©The Author(s) 2026.
World J Psychiatry. Feb 19, 2026; 16(2): 114736
Published online Feb 19, 2026. doi: 10.5498/wjp.v16.i2.114736
Published online Feb 19, 2026. doi: 10.5498/wjp.v16.i2.114736
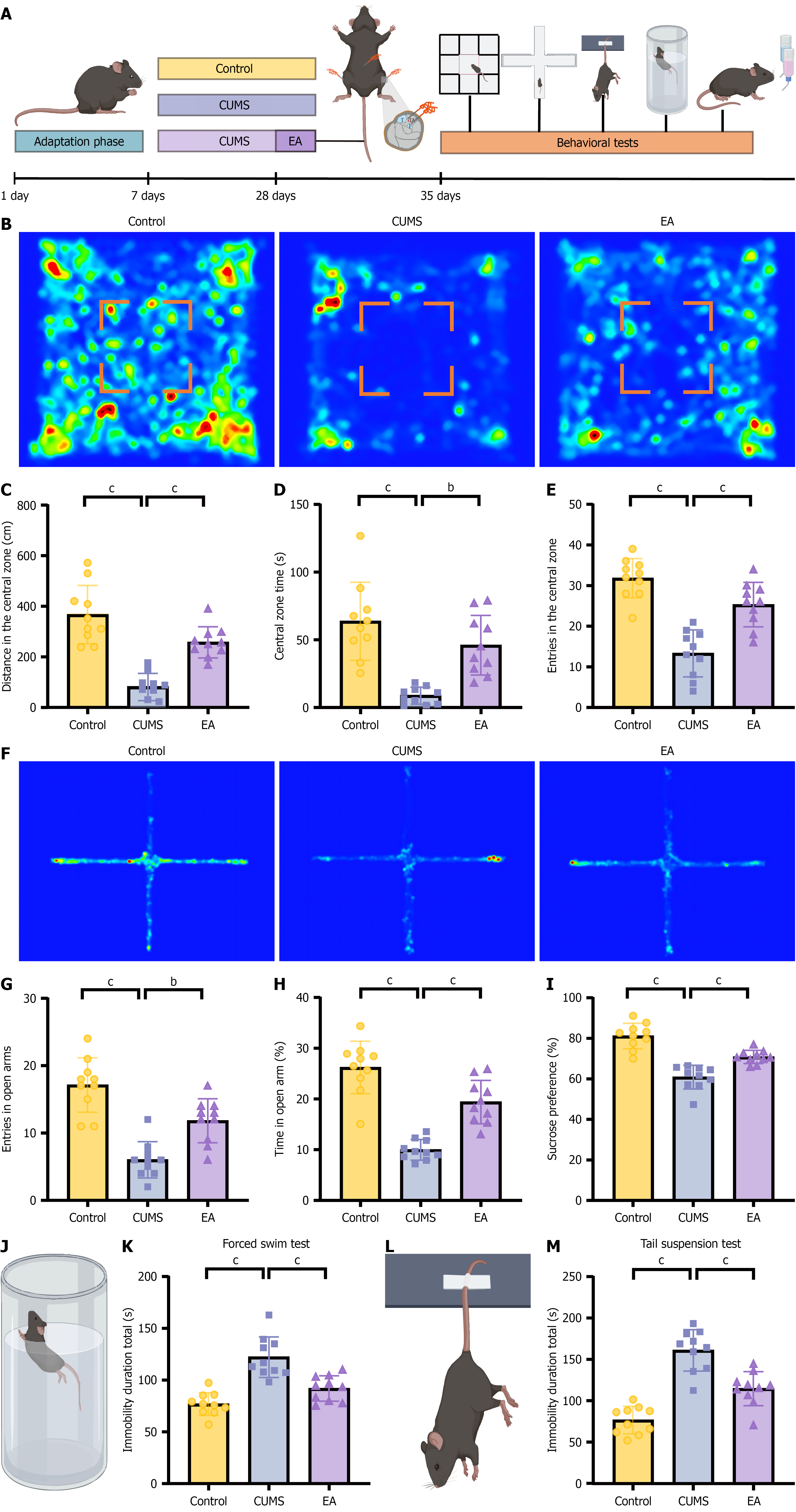
Figure 1 Electroacupuncture alleviates depression-like behavior in chronic unpredictable mild stress mice.
A: Experimental timeline outlining chronic unpredictable mild stress induction, electroacupuncture, and behavioral assays; B: Representative open-field locomotion heatmap; C-E: Open-field metrics: Distance traveled in the central zone (C), central-zone dwell time (D), and number of central-zone entries (E); F: Representative elevated plus maze heatmap; G and H: Quantification of number of entries in open-arm and percentage of time spent in open arms; I: Sucrose preference test results; J: Schematic of the forced swim test; K: Quantification of immobility duration in the forced swim test; L: Schematic of the tail suspension test; M: Quantification of immobility duration in the tail suspension test. Data are mean ± SE; statistics by one-way analysis of variance; n = 10 mice in each group. bP < 0.01, cP < 0.001. CUMS: Chronic unpredictable mild stress; EA: Electroacupuncture.
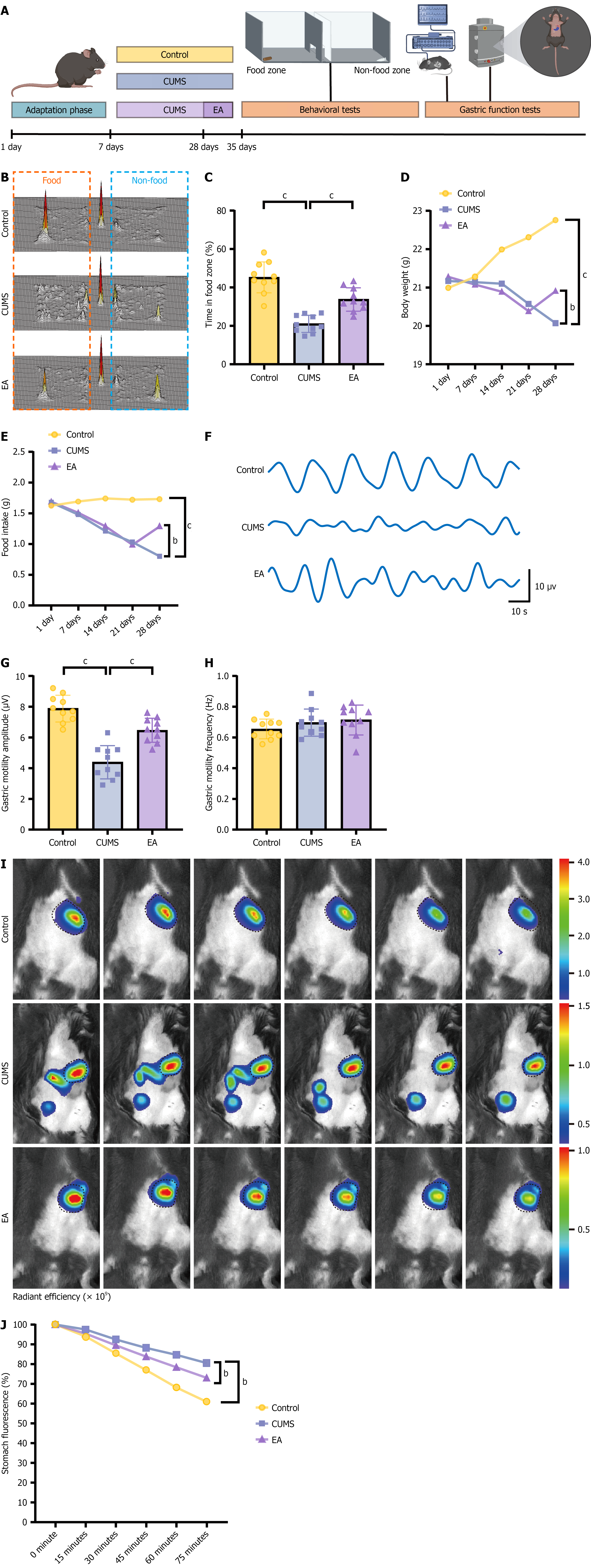
Figure 2 Electroacupuncture alleviates gastric dysfunction in chronic unpredictable mild stress mice.
A: Experimental timeline showing model preparation, electroacupuncture, behavioral testing, and gastric assessments; B: Representative three-dimensional heatmap of free-feeding behavior; C: Quantification of percentage of time spent in the feeding zone (free-feeding test); D: Average body weight changes over 28 days; E: Average 1-hour food intake over 28 days; F: Representative trace of gastric motility; G: Quantification of average periodic amplitude of gastric contractions; H: Quantification of mean gastric motility frequency; I: Representative fluorescent in vivo imaging of gastric emptying; J: Quantification of gastric emptying over 75 minutes (percent fluorescence loss relative to baseline at time 0). Data are mean ± SE; statistics by one-way or two-way analysis of variance as appropriate; n = 10 mice in each group. bP < 0.01, cP < 0.001. CUMS: Chronic unpredictable mild stress; EA: Electroacupuncture.
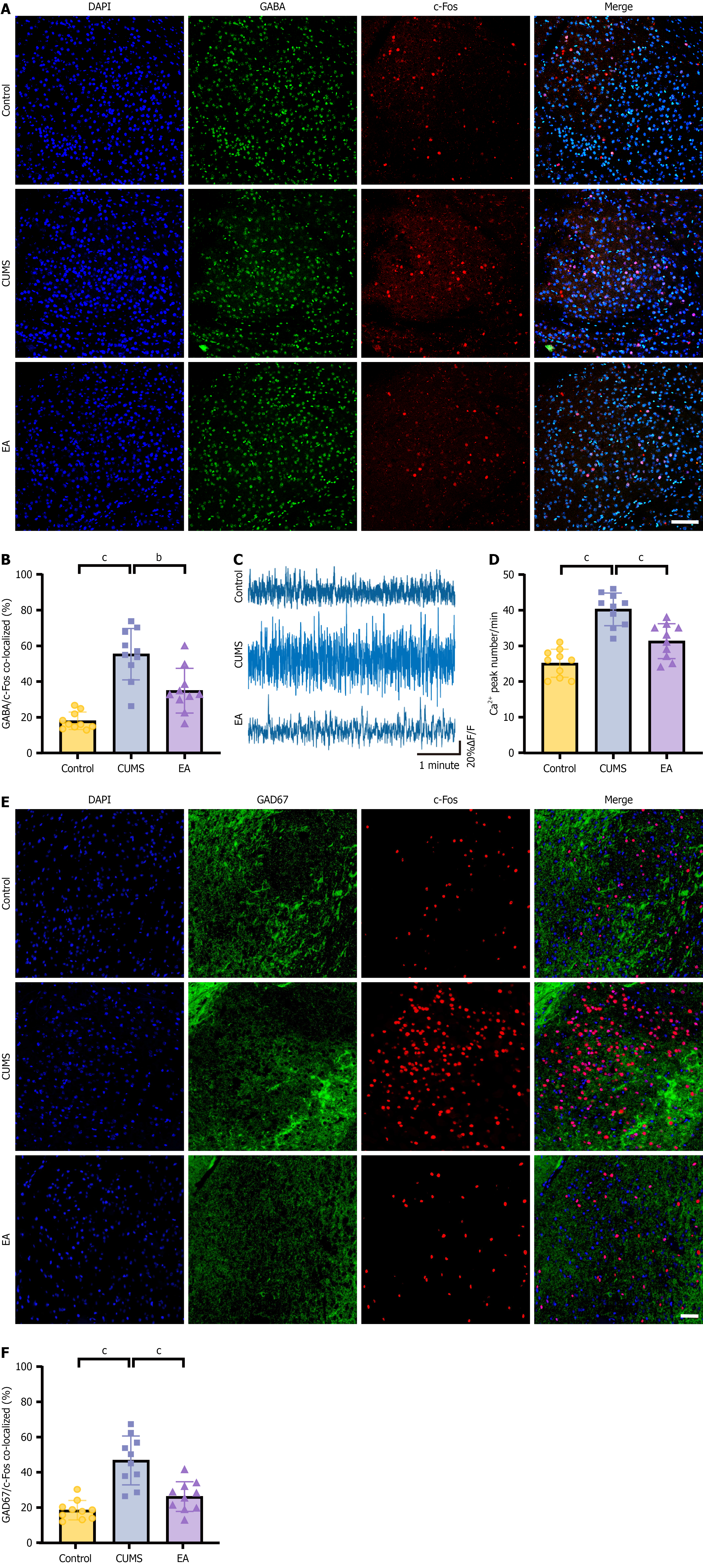
Figure 3 GABAergic neurons in the central amygdala contribute to depressive-like behavior and gastric dysfunction.
A: Representative im
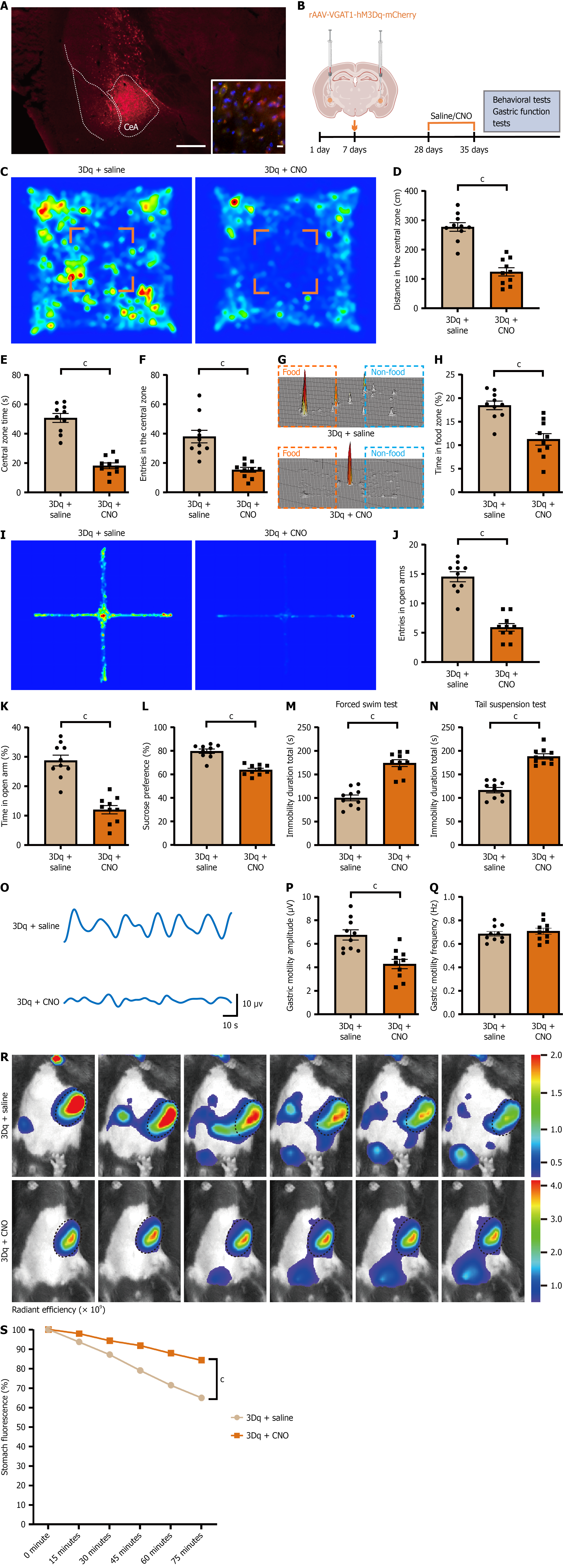
Figure 4 Activation of central amygdala GABAergic neurons induces depression-like behavior and gastric dysfunction.
A: Representative fluorescence image showing rAAV-VGAT1-hM3Dq-mCherry injection in the central amygdala. Scale bar, 2 mm; inset: GAD67/mCherry co-localization in the central amygdala (red: HM3Dq-mCherry; green: GAD67; blue: DAPI). Scale bar, 10 μm; B: Schematic diagram of the experimental process of virus injection; C: Representative heatmaps of open-field exploration in hM3Dq-expressing mice: Saline vs Clozapine-N-oxide; D-F: Open-field outcomes: Central-zone distance traveled (D), dwell time (E), and number of entries (F); G: Representative three-dimensional heatmap from the free-feeding test; H: Quantification of percentage of time spent in the food zone during the free-feeding test; I: Representative heatmap of the elevated plus maze test; J and K: Quantification of percentage of open-arm entries and time spent in open arms; L: Sucrose preference test results; M: Quantification of immobility duration in the forced swim test; N: Immobility duration in the tail-suspension test; O: Representative trace of gastric motility; P: Quantification of mean contraction amplitude of gastric motility; Q: Quantification of mean frequency of gastric motility; R: Representative in vivo fluorescence images of gastric emptying; S: Quantification of gastric emptying over 75 minutes (percent fluorescence loss relative to baseline at time 0). Data are mean ± SE; statistics by student’s t-test or two-way analysis of variance as appropriate; n = 10 mice in each group. cP < 0.001. CeA: Central amygdala; CNO: Clozapine N-oxide.
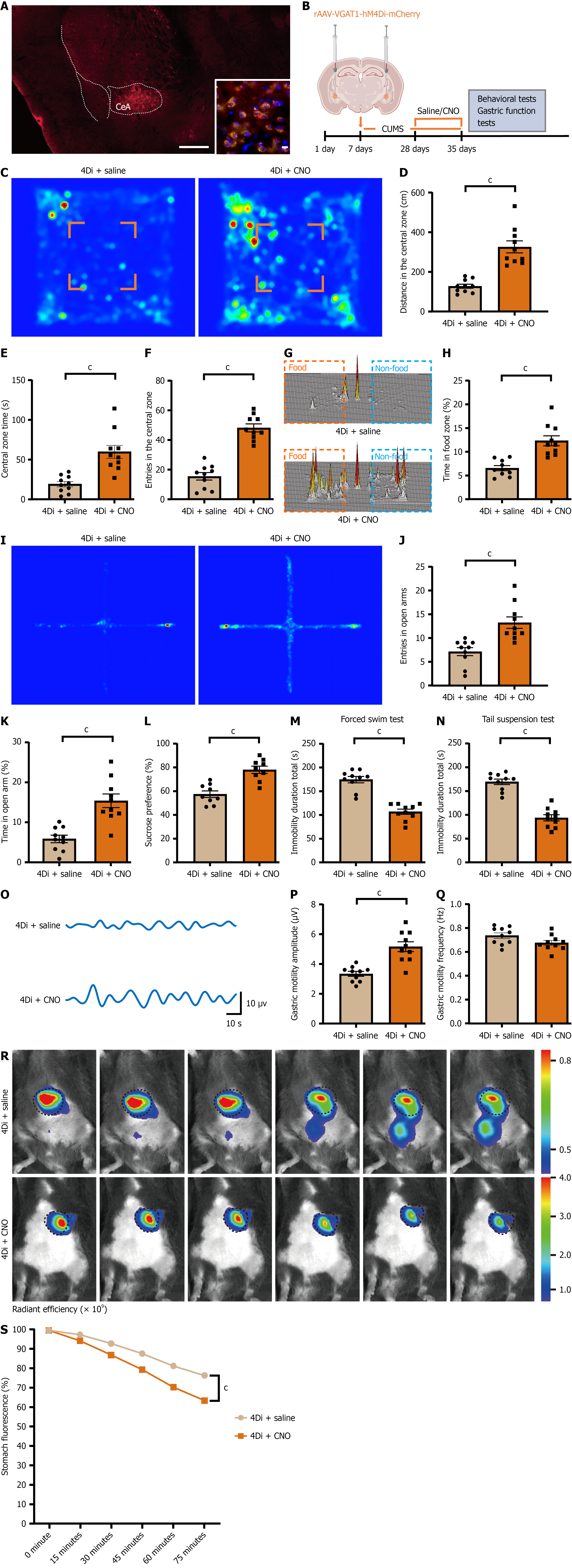
Figure 5 Inhibition of central amygdala GABAergic neurons alleviates depressive-like behavior and gastric dysfunction.
A: Representative fluorescence image showing rAAV-VGAT-hM4Di-mCherry injection in the central amygdala. Scale bar, 2 mm; inset: GAD67/mCherry co-localization in the central amygdala (red: HM4Di-mCherry; green: GAD67; blue: DAPI). Scale bar, 10 μm; B: Schematic of the viral injection protocol; C: Representative open-field heatmaps from hM4Di-expressing mice: Saline vs clozapine N-oxide; D-F: Open-field outcomes: Central-zone distance traveled (D), dwell time (E), and number of entries (F); G: Representative three-dimensional heatmap from the free-feeding test; H: Quantification of percentage of time spent in the food zone during the free-feeding test; I: Representative heatmap of the elevated plus maze test; J and K: Quantification of percentage of open-arm entries and time spent in open arms; L: Sucrose preference test results; M: Quantification of immobility duration in the forced swim test; N: Immobility duration in the tail-suspension test; O: Representative trace of gastric motility; P: Quantification of mean contraction amplitude of gastric motility; Q: Quantification of mean frequency of gastric motility; R: Representative in vivo fluorescence images of gastric emptying; S: Quantification of gastric emptying over 75 minutes (percent fluorescence loss relative to baseline at time 0). Data are mean ± SE; statistics by student’s t-test or two-way analysis of variance as appropriate; n = 10 mice in each group. cP < 0.001. CeA: Central amygdala; CNO: Clozapine N-oxide.
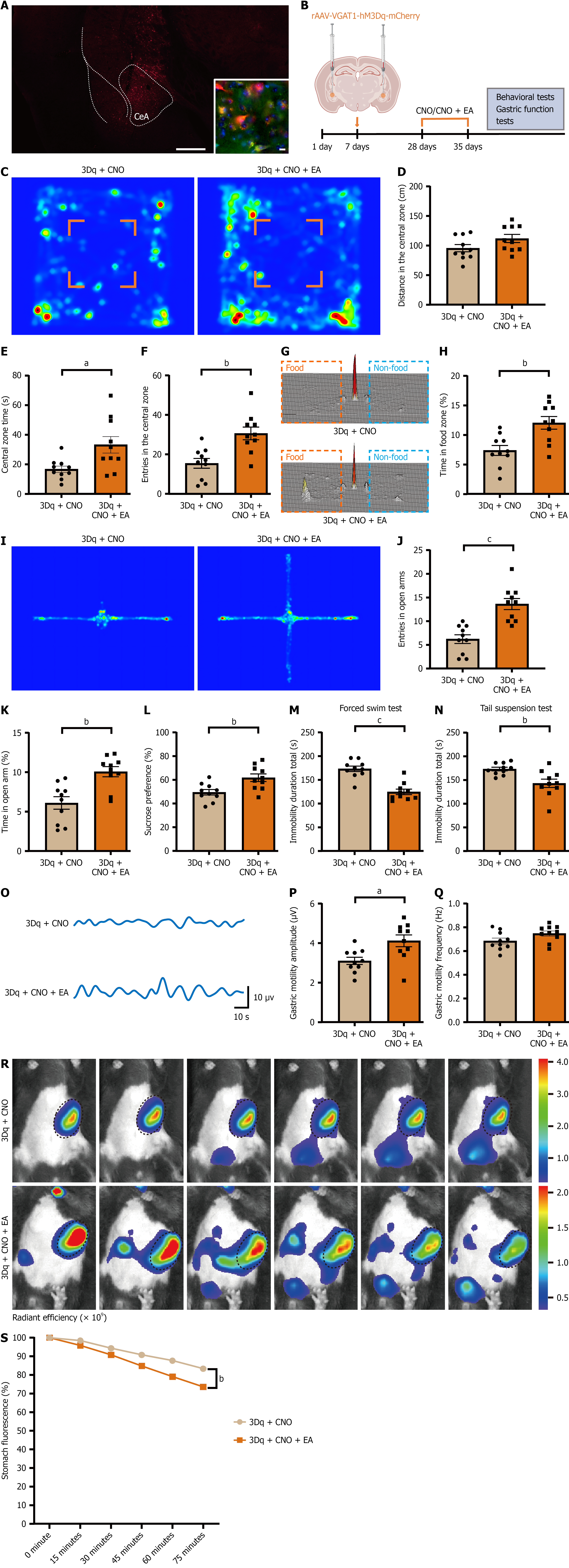
Figure 6 Electroacupuncture improves behavior and gastric motility in mice with chemogenically activated central amygdala GABAergic neurons.
A: Representative fluorescence image showing rAAV-VGAT-hM3Dq-mCherry injection in the central amygdala. Scale bar, 2 mm; inset: GAD67/mCherry co-localization in the central amygdala (red: HM3Dq-mCherry; green: GAD67; blue: DAPI). Scale bar, 10 μm; B: Schematic of the viral injection protocol and experimental timeline; C: Representative open-field heatmaps in hM3Dq-expressing mice under clozapine N-oxide alone and clozapine N-oxide + electroacupuncture conditions; D-F: Open-field outcomes: Central-zone distance traveled (D), dwell time (E), and number of entries (F); G: Representative three-dimensional heatmap of the free-feeding test; H: Quantification of percentage of time spent in the food zone during the free-feeding test; I: Representative heatmap of the elevated plus maze test; J and K: Quantification of percentage of open-arm entries and time spent in open arms; L: Sucrose preference test results; M: Quantification of immobility duration in the forced swim test; N: Immobility duration in the tail-suspension test; O: Representative trace of gastric motility; P: Quantification of mean contraction amplitude of gastric motility; Q: Quantification of mean frequency of gastric motility; R: Representative in vivo fluorescence images of gastric emptying; S: Quantification of gastric emptying over 75 minutes (percent fluorescence loss relative to baseline at time 0). Data are mean ± SE; statistics by student’s t-test or two-way analysis of variance as appropriate; n = 10 mice in each group. aP < 0.05, bP < 0.01, cP < 0.001. CeA: Central amygdala; CNO: Clozapine N-oxide; EA: Electroacupuncture.
- Citation: Ma HK, Zhu SL, Li XY, Yuan Y, Huang QY, Wang H, Shen GM, Wang XY. Electroacupuncture alleviates depression and gastrointestinal dysfunction by rebalancing GABAergic activity in the central amygdala. World J Psychiatry 2026; 16(2): 114736
- URL: https://www.wjgnet.com/2220-3206/full/v16/i2/114736.htm
- DOI: https://dx.doi.org/10.5498/wjp.v16.i2.114736