©The Author(s) 2025.
World J Psychiatry. Feb 19, 2025; 15(2): 101807
Published online Feb 19, 2025. doi: 10.5498/wjp.v15.i2.101807
Published online Feb 19, 2025. doi: 10.5498/wjp.v15.i2.101807
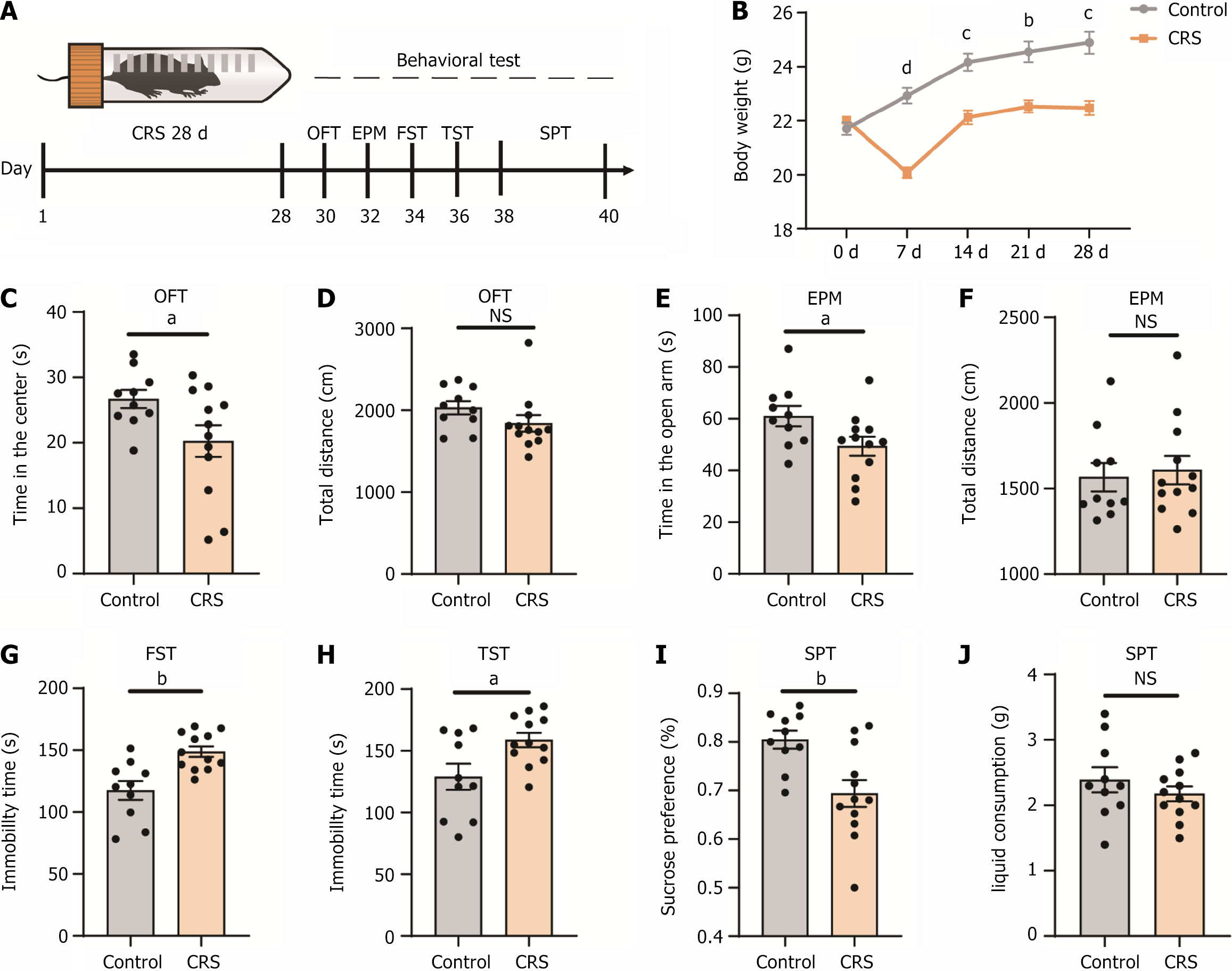
Figure 1 Chronic resistant stress induced anxiety-like and depression-like behaviors in mice.
A: Experimental strategy and timeline for behavioral assays; B: Bodyweight comparison over 4 weeks between mice in the chronic resistant stress (CRS) group and the control group; C and D: In the open field test, mice in the CRS group spent less time in the center compared to the control group. There was no change in the total distance traveled in both groups; E and F: In the elevated plus maze, mice in the CRS group spent less time in the open arms than the control group. The total distance traveled was similar between both groups; G: In the forced swim test, mice in the CRS group exhibited increased immobility time compared to the control group; H: In the tail suspension test, the mice in the CRS group showed longer immobility time compared with the control group; I and J: In the sucrose preference test, the mice in the CRS group had a significantly lower preference for sucrose than the control group. However, the total liquid consumption was the same for both groups. aP < 0.05; bP < 0.01; cP < 0.001; dP < 0.0001; n = 10 for control group; n = 12 for CRS group; NS: Not significant; CRS: Chronic resistant stress; GABA: Gamma-aminobutyric acid; OFT: Open field test; EPM: Elevated plus maze; FST: Forced swimming test; TST: Tail suspension test; SPT: Sucrose preference test.
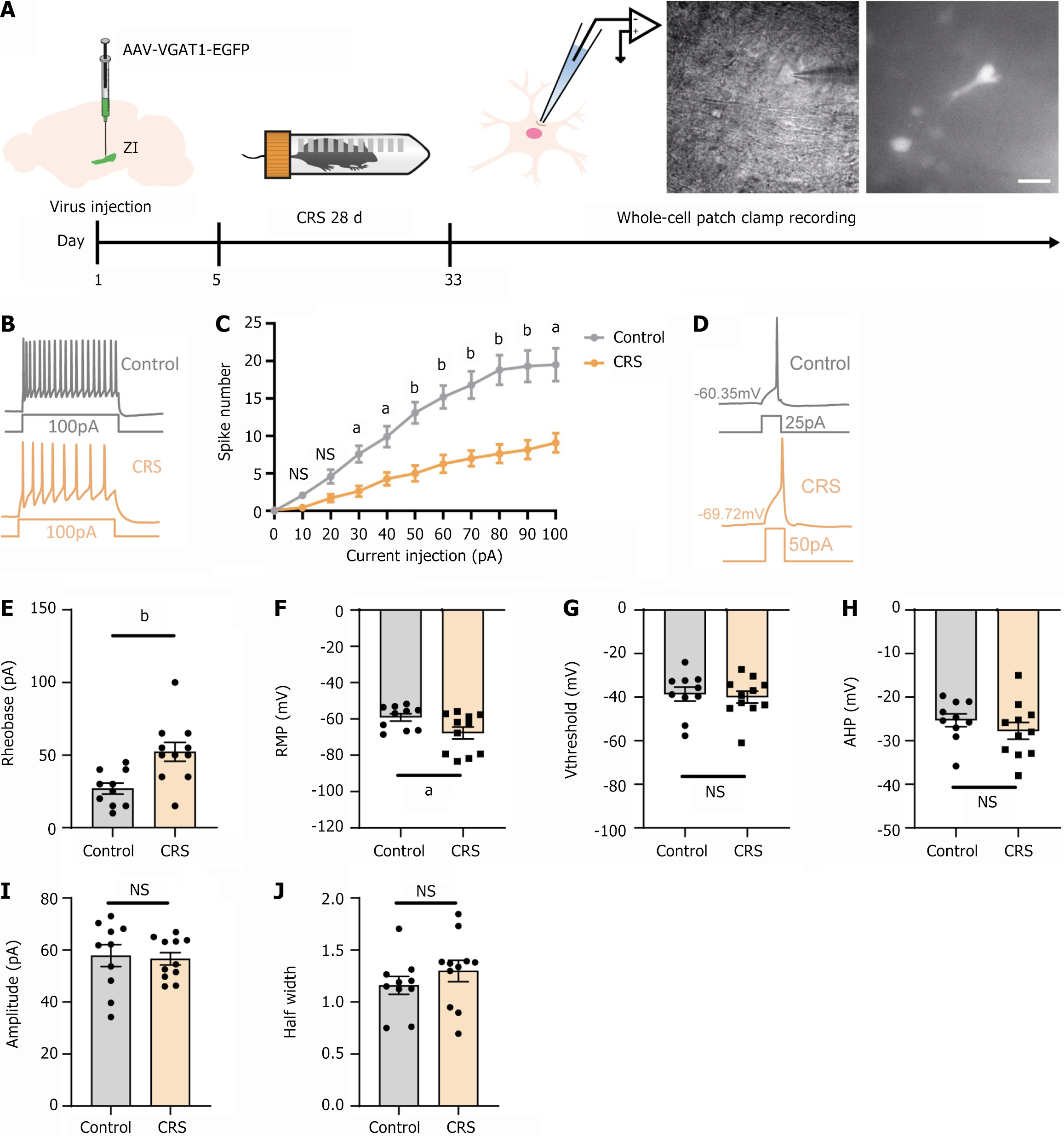
Figure 2 Effects of chronic resistant stress on the electrophysiological properties of the gamma-aminobutyric acid-ergic neurons in the zona incerta in mice.
A: Experimental timeline; B: Sample trace illustrating that chronic resistant stress (CRS) reduced the number of spikes in the zona incerta gamma-aminobutyric acid-ergic neurons in response to progressively depolarizing current injections, with a step size of 10 pA and a 400-millisecond duration (bottom); C: The relationship between the number of spikes and the amount of depolarizing current injected. CRS diminished repetitive action potential firing across the entire range of injected currents (10 pA to 100 pA); D: Sample traces indicated that CRS increased the rheobase current; E: CRS significantly increased the rheobase current; F: CRS resulted in hyperpolarization of the resting membrane potentials; G: CRS did not affect the action potential threshold; H: CRS had no impact on the afterhyperpolarization amplitude; I: CRS did not alter the amplitude of action potentials; J: CRS did not change the half-width of action potentials. aP < 0.05; bP < 0.01; control group, n = 10 cells from 3 mice; CRS group, n = 11 cells from 3 mice; Magnified scale bar = 20 µm; NS: Not significant; CRS: Chronic resistant stress.
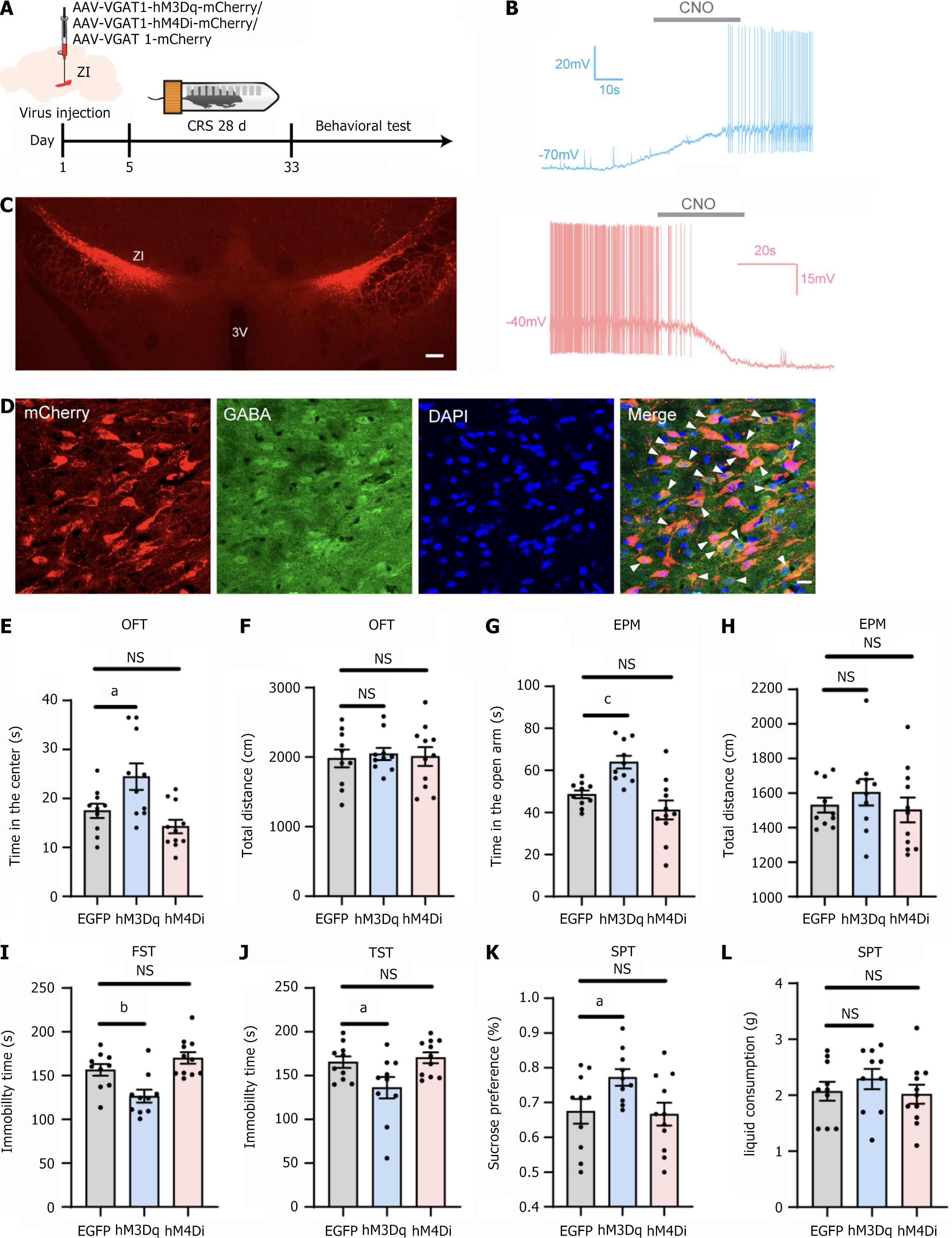
Figure 3 Effects of chemogenetic manipulation of gamma-aminobutyric acid-ergic neurons in the zona incerta on chronic resistant stress mice behavior.
A: Experimental strategy and timeline for behavioral assays; B: Bath application of clozapine-N-oxide (10 μM) induced neuronal discharge in neurons expressing hM3Dq-mCherry (clamped at -70 mV). It suppressed spiking in the hM4Di-mCherry group (clamped at -40 mV); C: The injection site of the virus in the zona incerta. Scale bars: 200 µm; D: Representative image of colocalization of neurons expressing mCherry and gamma-aminobutyric acid in the zona incerta. Blue: DAPI staining. Scale bars: 100 μm; E: Impact of chemogenetic manipulation on the time spent in the center of the open field test by mice in the chronic resistant stress (CRS) group; F: Impact of chemogenetic manipulation on the total distance traveled by mice in the CRS group in the open field test; G: Impact of chemogenetic manipulation on the time spent in the open arms of the elevated plus maze by mice in the CRS group; H: Impact of chemogenetic manipulation on the total distance traveled by mice in the CRS group in the elevated plus maze; I: Effect of chemogenetic manipulation on immobility time in the forced swim test for mice in the CRS group; J: Effect of chemogenetic manipulation on immobility time in the tail suspension test for mice in the CRS group; K and L: Effect of chemogenetic manipulation on sucrose preference and total liquid consumption in the sucrose preference test for mice in the CRS group. aP < 0.05; bP < 0.01; cP < 0.001; n = 12 for the EGFP group and hM3Dq group; n = 11 for hM4Di group; 3v: 3rd ventricle; NS: Not significant; CNO: Clozapine-N-oxide; GABA: Gamma-aminobutyric acid; OFT: Open field test; EPM: Elevated plus maze; FST: Forced swimming test; TST: Tail suspension test; SPT: Sucrose preference test.
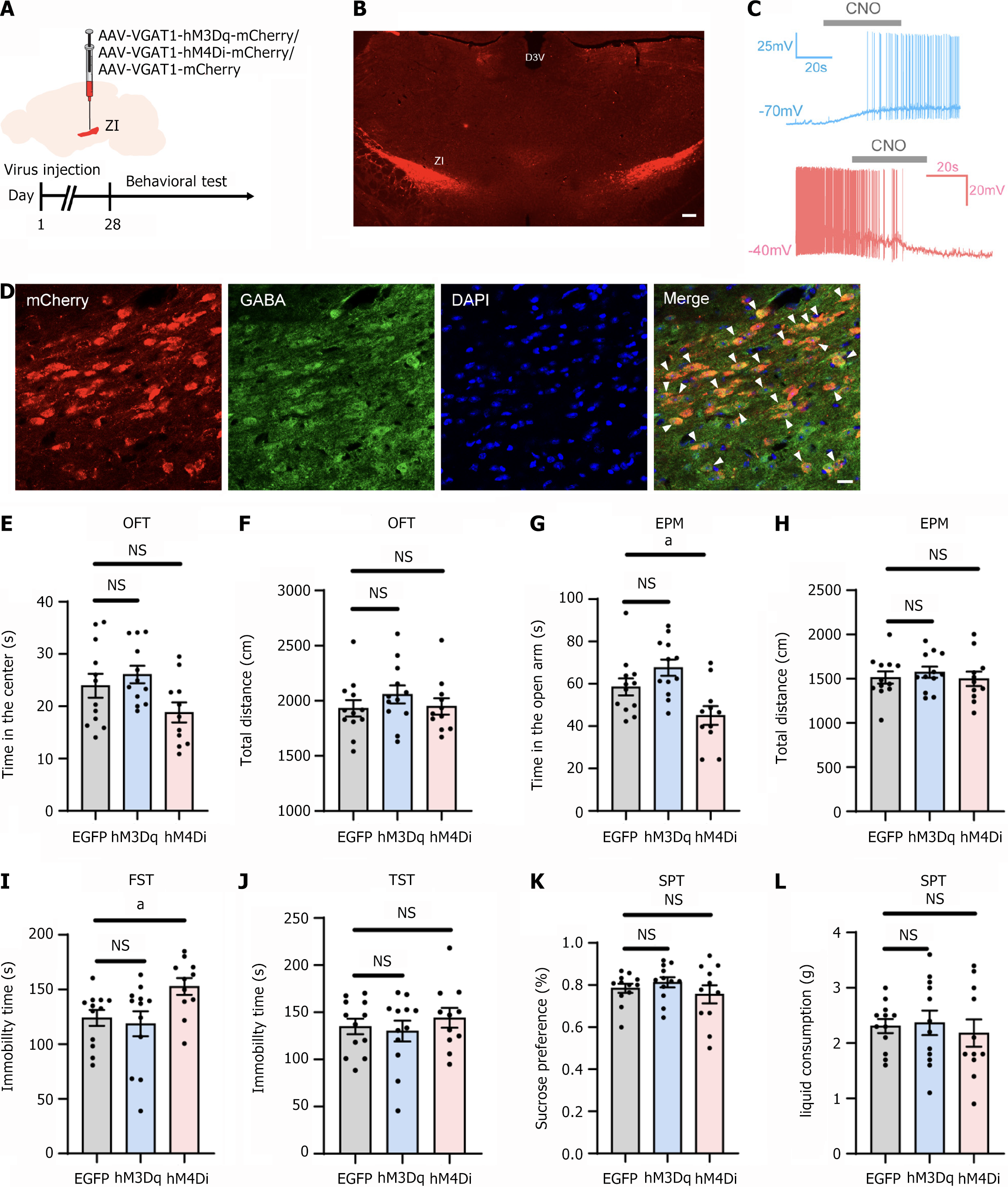
Figure 4 Effects of chemogenetic manipulation of the gamma-aminobutyric acid-ergic neurons in the zona incerta on normal mice behavior.
A: Experimental strategy and timeline for behavioral assays; B: The injection site of the virus in the zona incerta. Scale bars: 200 µm; C: Bath application of clozapine-N-oxide (10 μM) elicited neuronal discharge in neurons expressing hM3Dq-mCherry (clamped at -70 mV). It suppressed spiking in the hM4Di-mCherry group (clamped at -40 mV); D: Representative images of colocalization of neurons expressing mCherry and gamma-aminobutyric acid in the zona incerta. Blue: DAPI staining. Scale bars: 100 μm; E: Impact of chemogenetic manipulation on the time spent in the center of the open field test (OFT); F: Impact of chemogenetic manipulation on the total distance traveled in the OFT; G: Impact of chemogenetic manipulation on the time spent in the open arms of the elevated plus maze (EPM); H: Impact of chemogenetic manipulation on the total distance traveled in the EPM; I: Effect of chemogenetic manipulation on immobility time in the forced swim test; J: Effect of chemogenetic manipulation on immobility time in the tail suspension test; K and L: Effect of chemogenetic manipulation on sucrose preference and total liquid consumption in the sucrose preference test. aP < 0.05; n = 10 for the EGFP group and hM3Dq group; n = 11 for the hM4Di group; D3v: Dorsal 3rd ventricle; NS: Not significant; CNO: Clozapine-N-oxide; GABA: Gamma-aminobutyric acid; OFT: Open field test; EPM: Elevated plus maze; FST: Forced swimming test; TST: Tail suspension test; SPT: Sucrose preference test.
- Citation: Chen SH, Lan B, Zhang YY, Li GH, Qian YL, Hu MX, Tian YL, Zang WD, Cao J, Wang GH, Wang YG. Activation of zona incerta gamma-aminobutyric acid-ergic neurons alleviates depression-like and anxiety-like behaviors induced by chronic restraint stress. World J Psychiatry 2025; 15(2): 101807
- URL: https://www.wjgnet.com/2220-3206/full/v15/i2/101807.htm
- DOI: https://dx.doi.org/10.5498/wjp.v15.i2.101807