space of 3 ± 1 d if they were still hospitalised and alive. The maximum number of assessment was eight. Non-fasting blood was withdrawn the same days of the assessments. Bloods were centrifuged within ten minutes and then stored at -70 °C until analysis. Levels of IGF-I and GH were estimated with the ELISA method. Levels of IGF-I are measured in ng/ml and levels of GH in pg/mL.
Demographic data were collected from the computer of the hospital database. In addition at each time the following measurements/scales were administered.
The Montreal Cognitive Assessment (MoCA)[29] have been used for assessment of cognition. The maximum score in MoCA is 30 which indicates an intact cognition. In participants who were unable to complete all the sections due to a physical disability (e.g., visual impairment) MoCA results were standardized to give a maximum score of 30. To complete the MoCA it takes about 12-15 min.
The presence/absence of delirium was assessed with the CAM scale/algorithm[30]. The CAM is based in DSM-IIIR criteria for delirium. It asses four “cardinal” criteria for delirium.
To assess the severity of the underling physical illness the Acute Physiology and Chronic Health Evaluation II (APACHE-II)[31] (Acute Physiology and Chronic Health Evaluation II) and the APS subscale (Acute Physiology Score) were used. Higher scores in both scales indicate more illness severity.
Pre-existing dementia was assessed with two ways. First if it was documented clearly according to DSM-IV diagnostic criteria or if not the Short Informant Questionnaire of Cognitive Decline was used by interviewing the nearest relative. The cut-off point of ≥ 3.5[32] was used to define pre-existing dementia.
Informed consent was in writing using an earlier described method[33]. A separate consent in writing was asked for phlebotomy. The Sligo University Hospital Research Ethics Committee has graded Ethical approval for the project.
SPSS v23 was used for the analysis of the data. Continuous variables were presented as mean ± SD and categorical as counts and percentages. The Generalized Estimating Equations (GEE) method was used to analyse the effects of independent variables on delirium. GEE adjusts for correlations due to repeated assessments of each participant[34]. Because the dependent variable (delirium/no delirium) was binary the binominal distribution was used. To evaluate the fit of the model the Corrected Quasi Likelihood under Independence Model Criterion (QICC) value was used, (lower value – better fit). Because there were many missing values in the last 3 assessments (drop-outs) only the first 5 will be entered to the model.
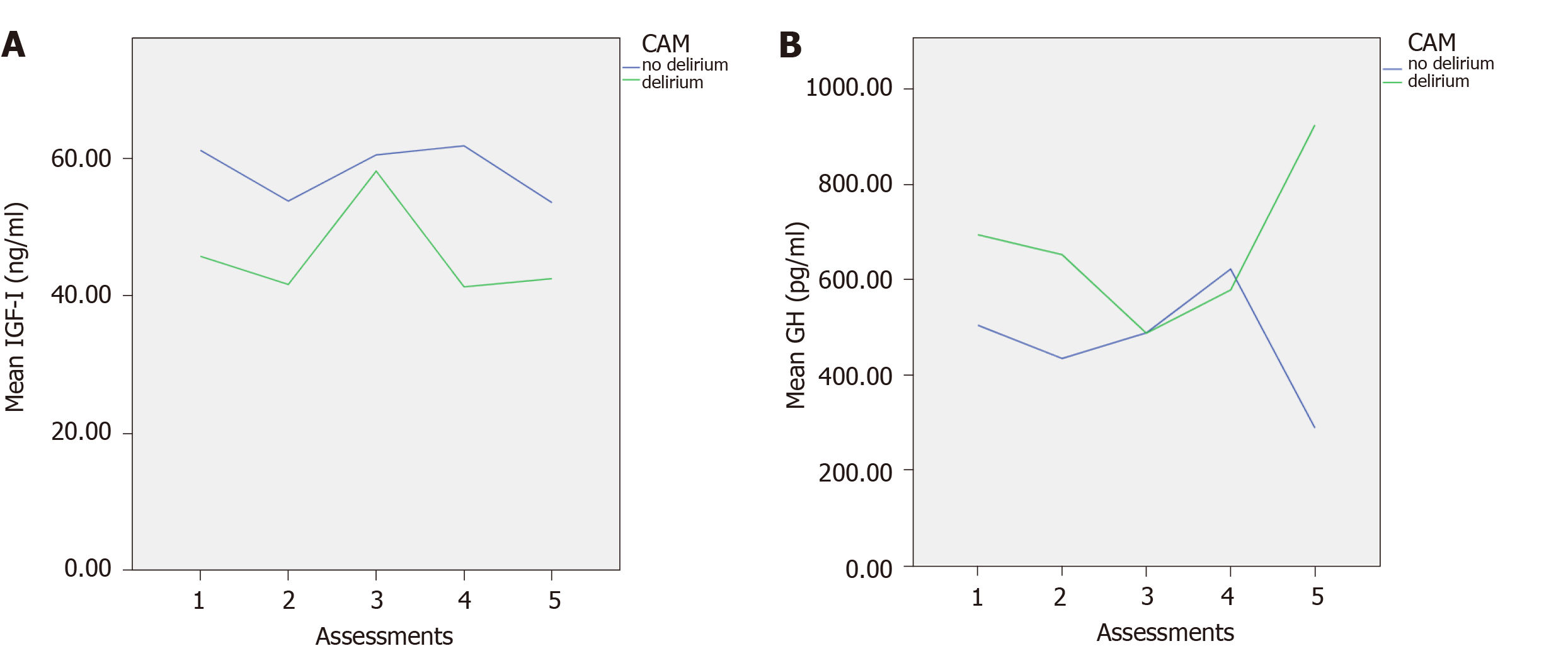
A total of 198 participants were analysed. The mean age of the participants was 80.63 ± 6.81; range 70-97. Of these 106 (53.5%) were males. Previous history of dementia was found in eighty six (43.4%). The characteristics of the two groups (delirium/ no delirium) at each of the five assessments, including means and standard deviations of the scales MoCA, and APACHE II scores, and IGF-I and GH levels at each assessment are shown in Table 1. Figure 1 shows the mean levels of IGF-I and GH across the assessments for those with and without delirium.
| Assessment | CAM (n) | MoCA | APACHE II | IGF-I (ng/mL) | GH (pg/mL) | |
| 1 | No delirium (173) | mean ± SD | 11.38 ± 7.90 | 8.59 ± 3.68 | 61.32 ± 22.95 | 503.95 ± 743.05 |
| Valid, n | 168 | 173 | 68 | 68 | ||
| Delirium (24) | mean ± SD | 3.78 ± 2.76 | 9.00 ± 3.86 | 45.88 ± 14.66 | 693.89 ± 462.15 | |
| Valid, n | 23 | 24 | 8 | 8 | ||
| 2 | No delirium (141) | mean ± SD | 10.42 ± 8.23 | 8.69 ± 3.69 | 53.93 ± 18.57 | 434.22 ± 561.88 |
| Valid, n | 134 | 140 | 49 | 49 | ||
| Delirium (11) | mean ± SD | 3.67 ± 3.43 | 11.00 ± 5.20 | 41.78 ± 13.98 | 652.14 ± 346.98 | |
| Valid, n | 9 | 11 | 5 | 5 | ||
| 3 | No delirium (90) | mean ± SD | 9.27 ± 7.58 | 8.77 ± 3.67 | 60.66 ± 18.88 | 487.98 ± 648.70 |
| Valid, n | 84 | 88 | 30 | 30 | ||
| Delirium (13) | mean ± SD | 3.90 ± 2.51 | 10.38 ± 3.69 | 58.29 ± 14.60 | 487.25 ± 507.84 | |
| Valid, n | 10 | 13 | 5 | 5 | ||
| 4 | No delirium (58) | mean ± SD | 10.63 ± 7.44 | 8.79 ± 3.55 | 61.97 ± 22.70 | 622.00 ± 790.09 |
| Valid, n | 56 | 58 | 18 | 18 | ||
| Delirium (8) | mean ± SD | 6.00 ± 1.83 | 9.00 ± 2.67 | 41.43 ± 8.13 | 577.94 ± 200.90 | |
| Valid, n | 7 | 8 | 2 | 2 | ||
| 5 | No delirium (46) | mean ± SD | 8.70 ± 7.20 | 8.80 ± 3.28 | 53.70 ± 14.37 | 288.27 ± 245.51 |
| Valid, n | 43 | 46 | 11 | 11 | ||
| Delirium (6) | mean ± SD | 2.33 ± 1.03 | 9.50 ± 3.33 | 42.62 ± 2.42 | 924.34 ± 677.88 | |
| Valid, n | 6 | 6 | 4 | 4 |
This was done by using the Little's MCAR test. The results of the test was not significant (MCAR, χ2 = 12.24, u: 9, P = 0.20) which indicates that the missing values were missing completely at random
Here we examined the effects of the independent variables age, gender, previous history of dementia (binary yes/no), APACHE II, MoCA scores, and the levels of IGF-I and GH on the dependent variable delirium/no delirium (binary). The most parsimonious model (lowest QICC value) is shown in Table 2.
| Parameter estimates | |||||||
| Parameter | B | SE | 95%CI | Hypothesis test | |||
| Lower | Upper | Wald χ2 | u | Sig. | |||
| GH (pg/ml) | -0.00111 | 0.0002 | -0.001 | 0 | 6.21 | 1 | 0.013 |
| IGF-1 (ng/ml) | 0.02 | 0.0074 | 0.005 | 0.034 | 7.196 | 1 | 0.007 |
| MoCA | 0.205 | 0.0586 | 0.09 | 0.32 | 12.231 | 1 | < 0.001 |
The results from the Table 2 shows that those with any delirium during the hospitalisation had significantly lower scores in the MoCA scale, lower levels of IGF-I and higher levels of GH compared to those without delirium. None of the other examined variables (age, gender, previous history of dementia or severity of physical illness (APACHE II) had any significant effect in the presence or absence of delirium as it was defined with CAM.
First of all, the results show that deficits in cognition as measured with the MoCA, are a significant independent predictor for the occurrence of any delirium (prevalent, incident, or fluctuating). This result is constantly found in all the studies which investigate delirium because disturbance in cognition is a central feature of delirium. Therefore this is a result which was expected. In addition the severity of the physical illness (as measured with APACHE II), previous history of dementia, and age did not have any effect on delirium. Severity of physical illness again is an expected finding since in our previous studies we did not find any effect and thus we generate the hypothesis that it is not the severity of insult that is important for causing the delirium but the reduced neuroprotection of the brain. Besides the lack of effect of age and previous history of dementia is easily explained by the more powerful predictor, scores in the MoCA.
Regarding the IGF-I, the results of the present study is in accordance with our previous study[13] in which we use similar methodology and longitudinal design but in a different population, different hospital and in a different country and also confirmed that low levels of circulating IGF-I are significantly linked with any delirium (incident or prevalence). Similar results have been reported form other research groups[35-39], but not from all[40-43]. However a resent meta-analysis[28] showed that lower levels of circulating IGF-I are associated with higher rates of delirium among older patients. There are many reasons for those discrepancies among the studies, (see also[28]). First of all different populations were studied. Some studies include populations with pre-existing dementia (e.g., the present study) while others excluded them[41,42]. A second reason perhaps is the setting where the study is conducted and the sample. Some of the studies were conducted in medical wards where the sample include populations with mainly medical illness while others in surgical wards in patients before and after surgery. Perhaps surgery is another stressor and perhaps pathophysiology which leads to delirium in those patients is different despite the end product being the same. In addition those studies in surgery wards have examined patients before and immediately after the surgery for delirium. However, it has been suggested that perhaps different mechanisms are underline the delirium that developed in the first 24 hours after surgery and in the delirium that developed the next one to three days after surgery[44]. Finally one important reason which can explain those discrepancies is the different scales/measurements/criteria that have been applied to define delirium. It has been shown that applying different criteria for delirium is influence significantly the rates and diagnosis of delirium and there is extensive discrepancy between the actual cases defined by each different system[45-48].
Furthermore, the present study for first time shows that GH is an important factor in the pathogenesis of delirium. However, because IGF-I and GH are correlated with a negative loop feedback we do not know which of them is more important in the pathogenesis of delirium. From the present study what we can conclude is that the somatotropic axis (IGF-I/GH) is disturbed during the delirium phase compared to those without delirium. Low IGF-I and high GH levels are related to delirium. To the best of our knowledge no previous studies have investigated the IGF-I/GH axis. Therefore we attempt to explain our hypothesis further.
The secretion of GH is age related and a decline of GH started between the ages of 18 to 25 years. This decrease of the GH concentration in the periphery is accompanied by a gradual decline of IGF-I as well[27,49]. In addition, lower levels of GH are correlated with frailty[27] and exogenous administration of GH can increase energy, and improve mood, concentration, and memory. Those improvements in cognition may are the results of a direct effect of GH in the brain[50,51]. Therefore, perhaps the increased levels of GH reflect a compensatory mechanism in the brain to recover through a direct effect of GH. However, this does not explain the low levels of IGF-I which interestingly have been proven to protect neurons directly[52]. Experimental studies in animals have shown that after administration of IGF-I in brain injuries there was an improvement in the outcomes (regarding behaviour and cognition)[53]. IGF-I has also been proposed as a treatment for Alzheimer’s disease because it play a crucial role in tau pathway and acetyl-choline which both connected with the occurrence of delirium[54]. Therefore a direct effect of IGF-I is more likely than a direct effect of GH. In the same line a second explanation has been suggested that in acute inflammation situations there is a GH resistance because the body prevents growth and energy storage in an attempt to keep the homeostasis. In those situations of acute inflammation the levels of IGF-I reduced, regardless of the increase of GH[41,55].
Taken together those suggestions provide a likely explanation that in delirious states GH is increased because of the disturbed IGF-I / GH axis and the lack of inhibitory mechanism of IGF-I.
Whatever mechanism is involved we cannot conclude from this study and it is important to notice that we measure levels in the periphery which may or may not reflect the brain levels of IGF-I and GH. However, we can conclude that in delirium the IGF-I/GH axis is disturbed, and that low levels of IGF-I together with high levels of GH and impaired cognition are independently significant predictors of delirium.
Limitations of the study
An obvious and common limitation of those kinds of studies including the present is the small sample size. However, the drop-outs and missing data of the study were completely missing at random so no biases have introduced in the study. A second limitation of the study is the lack of generalizability. The results of this study apply only in medically ill older people and not in other populations. Perhaps similar studies are needed also in other populations like surgical patients. There are ongoing collaborative studies examined the role of serum factors in postoperative delirium. Surgery induced delirium is a good model to separate the predisposal factors (preoperative) from the precipitating factors (post-operative) in the occurrence of delirium. Furthermore, the strengths of his study are the longitudinal design and the statistical analysis accompanied the design. By having this design and analysis we have included all the deliria during hospitalisation (prevalence, incident, fluctuated, and persistent) compared with the non-delirium states (including never delirium during hospitalisation and recovered delirium) across the time in the entire examined population.
As we noted above, further studies need to be done in different populations before we be asserted about the results. If the results are replicated in further studies this can lead to clinical trials for the treatment and / or prevention of delirium with small doses of IGF-I.
In conclusion, this study indicates that during delirium in older medically ill hospitalised patients the IGF-I/GH axis is disturbed but we do not know yet the mechanism behind it. However more studies are needed to confirm or disconfirm the above findings before we move further to clinical trials for treatment or prevention of delirium with small doses of IGF-I.
Delirium is a common disorder in elderly medical inpatients, in surgical wards, and Intensive care units with serious adverse outcomes.
To understand delirium is important to understand the underline mechanisms by which body and brain are linked and how brain responses to bodily homeostatic stress is mediated. We have notice from our previous research work that the severity of physical illness is not a risk factor for delirium at least in older populations and perhaps delirium is associated with deficits in the immunoreactivity of the brain (low cerebral reserve). Low levels of neuroprotective factors may possibly explain the onset of delirium rather than the actual trigger or “insult” factor. A number of studies have investigated the relationship between Insulin-like growth factor I (IGF-I) and delirium with conflicting results. A relevant also factor is the Growth Hormone (GH) which is related to IGF-I via negative feedback. Therefore in the present study we included also the GH.
To investigate the relationship of the occurrence of delirium during hospitalisation (prevalent and incident) with the serum levels of IGF-I and GH.
Observational, prospective, longitudinal study of older people who consecutively admitted to medical wards of a general hospital.
We found that low cognitive function, low levels of IGF-I and high levels of GH were significantly associated with any delirium (prevalence, incident, or fluctuating) during the study period.
The involvement of GH in delirium is a new finding from the present study. Also the finding of the low levels of IGF-I and the association of delirium confirms some of the previous studies. Those findings together with the association of cognitive decline with delirium strength the primary hypotheses that low brain reserves are possible the predisposing factor for delirium. Those findings needs further replication in other studies and especially in surgical samples
If the above findings are replicated in future studies then the next step is clinical trials with small doses of IGF-I for prevention of delirium.
| 1. | American Psychiatric Association. Task Force on DSM-IV. DSM-IV: Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994: 886. |
| 2. | Meagher DJ, Leonard M, Donnelly S, Conroy M, Adamis D, Trzepacz PT. A longitudinal study of motor subtypes in delirium: frequency and stability during episodes. J Psychosom Res. 2012;72:236-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 79] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 3. | Ryan DJ, O'Regan NA, Caoimh RÓ, Clare J, O'Connor M, Leonard M, McFarland J, Tighe S, O'Sullivan K, Trzepacz PT, Meagher D, Timmons S. Delirium in an adult acute hospital population: predictors, prevalence and detection. BMJ Open. 2013;3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 253] [Cited by in RCA: 275] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 4. | Adamis D, Macdonald AJ. Prevalence, Incidence (Epidemiology) and Associations (Risk Factors) of Delirium in Elderly Medical Inpatients. In: Newman KJ, Slater TC, editors. Delirium: Causes, Diagnosis and Treatment. Neuroscience Research Progress Psychiatry - Theory, Applications and Treatments. Hauppauge NY: Nova Science Publishers; 2012: 49-75. |
| 5. | Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383:911-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1954] [Cited by in RCA: 2524] [Article Influence: 210.3] [Reference Citation Analysis (0)] |
| 6. | Adamis D, Treloar A, Martin FC, Macdonald AJ. Recovery and outcome of delirium in elderly medical inpatients. Arch Gerontol Geriatr. 2006;43:289-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 79] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304:443-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1200] [Cited by in RCA: 1435] [Article Influence: 89.7] [Reference Citation Analysis (0)] |
| 8. | Adamis D, Meagher D, Treloar A, Dunne C, Larvin M, Martin FC, Macdonald AJ. Phenomenological and biological correlates of improved cognitive function in hospitalized elderly medical inpatients. Arch Gerontol Geriatr. 2014;59:593-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | MacLullich AM, Beaglehole A, Hall RJ, Meagher DJ. Delirium and long-term cognitive impairment. Int Rev Psychiatry. 2009;21:30-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 179] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 10. | Davis DH, Muniz Terrera G, Keage H, Rahkonen T, Oinas M, Matthews FE, Cunningham C, Polvikoski T, Sulkava R, MacLullich AM, Brayne C. Delirium is a strong risk factor for dementia in the oldest-old: a population-based cohort study. Brain. 2012;135:2809-2816. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 407] [Cited by in RCA: 384] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 11. | Maldonado JR. Acute Brain Failure: Pathophysiology, Diagnosis, Management, and Sequelae of Delirium. Crit Care Clin. 2017;33:461-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 227] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 12. | Adamis D, Meagher D. Insulin-like growth factor I and the pathogenesis of delirium: a review of current evidence. J Aging Res. 2011;2011:951403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Adamis D, Lunn M, Martin FC, Treloar A, Gregson N, Hamilton G, Macdonald AJ. Cytokines and IGF-I in delirious and non-delirious acutely ill older medical inpatients. Age Ageing. 2009;38:326-32; discussion 251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 14. | Adamis D. Cytokines in the elderly. In: Preedy VR, Hunter R, editors. Cytokines: Taylor & Francis; 2011: 79-92. [DOI] [Full Text] |
| 15. | Kim B, Feldman EL. Insulin resistance as a key link for the increased risk of cognitive impairment in the metabolic syndrome. Exp Mol Med. 2015;47:e149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 217] [Cited by in RCA: 249] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 16. | Kim B, Elzinga SE, Henn RE, McGinley LM, Feldman EL. The effects of insulin and insulin-like growth factor I on amyloid precursor protein phosphorylation in in vitro and in vivo models of Alzheimer's disease. Neurobiol Dis. 2019;132:104541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 17. | Labandeira-Garcia JL, Costa-Besada MA, Labandeira CM, Villar-Cheda B, Rodríguez-Perez AI. Insulin-Like Growth Factor-1 and Neuroinflammation. Front Aging Neurosci. 2017;9:365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 171] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 18. | Rodriguez-Perez AI, Borrajo A, Diaz-Ruiz C, Garrido-Gil P, Labandeira-Garcia JL. Crosstalk between insulin-like growth factor-1 and angiotensin-II in dopaminergic neurons and glial cells: role in neuroinflammation and aging. Oncotarget. 2016;7:30049-30067. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 55] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 19. | Nieto-Estévez V, Defterali Ç, Vicario-Abejón C. IGF-I: A Key Growth Factor that Regulates Neurogenesis and Synaptogenesis from Embryonic to Adult Stages of the Brain. Front Neurosci. 2016;10:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 211] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 20. | Wrigley S, Arafa D, Tropea D. Insulin-Like Growth Factor 1: At the Crossroads of Brain Development and Aging. Front Cell Neurosci. 2017;11:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 196] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 21. | Tien LT, Lee YJ, Pang Y, Lu S, Lee JW, Tseng CH, Bhatt AJ, Savich RD, Fan LW. Neuroprotective Effects of Intranasal IGF-1 against Neonatal Lipopolysaccharide-Induced Neurobehavioral Deficits and Neuronal Inflammation in the Substantia Nigra and Locus Coeruleus of Juvenile Rats. Dev Neurosci. 2017;39:443-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Qiu H, Yang JK, Chen C. Influence of insulin on growth hormone secretion, level and growth hormone signalling. Sheng Li Xue Bao. 2017;69:541-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 23. | Bergan-Roller HE, Sheridan MA. The growth hormone signaling system: Insights into coordinating the anabolic and catabolic actions of growth hormone. Gen Comp Endocrinol. 2018;258:119-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 104] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 24. | Barkan AL. Defining normalcy of the somatotropic axis: an attainable goal? Pituitary. 2007;10:135-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Koponen HJ, Leinonen E, Lepola U, Riekkinen PJ. A long-term follow-up study of cerebrospinal fluid somatostatin in delirium. Acta Psychiatr Scand. 1994;89:329-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Ross R, Miell J, Freeman E, Jones J, Matthews D, Preece M, Buchanan C. Critically ill patients have high basal growth hormone levels with attenuated oscillatory activity associated with low levels of insulin-like growth factor-I. Clin Endocrinol (Oxf). 1991;35:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 179] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 27. | Yeo AL, Levy D, Martin FC, Sönksen P, Sturgess I, Wheeler MM, Young A. Frailty and the biochemical effects of recombinant human growth hormone in women after surgery for hip fracture. Growth Horm IGF Res. 2003;13:361-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Li DJ, Tseng PT, Stubbs B, Chen TY, Lin PY, Chen SL, Thompson T, Adamis D, Chu CS. Low peripheral levels of insulin growth factor-1 are associated with high incidence of delirium among elderly patients: A systematic review and meta-analysis. Arch Gerontol Geriatr. 2018;77:13-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11622] [Cited by in RCA: 17365] [Article Influence: 826.9] [Reference Citation Analysis (0)] |
| 30. | Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3380] [Cited by in RCA: 3602] [Article Influence: 100.1] [Reference Citation Analysis (0)] |
| 31. | Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818-829. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10902] [Cited by in RCA: 11377] [Article Influence: 277.5] [Reference Citation Analysis (1)] |
| 32. | Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med. 1994;24:145-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 777] [Cited by in RCA: 896] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 33. | Adamis D, Martin FC, Treloar A, Macdonald AJ. Capacity, consent, and selection bias in a study of delirium. J Med Ethics. 2005;31:137-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 34. | Adamis D. Statistical methods for analysing longitudinal data in delirium studies. Int Rev Psychiatry. 2009;21:74-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 35. | Wilson K, Broadhurst C, Diver M, Jackson M, Mottram P. Plasma insulin growth factor-1 and incident delirium in older people. Int J Geriatr Psychiatry. 2005;20:154-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 36. | Morandi A, Gunther ML, Pandharipande PP, Jackson JC, Thompson JL, Shintani AK, Ely EW, Girard TD. Insulin-like growth factor-1 and delirium in critically ill mechanically ventilated patients: a preliminary investigation. Int Psychogeriatr. 2011;23:1175-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 37. | Çinar MA, Balikçi A, SertoÄŸlu E, Mehmet AK, Serdar MA, Özmenler KN. Role of CRP, TNF-a, and IGF-1 in Delirium Pathophysiology. Noro Psikiyatr Ars. 2014;51:376-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 38. | Egberts A, Wijnbeld EH, Fekkes D, van der Ploeg MA, Ziere G, Hooijkaas H, van der Cammen TJ, Mattace-Raso FU. Neopterin: a potential biomarker for delirium in elderly patients. Dement Geriatr Cogn Disord. 2015;39:116-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 39. | Shen H, Shao Y, Chen J, Guo J. Insulin-Like Growth Factor-1, a Potential Predicative Biomarker for Postoperative Delirium Among Elderly Patients with Open Abdominal Surgery. Curr Pharm Des. 2016;22:5879-5883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 40. | Lemstra AW, Kalisvaart KJ, Vreeswijk R, van Gool WA, Eikelenboom P. Pre-operative inflammatory markers and the risk of postoperative delirium in elderly patients. Int J Geriatr Psychiatry. 2008;23:943-948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 41. | Cerejeira J, Batista P, Nogueira V, Vaz-Serra A, Mukaetova-Ladinska EB. The stress response to surgery and postoperative delirium: evidence of hypothalamic-pituitary-adrenal axis hyperresponsiveness and decreased suppression of the GH/IGF-1 Axis. J Geriatr Psychiatry Neurol. 2013;26:185-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 42. | Yen TE, Allen JC, Rivelli SK, Patterson SC, Metcalf MR, Flink BJ, Mirrakhimov AE, Lagoo SA, Vail TP, Young CC, Moon RE, Trzepacz PT, Kwatra MM. Association between Serum IGF-I levels and Postoperative Delirium in Elderly Subjects Undergoing Elective Knee Arthroplasty. Sci Rep. 2016;6:20736. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 43. | Chu CS, Liang CK, Chou MY, Lin YT, Hsu CJ, Chu CL, Chou PH. Lack of Association between Pre-Operative Insulin-Like Growth Factor-1 and the Risk of Post-Operative Delirium in Elderly Chinese Patients. Psychiatry Investig. 2016;13:327-332. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 44. | Slor CJ, Witlox J, Adamis D, Jansen RWMM, Houdijk APJ, van Gool WA, de Jonghe JFM, Eikelenboom P. The trajectory of C-reactive protein serum levels in older hip fracture patients with postoperative delirium. Int J Geriatr Psychiatry. 2019;34:1438-1446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 45. | Laurila JV, Pitkala KH, Strandberg TE, Tilvis RS. Impact of different diagnostic criteria on prognosis of delirium: a prospective study. Dement Geriatr Cogn Disord. 2004;18:240-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 46. | Thomas C, Kreisel SH, Oster P, Driessen M, Arolt V, Inouye SK. Diagnosing delirium in older hospitalized adults with dementia: adapting the confusion assessment method to international classification of diseases, tenth revision, diagnostic criteria. J Am Geriatr Soc. 2012;60:1471-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 47. | Meagher DJ, Morandi A, Inouye SK, Ely W, Adamis D, Maclullich AJ, Rudolph JL, Neufeld K, Leonard M, Bellelli G, Davis D, Teodorczuk A, Kreisel S, Thomas C, Hasemann W, Timmons S, O'Regan N, Grover S, Jabbar F, Cullen W, Dunne C, Kamholz B, Van Munster BC, De Rooij SE, De Jonghe J, Trzepacz PT. Concordance between DSM-IV and DSM-5 criteria for delirium diagnosis in a pooled database of 768 prospectively evaluated patients using the delirium rating scale-revised-98. BMC Med. 2014;12:164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 48. | Adamis D, Rooney S, Meagher D, Mulligan O, McCarthy G. A comparison of delirium diagnosis in elderly medical inpatients using the CAM, DRS-R98, DSM-IV and DSM-5 criteria. Int Psychogeriatr. 2015;27:883-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 49. | Giustina A, Veldhuis JD. Pathophysiology of the neuroregulation of growth hormone secretion in experimental animals and the human. Endocr Rev. 1998;19:717-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 278] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 50. | Khorram O. Use of growth hormone and growth hormone secretagogues in aging: help or harm. Clin Obstet Gynecol. 2001;44:893-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 51. | Arwert LI, Veltman DJ, Deijen JB, van Dam PS, Drent ML. Effects of growth hormone substitution therapy on cognitive functioning in growth hormone deficient patients: a functional MRI study. Neuroendocrinology. 2006;83:12-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 52. | Gluckman PD, Guan J, Williams C, Scheepens A, Zhang R, Bennet L, Gunn A. Asphyxial brain injury--the role of the IGF system. Mol Cell Endocrinol. 1998;140:95-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 62] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 53. | Saatman KE, Contreras PC, Smith DH, Raghupathi R, McDermott KL, Fernandez SC, Sanderson KL, Voddi M, McIntosh TK. Insulin-like growth factor-1 (IGF-1) improves both neurological motor and cognitive outcome following experimental brain injury. Exp Neurol. 1997;147:418-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 143] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 54. | Lackey BR, Gray SL, Henricks DM. Actions and interactions of the IGF system in Alzheimer's disease: review and hypotheses. Growth Horm IGF Res. 2000;10:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 55. | Mesotten D, Van den Berghe G. Changes within the growth hormone/insulin-like growth factor I/IGF binding protein axis during critical illness. Endocrinol Metab Clin North Am. 2006;35:793-805, ix-ix. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: Irish Medical Council, No. 406822; and Royal Statistical Society, No. 020637.
Specialty type: Psychiatry
Country/Territory of origin: Ireland
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hosak L, Shiina A S-Editor: Ma YJ L-Editor: A P-Editor: Li JH