Published online Jul 19, 2020. doi: 10.5498/wjp.v10.i7.162
Peer-review started: February 13, 2020
First decision: April 3, 2020
Revised: May 25, 2020
Accepted: June 10, 2020
Article in press: June 10, 2020
Published online: July 19, 2020
Processing time: 154 Days and 13.1 Hours
Alzheimer’s disease (AD) is among the most prevalent forms of dementia in the world and neuropathological studies suggest similar high prevalence of mixed (AD + vascular) dementias. Approximately 25%-50% of individuals with AD develop psychosis sometime during their illness. The presence of psychosis in AD worsens outcomes. Currently there are no United States Food and Drug Administration (FDA) approved medications for the treatment of psychosis in AD. Pimavanserin, a novel atypical antipsychotic medication, was approved by the FDA for the treatment of hallucinations and delusions associated with Parkinson disease psychosis and is currently in clinical trials for the treatment of psychosis in AD.
To evaluate the existing literature regarding the use of pimavanserin for treating psychosis among individuals with AD.
A literature review of clinical studies of pimavanserin treatment for psychosis in individuals with AD was performed using the Preferred Reporting Items for Systematic Review and Meta-Analysis guidelines. Trials were identified by systematically searching PubMed, MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials, Web of Science, and Scopus through October 2019. The 5-point Jadad scoring system was used to assess the methodologic quality of the randomized placebo-controlled trials.
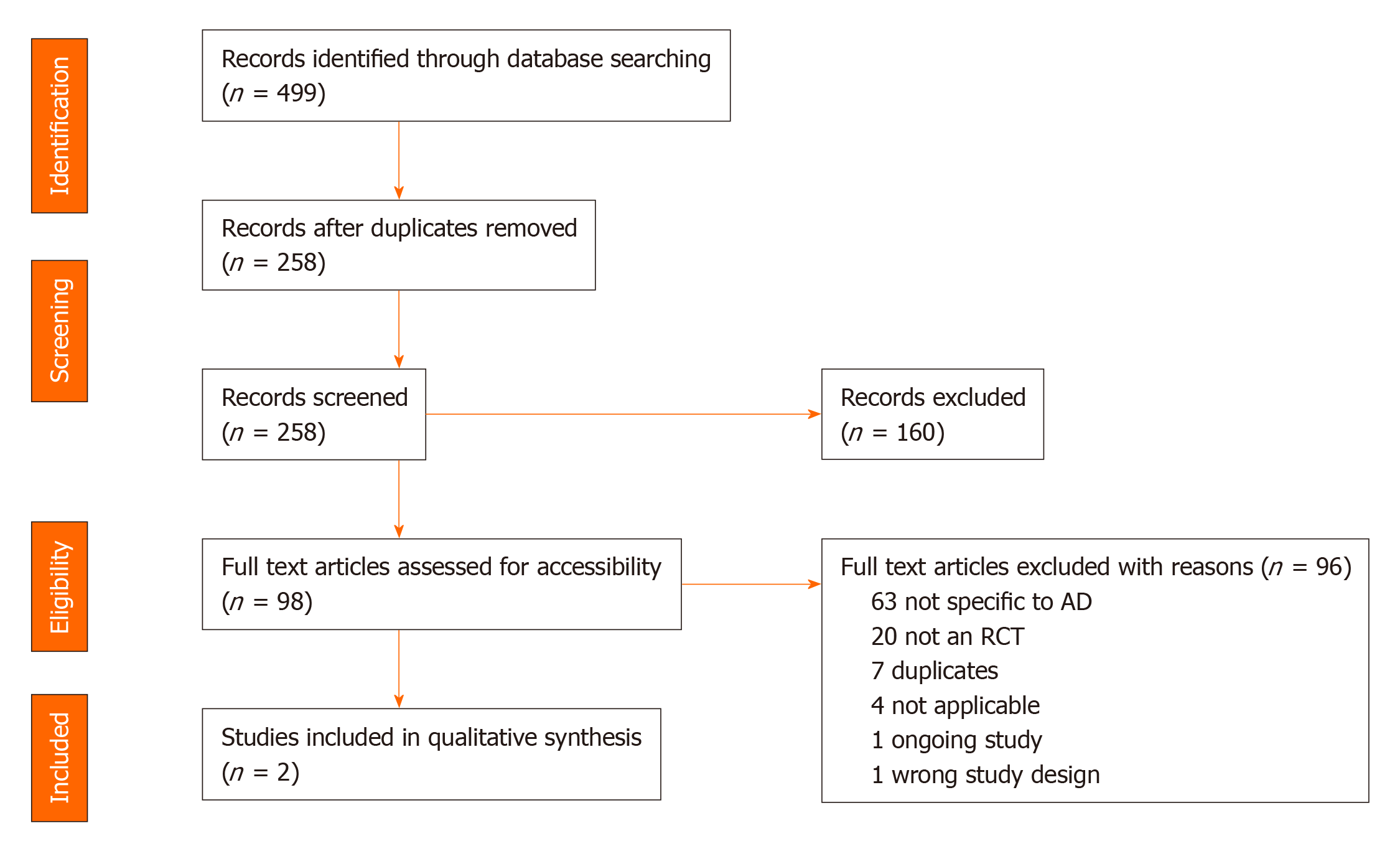
A total of 499 citations were retrieved and pooled in EndNote and de-duplicated to 258 citations. This set was uploaded to Covidence for screening. Two separate screeners (Srinivasan S and Tampi RR) evaluated the titles, abstracts, and full text of eligible articles. Of the identified 258 abstracts, 98 articles underwent full text review and 2 publications from 1 randomized controlled trial (RCT) were included in the final analysis. The quality of evidence was assessed to be of good methodologic quality, scoring 4 out of 5 using the 5-point Jadad questionnaire with the Jadad Scoring calculation. This systematic review found only one RCT that evaluated the use of pimavanserin for the treatment of psychosis among individuals with AD. This phase 2 trial resulted in two publications, the second of which was a subgroup analysis from the original study. The evidence from these two publications showed that pimavanserin improves psychotic symptoms among individuals with AD when compared to placebo at week 6.
Pimavanserin may be a pharmacologic consideration for the treatment for psychosis in AD. Additional RCTs are needed to assess the evidence of effectiveness before pimavanserin is considered a standard treatment.
Core tip: This systematic review was conducted to evaluate the evidence from randomized controlled clinical trials of pimavanserin for the treatment of psychosis in individuals with Alzheimer’s disease (AD). Behavioral disturbances including psychosis are prevalent in AD, and have a significant impact on management and outcomes. There are currently no United States Food and Drug Administration approved medications for the management of behavioral disturbance in AD. Based on the findings of our systematic review, pimavanserin, a novel atypical antipsychotic, may be a new pharmacologic consideration for treating psychosis in individuals with AD.
- Citation: Srinivasan S, Tampi RR, Balaram K, Kapoor A. Pimavanserin for the treatment of psychosis in Alzheimer’s disease: A literature review. World J Psychiatr 2020; 10(7): 162-174
- URL: https://www.wjgnet.com/2220-3206/full/v10/i7/162.htm
- DOI: https://dx.doi.org/10.5498/wjp.v10.i7.162
Alzheimer’s disease (AD) is among the most prevalent form of dementia, affecting over two-thirds of the 50 million individuals living with dementia globally[1]. The combination of AD and vascular processes, also known as mixed dementia appears to also have a high prevalence according to neuropathological studies. Furthermore, overlap in clinical presentations can blur the distinction between AD and vascular dementia[2]. While cognitive impairment is a prominent clinical manifestation of AD, behavioral changes, also known as behavioral and psychological symptoms of dementia (BPSD) frequently occur. BPSD can include agitation, aggression, anxiety, depressed mood, apathy, and psychosis (delusions, hallucinations, paranoia)[3]. Between 25%-50% of individuals with AD develop psychosis, with variability in course, duration, and severity of psychotic symptoms[4,5]. The impact of psychosis is far-reaching, affecting individuals and caregivers and is associated with higher rates of cognitive and functional decline, earlier time to institutionalization, higher treatment mortality, and caregiver burden respectively[6,7].
Available data indicates that psychosis in dementia occurs due to anatomical and biochemical changes within the brain[8]. Dysfunction of the adrenergic and serotonergic systems may also contribute to the behavioral symptoms of dementia[9]. Higher levels of norepinephrine in the substantia nigra and lower levels of serotonin in the presubiculum have been noted among individuals with psychosis in dementia when compared to non-psychotic individuals with dementia[10,11]. Neuropathologic changes that contribute to psychosis include the presence of neuritic plaques and tangles in the frontal and temporal cortices of these individuals[10,12,13]. Metabolic and perfusion imaging studies have demonstrated that psychosis in dementia correlates well with frontal, temporal and parietal lobe dysfunction[14-18]. A study of delusional misidentification symptoms (DMS) among individuals with AD found that individuals with DMS showed increased electroencephalograph delta-power over the right hemisphere and their computed tomography scans showed more severe right frontal lobe atrophy, and the number of their pyramidal cells in area CA1 was lower than in the patients without DMS[19]. There is also growing evidence that psychosis among individuals is higher among individuals who have relatives with AD and psychosis[20,21]. Psychosis is also more common among individuals with APOE3/4 genotype with more than threefold increase in the signs of depression and psychosis when compared with individuals with APOE 3/3 genotype or to control subjects[22]. Data also indicates that 5-HT2A receptor polymorphism 102-T/C and the 5-HT2C receptor polymorphism Cys23Ser are associated with the development of visual and auditory hallucinations among individuals with AD[23,24]. A study examining the association between selected polymorphisms in the dopamine receptor genes DRD1, DRD2, DRD3 and DRD4 and the presence of psychosis or aggressive behavior among individuals with AD found that psychosis and aggression were both significantly more frequent among the DRD1 B2/B2 homozygotes (P < 0.02), while psychosis was significantly more frequent in DRD3 1/1 or 2/2 homozygotes (P < 0.05)[25]. Another study found an association between the presence of psychotic symptoms and aggressive behavior and the DRD1 polymorphism and between the presence of psychosis, but not aggression and the DRD3 polymorphism[26]. Carriers of the DRD1 B2 allele were more likely to be aggressive or experience hallucinations whereas homozygous carriers of the DRD3 1 allele were more likely to experience delusions. These studies indicate that BPSD develops as a byproduct of the neurodegenerative disease process that manifests after a certain period, when the genetic factors assume greater significance in the brain[8].
Although non-pharmacological interventions are considered first line for BPSD, pharmacologic interventions may be warranted particularly when psychotic symptoms pose a potential threat to the individual or caregivers[27,28]. In the United States, there are currently no Federal Drug Administration (FDA)- approved medications for the treatment of psychosis in AD. While antipsychotic medications have been used to treat BPSD in individuals with AD, efficacy rates from randomized clinical trials have been shown to be modest[29,30]. In a systematic review conducted by Tampi et al[30] of 16 published meta-analyses evaluating antipsychotics in individuals with dementia, antipsychotics (convention and second generation/atypical) were found to demonstrate modest efficacy for the treatment of psychosis, agitation and aggression. However, significant limitations from an adverse effect profile were notable. The safety profile of antipsychotic medications must be considered, as they are associated with increased risk for death, cerebrovascular adverse events, sedation, falls, and pneumonia[29-32]. With these significant safety risk concerns, warnings regarding the use of antipsychotics in individuals with dementia-related psychosis have been issued by the United States FDA, the European Medicines Agency, and the United Kingdom Medicines and Healthcare Products Regulatory Agency[32]. Other pharmacologic agents, including cognitive enhancers (acetylcholinesterase inhibitors, memantine), glutamate modulators (dextromethorphan/quinidine), antidepressants and hormonal agents have been studied for agitation and aggression in AD with varying results and limited utility[7]. However, off-label use of antipsychotics, for dementia-related psychosis continues across different healthcare settings[33,34]. As a result, effective and safe pharmacological treatment of psychosis in AD remains an ongoing need.
Pimavanserin, an atypical antipsychotic medication, is a novel selective 5-HT2A inverse agonist with a low affinity for 5-HT2C receptors. Pimavanserin does not demonstrate clinically significant affinity to dopaminergic, histaminergic, muscarinic, or adrenergic receptors[35]. Subsequent to the results of a placebo-controlled six-week clinical trial, pimavanserin received FDA approval in 2016 for the treatment of hallucinations and delusions associated with Parkinson disease psychosis (PDP)[36,37].
There is emerging evidence that pimavanserin is being trialed for the treatment of dementia-related psychosis[37]. Although published reviews have examined the benefits and risks of antipsychotics for the treatment of psychosis in dementia, to our knowledge, this is the first review that systematically evaluates the evidence from literature on the use of pimavanserin for psychosis in AD from randomized controlled trials (RCTs). In accordance with PICOS format (participants, interventions, comparisons, outcomes, and study design), all identified studies of RCTs in adult patients with AD who received pimavanserin for the treatment of psychosis in AD were included.
A systematic literature search of clinical trials of pimavanserin was performed in accordance with the preferred reporting items for systematic reviews and meta-analyses guidelines[38]. Our study protocol was registered in the PROSPERO database of systematic reviews[39]. All identified studies of RCTs in adult patients with AD who received pimavanserin for the treatment of psychosis in AD were included. Studies of pimavanserin for the treatment of schizophrenia, dementia due to other etiologies, PDP, mood disorders, or other conditions were excluded. Literature involving nonhuman studies was also excluded.
We began our search with the Yale MeSH Analyzer (mesh.med.yale.edu), using key articles to refine the search strategy for the term pimavanserin. In each database we ran scoping queries followed by iterative refinement of the search strategy. Additional articles were identified by examining other systematic reviews, bibliographies, and pre-identified websites such as clinicaltrials.gov and publicly available internet searches (Google Scholar).
Literature searches were performed in the following databases from inception to October 25, 2019: PubMed, MEDLINE (Ovid), EMBASE (Ovid), Cochrane Central Register of Controlled Trials (Wiley), Web of Science Core Collection (Clarivate), and Scopus (Elsevier). No date or language restrictions were applied.
The databases were searched using both controlled vocabulary words and synonymous free text words for pimavanserin. The Cochrane highly sensitive search strategy was used to identify randomized trials in PubMed and Ovid databases[40]. The Cochrane RCT search strategy was adapted to identify trials in other electronic databases[41]. The search strategies were adjusted for the syntax appropriate for each database/platform. See supplementary material for MEDLINE search strategy (Supplementary Material).
The search retrieved a total of 499 references, which were pooled in EndNote and de-duplicated. This set was uploaded to Covidence for screening, which identified additional duplicates, leaving 258 for screening[42]. Two separate screeners (Srinivasan S and Tampi RR) evaluated the titles, abstracts, and full text of eligible articles. Of the identified 258 abstracts, 98 articles underwent full text review (Figure 1).
A total of two publications of pimavanserin among individuals with psychosis due to AD were identified and reviewed. Both were rated as being of good methodologic quality based on the five-point Jadad Score calculation[43]. This calculation assigns two points for randomization (one point for randomization and one point for description of system used to generate sequence of randomization), two points for blinding (description of, and method of blinding), and one point for description of withdrawals (Table 1). There was no funding for this review.
| Jadad score calculation | |
| Item | Score (Yes = 1) |
| Was the study described as randomized | 0/1 |
| Was the method used to generate sequence of randomization described and appropriate? | 0/1 |
| Was the study described as double-blind? | 0/1 |
| Was the method of blinding described and appropriate? | 0/1 |
| Was there a description of withdrawals and dropouts? | 0/1 |
We identified and reviewed a total of two publications of pimavanserin for the treatment of psychosis in individuals with AD from a single trial. The trial used the aforementioned Jadad score calculation and was rated as being of good methodological quality (score of 4/5). The publications are described individually below and a brief summary of both publications is outlined in Table 2.
| Ref. | Year | Number of participants | Age (yr) | Setting | Comparators | Duration |
| Ballard et al[44] | 2018 | 181 (pimavanserin n = 90; placebo n = 91) | ≥ 50 | Nursing homes | 17 mg × 2 tablets pimavanserin vs placebo (2 tablets) | 12 wk |
| Ballard et al[45] | 2019 | 181 (pimavanserin n = 90; placebo n = 91) | ≥ 50 | Nursing homes | 34 mg pimavanserin vs placebo | 12 wk |
The 2018 study by Ballard et al[44] was a phase 2, randomized, double-blind, placebo-controlled single center trial. This study was conducted at nursing homes across the United Kingdom. Pimavanserin was compared to placebo in individuals with possible or probable AD and psychotic symptoms (visual or auditory hallucinations, delusions or both). This trial was 12 wk in duration and participants were aged 50 years or older. After completing screening and baseline evaluations, trial participants were randomly assigned in a 1:1 ratio to receive either pimavanserin or placebo. Pimavanserin dosing was two 17 mg tablets daily and placebo was also dosed as two tablets daily. Participants had to have resided in the nursing home for at least 4 wk in order to be eligible for the study. The degree of psychotic symptoms meeting eligibility criteria included clinical severity warranting antipsychotic treatment, and participant score of ≥ 4 for frequency × severity of hallucinations or delusions domains of the Neuropsychiatric Inventory-Nursing Home version (NPI-NH) psychosis scale, or a total combined score of ≥ 6 for hallucinations and delusions. Groups were stratified based on baseline Mini-Mental State Examination (MMSE) total score of ≤ 6 or ≥ 6, and NPI-NH psychosis score of ≤ 12 or ≥ 12. Following screening, brief psychosocial therapy therapists evaluated participants during a 3-wk period and only individuals determined to require pharmacologic intervention progressed to study randomization, in an effort to minimize placebo response. This 3-wk period also served as a washout phase for participants who were taking antipsychotic medications.
After progressing through screening, individuals who met all study eligibility criteria at baseline (day 1) received a single dose of either pimavanserin (two 17 mg tablets) or placebo (two tablets). Participants continued to receive this regimen daily for 12 wk. Study visits were conducted at 2-wk intervals following baseline (day 1): days 15, 29, 43, 64, and 85, or at early termination. A telephone follow-up visit after the last dose of study medication was conducted for safety.
The primary outcome of this study was efficacy of pimavanserin vs placebo based on a change from baseline to week 6 in the NPI-NH psychosis score (hallucinations + delusions). Additional correlation analyses at week 6 included NPI-NH total score, NPI-NH agitation/aggression. AD Cooperative Study- Clinical Global Impression of Change (ADCS-CGIC), AD Cooperative Study- Activities of Daily Living (ADCS-ADL) total score, and the Cohen-Mansfield Agitation Inventory- Short Form (CMAI-SF) total score[45-48]. The assessment of behavioral symptoms at 6 wk and 12 wk constituted the secondary outcomes assessment. The ADCS-CGIC, NPI-NH agitation/aggression and sleep and nighttime domains, and the CMAI-SF total and subdomain scores were used as a measure of agitation. Additional subgroup analyses focused on stratified NPI- NH scores (< 12 or ≥ 12), baseline MMSE (< 6 or ≥ 6), sex, age (≤ 85 years or > 85 years). As well as for concomitant use of anti-dementia medication, selective serotonin reuptake inhibitor, and previous antipsychotic use. The MMSE was used to assess cognitive impairment, and the 1987 Unified Parkinson’s Disease Rating Scale (UPDRS) Part III was used to measure extrapyramidal symptoms[49,50].
All study participants who received pimavanserin or placebo were included in the safety analysis, with safety outcomes measured over 12 wk. An adverse event checklist (all reported adverse events, those leading to study discontinuation, serious adverse events, and mortality), physical examinations, vital signs and electrocardiogram, and laboratory tests were also conducted.
Out of 345 screened participants, 181 were randomized to receive pimavanserin (n = 90) vs placebo (n = 91). Three participants in the pimavanserin group did not have a post-baseline NPI-NH score, and were excluded from the analysis. Of the 178 participants who were included, 160 completed 6 wk of treatment and 140 completed 12 wk. Of 26% of participants (n = 23) in the pimavanserin group and 20% of those (n = 18) in the placebo group withdrew over the 12-wk study period. The mean MMSE score was 10.3 in the pimavanserin group and 9.8 in the placebo group.
More women (80%-82%) than men and white individuals were represented among study participants. The mean age (years) in both groups was balanced (85.6 years in the pimavanserin group and 86.1 years in the placebo group). More study participants had an NPI-NH psychosis score < 12 (60%-61%). The mean MMSE score was 10.3 in the pimavanserin group and 9.8 in the placebo group.
The investigators noted that the improvement in the primary outcome (change in NPI-NH psychosis score) at week 6 was higher in the pimavanserin group (39.5% reduction) vs the placebo group (19.3% reduction). They did not observe any statistically significant differences between pimavanserin and placebo for the additional correlational analyses at week 6 or at week 12. Although not statistically significant (P = 0.063), a numerical difference of 5 points in the NPI-NH total score was observed for pimavanserin compared with placebo at week 6.
In regard to discontinuation due to adverse events, the investigators reported a 9% dropout rate (n = 8 of 90) in the pimavanserin group and 12% (n = 11 of 91) in the placebo group. Falls, urinary tract infection, and agitation were the most common adverse events across both treatment groups. While the frequency of falls (23%) and urinary tract infections (22% vs 28%) were similar across both treatment groups, more participants receiving pimavanserin experienced agitation compared to placebo. Agitation (21% vs 14%), aggression (10% vs 4%), and peripheral edema (8% vs 2%) were more prevalent in the pimavanserin treated group than with placebo. The investigators also did not note differences in vital signs, clinical laboratory results or physical examinations between groups. There were no discontinuations due to QTc prolongation. Furthermore, the investigators found no evidence of decline in cognition, function, global outcome, or motor symptoms over the 12-wk study period.
A summary of the findings from the 2018 Ballard et al[44] study, adverse events, and strengths/limitations are included in Table 3.
| Ref. | Outcomes | Tolerability | Limitations |
| Ballard et al[44], 2018 | Primary outcome: At week 6: (1) Significant improvement in NPI-NH psychosis score (mean change was -3.76 points (SE 0.65) for pimavanserin group and -1.93 points (0.63) for placebo (mean difference -1.84 [95%CI: -3.64, -0.04]; P = 0.045) without negative effects on cognition or motor function; (2) Response (≥ 30% improvement) in 55% (pimavanserin) vs 37% (placebo); and (3) In NPI-NH < 12 subgroup: Mean change of the score from baseline to week 6 was -0.58 (95%CI: -2.10, 0.95) for pimavanserin vs -0.16 (-1.60 to 1.28) for placebo [mean difference -0.42 (95%CI: -2.52, 1.68)], Cohen’s d = –0.77; P = 0.694. At week 12: No significant advantage for pimavanserin vs placebo was observed for the overall study population [treatment difference -0.51 (95%CI: -2.23, 1.21); P = 0.561]. Secondary outcome: At weeks 6 and 12: No significant differences between placebo and pimavanserin for ADCS–CGIC, NPI–NH agitation/aggression, NPI–NH sleep and nighttime behavior disorders, and CMAI–SF | Adverse events (pimavanserin vs placebo). Most common: (1) Agitation (21% vs 14%); (2) Aggression (10% vs 4%); (3) Falls (21% vs 21%); (4) Urinary tract infection (20% vs 25%); and (5) Peripheral edema (8% vs 2%). Less common: (1) Weight loss (-0.7 kg vs -0.1 kg); (2) QTc prolongation (9.4 ms vs -0.2 ms); and (3) Death (4 vs 4) | Limitations: (1) Study was not sufficiently powered to control for secondary outcomes; (2) Limited number of participants in severe psychosis subcategory or prior history of antipsychotic use; (3) Biomarker confirmation for patients diagnosed with Alzheimer’s was not possible in the nursing home patients; (4) Possibility of trial participants inclusion of non-Alzheimer’s type and mixed dementia; (5) High attrition rate: 26% (pimavanserin) and 20% (placebo); and (6) Absence of active comparator to assess efficacy/tolerability between pimavanserin and other antipsychotics. Strengths: (1) Rigorous diagnosis of psychosis – high completion rate at week 12; (2) Participants assessed in the community care homes providing access to the elderly population to be included in the clinical study; and (3) Researchers were able to study frail, elderly participants in their “natural” environment |
This paper describes the outcomes of efficacy and tolerability of pimavanserin vs placebo in a subgroup of patients with severe psychosis associated with AD. Participants were part of the Phase 2 study summarized above and reported by Ballard et al[44] in 2018. The severity of psychosis was quantified by a cut-off score of ≥ 12 on the NPI-NH psychosis score. Participants in this subgroup analysis were nursing home residents with a baseline NPI-NH-NS score of ≥ 12, who were randomized to receive pimavanserin 34 mg or placebo daily over a 12-wk period. The primary endpoint at week 6 was the mean change from baseline on the NPI-NH-PS score. In addition, the investigators performed responder analyses, which was described as the observed proportions of individuals with an improvement from baseline at week 6. The investigators ascribed any missing values as non-responders.
The subgroup comprised of 57 participants (pimavanserin n = 27; placebo n = 30). Over 80% of participants in both pimavanserin and placebo groups were women. The average age range of participants was 85 years. A minority of participants in both groups had prior antipsychotic treatment (11.1% and 13.1%, pimavanserin vs placebo respectively). MMSE scores were similar across both pimavanserin and placebo subgroups (8.6 and 9.2 respectively) but were lower than in the overall study population described in the previous section.
In this subgroup, mean baseline NPI-NH psychosis scores were 15.3 (pimavanserin group) and 16.7 (placebo group). There was a statistically significant change in NPI-NH psychosis scores for pimavanserin vs placebo. Furthermore, the investigators reported that of 81% of study participants who had both hallucinations and delusions at baseline, pimavanserin was superior to placebo in treating these symptoms, with statistically significant improvement noted at week 6 for both domain scores (P = 0.046 for NPI-NH hallucinations, and P = 0.034 for NPI-NH delusions). While two-thirds of pimavanserin treated study participants had improvements in their NPI-NH psychosis score to < 6 (vs 32 % of placebo treated individuals) at week 6, 45.5% of both groups demonstrated this drop in NPI-NH score at week 12.
From a tolerability standpoint, there were no significant differences in the incidence of adverse events in the subgroup compared to the study population overall. Agitation, urinary tract infections and falls were the most common adverse events in the pimavanserin treated cohort. Over the 12-wk study period, the investigators noted that the change in MMSE score from baseline for the overall population across both treatment groups was minimal.
A summary of the findings from the 2019 Ballard et al[45] subgroup analysis, adverse events, and strengths/limitations are included in Table 4.
| Ref. | Outcomes | Tolerability | Limitations |
| Ballard et al[45], 2019 | For overall population: Adjusted mean change from baseline at week 6 (adjusted mean, MMRM analysis) for the NPI-NH psychosis score was -3.76 (0.65) for pimavanserin vs -1.93 (0.63) for placebo (delta = -1.84, 95% confidence interval (CI) [-3.64, -0.04], Cohen’s d = -0.32, P = 0.045); For patients with NPI-NH scores > 12: The mean change at week 6 was -10.15 (95%CI: -12.50, -7.80) for pimavanserin vs -5.72 (95%CI: -8.14, -3.30) for placebo (delta = -4.43 (95%CI: -7.81, -1.04), Cohen’s d effect size of -0.73, P = 0.011); In the more severe subgroup, pimavanserin was superior to placebo at week 6 in treating both hallucinations (P = 0.046) and delusions (P = 0.034); At week 6, 66.7% of those in the pimavanserin group improved to an NPI-NH psychosis score < 6 vs 32.0% of those in the placebo group (difference = 34.7%); At week 12, 45.5% of both pimavanserin and placebo-treated patients had an NPI-NH psychosis score < 6; The proportion with a baseline NPI-NH psychosis score ≥ 12 achieving a response was significantly (P < 0.05) greater with pimavanserin vs placebo | Incidence of aggression was 14.3% in the severe psychosis subgroup vs 10.0% in overall population; Incidence of agitation was 17.9% in severe subgroup and 21.1% in general population; Other side effects included falls, UTI, contusion, respiratory tract infections, anemia, edema, cellulitis, anxiety, increase in urea or potassium | Small sample size in subgroup; Subgroup analysis was secondary |
| 1 | 1 | 1 | 1 |
This review indicates that there is only one RCT that evaluated the use of pimavanserin for the treatment of psychosis among individuals with AD. This phase 2 trial resulted in two publications, the second of which was a subgroup analysis from the original study. The RCT was rated as having good methodological quality, scoring 4/5 on the Jadad score calculation. The evidence from these two publications indicates that pimavanserin improves psychotic symptoms among individuals with AD when compared to placebo at week 6. Additionally, among individuals with more severe psychotic symptoms (NPI-NH-NS score of ≥ 12), those individuals who were treated with pimavanserin had better outcomes than individuals receiving placebo. Pimavanserin was well tolerated in the study with the discontinuation rate due to adverse events being 9% in the pimavanserin group vs 12% in the placebo group. The investigators reported falls, urinary tract infection and agitation as the most common adverse events across both treatment groups with the frequency of falls (23%) and urinary tract infections (22% vs 28%) being similar across the treatment groups. Agitation (21% vs 14%), aggression (10% vs 4%) and peripheral edema (8% vs 2%) were more prevalent in the pimavanserin group when compared to placebo. The investigators also did not note any differences in vital signs, clinical laboratory results or physical examinations between the two groups. There were no discontinuations due to QTc prolongation. Additionally, treatment with pimavanserin did not result in decline in cognition, function, global outcome or motor symptoms over the 12-wk study period.
The strength of this review was the extensive review of multiple databases without any language restrictions. The limitation was that we restricted our review to only RCTs on the use of pimavanserin for the treatment of psychosis among individuals with AD. A review of clinicaltrials.gov found the SERENE study, conducted between 2016-2018 to evaluate the efficacy of pimavanserin 20 mg and 34 mg) vs placebo in the treatment of agitation and aggression in individuals with AD over 12 wk[51]. The primary outcome measure was change in the Cohen-Mansfield Agitation Inventory, while the secondary outcome measure was the Zarit Burden Interview, a measure of dementia caregiver stress[52]. While the original study design aimed to recruit 432 participants, 111 were randomized before recruitment was halted for business reasons. The investigators noted that the final study with 111 participants was no longer sufficiently powered to detect treatment effect.
Our review only found one RCT that met our inclusion criteria. The limitations of the study that we included in review were as follows: It was not sufficiently powered to control for secondary outcomes, there were limited number of participants in the severe psychosis subcategory or with a prior history of antipsychotic use, there were no biomarker confirmation for individuals with a diagnosis of AD who were living in the nursing homes and a high attrition rate among the pimavanserin (26%) and placebo (20%) groups. There strengths of the study were that there was a stringent criteria for the diagnosis of psychosis and there was participation from frail older adults living in community care homes in the study.
In a meta-analysis, Yasue et al[53] found that pimavanserin reduced the symptoms of hallucinations and delusions when compared to placebo [weighted mean differences (WMD) = -2.26, P = 0.005] among individuals with PDP. Additionally, pimavanserin was found to be superior to placebo in improving symptoms of hallucinations (WMD = -2.15, P = 0.001) and delusions (WMD = -1.32, P = 0.010) when considered independently. The authors did not find any significant difference between pimavanserin and placebo on the all-cause discontinuation rates for adverse events, death, Parkinson motor symptoms and the incidence of individual adverse events. Additionally, pimavanserin was found to be associated with less orthostatic hypotension when compared to placebo (risk ratio = 0.33, P = 0.008, number needed to harm = 17, P = 0.01). The investigators concluded that pimavanserin is beneficial for the treatment of symptoms of PDP and is well tolerated.
The data published by Institute for Safe Medication Practices indicated that there were a total 2236 adverse events reported from the use of pimavanserin in the 12 mo post-marketing observation period that ended March 2017, with hallucinations 487 (21.8%) drug ineffectiveness 333 (14.9%), confused state 258 (11.5%) and death 244 (10.9%) being the most commonly adverse events[54]. The United States FDA post-marketing review did not identify any new or unexpected safety findings with the use of pimavanserin[55]. In addition, the FDA did not find information that was inconsistent with the established safety profile for the drug concluding that the drug’s benefits outweigh its risks among individuals with PDP reporting hallucinations and delusions.
In conclusion, the data is limited given only one published RCT to date has examined the use of pimavanserin for the treatment of psychosis among individuals with AD. While evidence from this study suggests pimavanserin is effective and tolerated, more rigorous trials are needed to establish evidence of effectiveness. However, given the identified risks of using antipsychotics among individuals with dementia, caution should be advised when using newer antipsychotic medications among individuals with dementia[56]. Additional larger studies of longer duration of treatment with positive outcomes will be needed prior to pimavanserin being adopted as a standard treatment option among individuals with AD who have psychosis.
Alzheimer’s disease (AD) is among the most prevalent forms of dementia in the world. Approximately 25%-50% of individuals with AD develop psychosis sometime during their illness. The presence of psychosis in AD worsens outcomes. Currently there are no United States Food and Drug Administration (FDA) approved medications for the treatment of psychosis in AD.
Pimavanserin, a novel atypical antipsychotic medication, was approved by the FDA for the treatment of hallucinations and delusions associated with Parkinson disease psychosis and is currently in clinical trials for the treatment of psychosis in AD.
This review evaluates the existing literature regarding the use of pimavanserin to treat psychosis among individuals with AD.
A literature review of clinical studies of pimavanserin treatment for psychosis in individuals with AD was performed using the preferred reporting items for systematic review and meta-analysis guidelines. Trials were identified by systematically searching PubMed, MEDLINE, EMBASE, Cochrane Central Register of Controlled Trials, Web of Science, and Scopus through October 2019. The 5-point Jadad scoring system was used to assess the methodologic quality of the randomized placebo-controlled trials.
This systematic review found only one randomized controlled trial (RCT) that evaluated the use of pimavanserin for the treatment of psychosis among individuals with AD. This phase 2 trial resulted in two publications, the second of which was a subgroup analysis from the original study. The evidence from these two publications showed that pimavanserin improves psychotic symptoms among individuals with AD when compared to placebo at week 6.
Limited evidence indicates that pimavanserin may be a pharmacologic consideration for the treatment for psychosis in AD. Additional RCTs are needed to assess the evidence of effectiveness before pimavanserin is considered a standard treatment.
Additional RCTs are needed to assess the evidence of effectiveness before pimavanserin would be considered a standard treatment for psychosis in AD.
The authors would like to gratefully acknowledge Funaro MC (MS, MLS) for her assistance with the literature and database search for this scientific review.
| 1. | Alzheimer’s disease International. World Alzheimer’s Report 2018. [published September. 2018;cited 24 May 2020] Available from: https://www.alz.co.uk/research/world-report-2018. |
| 2. | Custodio N, Montesinos R, Lira D, Herrera-Pérez E, Bardales Y, Valeriano-Lorenzo L. Mixed dementia: A review of the evidence. Dement Neuropsychol. 2017;11:364-370. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 89] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 3. | Steinberg M, Shao H, Zandi P, Lyketsos CG, Welsh-Bohmer KA, Norton MC, Breitner JC, Steffens DC, Tschanz JT; Cache County Investigators. Point and 5-year period prevalence of neuropsychiatric symptoms in dementia: the Cache County Study. Int J Geriatr Psychiatry. 2008;23:170-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 563] [Cited by in RCA: 538] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 4. | Murray PS, Kumar S, Demichele-Sweet MA, Sweet RA. Psychosis in Alzheimer's disease. Biol Psychiatry. 2014;75:542-552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 148] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 5. | Zhao QF, Tan L, Wang HF, Jiang T, Tan MS, Tan L, Xu W, Li JQ, Wang J, Lai TJ, Yu JT. The prevalence of neuropsychiatric symptoms in Alzheimer's disease: Systematic review and meta-analysis. J Affect Disord. 2016;190:264-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 699] [Cited by in RCA: 634] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 6. | Zahodne LB, Ornstein K, Cosentino S, Devanand DP, Stern Y. Longitudinal relationships between Alzheimer disease progression and psychosis, depressed mood, and agitation/aggression. Am J Geriatr Psychiatry. 2015;23:130-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 107] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 7. | Porsteinsson AP, Antonsdottir IM. An update on the advancements in the treatment of agitation in Alzheimer's disease. Expert Opin Pharmacother. 2017;18:611-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 8. | McIlroy S, Craig D. Neurobiology and genetics of behavioural syndromes of Alzheimer's disease. Curr Alzheimer Res. 2004;1:135-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Cummings JL, Back C. The cholinergic hypothesis of neuropsychiatric symptoms in Alzheimer's disease. Am J Geriatr Psychiatry. 1998;6:S64-S78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 150] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 10. | Zubenko GS, Moossy J, Martinez AJ, Rao G, Claassen D, Rosen J, Kopp U. Neuropathologic and neurochemical correlates of psychosis in primary dementia. Arch Neurol. 1991;48:619-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 169] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 11. | Victoroff J, Zarow C, Mack WJ, Hsu E, Chui HC. Physical aggression is associated with preservation of substantia nigra pars compacta in Alzheimer disease. Arch Neurol. 1996;53:428-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Farber NB, Rubin EH, Newcomer JW, Kinscherf DA, Miller JP, Morris JC, Olney JW, McKeel DW. Increased neocortical neurofibrillary tangle density in subjects with Alzheimer disease and psychosis. Arch Gen Psychiatry. 2000;57:1165-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 124] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Tekin S, Mega MS, Masterman DM, Chow T, Garakian J, Vinters HV, Cummings JL. Orbitofrontal and anterior cingulate cortex neurofibrillary tangle burden is associated with agitation in Alzheimer disease. Ann Neurol. 2001;49:355-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 195] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 14. | Kotrla KJ, Chacko RC, Harper RG, Doody R. Clinical variables associated with psychosis in Alzheimer's disease. Am J Psychiatry. 1995;152:1377-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 37] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 15. | Starkstein SE, Vázquez S, Petracca G, Sabe L, Migliorelli R, Tesón A, Leiguarda R. A SPECT study of delusions in Alzheimer's disease. Neurology. 1994;44:2055-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 64] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 16. | Mentis MJ, Weinstein EA, Horwitz B, McIntosh AR, Pietrini P, Alexander GE, Furey M, Murphy DG. Abnormal brain glucose metabolism in the delusional misidentification syndromes: a positron emission tomography study in Alzheimer disease. Biol Psychiatry. 1995;38:438-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 78] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 17. | Sultzer DL, Mahler ME, Mandelkern MA, Cummings JL, Van Gorp WG, Hinkin CH, Berisford MA. The relationship between psychiatric symptoms and regional cortical metabolism in Alzheimer's disease. J Neuropsychiatry Clin Neurosci. 1995;7:476-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 112] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 18. | Hirono N, Mori E, Ishii K, Kitagaki H, Sasaki M, Ikejiri Y, Imamura T, Shimomura T, Ikeda M, Yamashita H. Alteration of regional cerebral glucose utilization with delusions in Alzheimer's disease. J Neuropsychiatry Clin Neurosci. 1998;10:433-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Förstl H, Besthorn C, Burns A, Geiger-Kabisch C, Levy R, Sattel A. Delusional misidentification in Alzheimer's disease: a summary of clinical and biological aspects. Psychopathology. 1994;27:194-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 56] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Sweet RA, Nimgaonkar VL, Devlin B, Lopez OL, DeKosky ST. Increased familial risk of the psychotic phenotype of Alzheimer disease. Neurology. 2002;58:907-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 65] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 21. | Bacanu SA, Devlin B, Chowdari KV, DeKosky ST, Nimgaonkar VL, Sweet RA. Heritability of psychosis in Alzheimer disease. Am J Geriatr Psychiatry. 2005;13:624-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 40] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Ramachandran G, Marder K, Tang M, Schofield PW, Chun MR, Devanand DP, Stern Y, Mayeux R. A preliminary study of apolipoprotein E genotype and psychiatric manifestations of Alzheimer's disease. Neurology. 1996;47:256-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 62] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 23. | Sweet RA, Nimgaonkar VL, Kamboh MI, Lopez OL, Zhang F, DeKosky ST. Dopamine receptor genetic variation, psychosis, and aggression in Alzheimer disease. Arch Neurol. 1998;55:1335-1340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 97] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Holmes C, Smith H, Ganderton R, Arranz M, Collier D, Powell J, Lovestone S. Psychosis and aggression in Alzheimer's disease: the effect of dopamine receptor gene variation. J Neurol Neurosurg Psychiatry. 2001;71:777-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 74] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Holmes C, Arranz MJ, Powell JF, Collier DA, Lovestone S. 5-HT2A and 5-HT2C receptor polymorphisms and psychopathology in late onset Alzheimer's disease. Hum Mol Genet. 1998;7:1507-1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 135] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Sweet RA, Pollock BG, Sukonick DL, Mulsant BH, Rosen J, Klunk WE, Kastango KB, DeKosky ST, Ferrell RE. The 5-HTTPR polymorphism confers liability to a combined phenotype of psychotic and aggressive behavior in Alzheimer disease. Int Psychogeriatr. 2001;13:401-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 71] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Kales HC, Lyketsos CG, Miller EM, Ballard C. Management of behavioral and psychological symptoms in people with Alzheimer's disease: an international Delphi consensus. Int Psychogeriatr. 2019;31:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 167] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 28. | Gerlach LB, Kales HC. Managing Behavioral and Psychological Symptoms of Dementia. Psychiatr Clin North Am. 2018;41:127-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 60] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 29. | Ballard CG, Gauthier S, Cummings JL, Brodaty H, Grossberg GT, Robert P, Lyketsos CG. Management of agitation and aggression associated with Alzheimer disease. Nat Rev Neurol. 2009;5:245-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 250] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 30. | Tampi RR, Tampi DJ, Balachandran S, Srinivasan S. Antipsychotic use in dementia: a systematic review of benefits and risks from meta-analyses. Ther Adv Chronic Dis. 2016;7:229-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 186] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 31. | Ballard C, Howard R. Neuroleptic drugs in dementia: benefits and harm. Nat Rev Neurosci. 2006;7:492-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 195] [Cited by in RCA: 195] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 32. | Mittal V, Kurup L, Williamson D, Muralee S, Tampi RR. Risk of cerebrovascular adverse events and death in elderly patients with dementia when treated with antipsychotic medications: a literature review of evidence. Am J Alzheimers Dis Other Demen. 2011;26:10-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 33. | Zuidema SU, Johansson A, Selbaek G, Murray M, Burns A, Ballard C, Koopmans RT. A consensus guideline for antipsychotic drug use for dementia in care homes. Bridging the gap between scientific evidence and clinical practice. Int Psychogeriatr. 2015;27:1849-1859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 34. | Gellad WF, Aspinall SL, Handler SM, Stone RA, Castle N, Semla TP, Good CB, Fine MJ, Dysken M, Hanlon JT. Use of antipsychotics among older residents in VA nursing homes. Med Care. 2012;50:954-960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | Acadia Pharmaceuticals, Inc. Nuplazid (pimavanserin) prescribing information. San Diego, California, 2016. [cited 24 May 2020]. Available from: https://www.acadia-pharm.com/product/. |
| 36. | Cummings J, Isaacson S, Mills R, Williams H, Chi-Burris K, Corbett A, Dhall R, Ballard C. Pimavanserin for patients with Parkinson's disease psychosis: a randomised, placebo-controlled phase 3 trial. Lancet. 2014;383:533-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 464] [Cited by in RCA: 493] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 37. | U.S. Food and Drug Administration. Nuplazid (pimavanserin). 2016 [cited 28 October 2019]. Available from: URL: https://www.accessdata.fda.gov/drugsatfda_docs/nda/2016/207318orig1s000toc.cfm. |
| 38. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264-269, W64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21613] [Cited by in RCA: 18559] [Article Influence: 1091.7] [Reference Citation Analysis (0)] |
| 39. | Muchie KF. PROSPERO International prospective register of systematic reviews. 2018. |
| 40. | Lefebvre C, Manheimer E, Glanville J. In: Higgins J, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Wiley Online Library; Searching for studies, 2011. |
| 41. | Cochrane Work. Trusted evidence. Informed decisions.. Better health. 2011 [cited 28 October 2019] Available from: https://work.cochrane.org/pubmed. |
| 42. | Covidence. Covidence systematic review software, Veritas Health Innovation, Melbourne, Australia. [cited 28 October 2019]. Available from: www.covidence.org. |
| 43. | Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12275] [Cited by in RCA: 13048] [Article Influence: 434.9] [Reference Citation Analysis (3)] |
| 44. | Ballard C, Banister C, Khan Z, Cummings J, Demos G, Coate B, Youakim JM, Owen R, Stankovic S; ADP Investigators. Evaluation of the safety, tolerability, and efficacy of pimavanserin versus placebo in patients with Alzheimer's disease psychosis: a phase 2, randomised, placebo-controlled, double-blind study. Lancet Neurol. 2018;17:213-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 127] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 45. | Ballard C, Youakim JM, Coate B, Stankovic S. Pimavanserin in Alzheimer's Disease Psychosis: Efficacy in Patients with More Pronounced Psychotic Symptoms. J Prev Alzheimers Dis. 2019;6:27-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 46. | Schneider LS, Olin JT, Doody RS, Clark CM, Morris JC, Reisberg B, Schmitt FA, Grundman M, Thomas RG, Ferris SH. Validity and reliability of the Alzheimer's Disease Cooperative Study-Clinical Global Impression of Change. The Alzheimer's Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11 Suppl 2:S22-S32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 452] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 47. | Galasko D, Bennett D, Sano M, Ernesto C, Thomas R, Grundman M, Ferris S. An inventory to assess activities of daily living for clinical trials in Alzheimer's disease. The Alzheimer's Disease Cooperative Study. Alzheimer Dis Assoc Disord. 1997;11 Suppl 2:S33-S39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 706] [Cited by in RCA: 802] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 48. | Cohen-Mansfield J, Marx MS, Rosenthal AS. A description of agitation in a nursing home. J Gerontol. 1989;44:M77-M84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 874] [Cited by in RCA: 905] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 49. | Folstein MF, Folstein SE, McHugh PR. "Mini-mental state". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56757] [Cited by in RCA: 61894] [Article Influence: 1213.6] [Reference Citation Analysis (0)] |
| 50. | Fahn S, Elton RL, the Members of the UPDRS Development Committee; Fahn S, Marsden CD, Calne DB, Goldstein M. Unified Parkinson’s disease rating scale. Fahn S, Marsden CD, Calne DB, Goldstein M. Recent developments in Parkinson’s disease. Florham Park, NJ: Macmillan Health Care Information 1987; 153-163. |
| 51. | ACADIA Pharmaceuticals Inc. Study to Examine the Safety and Efficacy of Pimavanserin for the Treatment of Agitation and Aggression in Alzheimer’s Disease (SERENE). [accessed 30 December 2019]. In: ClinicalTrials.gov [Internet]. Bethesda (MD): U.S. National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/study/NCT02992132?show_locs=Y ClinicalTrials.gov Identifier: NCT02992132/. |
| 52. | Zarit SH, Reever KE, Bach-Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist. 1980;20:649-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3184] [Cited by in RCA: 3493] [Article Influence: 75.9] [Reference Citation Analysis (0)] |
| 53. | Yasue I, Matsunaga S, Kishi T, Fujita K, Iwata N. Serotonin 2A Receptor Inverse Agonist as a Treatment for Parkinson's Disease Psychosis: A Systematic Review and Meta-analysis of Serotonin 2A Receptor Negative Modulators. J Alzheimers Dis. 2016;50:733-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 54. | Institute for Safe Medication Practices. Hallucinations and Pimavanserin (NUPLAZID), a New Kind of Drug for Psychosis. 2017 [cited 24 December 2019]. Available from: URL: https://www.ismp.org/sites/default/files/attachments/2018-01/2017Q1_0.pdf. |
| 55. | U.S. Food and Drug Administration. FDA Analysis Finds No New or Unexpected Safety Risks Associated with Nuplazid (Pimavanserin), a Medication to Treat the Hallucinations and Delusions of Parkinsonâs Disease Psychosis. 2018 September [cited 24 December 2019]. Available from: URL: https://www.ismp.org/sites/default/files/attachments/2018-01/2017Q1_0.pdf. |
| 56. | Farlow MR, Shamliyan TA. Benefits and harms of atypical antipsychotics for agitation in adults with dementia. Eur Neuropsychopharmacol. 2017;27:217-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Psychiatry
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hosak L, Seeman MV, Shiina A S-Editor: Yan JP L-Editor: A E-Editor: Qi LL