INTRODUCTION
Tuberculosis (TB) is a major global health challenge, affecting millions of people and causing a substantial number of deaths worldwide[1,2]. About one-third of the global population is infected with TB. According to the World Health Organization (WHO), approximately 10.8 million new cases of TB were diagnosed in 2023 with over 1.25 million deaths worldwide[3]. Despite the availability of effective treatment, the disease predominantly impacts developing countries. The WHO South-East Asia region accounts for 45% of global TB cases followed by the African Region (24%) and the Western Pacific Region (17%)[3].
The Bacillus Calmette-Guérin (BCG) vaccine is currently the only licensed vaccine against TB[4]. Although the BCG vaccine is effective against TB in infants and children, its efficacy against adult pulmonary TB is limited[5]. The low effectiveness of the BCG vaccine is influenced by factors such as variations in BCG strains, differences in immune responses among populations, and exposure to environmental mycobacteria[6]. The limitations of the current BCG vaccine, especially in adults, highlight the need for more effective TB vaccination strategies. New strategies aim to provide long-term protection while addressing the rising threat of drug-resistant mycobacterium TB (Mtb) strains and the high prevalence of human immunodeficiency virus (HIV)[7]. Some novel vaccine candidates are being developed to overcome these challenges, such as live attenuated Mtb vaccines, recombinant BCG, DNA vaccines, and subunit vaccines. In addition, messenger RNA (mRNA) vaccines are also considered a promising approach for treating and preventing TB[8].
The mRNA vaccines are emerging as potential next-generation TB vaccines, currently in preclinical stages or clinical trials. The coronavirus disease 2019 (COVID-19) pandemic accelerated the development of mRNA vaccines and highlighted their ability to rapidly generate strong immune responses. The first two authorized mRNA vaccines demonstrated over 90% efficacy in preventing symptomatic COVID-19 infection[9]. Recent advancements in mRNA vaccine technology, spurred by the success of COVID-19 vaccines, have opened new avenues for TB vaccine development. The mRNA vaccine technology offers a versatile platform with many advantages compared to traditional vaccines. The mRNA vaccines stand out due to their quickly adjustable designs, high potency, rapid development, low severity of side effects, and cost-effective production. These advantages position mRNA vaccines as a promising treatment strategy for TB[10]. This narrative review aims to explore the current state of mRNA vaccines and their advantages for TB. This narrative review summarizes recent research advancements, identifies current challenges, and discusses prospects in the development of mRNA vaccines specifically for TB.
HOST IMMUNE RESPONSE TO TB
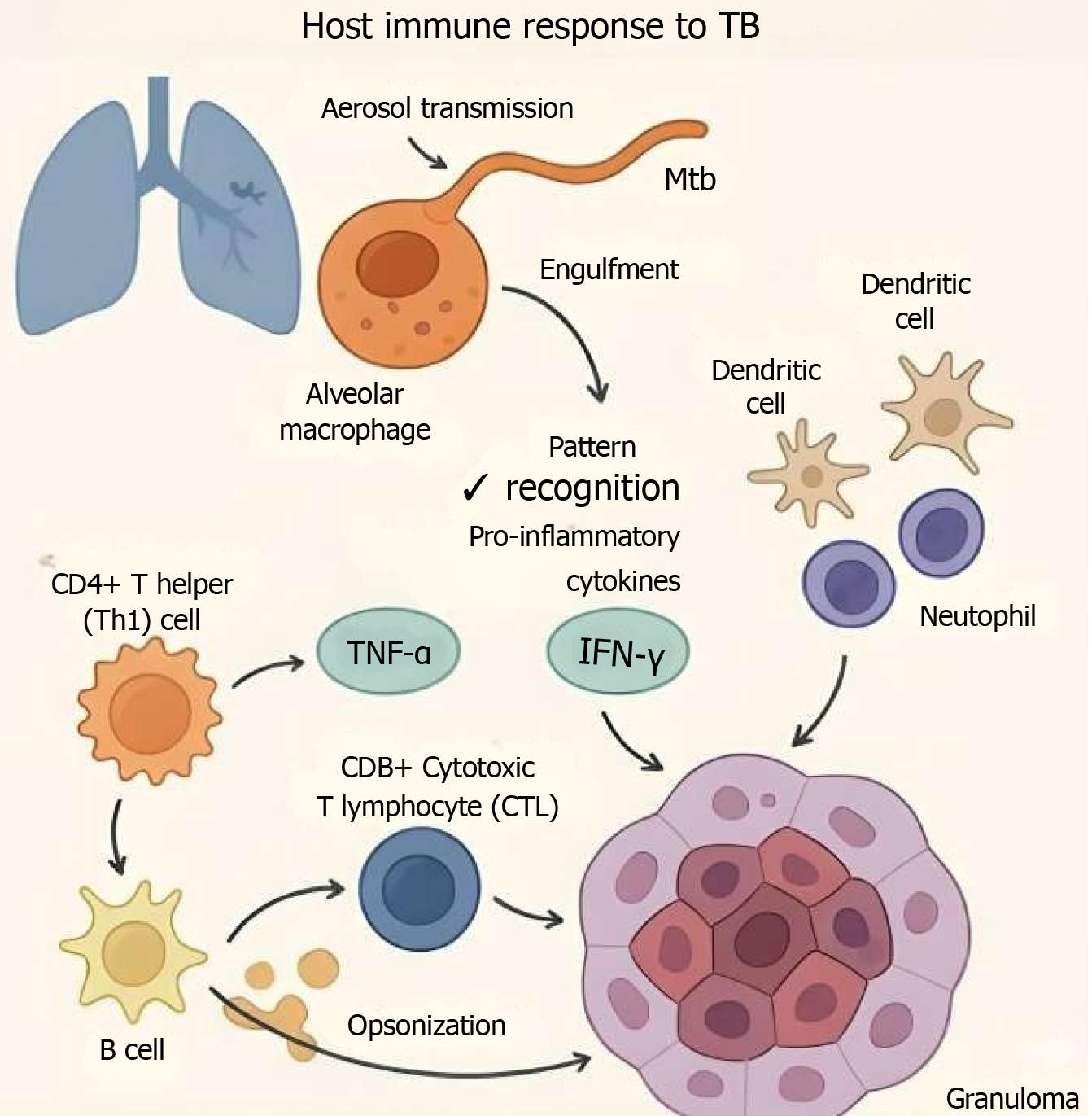
Following aerosol transmission, Mtb is engulfed by alveolar macrophages, dendritic cells, and neutrophils in the lungs. Macrophages act as the primary site of infection and as antigen-presenting cells (APCs), initiating the adaptive immune response[11]. Pattern recognition receptors such as toll-like receptors and C-type lectin receptors detect Mtb and activate signaling cascades that lead to the production of pro-inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α) and interferon-gamma (IFN-γ)[12]. These cytokines are critical in orchestrating granuloma formation and limiting bacterial spread.
Activation of CD4+ T helper (Th1) cells is essential for producing IFN-γ, which enhances macrophage bactericidal activity. CD8+ cytotoxic T lymphocytes also play a crucial role by killing infected cells and contributing to the containment of intracellular bacteria[13]. B cells and antibody responses, though historically underemphasized, are now recognized to contribute to TB control via opsonization, neutralization, and modulation of cellular immunity[14].
A hallmark of TB immunopathogenesis is the formation of granulomas, an organized immune cell structure designed to contain Mtb. Granulomas are dynamic and can either lead to sterilization, persistence (latency), or eventual breakdown and disease progression[15].
IMPLICATIONS FOR BCG VACCINE EFFICACY
The partial and inconsistent protection offered by the BCG vaccine, especially against adult pulmonary TB, is largely attributable to Mtb’s immune evasion strategies and the complexity of human TB pathogenesis. BCG is effective in protecting infants against severe forms like TB meningitis, but it fails to reliably prevent adult pulmonary TB due to the following reasons: (1) Antigenic mismatch: BCG lacks key Mtb virulence antigens such as ESAT-6 and culture filtrate protein-10 (encoded in the region of difference 1 region), which are critical for inducing protective immunity against pulmonary disease[20]; (2) Weak induction of CD8+ T-cell responses: BCG primarily induces CD4+ T-cell responses. Its limited ability to stimulate robust cytotoxic CD8+ T-cell responses essential for targeting intracellular Mtb, compromises its effectiveness[21]; and (3) Lack of durability and waning immunity: Immunity conferred by BCG tends to wane after adolescence. In areas with high Mtb exposure, repeated environmental sensitization can mask or block vaccine-induced responses[22].
Understanding these immunological limitations is critical for guiding the development of new TB vaccines. The mRNA vaccine platforms have the potential to overcome many of these issues by delivering multiple, well-conserved Mtb antigens and promoting both CD4+ and CD8+ T-cell responses, along with long-lived memory populations. Moreover, self-adjuvanted or nanoparticle-encapsulated mRNA vaccines may improve antigen processing and presentation, enhancing immune recognition even in the face of Mtb’s evasion strategies.
The mechanism of mRNA vaccines in immunization
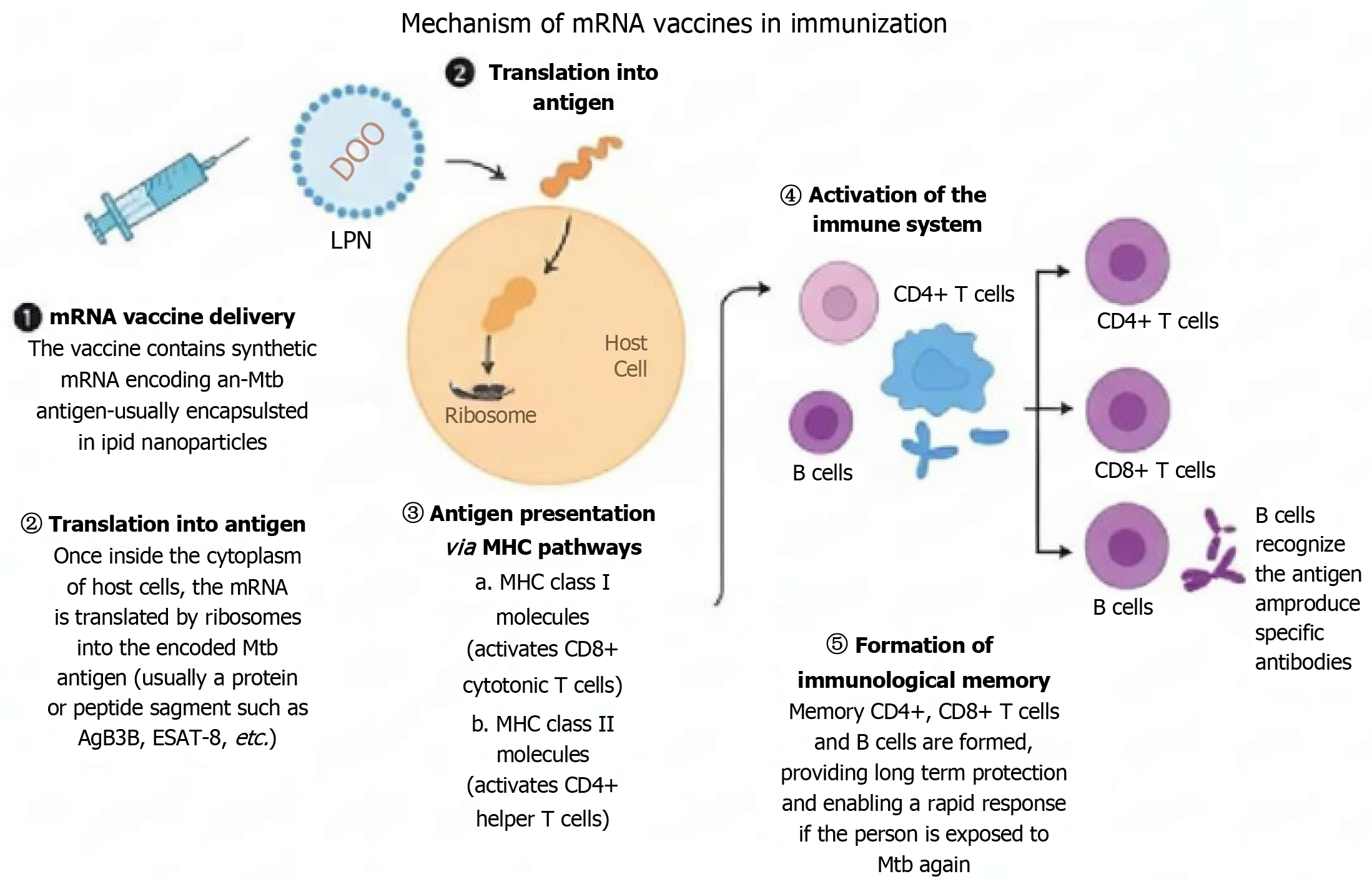
The basic mechanism of mRNA vaccines involves using synthetic mRNA to instruct body cells to produce a specific protein that triggers an immune response[23]. This prepares the immune system to recognize and fight off a particular pathogen in the future. The mRNA vaccines work by delivering mRNA that encodes tumor antigens or other target antigens to host cells. Once inside the cells, the mRNA is translated by the host's cellular machinery into the target protein antigen. These protein antigens are then displayed on the cell’s surface and are subsequently recognized by the immune system. The immune system initiates an immune response creating antibodies and activating T-cells specific to the pathogen[24]. This process also forms memory cells that provide a rapid and strong immune response against future infections by the pathogen[25]. Moreover, these vaccines are typically delivered as nucleoside-modified mRNA encapsulated in lipid nanoparticles (LNPs) which protect the mRNA from degradation and enhance its immunogenicity[26].
The effective prevention of TB relies on a strong T-cell response against Mtb, the causative agent of TB. The mRNA vaccines work by instructing host cells to produce specific Mtb antigens, effectively triggering both T-cell and B-cell responses. The mRNA vaccines provide a dual immune response i.e. cell-mediated and humoral immunity[27,28]. The cell-mediated response is driven by T cells of the immune system. The T-cells, particularly CD4+ and CD8+ T cells, target and eradicate intracellular pathogens like Mtb[29]. CD4+ T cells, also known as Th cells, facilitate the cytotoxic functions of CD8+ T cells and the activation of B cells. CD8+ T cells are directly involved in eliminating infected cells, a critical step in controlling TB. The activation of B cells produces antibodies to neutralize extracellular pathogens. Together, these mechanisms offer comprehensive protection against TB. A key advantage of mRNA vaccines is their ability to generate memory T cells. Upon re-exposure to Mtb, these cells prepare the immune system to respond rapidly and robustly. By promoting long-term immunity, memory T cells help control bacterial replication and prevent reinfection[30].
The immunogenicity of mRNA vaccines offers several advantages over traditional TB vaccines, such as BCG. BCG vaccine utilizes weakened live bacteria to elicit a T-cell-mediated immune response. However, it is not always sufficient to prevent TB infection in adults[31]. On the other hand, mRNA vaccines induce robust humoral (antibody-mediated) and cellular immunity, with a pronounced focus on T-cell activation. This is similar to the high immunogenicity observed in mRNA vaccines for COVID-19, which produce strong antibody and T-cell responses[32]. Recent studies have highlighted the superior immunogenicity of mRNA vaccines compared to BCG[33]. For example, an mRNA vaccine encoding ESAT-6 epitopes (5'-TPL-ESAT-6-3'-Mod) demonstrated higher efficacy compared to BCG in stimulating an adaptive immune response and protecting against Mtb infection in mice[34].
The primary types of mRNA vaccines under consideration are conventional non-amplifying mRNA vaccines and self-amplifying mRNA (saRNA) vaccines[35]. Conventional mRNA encodes only the target antigen while saRNA includes additional sequences that allow it to replicate, potentially enhancing immune responses with smaller doses[36]. The mRNA vaccines have emerged as a promising technology for combating complex pathogens like Mtb. These vaccines offer precise and adaptable immune targeting, efficient stimulation of immune responses, and rapid development. They also provide advantages such as high efficacy, relatively low side effects, and cost-effectiveness. These features position mRNA as a promising platform for TB prevention, addressing limitations associated with traditional vaccines like BCG (Figure 2).
Figure 2 The mechanism of messenger RNA vaccines in immunization.
ESAT: Early secreted antigenic target; LPN: Licensed practical nurses; MHC: Major histocompatibility complex; mRNA: Messenger RNA; Mtb: Mycobacterium tuberculosis.
Development of mRNA vaccines for TB
Efforts to develop mRNA vaccines for TB gained momentum following the success of mRNA-based COVID-19 vaccines. These initiatives focus on identifying and utilizing antigens specific to Mtb to induce targeted immune responses. Preclinical studies have demonstrated the potential of RNA-based vaccines for TB. These vaccines have shown feasibility in development, elicited enhanced immune responses, and yielded improved outcomes in animal models[37]. A notable milestone is the establishment of mycobacterium-specific mRNA constructs, which are undergoing preclinical testing to assess their immunogenicity and safety. A key study by Lorenzi et al[8] illustrated the efficacy of mRNA vaccines for combating TB. The research demonstrated that intranasal administration of an mRNA vaccine encoding the mycobacterium leprae heat shock protein 65 provided significant protection against Mtb challenge in mice. This vaccine stimulated specific cytokine production and activated APCs within the lungs, critical for an effective immune response. These findings highlighted the broader potential of mRNA vaccines for infectious diseases, including TB. Another promising development involves a replicating RNA vaccine formulated in nanostructured lipids. Preclinical data indicate that this formulation effectively induces both cell-mediated and humoral immune responses while significantly reducing bacterial burden in animal models. Such advances underscore the versatility and potential of mRNA vaccine platforms in addressing TB.
Preclinical and clinical research on mRNA TB vaccines
Preclinical evidence from animal models: Preclinical research and animal models are essential tools in TB mRNA vaccine research and testing. They provide valuable insights into the pathogenesis of Mtb, immune response mechanisms, and the efficacy of vaccines[38]. These models have facilitated the development of innovative vaccine platforms like DNA and mRNA vaccines and have also been pivotal in evaluating their effectiveness against drug-resistant TB strains[39]. Preclinical studies have shown promising results for mRNA vaccines against TB, demonstrating enhanced immune responses and improved outcomes in animal models. Shepelkova et al[34] found that an mRNA vaccine encoding ESAT-6 epitopes exhibited greater efficacy in mice compared to the BCG vaccine. Similarly, Vasileva et al[31] demonstrated that a multi-epitope mRNA vaccine elicited a stronger T-cell response in mice than a full-length mRNA vaccine, highlighting the advantages of targeted antigen design. Additionally, Mishra et al[40] developed a self-adjuvanted mRNA vaccine expressing Mtb antigens, which significantly reduced the Mtb burden and enhanced both innate and adaptive immunity in mice. These findings underscore the potential of mRNA vaccines as next-generation tools for TB prevention, offering improved efficacy and immune activation compared to traditional approaches.
Limitations of animal models in reflecting human TB pathogenesis: Animal models are invaluable in vaccine research but often face limitations in accurately translating outcomes to humans. While mRNA vaccines for TB have shown promise in animal models, translating these results to humans presents obstacles, including differences in immune responses, TB pathogenesis, poor experimental design, inappropriate data analysis, and misuse of statistics[41]. One of the primary obstacles is the inability of animal models to replicate latent TB infection and post-primary TB, which are key stages of human TB pathogenesis. Human TB is characterized by structured granulomas, persistent latent infection, and reactivation potential, none of which are reliably modeled in animals. Mice typically fail to form structured granulomas and either clear the infection or develop acute disease, limiting the study of long-term immunity and reactivation[42]. Post-primary TB, the main driver of human-to-human transmission and a hallmark of adult disease, involves cavitation and chronic lung pathology. These features are rarely seen in animal models, even in advanced systems like guinea pigs or non-human primate[43,44]. Additionally, human immune responses to Mtb are highly complex, and the pathogen's ability to establish latent infections further complicates vaccine development[45]. Animal models, particularly mice, often do not fully replicate the human immune response to Mtb, leading to discrepancies in vaccine efficacy between preclinical models and human trials[46]. For instance, cytokine signaling (e.g., IL-2, IL-17) and memory T-cell formation behave differently across species, affecting the predictive value of immunogenicity data[47]. Vaccine responses that appear effective in animals may not elicit comparable immunity or protection in humans, especially in the context of TB’s complex intracellular lifestyle. Furthermore, TB vaccine trials also face challenges due to the long follow-up periods required to assess efficacy, especially in preventing latent infections from progressing to active disease. These extended timelines make trial outcomes more variable, necessitating careful selection of trial sites with high TB incidence to ensure statistically significant results. Despite these limitations, animal models remain invaluable in TB research, providing critical insights into Mtb pathogenesis, drug development, and vaccine efficacy[48]. To address their limitations, researchers now advocate for the complementary use of multiple models, such as mice for immunogenicity, guinea pigs for disease progression, and non-human primates for latency and pathology[49]. Emerging approaches like humanized mouse models, computational simulations, and biomarker-guided clinical validation are also being explored to better bridge preclinical and clinical data.
CLINICAL TRANSLATION AND EARLY HUMAN TRIALS
Human clinical trials for mRNA TB vaccines are still in the early stages, with efforts primarily focused on evaluating safety, immunogenicity, and preliminary efficacy. In 2023, BioNTech initiated a phase 1 randomized, controlled, dose-finding trial for BNT164, the first mRNA vaccine candidate for TB to enter clinical evaluation. This trial aims to assess the vaccine’s safety and immune response in humans, marking a significant milestone in TB vaccine development[50]. The path to regulatory approval is rigorous, particularly given the unique technical and ethical challenges associated with TB. TB disproportionately affects populations in low-income countries, necessitating careful consideration of trial locations, control group selection, and informed consent to ensure ethical responsibility[51]. Additionally, safety assessments and compliance with regulatory standards are critical, further complicating the development and approval process. Despite these hurdles, progress is being made, offering hope for innovative solutions to address this high-burden disease.
BIOLOGICAL AND PATHOGEN-SPECIFIC CHALLENGES
The development of vaccines for TB faces significant biological and pathogen-specific challenges, primarily due to the complex nature of Mtb and its ability to evade the immune system. Mtb has evolved sophisticated mechanisms to persist within the host. These mechanisms include manipulating the phagosomal environment in macrophages, engaging pattern recognition receptors, and modulating cytokine production[52]. Additionally, the pathogen's genetic complexity, with approximately 4000 genes, makes selecting the ideal antigens for vaccine formulation a substantial hurdle.
One of the key challenges in TB vaccine development is identifying immune correlates of protection. The different stages of TB disease and the complex immune response required to combat Mtb slow the progress of vaccine candidates. Effective TB vaccines need to elicit a strong T-cell response, particularly involving CD4+ and CD8+ T cells. Designing mRNA vaccines that can trigger such responses is complex and requires precise epitope selection. Furthermore, TB vaccines must induce a durable immune response, which is particularly difficult due to Mtb's ability to establish latent infections. With over 2 billion people worldwide harboring latent TB, this represents a significant challenge in TB control[53]. Current mRNA vaccine designs must address both active and latent TB cases, highlighting the urgent need for novel vaccination strategies to effectively target all stages of the disease[54].
Scientific and technical challenges
Developing mRNA vaccines for TB presents unique challenges, particularly due to the inherent instability of mRNA molecules and the complexities of designing TB-specific antigens. The mRNA is naturally unstable and highly susceptible to enzymatic degradation, making its delivery and storage difficult[55]. This instability requires advanced stabilization methods, such as encapsulation in LNPs, which protect mRNA from degradation and facilitate its delivery into cells. Thus, mRNA’s large size, instability, and need for cold-chain storage complicate distribution, especially in low-resource, high-TB-burden regions.
Efficient intracellular delivery of mRNA remains a major hurdle. While non-viral delivery systems are being explored, they must ensure mRNA stability and proper translation within the target cells. Recent advances in mRNA vaccine technology have improved stability and delivery, but challenges remain, including immune activation and the need for precise antigen selection[56]. For TB, a successful vaccine must induce durable T-cell-mediated immunity[57], as TB infection often involves prolonged or latent phases where Mtb remains dormant within the host. A robust and long-lasting T-cell memory is essential to protect against re-exposure to TB. The mRNA vaccines, with their potent immunogenicity, are being explored to specifically encode Mtb antigens that can stimulate strong and enduring T-cell responses. However, overcoming the barriers to reliably inducing protective T-cell immunity remains one of the key challenges in developing an effective TB vaccine[58].
Logistical and financial barriers
Large-scale production of mRNA vaccines presents significant logistical and financial challenges, particularly for global distribution[59]. The manufacturing process is complex and resource-intensive, with high production costs and stringent cold-chain requirements to maintain stability[60]. These vaccines typically require ultra-cold storage temperatures, which complicates their distribution in resource-poor countries, where TB is most prevalent[61]. Adapting mRNA vaccine production for TB in such settings poses additional economic and infrastructural hurdles, making affordability a critical consideration for widespread implementation. Furthermore, funding for TB vaccine research has historically been insufficient due to limited commercial incentives and fears of inadequate returns on investment[62,63]. Despite the global health burden of TB, private firms have conducted minimal research, leaving vaccine development underfunded, particularly in low-income, high-burden regions. These challenges underscore the need for increased investment and innovative approaches to make mRNA vaccines for TB accessible and sustainable worldwide.
Prospective benefits of mRNA vaccines for TB prevention
The development of mRNA vaccines for TB offers several potential benefits that could revolutionize TB prevention.
Improved immunogenicity: The mRNA vaccines offer a more tailored and effective approach than traditional vaccines like BCG, demonstrating superior immunogenicity in recent studies. They are particularly adept at inducing both humoral (antibody-mediated) and cellular (T-cell-mediated) immune responses, which are critical for controlling TB due to the intracellular nature of Mtb. Robust T-cell responses, essential for combating Mtb, are a key advantage of mRNA vaccines. This dual stimulation of immune pathways makes mRNA vaccines highly promising for TB control, offering enhanced and specific immune activation compared to BCG or adenoviral vector-based vaccines.
Rapid and scalable production: The mRNA vaccines present a compelling alternative to conventional vaccines, offering the advantage of rapid development and adaptability. Their ability to be designed and produced swiftly makes them particularly valuable for responding to global health emergencies, such as TB outbreaks or the emergence of drug-resistant strains. The success of mRNA vaccines during the COVID-19 pandemic has demonstrated the feasibility of scaling production efficiently, paving the way for accelerated deployment to high-burden TB regions[64]. This platform not only addresses global health disparities but also holds promise for improving vaccine supply and accessibility worldwide.
Adaptability and customization: The mRNA vaccines offer a transformative approach to TB prevention, with their ability to be rapidly modified to address emerging strains, including drug-resistant variants. This adaptability is particularly critical given the high mutation rate of Mtb. Unlike traditional vaccines, mRNA platforms allow for the precise inclusion of multiple TB antigens or epitopes, enabling a more robust and comprehensive immune response. This flexibility could overcome the limitations of the BCG vaccine, which demonstrates inconsistent efficacy across regions and populations. By facilitating swift adjustments to vaccine formulations, mRNA technology ensures preparedness against evolving TB strains, making it a powerful tool in global TB control efforts[65].
Safer profile: The safety profile of mRNA TB vaccines has been a focal point of clinical and preclinical studies. These studies consistently demonstrate that these vaccines are well-tolerated with mostly mild to moderate side effects. Common adverse reactions include localized pain, redness, and swelling at the injection site. Other transient systemic symptoms include fever, fatigue, and muscle or joint aches, all of which are self-limiting. Unlike live-attenuated vaccines, mRNA vaccines eliminate the risks of uncontrolled replication or reactivation. This quality makes them particularly suitable for immunocompromised populations, such as those with HIV, who are at higher risk for TB but have limited vaccine options. Drawing on the successful safety record of mRNA vaccines developed for COVID-19, early results for mRNA TB vaccines suggest a similarly favorable safety profile. However, the long-term effects of these vaccines remain uncertain, necessitating robust post-market surveillance and follow-up studies to ensure their continued safety and efficacy.
Implications for global health: The development of mRNA vaccines for TB represents a transformative opportunity for global health. These vaccines demonstrate the potential to significantly reduce TB incidence and mortality, especially in high-burden, low-income regions where the disease remains a leading cause of death. An effective mRNA TB vaccine could help bridge disparities in prevention and control efforts between high-income and low-income nations, addressing critical gaps in global TB management. By reducing transmission and preventing drug-resistant TB cases, these vaccines could substantially lower mortality rates in endemic areas. Additionally, leveraging mRNA platforms for combination vaccines offers the possibility of tackling TB alongside co-infections like HIV, which frequently coexist in affected populations, further amplifying the impact on public health.
Future public health strategies: The mRNA vaccines hold a significant role in advancing TB eradication programs by offering unparalleled safety, adaptability, and efficacy. Their integration into existing TB control frameworks, especially in areas where the BCG vaccine has limited effectiveness, could transform global prevention efforts. The mRNA technology also enables the development of multi-component vaccines, combining TB prevention with protection against other respiratory pathogens, thus streamlining immunization strategies. The integration of effective vaccines, improved drugs, and diagnostics could substantially lower TB incidence. However, achieving eradication will require innovative delivery approaches and interventions targeting latent infections to address the disease's complex nature comprehensively[66].
Innovations and future directions in mRNA TB vaccine research
The development of mRNA vaccines for TB is benefiting from significant technological advancements and novel approaches. Recent advancements in mRNA technology have revolutionized biomedicine, particularly in vaccine development and immunotherapy[67].
Innovations in LNPs, the leading delivery system for mRNA vaccines, have enhanced mRNA stability, protected it from degradation, and improved cellular uptake. LNPs now include optimized components such as ionizable lipids, polyethylene glycol, and cholesterol, with ongoing modifications to increase efficacy and minimize side effects[68]. Concurrently, advancements in mRNA stability have further strengthened vaccine performance. Structural modifications to the 5' cap, untranslated regions, coding regions, and poly(A) tails have enhanced mRNA translation efficiency and immune response induction[69]. Additionally, techniques such as sequence modifications and complexation with protamine have improved mRNA stability and immunogenicity[70], paving the way for more robust and effective mRNA vaccines. Emerging technologies in mRNA vaccine platforms are also paving the way for broader applications. Encapsulation techniques, including novel nanoparticle designs and hybrid delivery systems, show promise in increasing vaccine potency and accessibility[71]. Multi-antigen and multistage mRNA vaccines targeting different phases of TB infection, such as active and latent forms, are being explored[72,73]. These innovations aim to induce robust immune responses, including long-term protection against TB.
Research is also focusing on identifying biomarkers to monitor vaccine efficacy and immune responses in clinical trials. These biomarkers are critical for evaluating the success of mRNA TB vaccines, offering valuable insights into vaccine-induced immunity. Techniques like peripheral blood analysis and enzyme-linked immunosorbent assay are widely used to measure humoral responses[74], while ongoing research aims to discover markers predictive of long-term immunity against TB. Such advancements could enhance mRNA vaccine designs, ensuring durable protection and increasing their overall effectiveness in TB control.
Future directions in TB vaccine research emphasize the transformative potential of mRNA technology to address global health challenges. Inhalable mRNA vaccines are under exploration, offering non-invasive delivery options ideal for respiratory diseases, including TB[75]. Multi-pathogen vaccines could combine protection against TB and other respiratory infections, addressing co-infection challenges and reducing logistical burdens, particularly in high-burden regions. The adaptability of mRNA platforms also enables combination strategies targeting TB-HIV co-infections, providing safer and more effective immunity for immunocompromised populations[76]. Clinical trials in TB-endemic regions are critical to assess the efficacy of these vaccines across diverse populations and environmental conditions, with a focus on equitable access through collaboration with low-income countries. The mRNA’s modularity supports personalized vaccination approaches, tailoring formulations for high-risk groups like children or immunocompromised individuals. Advanced delivery techniques, including aerosol-based systems, promise to enhance vaccine administration. Studies on long-term immunity and booster needs will ensure sustained protection. Establishing standardized manufacturing and regulatory frameworks is essential to scale these innovations safely, signaling a pivotal shift in TB vaccine development and global disease management.
Insights from the WHO meeting on mRNA-based TB vaccine development
The WHO mRNA Technology Transfer Programme meeting held in April 2023 highlighted the promise of mRNA-based platforms for TB vaccine development. Experts, policymakers, and industry representatives collectively acknowledged mRNA’s potential as a complementary tool alongside other vaccine technologies. While success in COVID-19 vaccination underscores the platform's safety and efficacy, rigorous testing in animal models and human trials is crucial to confirm its suitability for TB. The meeting emphasized open data sharing and cross-disciplinary collaboration to accelerate vaccine evaluation. Moreover, enhancing research and manufacturing capacity in low-income and middle-income countries was identified as a priority to ensure equitable access and build trust in mRNA-based solutions. Despite logistical challenges, advancements in antigen selection, immune response studies, and pandemic-driven investment in infrastructure have positioned mRNA vaccines as a viable option for addressing the global TB burden. Recommendations from the meeting advocate for targeted research, consensus-building on antigen selection, and robust community engagement to overcome barriers to TB vaccine development and access[77].