Published online May 27, 2014. doi: 10.5496/wjmg.v4.i2.19
Revised: February 25, 2014
Accepted: March 13, 2014
Published online: May 27, 2014
Processing time: 152 Days and 19.6 Hours
Although safe and effective vaccines against hepatitis B virus (HBV) have been available for three decades, HBV infection remains the leading cause of chronic hepatitis, cirrhosis and hepatocellular carcinoma (HCC) worldwide, especially in Asian countries. HBV has been classified into at least 9 genotypes according to the molecular evolutionary analysis of the genomic DNA sequence and shown to have a distinct geographical distribution. Novel HBV genotypes/subgenotypes have been reported, especially from Southeast Asian countries. The clinical characteristics and therapeutic effectiveness of interferon (IFN) and nucleos(t)ide analogues vary among different HBV genotypes. Mutations at T1653C in subgenotype C2 from Japan and South Korea, C/A1753T and C1858T in subgenotype C1 from Vietnam, and C1638T and T1753V in subgenotype B3 from Indonesia were reported to be associated with advanced liver diseases including HCC. Genotype distribution in Japan has been changed by an increasing ratio of subgenotype A2 in chronic hepatitis B. While a large number of epidemiological and clinical studies have been reported from Asian countries, most of the studies were conducted in developed countries such as Taiwan, China, South Korea and Japan. In this review, the most recent publications on the geographical distribution of genetic variants of HBV and related issues such as disease progression and therapy in Asia are updated and summarized.
Core tip: Chronic hepatitis B virus (HBV) infection usually progresses to liver cirrhosis and hepatocellular carcinoma. The variation of the HBV genotype is related to the geographical distribution. Also, the clinical characteristics and therapeutic effectiveness of interferon and nucleos(t)ide analogue vary among different HBV genotypes. A large number of epidemiological and clinical studies have been reported from Asian countries. However, most of the studies were conducted in developed countries such as Taiwan, China, South Korea and Japan. In this review, epidemiologically and clinically important aspects of HBV genotypes/subgenotypes found in East and Southeast Asian countries are updated and summarized.
- Citation: Utsumi T, Yano Y, Hotta H. Molecular epidemiology of hepatitis B virus in Asia. World J Med Genet 2014; 4(2): 19-26
- URL: https://www.wjgnet.com/2220-3184/full/v4/i2/19.htm
- DOI: https://dx.doi.org/10.5496/wjmg.v4.i2.19
Although safe and effective vaccines against Hepatitis B virus (HBV) have been available for more than three decades, HBV infection remains a burden to global public health, resulting in 600000 to 1 million deaths per year worldwide[1]. Two billion people are estimated to be exposed to HBV infection once in their life and it causes a wide spectrum of liver disease, including acute or fulminant hepatitis, inactive carrier state, reactivation, chronic hepatitis, cirrhosis and hepatocellular carcinoma (HCC)[2]. More than 420 million individuals in the world are estimated to have chronic HBV infections; 15%-40% of them are at risk of death due to liver failure or HCC[3]. The prevalence of HBV infection varies markedly in different geographical areas of the world. Overall, approximately 45% of the global population live in areas of high HBV prevalence, such as sub-Saharan Africa, the Pacific and particularly Asia[4].
HBV has been classified into at least 9 genotypes (A through H and J) and shown to have a distinct geographical distribution[5,6]. In Asia, HBV genotypes B and C are prevalent, with genotype C having been shown to cause more serious liver diseases than genotype B. High prevalence of HBV mutants with various forms, such as the pre-S mutants, basal core promoter (BCP) mutants, YMDD motif mutants and vaccine escape mutants[7,8], were seen in Asia and these were found to be related to severe liver diseases and resistance to treatment and prevention. This article provides an overview of the molecular-based epidemiology of HBV in Asian countries.
HBV contains a partially double-stranded DNA genome of approximately 3200 base pairs. HBV replicates via a RNA intermediate anti-genome sequence, which encodes a potentially error-prone polymerase without proof-reading activity. The error frequencies are similar to those of retroviruses and other RNA viruses. The HBV genome encodes viral proteins through four open and partially overlapping reading frames: surface (S), core (C), polymerase (P) and X genes. This unusual genomic structure can compress a large amount of information into short sequences but implies a constrained evolution for the virus. This constraint can be reflected on the calculated rate of substitution, 10-5 per site per year, slower than the rate displayed by the retroviruses of around 10-3 per site per year[9].
The prevalence of chronic HBV infection varies greatly in different parts of the world and can be categorized as high (≥ 8%), intermediate (2%-7%) and low (< 2%) endemicity. Table 1 shows the prevalence of hepatitis B surface antigen (HBsAg)-positive individuals in the general population of Southeast Asia and East Asia. HBV infection is highly endemic in Myanmar[10]; has intermediate to high endemicity in Indonesia[11-13], Cambodia[10,14], Thailand[15,16], the Philippines[17-19], Vietnam[20,21] and Laos[10,24]; low to high endemicity in Malaysia[22,23] and China[25-27]; and intermediate endemicity in Singapore[28,29], Brunei[30,31] and South Korea[32,33]. Japan is the only country with low endemicity of HBV infection in Asia[22].
| Country | HBsAg positivity (%) | Ref. |
| Southeast Asia | ||
| Brunei | 4.7 | Sebastian et al[30] |
| 6.0 | Alexander et al[31] | |
| Cambodia | 7.7 | OI et al[14] |
| 10.8 | Sa-Nguanmoo et al[10] | |
| Indonesia | 3.5-9.1 | Hasan[11] |
| 4.9 | Achwan et al[12] | |
| 2.1-10.5 | Lusida et al[13] | |
| Laos | 6.9 | Jutavijittum et al[24] |
| 8.7 | Sa-Nguanmoo et al[10] | |
| Malaysia | 3.0-5.0 | Merican et al[22] |
| 0.5-1.8 | Yousuf et al[23] | |
| Myanmar | 9.7 | Sa-Nguanmoo et al[10] |
| Philippines | 10.0 | Lingao et al[17] |
| 2.0-16.0 | Lansang et al[18] | |
| 16.7 | Wong et al[19] | |
| Singapore | 3.6-4.0 | James et al[28] |
| 2.7-4.0 | Ang et al[29] | |
| Thailand | 4.0 | Suwannakarn et al[15] |
| 13.8 | Louisirirotchanakul et al[16] | |
| Vietnam | 11.4 | Viet et al[20] |
| 7.5 | Reekie et al[21] | |
| East Asia | ||
| China | 2.4 | Ting-Lu et al[25] |
| 1.0 | Liu et al[26] | |
| 10.6 | Chen et al[27] | |
| Japan | 0.8 | Merican et al[22] |
| South Korea | 3.0-4.0, 6.0 | Kim et al[32] |
| 6.0 | Hyun et al[33] |
HBV infection is highly endemic in developing regions with a large population such as Southeast Asia and China, where at least 8% of the population are HBV chronic carriers. For example, in Indonesia, which consists of thousands of islands with many ethnicities, the endemicity of HBV infection greatly varies even within the country. The wide range of the HBV prevalence is largely related to differences in age at the time of infection[3].
HBV is currently grouped into at least 9 genotypes (A through H and J, with I still being controversial)[6,34,35], based on a full genome diversity of more than 8% at the nucleotide (nt) level, and phylogenetic analyses have shown that most of the genotypes can be further divided into subgenotypes differing by at least 4% of their full genome sequences. The prevalence of each HBV genotype and subgenotype varies in different geographical regions and is strongly associated with ethnicity[36].
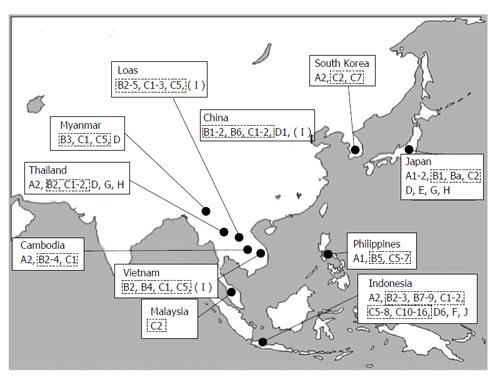
Genotype A is highly prevalent in Sub-Saharan Africa (A1 or Aa: a for Africa), Northern Europe (A2 or Ae; e stands for Europe) and Western Africa (A3). Genotypes B and C are the major HBV genotypes circulating in East and Southeast Asia[37] (Figure 1) and co-infection has led to a frequent occurrence of recombination between these two genotypes[38,39]. Subgenotype B1 (or Bj; j for Japan) is found almost exclusively in Japan and B2 (or Ba; a for Asia) is found in the rest of Asia[40,41], but mainly in China and Vietnam. B1 is not a recombinant while B2 is considered to be B/C recombinants with the precore and core genes from genotype C. B3 is mostly found in Indonesia[42] while B4 is in Vietnam[5]. B5 was initially reported in 2006 from the Philippines[43]. B6 was identified in 2007 from the Arctic[39]. B7 to B9 were isolated in eastern Indonesia during the years 2007 to 2011[44-46]. C1 (or Cs: s for Southeast Asia) is the dominant strain in Southeast Asia and southern China, while C2 (or Ce: e for East Asia) is found mainly in East Asia (South Korea and Japan) and the northern part of China, C3 in Oceania[47] and C4 in the Aborigines from Australia[48]. C5 was initially reported in 2006 from the Philippines with B5[44]. C6 was identified from a Papuan population in Indonesia[13,49] and the Philippines[50] in 2008. Surprisingly, ten novel subgenotypes (C7 to C16) were isolated in Indonesia during 2009 to 2012[45,51-54]. Subgenotypes D1 to D4 of genotype D are widely distributed globally[5], D5 in India[55] and D6 in Papua, Indonesia[13]. Genotype E is found mainly in sub-Saharan Africa. Genotypes F and H are found mainly in South and Central America, respectively. Genotype G has been found in Europe, United States and Japan. Genotype I was originally identified in Laos[56], Vietnam and Southern China. However, this classification is still controversial as the sequence divergence hovers at but is slightly less than 8%, with a close relationship to genotype C[35]. Genotype J was found in a Japanese soldier who was thought to have been infected in the forests in Kalimantan, Indonesia, during World War II[57]. Thus, novel HBV genotypes and novel subgenotypes have been found in Southeast Asia, especially in Laos, Vietnam, the Philippines and Indonesia, all consisting of many islands and ethnic groups. In addition to genotypes B and C which are common in Asia, an increasing rate of infection with rare HBV genotypes, such as genotypes A, D, E, G and H, has been recognized throughout Asia. Globalization may yield HBV strains of possible novel genotypes containing novel nucleotide sequences in the precore/core region[58]. The distribution of genotypes/subgenotypes varies even in different regions of a country, as observed in Indonesia, which may partly be related to the ethnic origin of the infected patients.
Chronic HBV infections usually progress to liver cirrhosis and HCC. Several studies revealed that the presence of hepatitis B e antigen (HBeAg) and high levels of HBV DNA were independent risk factors for the development of liver cirrhosis and HCC[59-62]. HBV genotypes are also related to the clinical characteristics[63]. In northeast Asian countries, where genotypes B and C are prevalent, the dominant mode of transmission is vertical (mother-to-child). A large number of studies have shown that genotype B is associated with HBeAg seroconversion at an earlier age, more sustained remission after HBeAg seroconversion, less active hepatic necroinflammation, a slower rate of progression to cirrhosis, and a lower rate of HCC development compared to genotype C[59,64-67]. On the other hand, genotypes D and A are prevalent in the southwest Asian countries, such as India and Pakistan[68]. The transmission route among Pakistanis, including Afghan refugees, is not only vertical transmission but also through unsterilized materials and intravenous drug use[69,70]. Reports concerning the risk factors of advanced liver diseases are still limited in those countries.
Mutations in the viral genome, including the X region, are also important factors in association with disease progression. A study from Taiwan revealed that the precore G1896A wild-type and the BCP A1762T/G1764A mutation were strongly associated with HCC development among genotype C[71]. A study from north India also showed that the BCP A1762T/G1764A mutation was associated with progressive liver diseases among genotype D[72]. In Japan and South Korea, the T1653C mutation was reported as a predictive factor for the development of advanced liver diseases in HBV genotype C2 infection[73,74]. Whereas the C/A1753T and C1858T mutations were associated with advanced liver diseases in genotype C1 infection in Vietnam, C1638T and T1753V were independent risk factors for advanced liver diseases in genotype B3 infection in Indonesia[42,75]. In addition, several studies from Taiwan and Japan showed that the pre-S mutation also contributed to the progressive liver disease and HCC[76,77]. The progression from acute hepatitis to chronic infection occurs more frequently in genotype A (23%) compared with genotypes B (11%) and C (7%)[78]. This might change genotype distribution in the future. In Japan, indeed, the prevalence of genotype A in chronic hepatitis B increased from 1.7% to 3.5% during the period between 2000 and 2006[79].
The purpose of antiviral therapy for chronic hepatitis B is the sustained suppression of HBV replication, biochemical remission, HBeAg seroconversion and ultimately HBsAg seroconversion. The annual rate of spontaneous HBsAg seroclearance is approximately 0.4%-2.3%, and the HBsAg seroclearance rates of genotypes A and B are higher than that of genotypes C and D[80,81].
Interferon (IFN) and nucleos(t)ide analogues (NA) are commonly used for the treatment of chronic hepatitis B. Antiviral regimens for chronic hepatitis B are decided based on the age, HBV-DNA viral load, alanine aminotransferase (ALT) levels and the degree of fibrosis. In general, younger patients with high ALT levels are recommended to be treated with IFN therapy and older and/or clinically advanced patients with NA. Due to the economic growth, the treatment of chronic hepatitis B has become universal in most developed and developing Asian countries. However, most of the clinical studies about antiviral therapy were reported from developed countries, with few studies being reported from developing countries. IFN has antiviral, antiproliferative and immunomodulatory effects. The response to IFN treatment is poorer in Asian patients compared with Caucasian patients, which may be due partly to the difference in the genotype distribution[82]. It was shown that patients infected with HBV genotypes A and B showed better response than those with genotypes C and D[83-87]. A meta-analysis also revealed that IFN therapy was more effective in patients infected with genotype A than in those with genotype D, and also more effective in genotype B than in genotype C infection[88].
Currently, lamivudine, adefovir, entecavir, telbivudine and tenofovir have been approved for the treatment of chronic hepatitis B (Table 2). Lamivudine (Zeffix®) was first introduced in 1999 and the clinical efficacy was shown by a long-term follow-up study[93,94]. However, drug-resistant mutations, especially multidrug-resistant mutations, are the major concern with patients receiving long-term NA treatment. It was reported that the drug resistance against lamivudine monotherapy reached 70% after 4 years of treatment[95,96]. Entecavir (Baraclude®) is widely used and a first-line drug in many Asian countries, including China, South Korea, Thailand, Hong Kong and Japan. Entecavir is still expensive but the occurrence of drug resistance is very low for naïve patients. However, the chemical structure of entecavir is similar to lamivudine, which resulted in the cross-resistance between lamivudine and entecavir. Recent long-term follow up studies conducted in South Korea and Hong Kong revealed that entecavir reduced liver-related death and HCC[97,98]. Adefovir (Hepsera®) is effective against lamivudine-resistant mutants and add-on therapy of adefovir and lamivudine is common for suppression of lamivudine-resistant mutants. Tenofovir (Viread®) and telbivudine (Sebivo®) are also safe and effective drugs but their introduction to clinical use is still limited. Telbivudine has recently been approved and is being used as a first-line drug in Indonesia. Unlike IFN therapy, meta-analysis revealed no significant difference between genotypes and response to NA[88]. However, as entecavir and telbivudine were introduced recently in developing countries, further studies will be needed to assess their efficacy against the different HBV genotypes/subgenotypes prevailing in those countries.
| Lamivudine | Adefovir | Entecavir | Telbivudine | Tenofovir | Ref. | |
| Analogue type | Nucleoside | Nucleotide | Nucleoside | Nucleoside | Nucleotide | |
| Introduction (yr) | 1999 | 2002 | 2005 | 2006 | 2008 | |
| Product name (company) | Zefix (GSK) | Hepsera (Gilead) | Baraclude (BMS) | Sebivo (Novartis) | Viread (Gilead) | |
| Dose | 100 mg | 10 mg | 0.5 mg | 600 mg | 300 mg | |
| Once daily | Once daily | Once daily | Once daily | Once daily | ||
| Advantage | Low cost | Effective for HIV coinfection | Possible for pregnancy | Effective for HIV coinfection | [89] | |
| Disadvantage | High rate of drug resistance | Renal dysfunction Fanconi anemia | Not recommend for pregnancy | Renal dysfunction | Renal dysfunction Fanconi anemia | |
| Undetectable HBV-DNA | ||||||
| HBeAg positive | 36% | 21% | 67% | 60% | 76% | [90] |
| HBeAg negative | 89% | 72% | 90% | 88% | 93% | |
| HBeAg seroconversion | 22% | 12% | 21% | 23% | 21% | [91] |
| Drug-resistance | 24% | 0% | 0.2% | 4% | 0% | [92] |
| Drug-resistant mutation | V173l, L180M, A181T, M204V/I | A181V/T, N236T | I169T, L180M, T184A/F/L/S, S202G/I, M204V, M250V | M204V/I | A181V/T, N236T |
HBV is widespread in Asian countries and contributes to the mortality from HCC. To reduce HBV infection and HCC mortality, appropriate national immunization programs are required in HBV-endemic countries, including Japan. Although HBV infection is predominant and a number of novel genotypes/subgenotypes have been discovered in Asian countries, studies have not been sufficient regarding disease prognosis and antiviral treatment. It is possible that certain genotypes or variants of HBV prevailing in these regions possess stronger pathogenicity and are associated with more severe outcomes of liver diseases. The studies on HBV genotypes related to their pathogenicity in chronic liver diseases, including liver cirrhosis and HCC, and their effects on treatment outcome are awaited with great interest, especially in Southeast Asia, which is the most endemic region of HBV in Asia with unique HBV genotypes/subgenotypes.
| 1. | Kao JH, Chen DS. Global control of hepatitis B virus infection. Lancet Infect Dis. 2002;2:395-403. [PubMed] |
| 2. | Mizokami M. C hanging concept of hepatitis B virus. ISBT Science Series. 2009;4:192. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | Lok AS. Chronic hepatitis B. N Engl J Med. 2002;346:1682-1683. [PubMed] |
| 4. | Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11:97-107. [PubMed] |
| 5. | Norder H, Couroucé AM, Coursaget P, Echevarria JM, Lee SD, Mushahwar IK, Robertson BH, Locarnini S, Magnius LO. Genetic diversity of hepatitis B virus strains derived worldwide: genotypes, subgenotypes, and HBsAg subtypes. Intervirology. 2004;47:289-309. [PubMed] |
| 6. | Locarnini S, Littlejohn M, Aziz MN, Yuen L. Possible origins and evolution of the hepatitis B virus (HBV). Semin Cancer Biol. 2013;23:561-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 7. | Utsumi T, Yano Y, Lusida MI, Nasronudin M, Juniastuti H, Hayashi Y. Detection of highly prevalent hepatitis B virus co-infection with HIV in Indonesia. Hepatol Res. 2013;43:1032-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Utsumi T, Yano Y, Lusida MI, Amin M, Soetjipto H, Hayashi Y. Serologic and molecular characteristics of hepatitis B virus among school children in East Java, Indonesia. Am J Trop Med Hyg. 2010;83:189-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 9. | Mizokami M, Orito E, Ohba K, Ikeo K, Lau JY, Gojobori T. Constrained evolution with respect to gene overlap of hepatitis B virus. J Mol Evol. 1997;44 Suppl 1:S83-S90. [PubMed] |
| 10. | Sa-Nguanmoo P, Tangkijvanich P, Thawornsuk N, Vichaiwattana P, Prianantathavorn K, Theamboonlers A, Tanaka Y, Poovorawan Y. Molecular epidemiological study of hepatitis B virus among migrant workers from Cambodia, Laos, and Myanmar to Thailand. J Med Virol. 2010;82:1341-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Hasan I. Epidemiology of hepatitis B. Acta Med Indones. 2005;37:231-234. [PubMed] |
| 12. | Achwan WA, Muttaqin Z, Zakaria E, Depamede SA, Mulyanto S, Tsuda F, Takahashi K, Abe N, Mishiro S. Epidemiology of hepatitis B, C, and E viruses and human immunodeficiency virus infections in Tahuna, Sangihe-Talaud Archipelago, Indonesia. Intervirology. 2007;50:408-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Lusida MI, Nugrahaputra VE, Soetjipto R, Nagano-Fujii M, Sasayama M, Utsumi T, Hotta H. Novel subgenotypes of hepatitis B virus genotypes C and D in Papua, Indonesia. J Clin Microbiol. 2008;46:2160-2166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 70] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Ol HS, Bjoerkvoll B, Sothy S, Van Heng Y, Hoel H, Husebekk A, Gutteberg T, Larsen S, Husum H. Prevalence of hepatitis B and hepatitis C virus infections in potential blood donors in rural Cambodia. Southeast Asian J Trop Med Public Health. 2009;40:963-971. [PubMed] |
| 15. | Suwannakarn K, Tangkijvanich P, Thawornsuk N, Theamboonlers A, Tharmaphornpilas P, Yoocharoen P, Chongsrisawat V, Poovorawan Y. Molecular epidemiological study of hepatitis B virus in Thailand based on the analysis of pre-S and S genes. Hepatol Res. 2008;38:244-251. [PubMed] |
| 16. | Louisirirotchanakul S, Olinger CM, Arunkaewchaemsri P, Poovorawan Y, Kanoksinsombat C, Thongme C, Sa-Nguanmoo P, Krasae S, Theamboonlert A, Oota S. The distribution of hepatitis B virus genotypes in Thailand. J Med Virol. 2012;84:1541-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Lingao AL, Torres NT, Muñoz N, Lansang MA, West SK, Bosch FX, Domingo EO. Mother to child transmission of hepatitis B virus in the Philippines. Infection. 1989;17:275-279. [PubMed] |
| 18. | Lansang MA. Epidemiology and control of hepatitis B infection: a perspective from the Philippines, Asia. Gut. 1996;38 Suppl 2:S43-S47. [PubMed] |
| 19. | Wong SN, Ong JP, Labio ME, Cabahug OT, Daez ML, Valdellon EV, Sollano JD, Arguillas MO. Hepatitis B infection among adults in the philippines: A national seroprevalence study. World J Hepatol. 2013;5:214-219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 20. | Viet L, Lan NT, Ty PX, Björkvoll B, Hoel H, Gutteberg T, Husebekk A, Larsen S, Skjerve E, Husum H. Prevalence of hepatitis B & hepatitis C virus infections in potential blood donors in rural Vietnam. Indian J Med Res. 2012;136:74-81. [PubMed] |
| 21. | Reekie J, Gidding HF, Kaldor JM, Liu B. Country of birth and other factors associated with hepatitis B prevalence in a population with high levels of immigration. J Gastroenterol Hepatol. 2013;28:1539-1544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Merican I, Guan R, Amarapuka D, Alexander MJ, Chutaputti A, Chien RN, Hasnian SS, Leung N, Lesmana L, Phiet PH. Chronic hepatitis B virus infection in Asian countries. J Gastroenterol Hepatol. 2000;15:1356-1361. [PubMed] |
| 23. | Yousuf R, Rapiaah M, Ahmed SA, Rosline H, Salam A, Selamah S, Roshan TM. Trends in hepatitis B virus infection among blood donors in Kelantan, Malaysia: a retrospective study. Southeast Asian J Trop Med Public Health. 2007;38:1070-1074. [PubMed] |
| 24. | Jutavijittum P, Yousukh A, Samountry B, Samountry K, Ounavong A, Thammavong T, Keokhamphue J, Toriyama K. Seroprevalence of hepatitis B and C virus infections among Lao blood donors. Southeast Asian J Trop Med Public Health. 2007;38:674-679. [PubMed] |
| 25. | Ting-Lu Z, Zhi-Ping X, Hong-Yu L, Chang-Hong G, Liang Y, Qiang D, Kai-Ling X, Yan-Ming M, Yue-He D, Ling-Yang Z. A community-based sero-epidemiological study of hepatitis B infection in Lianyungang, China, 2010. Western Pac Surveill Response J. 2012;3:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Liu GC, Sui GY, Liu GY, Zheng Y, Deng Y, Gao YY, Wang L. A Bayesian meta-analysis on prevalence of hepatitis B virus infection among Chinese volunteer blood donors. PLoS One. 2013;8:e79203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Chen P, Yu C, Ruan B, Yang S, Ren J, Xu W, Luo Z, Li L. Prevalence of hepatitis B in insular regions of southeast China: a community-based study. PLoS One. 2013;8:e56444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 28. | James L, Fong CW, Foong BH, Wee MK, Chow A, Shum E, Chew SK. Hepatitis B Seroprevalence Study 1999. Singapore Med J. 2001;42:420-424. [PubMed] |
| 29. | Ang LW, Cutter J, James L, Goh KT. Seroepidemiology of hepatitis B virus infection among adults in Singapore: a 12-year review. Vaccine. 2013;32:103-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Sebastian VJ, Bhattacharya S, Ray S, Ahmad MZ. Hepatitis-B surface antigen and VDRL in healthy blood donors of Brunei Darussalam. Singapore Med J. 1989;30:568-570. [PubMed] |
| 31. | Alexander MJ, Sinnatamby AS, Rohaimah MJ, Harun AH, Ng JS. Incidence of hepatitis B infection in Brunei Darussalam--analysis of racial distribution. Ann Acad Med Singapore. 1990;19:344-346. [PubMed] |
| 32. | Kim H, Shin AR, Chung HH, Kim MK, Lee JS, Shim JJ, Kim BH. Recent trends in hepatitis B virus infection in the general Korean population. Korean J Intern Med. 2013;28:413-419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 33. | Hyun HJ, Shim JJ, Kim JW, Lee JS, Lee CK, Jang JY, Kim BH. The Prevalence of Elevated Alanine Transaminase and its Possible Causes in the General Korean Population. J Clin Gastroenterol. 2013;Epub ahead of print. [PubMed] |
| 34. | Roman S, Panduro A. HBV endemicity in Mexico is associated with HBV genotypes H and G. World J Gastroenterol. 2013;19:5446-5453. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 44] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | Seeger C, Zoulim F, Mason WS. Hepadnaviruses. Fields Virology. Philadelphia: Lippincott, Williams and Wilkins 2013; 2185-2221. |
| 36. | Lindh M, Andersson AS, Gusdal A. Genotypes, nt 1858 variants, and geographic origin of hepatitis B virus--large-scale analysis using a new genotyping method. J Infect Dis. 1997;175:1285-1293. [PubMed] |
| 37. | Norder H, Hammas B, Lee SD, Bile K, Couroucé AM, Mushahwar IK, Magnius LO. Genetic relatedness of hepatitis B viral strains of diverse geographical origin and natural variations in the primary structure of the surface antigen. J Gen Virol. 1993;74:1341-1348. [PubMed] |
| 38. | Sugauchi F, Orito E, Ichida T, Kato H, Sakugawa H, Kakumu S, Ishida T, Chutaputti A, Lai CL, Ueda R. Hepatitis B virus of genotype B with or without recombination with genotype C over the precore region plus the core gene. J Virol. 2002;76:5985-5992. [PubMed] |
| 39. | Sakamoto T, Tanaka Y, Simonetti J, Osiowy C, Borresen ML, Koch A, Kurbanov F, Sugiyama M, Minuk GY, McMahon BJ. Classification of hepatitis B virus genotype B into 2 major types based on characterization of a novel subgenotype in Arctic indigenous populations. J Infect Dis. 2007;196:1487-1492. [PubMed] |
| 40. | Orito E, Ichida T, Sakugawa H, Sata M, Horiike N, Hino K, Okita K, Okanoue T, Iino S, Tanaka E. Geographic distribution of hepatitis B virus (HBV) genotype in patients with chronic HBV infection in Japan. Hepatology. 2001;34:590-594. [PubMed] |
| 41. | Chan HL. JGH Foundation emerging leadership lecture. Significance of hepatitis B virus genotypes and mutations in the development of hepatocellular carcinoma in Asia. J Gastroenterol Hepatol. 2011;26:8-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 42. | Heriyanto DS, Yano Y, Utsumi T, Anggorowati N, Rinonce HT, Lusida MI, Soetjipto C, Ratnasari N, Maduseno S, Purnama PB. Mutations within enhancer II and BCP regions of hepatitis B virus in relation to advanced liver diseases in patients infected with subgenotype B3 in Indonesia. J Med Virol. 2012;84:44-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 43. | Sakamoto T, Tanaka Y, Orito E, Co J, Clavio J, Sugauchi F, Ito K, Ozasa A, Quino A, Ueda R. Novel subtypes (subgenotypes) of hepatitis B virus genotypes B and C among chronic liver disease patients in the Philippines. J Gen Virol. 2006;87:1873-1882. [PubMed] |
| 44. | Nurainy N, Muljono DH, Sudoyo H, Marzuki S. Genetic study of hepatitis B virus in Indonesia reveals a new subgenotype of genotype B in east Nusa Tenggara. Arch Virol. 2008;153:1057-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 45. | Mulyanto SN, Surayah K, Tsuda F, Ichiyama K, Takahashi M, Okamoto H. A nationwide molecular epidemiological study on hepatitis B virus in Indonesia: identification of two novel subgenotypes, B8 and C7. Arch Virol. 2009;154:1047-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 46. | Thedja MD, Muljono DH, Nurainy N, Sukowati CH, Verhoef J, Marzuki S. Ethnogeographical structure of hepatitis B virus genotype distribution in Indonesia and discovery of a new subgenotype, B9. Arch Virol. 2011;156:855-868. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 47. | Utsumi T, Yano Y, Truong BX, Tanaka Y, Mizokami M, Seo Y, Kasuga M, Kawabata M, Hayashi Y. Molecular epidemiological study of hepatitis B virus infection in two different ethnic populations from the Solomon Islands. J Med Virol. 2007;79:229-235. [PubMed] |
| 48. | Sugauchi F, Mizokami M, Orito E, Ohno T, Kato H, Suzuki S, Kimura Y, Ueda R, Butterworth LA, Cooksley WG. A novel variant genotype C of hepatitis B virus identified in isolates from Australian Aborigines: complete genome sequence and phylogenetic relatedness. J Gen Virol. 2001;82:883-892. [PubMed] |
| 49. | Utsumi T, Lusida MI, Yano Y, Nugrahaputra VE, Amin M, Juniastuti Y, Hotta H. Complete genome sequence and phylogenetic relatedness of hepatitis B virus isolates in Papua, Indonesia. J Clin Microbiol. 2009;47:1842-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 50. | Cavinta L, Sun J, May A, Yin J, von Meltzer M, Radtke M, Barzaga NG, Cao G, Schaefer S. A new isolate of hepatitis B virus from the Philippines possibly representing a new subgenotype C6. J Med Virol. 2009;81:983-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 51. | Mulyanto SN, Surayah K, Tjahyono AA, Jirintai S, Takahashi M, Okamoto H. Identification and characterization of novel hepatitis B virus subgenotype C10 in Nusa Tenggara, Indonesia. Arch Virol. 2010;155:705-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 52. | Mulyanto SN, Wahyono A, Jirintai S, Takahashi M, Okamoto H. Analysis of the full-length genomes of novel hepatitis B virus subgenotypes C11 and C12 in Papua, Indonesia. J Med Virol. 2011;83:54-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 53. | Utsumi T, Nugrahaputra VE, Amin M, Hayashi Y, Hotta H, Lusida MI. Another novel subgenotype of hepatitis B virus genotype C from papuans of Highland origin. J Med Virol. 2011;83:225-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 54. | Mulyanto P, Depamede SN, Wahyono A, Jirintai S, Nagashima S, Takahashi M, Nishizawa T, Okamoto H. Identification of four novel subgenotypes (C13-C16) and two inter-genotypic recombinants (C12/G and C13/B3) of hepatitis B virus in Papua province, Indonesia. Virus Res. 2012;163:129-140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 55. | Banerjee A, Kurbanov F, Datta S, Chandra PK, Tanaka Y, Mizokami M, Chakravarty R. Phylogenetic relatedness and genetic diversity of hepatitis B virus isolates in Eastern India. J Med Virol. 2006;78:1164-1174. [PubMed] |
| 56. | Olinger CM, Jutavijittum P, Hübschen JM, Yousukh A, Samountry B, Thammavong T, Toriyama K, Muller CP. Possible new hepatitis B virus genotype, southeast Asia. Emerg Infect Dis. 2008;14:1777-1780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 139] [Cited by in RCA: 145] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 57. | Tatematsu K, Tanaka Y, Kurbanov F, Sugauchi F, Mano S, Maeshiro T, Nakayoshi T, Wakuta M, Miyakawa Y, Mizokami M. A genetic variant of hepatitis B virus divergent from known human and ape genotypes isolated from a Japanese patient and provisionally assigned to new genotype J. J Virol. 2009;83:10538-10547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 338] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 58. | Uchida Y, Kouyama JI, Naiki K, Sugawara K, Inao M, Nakayama N, Mochida S. Novel hepatitis B virus strain developing due to recombination between genotypes H and B strains isolated from a Japanese patient. Hepatol Res. 2013;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 59. | Yu MW, Yeh SH, Chen PJ, Liaw YF, Lin CL, Liu CJ, Shih WL, Kao JH, Chen DS, Chen CJ. Hepatitis B virus genotype and DNA level and hepatocellular carcinoma: a prospective study in men. J Natl Cancer Inst. 2005;97:265-272. [PubMed] |
| 60. | Yang HI, Lu SN, Liaw YF, You SL, Sun CA, Wang LY, Hsiao CK, Chen PJ, Chen DS, Chen CJ, Taiwan Community-Based Cancer Screening Project Group. Hepatitis B e antigen and the risk of hepatocellular carcinoma. N Engl J Med. 2002;347:168-174. [PubMed] |
| 61. | Iloeje UH, Yang HI, Su J, Jen CL, You SL, Chen CJ, Risk Evaluation of Viral Load Elevation and Associated Liver Disease/Cancer-In HBV (the REVEAL-HBV) Study Group. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology. 2006;130:678-686. [PubMed] |
| 62. | Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA. 2006;295:65-73. [PubMed] |
| 63. | Ogawa M, Hasegawa K, Naritomi T, Torii N, Hayashi N. Clinical features and viral sequences of various genotypes of hepatitis B virus compared among patients with acute hepatitis B. Hepatol Res. 2002;23:167-177. [PubMed] |
| 64. | Chan HL, Hui AY, Wong ML, Tse AM, Hung LC, Wong VW, Sung JJ. Genotype C hepatitis B virus infection is associated with an increased risk of hepatocellular carcinoma. Gut. 2004;53:1494-1498. [PubMed] |
| 65. | Chu CJ, Hussain M, Lok AS. Hepatitis B virus genotype B is associated with earlier HBeAg seroconversion compared with hepatitis B virus genotype C. Gastroenterology. 2002;122:1756-1762. [PubMed] |
| 66. | Chu CM, Liaw YF. Genotype C hepatitis B virus infection is associated with a higher risk of reactivation of hepatitis B and progression to cirrhosis than genotype B: a longitudinal study of hepatitis B e antigen-positive patients with normal aminotransferase levels at baseline. J Hepatol. 2005;43:411-417. [PubMed] |
| 67. | Sumi H, Yokosuka O, Seki N, Arai M, Imazeki F, Kurihara T, Kanda T, Fukai K, Kato M, Saisho H. Influence of hepatitis B virus genotypes on the progression of chronic type B liver disease. Hepatology. 2003;37:19-26. [PubMed] |
| 68. | Idrees M, Khan S, Riazuddin S. Common genotypes of hepatitis B virus. J Coll Physicians Surg Pak. 2004;14:344-347. [PubMed] |
| 69. | Masood Z, Jawaid M, Khan RA, Rehman S. Screening for hepatitis B and C: A routine preoperative investigation. Pak J Med Sci. 2005;21:455-459. |
| 70. | Strathdee SA, Zafar T, Brahmbhatt H, Baksh A, ul Hassan S. Rise in needle sharing among injection drug users in Pakistan during the Afghanistan war. Drug Alcohol Depend. 2003;71:17-24. [PubMed] |
| 71. | Yang HI, Yeh SH, Chen PJ, Iloeje UH, Jen CL, Su J, Wang LY, Lu SN, You SL, Chen DS, Liaw YF, Chen CJ, REVEAL-HBV Study Group. Associations between hepatitis B virus genotype and mutants and the risk of hepatocellular carcinoma. J Natl Cancer Inst. 2008;100:1134-1143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 447] [Cited by in RCA: 485] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 72. | Sharma S, Sharma B, Singla B, Chawla YK, Chakraborti A, Saini N, Duseja A, Das A, Dhiman RK. Clinical significance of genotypes and precore/basal core promoter mutations in HBV related chronic liver disease patients in North India. Dig Dis Sci. 2010;55:794-802. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 73. | Tanaka Y, Mukaide M, Orito E, Yuen MF, Ito K, Kurbanov F, Sugauchi F, Asahina Y, Izumi N, Kato M. Specific mutations in enhancer II/core promoter of hepatitis B virus subgenotypes C1/C2 increase the risk of hepatocellular carcinoma. J Hepatol. 2006;45:646-653. [PubMed] |
| 74. | Kim JK, Chang HY, Lee JM, Baatarkhuu O, Yoon YJ, Park JY, Kim do Y, Han KH, Chon CY, Ahn SH. Specific mutations in the enhancer II/core promoter/precore regions of hepatitis B virus subgenotype C2 in Korean patients with hepatocellular carcinoma. J Med Virol. 2009;81:1002-1008. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 75. | Truong BX, Yano Y, Seo Y, Phuong TM, Tanaka Y, Kato H, Miki A, Utsumi T, Azuma T, Trach NK. Variations in the core promoter/pre-core region in HBV genotype C in Japanese and Northern Vietnamese patients. J Med Virol. 2007;79:1293-1304. [PubMed] |
| 76. | Liu S, Zhang H, Gu C, Yin J, He Y, Xie J, Cao G. Associations between hepatitis B virus mutations and the risk of hepatocellular carcinoma: a meta-analysis. J Natl Cancer Inst. 2009;101:1066-1082. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 279] [Cited by in RCA: 332] [Article Influence: 19.5] [Reference Citation Analysis (1)] |
| 77. | Sugauchi F, Ohno T, Orito E, Sakugawa H, Ichida T, Komatsu M, Kuramitsu T, Ueda R, Miyakawa Y, Mizokami M. Influence of hepatitis B virus genotypes on the development of preS deletions and advanced liver disease. J Med Virol. 2003;70:537-544. [PubMed] |
| 78. | Suzuki Y, Kobayashi M, Ikeda K, Suzuki F, Arfase Y, Akuta N, Hosaka T, Saitoh S, Kobayashi M, Someya T. Persistence of acute infection with hepatitis B virus genotype A and treatment in Japan. J Med Virol. 2005;76:33-39. [PubMed] |
| 79. | Matsuura K, Tanaka Y, Hige S, Yamada G, Murawaki Y, Komatsu M, Kuramitsu T, Kawata S, Tanaka E, Izumi N. Distribution of hepatitis B virus genotypes among patients with chronic infection in Japan shifting toward an increase of genotype A. J Clin Microbiol. 2009;47:1476-1483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 98] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 80. | Sánchez-Tapias JM, Costa J, Mas A, Bruguera M, Rodés J. Influence of hepatitis B virus genotype on the long-term outcome of chronic hepatitis B in western patients. Gastroenterology. 2002;123:1848-1856. [PubMed] |
| 81. | Yuen MF, Wong DK, Sablon E, Tse E, Ng IO, Yuan HJ, Siu CW, Sander TJ, Bourne EJ, Hall JG. HBsAg seroclearance in chronic hepatitis B in the Chinese: virological, histological, and clinical aspects. Hepatology. 2004;39:1694-1701. [PubMed] |
| 82. | Lok AS, Lai CL, Wu PC, Leung EK. Long-term follow-up in a randomised controlled trial of recombinant alpha 2-interferon in Chinese patients with chronic hepatitis B infection. Lancet. 1988;2:298-302. [PubMed] |
| 83. | Janssen HL, van Zonneveld M, Senturk H, Zeuzem S, Akarca US, Cakaloglu Y, Simon C, So TM, Gerken G, de Man RA, Niesters HG, Zondervan P, Hansen B, Schalm SW, HBV 99-01 Study Group; Rotterdam Foundation for Liver Research. Pegylated interferon alfa-2b alone or in combination with lamivudine for HBeAg-positive chronic hepatitis B: a randomised trial. Lancet. 2005;365:123-129. [PubMed] |
| 84. | Perrillo RP, Schiff ER, Davis GL, Bodenheimer HC, Lindsay K, Payne J, Dienstag JL, O’Brien C, Tamburro C, Jacobson IM. A randomized, controlled trial of interferon alfa-2b alone and after prednisone withdrawal for the treatment of chronic hepatitis B. The Hepatitis Interventional Therapy Group. N Engl J Med. 1990;323:295-301. [PubMed] |
| 85. | Lok AS, Wu PC, Lai CL, Lau JY, Leung EK, Wong LS, Ma OC, Lauder IJ, Ng CP, Chung HT. A controlled trial of interferon with or without prednisone priming for chronic hepatitis B. Gastroenterology. 1992;102:2091-2097. [PubMed] |
| 86. | Wai CT, Chu CJ, Hussain M, Lok AS. HBV genotype B is associated with better response to interferon therapy in HBeAg(+) chronic hepatitis than genotype C. Hepatology. 2002;36:1425-1430. [PubMed] |
| 87. | Erhardt A, Blondin D, Hauck K, Sagir A, Kohnle T, Heintges T, Häussinger D. Response to interferon alfa is hepatitis B virus genotype dependent: genotype A is more sensitive to interferon than genotype D. Gut. 2005;54:1009-1013. [PubMed] |
| 88. | Wiegand J, Hasenclever D, Tillmann HL. Should treatment of hepatitis B depend on hepatitis B virus genotypes? A hypothesis generated from an explorative analysis of published evidence. Antivir Ther. 2008;13:211-220. [PubMed] |
| 89. | Deng M, Zhou X, Gao S, Yang SG, Wang B, Chen HZ, Ruan B. The effects of telbivudine in late pregnancy to prevent intrauterine transmission of the hepatitis B virus: a systematic review and meta-analysis. Virol J. 2012;9:185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 54] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 90. | Lai CL, Yuen MF. Chronic hepatitis B--new goals, new treatment. N Engl J Med. 2008;359:2488-2491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 86] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 91. | Lau GK. Current treatments for patients with HBeAg-positive chronic hepatitis B virus infection: a comparison focusing on HBeAg seroconversion. Liver Int. 2010;30:512-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 92. | European Association For The Study Of The Liver. EASL Clinical Practice Guidelines: management of chronic hepatitis B. J Hepatol. 2009;50:227-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1152] [Cited by in RCA: 1156] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 93. | Eun JR, Lee HJ, Kim TN, Lee KS. Risk assessment for the development of hepatocellular carcinoma: according to on-treatment viral response during long-term lamivudine therapy in hepatitis B virus-related liver disease. J Hepatol. 2010;53:118-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 94. | Kurokawa M, Hiramatsu N, Oze T, Yakushijin T, Miyazaki M, Hosui A, Miyagi T, Yoshida Y, Ishida H, Tatsumi T. Long-term effect of lamivudine treatment on the incidence of hepatocellular carcinoma in patients with hepatitis B virus infection. J Gastroenterol. 2012;47:577-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 95. | Lok AS, Zoulim F, Locarnini S, Bartholomeusz A, Ghany MG, Pawlotsky JM, Liaw YF, Mizokami M, Kuiken C, Hepatitis B Virus Drug Resistance Working Group. Antiviral drug-resistant HBV: standardization of nomenclature and assays and recommendations for management. Hepatology. 2007;46:254-265. [PubMed] |
| 96. | Chang TT, Lai CL, Chien RN, Guan R, Lim SG, Lee CM, Ng KY, Nicholls GJ, Dent JC, Leung NW. Four years of lamivudine treatment in Chinese patients with chronic hepatitis B. J Gastroenterol Hepatol. 2004;19:1276-1282. [PubMed] |
| 97. | Jin YJ, Shim JH, Lee HC, Yoo DJ, Kim KM, Lim YS, Suh DJ. Suppressive effects of entecavir on hepatitis B virus and hepatocellular carcinoma. J Gastroenterol Hepatol. 2011;26:1380-1388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 98. | Wong GL, Chan HL, Mak CW, Lee SK, Ip ZM, Lam AT, Iu HW, Leung JM, Lai JW, Lo AO. Entecavir treatment reduces hepatic events and deaths in chronic hepatitis B patients with liver cirrhosis. Hepatology. 2013;58:1537-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 403] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
P- Reviewers: Narciso-Schiavon JL, Sandblom G S- Editor: Zhai HH L- Editor: Roemmele A E- Editor: Liu SQ