Published online Nov 25, 2015. doi: 10.5495/wjcid.v5.i4.59
Peer-review started: July 15, 2015
First decision: August 25, 2015
Revised: August 28, 2015
Accepted: October 16, 2015
Article in press: October 19, 2015
Published online: November 25, 2015
Processing time: 139 Days and 16.5 Hours
Hepatocellular carcinoma (HCC) is the third most frequent oncological cause of death worldwide, principally a consequence of hepatitis C virus (HCV) infection and its prognosis is mostly poor. For early identification and surveillance of HCV patients with liver disease progression, the availability of suitable diagnostic and prognostic biomarkers is still an unmet clinical need. Alfa-fetoprotein together with imaging techniques is commonly used, however its specificity and sensitivity are not satisfactory. Several clinical and serological data have been proposed to define the risk of disease progression in HCV infected patients and new biomarkers have been proposed, including post-transcriptionally modified molecules and genetic biomarkers. The present editorial article attempts to summarize the current knowledge on the new promising tools for effective early diagnosis of HCV-related liver disease progression and for the surveillance of HCC.
Core tip: Hepatitis C virus (HCV) infection is a major cause of cirrhosis and hepatocellular carcinoma (HCC), leading to liver failure and/or liver transplantation. The current knowledge on the new promising biomarkers, able to predict the progression of HCV-related liver disease and HCC, has been the focus of this editorial.
- Citation: Biasiolo A, Martini A, Pontisso P. New biomarkers for clinical management of hepatitis C virus infected patients. World J Clin Infect Dis 2015; 5(4): 59-66
- URL: https://www.wjgnet.com/2220-3176/full/v5/i4/59.htm
- DOI: https://dx.doi.org/10.5495/wjcid.v5.i4.59
To date, the natural history of hepatitis C virus (HCV) infection is still difficult to define because of the insidious onset of the disease and the absence or paucity of symptoms during the generally prolonged (20-40 years) chronic phase of the illness. Moreover, the methodologic differences used to study the clinical course of disease (prospective-retrospective cohort, community-based or cross-sectional studies), in addition to the different selected study populations (patients referring to specialist liver clinics or tertiary care centers, blood donors) or different phases of the disease, determine heterogeneous results in terms of rates of disease progression[1-6]. The ideal study to define the natural history of HCV infection would be to closely monitor a representative group of patients from the onset of the acute infection, refrain from treating their liver disease, and then monitor their untreated course to a liver disease end point and/or death, whether from liver disease or from other causes. Since such kind of study cannot be performed, reliance must be placed on surrogate markers of disease progression.
The long-term sequelae of HCV infection, include the transition from not perceivable acute to chronic hepatitis up to cirrhosis, which may progress to end-stage liver disease and/or to hepatocellular carcinoma (HCC), frequently leading to liver transplantation or death[7-11]. The recent Global Burden of Disease project estimated that in 2010, among 170 million people with chronic HCV, more than 30 million suffer from cirrhosis and the incidence of HCC is about 1-2 million new cases/year. The actual estimated incidence has been markedly decreased, and this is mainly attributable to the employment of a safe transfusion screening policy, which has markedly decreased the number of new infection. Several reports have identified that among persons with chronic hepatitis C (CHC), cirrhosis has developed in 20% and HCC in 1%-5%, approximately 20 years after disease onset. These data indicate that not all persons with CHC will develop cirrhosis or complications of the disease. The detection of specific markers, able to predict the progression of the disease, has been therefore the focus of this editorial.
A long-lasting elevated necroinflammatory activity seems to play a crucial role in the progression of the liver disease, as supported by data from patients with persistently normal transaminase levels[12-14]. The biochemistry profile is indeed only partially indicative to predict the disease’s progression and the clinical outcome might be modified by different variables and many factors, either virus-related, host-related and environmental associated.
The role of viral-dependent factors as viral load and genotype is still debated and controversial. Several studies have evaluated the relationship between serum concentration of HCV-RNA and liver disease severity with conflicting results. While some reports have demonstrated a positive correlation between HCV-RNA load and histopathological abnormalities[15-17], others have found no association with hepatic inflammation[18] or liver fibrosis[19,20]. These studies were conducted as cross-sectional design, resulting in limitations of casual temporality or including a particular type of person [i.e., drug users coinfected with human immunodeficiency virus (HIV)], thus the generalization of the obtained results to the population in the community was limited[21].
Regardless from the evidence that genotype 1b was reported to be more associated with the development of HCC than genotype 2[22] and with a poor outcome of disease[22-24], no data are available, so far, for other HCV genotypes. It should be noted that most of the studies were cross-sectional or included patients enrolled in clinical trials[25-29]. In a prospective study, Martinot-Peignoux et al[25], followed 163 patients with liver cirrhosis for seventeen years and reported that HCV genoptype 1b was a major risk factor for HCC development. This genotype was confirmed to have three times higher risk of liver tumor development, compared with patients infected with other genotypes[26]. Within the community based study REVEAL-HCV study cohort, that recruited 1095 subjects seropositive for antibodies against HCV followed for fifteen years, the multivariate analysis selected serum HCV-RNA, alanine aminotransferase (ALT) levels and HCV genotype as independent risk predictors of HCC. These seromarkers have been proposed as pretreatment markers in clinical decision to classify high-risk patients who need particular clinical care[30].
The presence of other viral coinfections (i.e., HIV, HBV) speeds up the clinical course of the disease with a negative impact on the natural history of HCV[31,32]. The progression to cirrhosis is higher in HCV-HIV coinfected patients[33,34] and is associated to other complications, such as hematologic disorders[35], kidney disease[36], cardiovascular disease[37] and neurologic abnormalities[38]. In addition, the coinfection with HBV determines a more severe liver injury in terms of progression of fibrosis, liver cirrhosis and hepatic decompensation[39,40].
Among the host-related factors, age at infection, male gender and heavy alcohol intake seem to influence the outcome of CHC[41-43]. Freeman et al[44], using an ecologic analysis to estimate relative risk of cirrhosis progression across four study methodologies, have found an association between male sex (RR = 1.08) with heavy alcohol consumption (RR = 1.61) and elevated serum ALT levels (RR = 1.23) with higher histological activity index.
In the last few years, another important aspect, represented by the presence of comorbidities, was shown to impact on the evolution of chronic HCV infection. Hepatic steatosis, obesity and insulin resistance must be considered important determinants of liver disease progression, although the relationship between these metabolic factors and clinical outcomes is still complicated. In a long-term peginterferon treatment (HALT-C study) the modifiable risk factors for liver disease progression were studied, and insulin resistance was found strongly associated with clinical outcomes[45]. Similar results were found in a large scale community-based study in which an association between diabetes and HCC was observed[46]. However, the association between HCV infection and the development of diabetes remains controversial[47,48].
Major advances in genetics during the last decade allow the identification of specific markers associated with viral response and consequently with HCV infection outcomes. Among them, IL28B gene variants (rs12979860 and rs8099917) have been strongly associated with favourable response to standard antiviral treatment in patients with CHC[49-51]. These treatment response findings were confirmed by many studies in different populations such as HCV-1 patients[52-57] and reproduced in several meta-analyses[58-60]. Therefore, both host and virus factors are important determinants of liver diseases outcome.
The most relevant consequence of HCV infection is HCC development[61]. This primary liver tumor is the third leading cause of cancer deaths worldwide, with an incidence in United States more than doubled in the past 25 years[62]. Screening strategies for detection of early HCC have relied primarily on radiologic imaging[63,64] and serum biomarkers[65]. Over the past two decades, considerable number of studies have been published in order to identify suitable biomarkers, however the results are frequently contradictory. For this reason, tumor marker levels are not included in the screening recommendations of international guidelines[66,67]. In this setting, α-fetoprotein (AFP) is the most commonly used biomarker, but its sensitivity and specificity in detecting HCC is poor. AFP levels are often increased in patients with cirrhosis without HCC[68,69] and the positive rate of AFP in HCC is about 60%, making it a diagnostic limitation.
Other tumor biomarkers have been proposed to complement or replace AFP in HCC detection. In clinical practice, Lens culinaris agglutinin A - reactive fraction of α-AFP (α-AFP-L3), a glycoform of AFP[70,71], and des-γ-carboxyprothrombin (DCP), an abnormal prothrombin molecule generated by the absence of vitamin K responsible of an insufficient post-traslational carboxylation of the prothrombin precursor in malignant cells[72,73] have been used. These biomarkers represent independent tumor proteins and, as reported in several studies, they may be complementary in the detection of HCC[74-76]. Few prospective studies have been addressed to evaluate the usefulness of these new biomarkers in terms of prognosis. Sterling et al[77], in an ancillary study of the prospective HALT-C trial including 855 patients, demonstrated that mild-moderate elevation of total AFP and DCP, but not AFP-L3 occurs in patients with CHC and advanced fibrosis in absence of HCC. However, marked increase of these biomarkers were uncommon in subjects without HCC, although several factors other than HCC, such as gender, age, race and the presence of more advanced liver disease, could be responsible for these increased values. Since sensitivity, specificity and predictive values of these biomarkers were low, the authors concluded that they are poor predictors of HCC development. More recently, the usefulness of these three biomarkers as diagnostic tool for HCC[78] and as predictors of outcome in patients with HCC has been reviewed. The combination of these biomarkers resulted to provide a good predictive ability of survival after diagnosis and when considered at time of diagnosis, together with serum albumin and birilubin levels, they could be used for HCC staging and to predict HCC prognosis[79].
Among the new class of genetic biomarkers, reflecting the presence of circulating HCC cells, serum h-TERT mRNA detection showed higher sensitivity and specificity compared with AFP-mRNA in HCC patients (90% and 85% vs 69% and 50%, respectively) and a close correlation with tumor size and number also in early tumor stage[80]. Circulating h-TERT mRNA was indeed detectable in small size tumors, indicating that h-TERT mRNA was up-regulated during rapid proliferation of the tumor, at the early phase of oncogenesis-dedifferentiation.
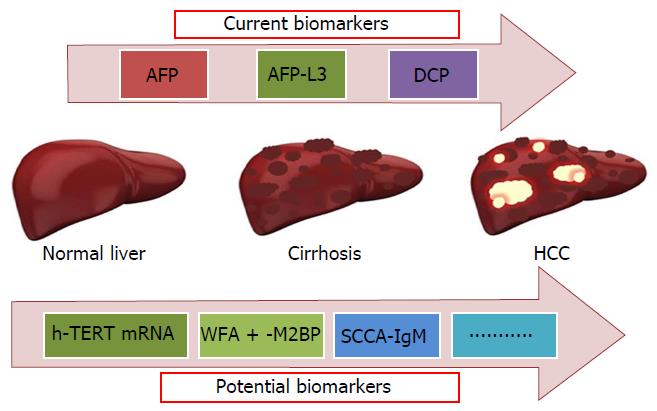
In the last 10 years a large number of new molecules potentially clinically useful as markers of liver disease progression in HCV infected patients has been identified (Figure 1). One of them is Wisteria floribunda agglutinin-positive human Mac-2-binding protein (WFA + -M2BP), a liver fibrosis glycobiomarker with a unique fibrosis-related glyco-alteration. Using fully automated immunoassay Yamasaki et al[81]. tested serum samples of 707 patients infected with HCV and found increased serum WFA + -M2BP levels in parallel with the progression of liver fibrosis stage. In each distinctive stage of fibrosis, the risk of HCC development was increased, according to elevation of WFA + -M2BP. The diagnostic performance of this protein, based on the AUROC values, was superior to that of AFP for predicting the development of HCC at 3, 5, and 7 years. The WFA + -M2BP values are proposed as noninvasive predictors of HCC development and could be considered a surrogate marker of liver fibrosis to be added to FibroScan tecnique.
Several studies have demonstrated in recent years that tumor released antigens can react with natural IgM class of immunoglobulins and form circulating immune complexes in different human tumors. The circulating immune complex composed of squamous cell carcinoma antigen (SCCA) linked with IgM (SCCA-IgM) has been recently discovered as a promising tool to identify patients with progressive liver disease in HCV infected patients. SCCA-IgM complexes were undetectable in the sera of healthy subjects, but the detection rates and the levels consistently increased with liver disease progression[82]. In another study, SCCA-IgM complexes were detectable in 33% of the patients with chronic hepatitis[83] and in this study a significant increase over time of the immune complex levels was observed in patients with significant increase of liver fibrosis within a time frame of four years, but not in those without histologic progression, suggesting that monitoring the immune complex over time allowed to identify patients at higher risk of cirrhosis progression. In agreement with these findings, significant decrease of the immune complex was observed in sera of patients with HCV infection and persistent virologic response to antiviral therapy[84,85]. The positivity of SCCA-IgM has been found correlated with histologic non-alcoholic steatohepatitis (NASH) in patients with CHC[86]. It is worth to note that NASH has been recognized as risk factor of liver disease worsening and of HCC development[87]. On the basis of these considerations, it is likely that patients with HCV infection and SCCA-IgM positivity present a more fibrogenic and tumorigenic liver condition that should be accurately monitored and therapeutically managed, if possible.
In line with these results, the behaviour profile of SCCA-IgM was different in patient with early HCV-related cirrhosis with or without HCC progression. In a longitudinal, retrospective study a progressive increase of this immune complex was described in the majority of the patients with histological diagnosis of cirrhosis C who developed HCC after at least one year from the end of the study, while the levels of this biomarker remained unchanged or decreased in the majority of the patients without evidence of HCC development during the same time interval. Conversely, the increase of AFP, which was chosen as reference biomarker, was not significantly different between the two groups. The diagnostic accuracy, measured as AUROC values, was higher for SCCA-IgM than for AFP and the former biomarker performed better to identify cirrhotic patients at higher risk of HCC development[88]. This behaviour was observed at least one year before clinical diagnosis of HCC, suggesting that this preclinical phase might become a suitable window to specifically address new potentially effective therapies. A multicenter study, performed in HCV infected patients with overt cirrhosis, started from an opposite approach and demonstrated that the SCCA-IgM value ≤ 200 AU/mL accurately identifies patients with low risk of HCC development in the subsequent year (sensitivity 75%, specificity 62%). On the basis of the obtained results the authors concluded that this biomarker might be utilized to tailor surveillance timing[89].
Identifying new factors that could influence clinical outcome of HCV infection is important in order to counsel individuals regarding prognosis and to facilitate decisions related to clinical management. This point is crucial mostly in the current scenarios of new antiviral treatments that include various direct acting antiviral drugs[90,91]. Given the high cost of treatment and the increased possibility of adverse events, identification of factors predicting sustained virological response to individualize HCV therapy in clinical decision-making is urgent. While prioritizing treatment to patients who are at risk of future problems seems the optimal solution to deliver most benefits at the lowest costs, the problem still lies in the identification of those patients who should be included in the target population[92]. In this context, the above new biomarkers might become useful tools, as part of personalized medicine, for the surveillance of HCV infected patients with chronic hepatitis and/or cirrhosis, in order to better define follow up timing and suitable therapeutic management of the patients at higher risk of liver disease worsening.
| 1. | Alberti A, Chemello L, Benvegnù L. Natural history of hepatitis C. J Hepatol. 1999;31 Suppl 1:17-24. [PubMed] |
| 2. | Mattsson L, Sönnerborg A, Weiland O. Outcome of acute symptomatic non-A, non-B hepatitis: a 13-year follow-up study of hepatitis C virus markers. Liver. 1993;13:274-278. [PubMed] |
| 3. | Hopf U, Möller B, Küther D, Stemerowicz R, Lobeck H, Lüdtke-Handjery A, Walter E, Blum HE, Roggendorf M, Deinhardt F. Long-term follow-up of posttransfusion and sporadic chronic hepatitis non-A, non-B and frequency of circulating antibodies to hepatitis C virus (HCV). J Hepatol. 1990;10:69-76. [PubMed] |
| 4. | Tremolada F, Casarin C, Alberti A, Drago C, Tagger A, Ribero ML, Realdi G. Long-term follow-up of non-A, non-B (type C) post-transfusion hepatitis. J Hepatol. 1992;16:273-281. [PubMed] |
| 5. | Rai R, Wilson LE, Astemborski J, Anania F, Torbenson M, Spoler C, Vlahov D, Strathdee SA, Boitnott J, Nelson KE. Severity and correlates of liver disease in hepatitis C virus-infected injection drug users. Hepatology. 2002;35:1247-1255. [PubMed] |
| 6. | Niederau C, Lange S, Heintges T, Erhardt A, Buschkamp M, Hürter D, Nawrocki M, Kruska L, Hensel F, Petry W. Prognosis of chronic hepatitis C: results of a large, prospective cohort study. Hepatology. 1998;28:1687-1695. [PubMed] |
| 7. | Fattovich G, Pantalena M, Zagni I, Realdi G, Schalm SW, Christensen E. Effect of hepatitis B and C virus infections on the natural history of compensated cirrhosis: a cohort study of 297 patients. Am J Gastroenterol. 2002;97:2886-2895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Benvegnù L, Gios M, Boccato S, Alberti A. Natural history of compensated viral cirrhosis: a prospective study on the incidence and hierarchy of major complications. Gut. 2004;53:744-749. [PubMed] |
| 9. | Serfaty L, Aumaître H, Chazouillères O, Bonnand AM, Rosmorduc O, Poupon RE, Poupon R. Determinants of outcome of compensated hepatitis C virus-related cirrhosis. Hepatology. 1998;27:1435-1440. [PubMed] |
| 10. | Fattovich G, Giustina G, Degos F, Tremolada F, Diodati G, Almasio P, Nevens F, Solinas A, Mura D, Brouwer JT. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology. 1997;112:463-472. [PubMed] |
| 11. | Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006;45:529-538. [PubMed] |
| 12. | Persico M, Persico E, Suozzo R, Conte S, De Seta M, Coppola L, Palmentieri B, Sasso FC, Torella R. Natural history of hepatitis C virus carriers with persistently normal aminotransferase levels. Gastroenterology. 2000;118:760-764. [PubMed] |
| 13. | Rossi S, De Filippi F, Saibeni S, Persico M, Bollani S, Camera A, Rizzolo L, Maria Croce A, Bruno S. A 15-Yr Prospective Histological Follow-Up Study in Patients With Persistently Normal Aminotransferase Levels (PNAL) Carrying HCV Infection. Am J Gastroenterol. 2007;102:2604-2606. [PubMed] |
| 14. | Rumi MG, De Filippi F, Donato MF, Del Ninno E, Colombo M. Progressive hepatic fibrosis in healthy carriers of hepatitis C virus with a transaminase breakthrough. J Viral Hepat. 2002;9:71-74. [PubMed] |
| 15. | Fanning L, Kenny E, Sheehan M, Cannon B, Whelton M, O’Connell J, Collins JK, Shanahan F. Viral load and clinicopathological features of chronic hepatitis C (1b) in a homogeneous patient population. Hepatology. 1999;29:904-907. [PubMed] |
| 16. | Lau JY, Davis GL, Kniffen J, Qian KP, Urdea MS, Chan CS, Mizokami M, Neuwald PD, Wilber JC. Significance of serum hepatitis C virus RNA levels in chronic hepatitis C. Lancet. 1993;341:1501-1504. [PubMed] |
| 17. | Naito M, Hayashi N, Hagiwara H, Hiramatsu N, Kasahara A, Fusamoto H, Kamada T. Serum hepatitis C virus RNA quantity and histological features of hepatitis C virus carriers with persistently normal ALT levels. Hepatology. 1994;19:871-875. [PubMed] |
| 18. | De Moliner L, Pontisso P, De Salvo GL, Cavalletto L, Chemello L, Alberti A. Serum and liver HCV RNA levels in patients with chronic hepatitis C: correlation with clinical and histological features. Gut. 1998;42:856-860. [PubMed] |
| 19. | Duvoux C, Pawlotsky JM, Bastie A, Cherqui D, Soussy CJ, Dhumeaux D. Low HCV replication levels in end-stage hepatitis C virus-related liver disease. J Hepatol. 1999;31:593-597. [PubMed] |
| 20. | Lagging LM, Garcia CE, Westin J, Wejstål R, Norkrans G, Dhillon AP, Lindh M. Comparison of serum hepatitis C virus RNA and core antigen concentrations and determination of whether levels are associated with liver histology or affected by specimen storage time. J Clin Microbiol. 2002;40:4224-4229. [PubMed] |
| 21. | Hisada M, Chatterjee N, Kalaylioglu Z, Battjes RJ, Goedert JJ. Hepatitis C virus load and survival among injection drug users in the United States. Hepatology. 2005;42:1446-1452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Raimondi S, Bruno S, Mondelli MU, Maisonneuve P. Hepatitis C virus genotype 1b as a risk factor for hepatocellular carcinoma development: a meta-analysis. J Hepatol. 2009;50:1142-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 171] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 23. | Bruno S, Zuin M, Crosignani A, Rossi S, Zadra F, Roffi L, Borzio M, Redaelli A, Chiesa A, Silini EM. Predicting mortality risk in patients with compensated HCV-induced cirrhosis: a long-term prospective study. Am J Gastroenterol. 2009;104:1147-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 120] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 24. | Bruno S, Crosignani A, Facciotto C, Rossi S, Roffi L, Redaelli A, de Franchis R, Almasio PL, Maisonneuve P. Sustained virologic response prevents the development of esophageal varices in compensated, Child-Pugh class A hepatitis C virus-induced cirrhosis. A 12-year prospective follow-up study. Hepatology. 2010;51:2069-2076. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 140] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 25. | Martinot-Peignoux M, Roudot-Thoraval F, Mendel I, Coste J, Izopet J, Duverlie G, Payan C, Pawlotsky JM, Defer C, Bogard M. Hepatitis C virus genotypes in France: relationship with epidemiology, pathogenicity and response to interferon therapy. The GEMHEP. J Viral Hepat. 1999;6:435-443. [PubMed] |
| 26. | Bruno S, Crosignani A, Maisonneuve P, Rossi S, Silini E, Mondelli MU. Hepatitis C virus genotype 1b as a major risk factor associated with hepatocellular carcinoma in patients with cirrhosis: a seventeen-year prospective cohort study. Hepatology. 2007;46:1350-1356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 150] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 27. | Fattovich G, Ribero ML, Pantalena M, Diodati G, Almasio P, Nevens F, Tremolada F, Degos F, Rai J, Solinas A. Hepatitis C virus genotypes: distribution and clinical significance in patients with cirrhosis type C seen at tertiary referral centres in Europe. J Viral Hepat. 2001;8:206-216. [PubMed] |
| 28. | Kobayashi M, Tanaka E, Sodeyama T, Urushihara A, Matsumoto A, Kiyosawa K. The natural course of chronic hepatitis C: a comparison between patients with genotypes 1 and 2 hepatitis C viruses. Hepatology. 1996;23:695-699. [PubMed] |
| 29. | Silini E, Bottelli R, Asti M, Bruno S, Candusso ME, Brambilla S, Bono F, Iamoni G, Tinelli C, Mondelli MU. Hepatitis C virus genotypes and risk of hepatocellular carcinoma in cirrhosis: a case-control study. Gastroenterology. 1996;111:199-205. [PubMed] |
| 30. | Lee MH, Yang HI, Yuan Y, L’Italien G, Chen CJ. Epidemiology and natural history of hepatitis C virus infection. World J Gastroenterol. 2014;20:9270-9280. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 66] [Reference Citation Analysis (1)] |
| 31. | Soto B, Sánchez-Quijano A, Rodrigo L, del Olmo JA, García-Bengoechea M, Hernández-Quero J, Rey C, Abad MA, Rodríguez M, Sales Gilabert M. Human immunodeficiency virus infection modifies the natural history of chronic parenterally-acquired hepatitis C with an unusually rapid progression to cirrhosis. J Hepatol. 1997;26:1-5. [PubMed] |
| 32. | Pontisso P, Gerotto M, Benvegnù L, Chemello L, Alberti A. Coinfection by hepatitis B virus and hepatitis C virus. Antivir Ther. 1998;3:137-142. [PubMed] |
| 33. | Kirk GD, Lesi OA, Mendy M, Akano AO, Sam O, Goedert JJ, Hainaut P, Hall AJ, Whittle H, Montesano R. The Gambia Liver Cancer Study: Infection with hepatitis B and C and the risk of hepatocellular carcinoma in West Africa. Hepatology. 2004;39:211-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 153] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 34. | Thompson MA, Mugavero MJ, Amico KR, Cargill VA, Chang LW, Gross R, Orrell C, Altice FL, Bangsberg DR, Bartlett JG. Guidelines for improving entry into and retention in care and antiretroviral adherence for persons with HIV: evidence-based recommendations from an International Association of Physicians in AIDS Care panel. Ann Intern Med. 2012;156:817-833, W-284, W-285, W-286, W-287, W-288, W-289, W-290, W-291, W-292, W-293, W-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 445] [Cited by in RCA: 484] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 35. | Lapinski TW, Parfieniuk A, Rogalska-Plonska M, Czajkowska J, Flisiak R. Prevalence of cryoglobulinaemia in hepatitis C virus- and hepatitis C virus/human immunodeficiency virus-infected individuals: implications for renal function. Liver Int. 2009;29:1158-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 36. | Wyatt CM, Malvestutto C, Coca SG, Klotman PE, Parikh CR. The impact of hepatitis C virus coinfection on HIV-related kidney disease: a systematic review and meta-analysis. AIDS. 2008;22:1799-1807. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 84] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 37. | Bedimo R, Westfall AO, Mugavero M, Drechsler H, Khanna N, Saag M. Hepatitis C virus coinfection and the risk of cardiovascular disease among HIV-infected patients. HIV Med. 2010;11:462-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 38. | Aronow HA, Weston AJ, Pezeshki BB, Lazarus TS. Effects of coinfection with HIV and hepatitis C virus on the nervous system. AIDS Read. 2008;18:43-48. [PubMed] |
| 39. | Zarski JP, Bohn B, Bastie A, Pawlotsky JM, Baud M, Bost-Bezeaux F, Tran van Nhieu J, Seigneurin JM, Buffet C, Dhumeaux D. Characteristics of patients with dual infection by hepatitis B and C viruses. J Hepatol. 1998;28:27-33. [PubMed] |
| 40. | Fattovich G, Tagger A, Brollo L, Giustina G, Pontisso P, Realdi G, Alberti A, Ruol A. Hepatitis C virus infection in chronic hepatitis B virus carriers. J Infect Dis. 1991;163:400-402. [PubMed] |
| 41. | Minola E, Prati D, Suter F, Maggiolo F, Caprioli F, Sonzogni A, Fraquelli M, Paggi S, Conte D. Age at infection affects the long-term outcome of transfusion-associated chronic hepatitis C. Blood. 2002;99:4588-4591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 103] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 42. | Wiley TE, McCarthy M, Breidi L, McCarthy M, Layden TJ. Impact of alcohol on the histological and clinical progression of hepatitis C infection. Hepatology. 1998;28:805-809. [PubMed] |
| 43. | Powell EE, Edwards-Smith CJ, Hay JL, Clouston AD, Crawford DH, Shorthouse C, Purdie DM, Jonsson JR. Host genetic factors influence disease progression in chronic hepatitis C. Hepatology. 2000;31:828-833. [PubMed] |
| 44. | Freeman AJ, Law MG, Kaldor JM, Dore GJ. Predicting progression to cirrhosis in chronic hepatitis C virus infection. J Viral Hepat. 2003;10:285-293. [PubMed] |
| 45. | Everhart JE, Lok AS, Kim HY, Morgan TR, Lindsay KL, Chung RT, Bonkovsky HL, Ghany MG. Weight-related effects on disease progression in the hepatitis C antiviral long-term treatment against cirrhosis trial. Gastroenterology. 2009;137:549-557. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 104] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 46. | Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Diabetes increases the risk of hepatocellular carcinoma in the United States: a population based case control study. Gut. 2005;54:533-539. [PubMed] |
| 47. | Ruhl CE, Menke A, Cowie CC, Everhart JE. Relationship of hepatitis C virus infection with diabetes in the U.S. population. Hepatology. 2014;60:1139-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 48. | Mason AL, Lau JY, Hoang N, Qian K, Alexander GJ, Xu L, Guo L, Jacob S, Regenstein FG, Zimmerman R. Association of diabetes mellitus and chronic hepatitis C virus infection. Hepatology. 1999;29:328-333. [PubMed] |
| 49. | Suppiah V, Moldovan M, Ahlenstiel G, Berg T, Weltman M, Abate ML, Bassendine M, Spengler U, Dore GJ, Powell E. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nat Genet. 2009;41:1100-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1505] [Cited by in RCA: 1506] [Article Influence: 88.6] [Reference Citation Analysis (1)] |
| 50. | Tanaka Y, Nishida N, Sugiyama M, Kurosaki M, Matsuura K, Sakamoto N, Nakagawa M, Korenaga M, Hino K, Hige S. Genome-wide association of IL28B with response to pegylated interferon-alpha and ribavirin therapy for chronic hepatitis C. Nat Genet. 2009;41:1105-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1779] [Cited by in RCA: 1777] [Article Influence: 104.5] [Reference Citation Analysis (1)] |
| 51. | Ge D, Fellay J, Thompson AJ, Simon JS, Shianna KV, Urban TJ, Heinzen EL, Qiu P, Bertelsen AH, Muir AJ. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature. 2009;461:399-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2776] [Cited by in RCA: 2734] [Article Influence: 160.8] [Reference Citation Analysis (1)] |
| 52. | Thompson AJ, Muir AJ, Sulkowski MS, Ge D, Fellay J, Shianna KV, Urban T, Afdhal NH, Jacobson IM, Esteban R. Interleukin-28B polymorphism improves viral kinetics and is the strongest pretreatment predictor of sustained virologic response in genotype 1 hepatitis C virus. Gastroenterology. 2010;139:120-129.e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 535] [Article Influence: 33.4] [Reference Citation Analysis (1)] |
| 53. | McCarthy JJ, Li JH, Thompson A, Suchindran S, Lao XQ, Patel K, Tillmann HL, Muir AJ, McHutchison JG. Replicated association between an IL28B gene variant and a sustained response to pegylated interferon and ribavirin. Gastroenterology. 2010;138:2307-2314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 246] [Cited by in RCA: 261] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 54. | Montes-Cano MA, García-Lozano JR, Abad-Molina C, Romero-Gómez M, Barroso N, Aguilar-Reina J, Núñez-Roldán A, González-Escribano MF. Interleukin-28B genetic variants and hepatitis virus infection by different viral genotypes. Hepatology. 2010;52:33-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 166] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 55. | Hayes CN, Kobayashi M, Akuta N, Suzuki F, Kumada H, Abe H, Miki D, Imamura M, Ochi H, Kamatani N. HCV substitutions and IL28B polymorphisms on outcome of peg-interferon plus ribavirin combination therapy. Gut. 2011;60:261-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 56. | Venegas M, Villanueva RA, González K, Brahm J. IL28B polymorphisms associated with therapy response in Chilean chronic hepatitis C patients. World J Gastroenterol. 2011;17:3636-3639. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 57. | Cieśla A, Bociąga-Jasik M, Sobczyk-Krupiarz I, Głowacki MK, Owczarek D, Cibor D, Sanak M, Mach T. IL28B polymorphism as a predictor of antiviral response in chronic hepatitis C. World J Gastroenterol. 2012;18:4892-4897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 58. | Jia Z, Ding Y, Tian S, Niu J, Jiang J. Test of IL28B polymorphisms in chronic hepatitis C patients treated with PegIFN and ribavirin depends on HCV genotypes: results from a meta-analysis. PLoS One. 2012;7:e45698. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 59. | Luo Y, Jin C, Ling Z, Mou X, Zhang Q, Xiang C. Association study of IL28B: rs12979860 and rs8099917 polymorphisms with SVR in patients infected with chronic HCV genotype 1 to PEG-INF/RBV therapy using systematic meta-analysis. Gene. 2013;513:292-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 60. | Jiménez-Sousa MA, Fernández-Rodríguez A, Guzmán-Fulgencio M, García-Álvarez M, Resino S. Meta-analysis: implications of interleukin-28B polymorphisms in spontaneous and treatment-related clearance for patients with hepatitis C. BMC Med. 2013;11:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 73] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 61. | El-Serag HB. Hepatocellular carcinoma: recent trends in the United States. Gastroenterology. 2004;127:S27-S34. [PubMed] |
| 62. | Everhart JE, Ruhl CE. Burden of digestive diseases in the United States Part III: Liver, biliary tract, and pancreas. Gastroenterology. 2009;136:1134-1144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 606] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 63. | Ikeda K, Saitoh S, Koida I, Tsubota A, Arase Y, Chayama K, Kumada H. Imaging diagnosis of small hepatocellular carcinoma. Hepatology. 1994;20:82-87. [PubMed] |
| 64. | Takayasu K, Furukawa H, Wakao F, Muramatsu Y, Abe H, Terauchi T, Winter TC, Sakamoto M, Hirohashi S. CT diagnosis of early hepatocellular carcinoma: sensitivity, findings, and CT-pathologic correlation. AJR Am J Roentgenol. 1995;164:885-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 97] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 65. | Oka H, Tamori A, Kuroki T, Kobayashi K, Yamamoto S. Prospective study of alpha-fetoprotein in cirrhotic patients monitored for development of hepatocellular carcinoma. Hepatology. 1994;19:61-66. [PubMed] |
| 66. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6629] [Article Influence: 441.9] [Reference Citation Analysis (1)] |
| 67. | European Association For The Study Of The Liver, European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4562] [Article Influence: 325.9] [Reference Citation Analysis (4)] |
| 68. | Bayati N, Silverman AL, Gordon SC. Serum alpha-fetoprotein levels and liver histology in patients with chronic hepatitis C. Am J Gastroenterol. 1998;93:2452-2456. [PubMed] |
| 69. | Di Bisceglie AM, Sterling RK, Chung RT, Everhart JE, Dienstag JL, Bonkovsky HL, Wright EC, Everson GT, Lindsay KL, Lok AS. Serum alpha-fetoprotein levels in patients with advanced hepatitis C: results from the HALT-C Trial. J Hepatol. 2005;43:434-441. [PubMed] |
| 70. | Oka H, Saito A, Ito K, Kumada T, Satomura S, Kasugai H, Osaki Y, Seki T, Kudo M, Tanaka M. Multicenter prospective analysis of newly diagnosed hepatocellular carcinoma with respect to the percentage of Lens culinaris agglutinin-reactive alpha-fetoprotein. J Gastroenterol Hepatol. 2001;16:1378-1383. [PubMed] |
| 71. | Taketa K, Endo Y, Sekiya C, Tanikawa K, Koji T, Taga H, Satomura S, Matsuura S, Kawai T, Hirai H. A collaborative study for the evaluation of lectin-reactive alpha-fetoproteins in early detection of hepatocellular carcinoma. Cancer Res. 1993;53:5419-5423. [PubMed] |
| 72. | Mita Y, Aoyagi Y, Yanagi M, Suda T, Suzuki Y, Asakura H. The usefulness of determining des-gamma-carboxy prothrombin by sensitive enzyme immunoassay in the early diagnosis of patients with hepatocellular carcinoma. Cancer. 1998;82:1643-1648. [PubMed] |
| 73. | Ishii M, Gama H, Chida N, Ueno Y, Shinzawa H, Takagi T, Toyota T, Takahashi T, Kasukawa R. Simultaneous measurements of serum alpha-fetoprotein and protein induced by vitamin K absence for detecting hepatocellular carcinoma. South Tohoku District Study Group. Am J Gastroenterol. 2000;95:1036-1040. [PubMed] |
| 74. | Marrero JA, Feng Z, Wang Y, Nguyen MH, Befeler AS, Roberts LR, Reddy KR, Harnois D, Llovet JM, Normolle D. Alpha-fetoprotein, des-gamma carboxyprothrombin, and lectin-bound alpha-fetoprotein in early hepatocellular carcinoma. Gastroenterology. 2009;137:110-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 583] [Cited by in RCA: 593] [Article Influence: 34.9] [Reference Citation Analysis (9)] |
| 75. | Volk ML, Hernandez JC, Su GL, Lok AS, Marrero JA. Risk factors for hepatocellular carcinoma may impair the performance of biomarkers: a comparison of AFP, DCP, and AFP-L3. Cancer Biomark. 2007;3:79-87. [PubMed] |
| 76. | Lok AS, Sterling RK, Everhart JE, Wright EC, Hoefs JC, Di Bisceglie AM, Morgan TR, Kim HY, Lee WM, Bonkovsky HL. Des-gamma-carboxy prothrombin and alpha-fetoprotein as biomarkers for the early detection of hepatocellular carcinoma. Gastroenterology. 2010;138:493-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 457] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 77. | Sterling RK, Wright EC, Morgan TR, Seeff LB, Hoefs JC, Di Bisceglie AM, Dienstag JL, Lok AS. Frequency of elevated hepatocellular carcinoma (HCC) biomarkers in patients with advanced hepatitis C. Am J Gastroenterol. 2012;107:64-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 78. | Johnson PJ, Pirrie SJ, Cox TF, Berhane S, Teng M, Palmer D, Morse J, Hull D, Patman G, Kagebayashi C. The detection of hepatocellular carcinoma using a prospectively developed and validated model based on serological biomarkers. Cancer Epidemiol Biomarkers Prev. 2014;23:144-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 252] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 79. | Toyoda H, Kumada T, Tada T, Sone Y, Kaneoka Y, Maeda A. Tumor Markers for Hepatocellular Carcinoma: Simple and Significant Predictors of Outcome in Patients with HCC. Liver Cancer. 2015;4:126-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 125] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 80. | Miura N, Osaki Y, Nagashima M, Kohno M, Yorozu K, Shomori K, Kanbe T, Oyama K, Kishimoto Y, Maruyama S. A novel biomarker TERTmRNA is applicable for early detection of hepatoma. BMC Gastroenterol. 2010;10:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 81. | Yamasaki K, Tateyama M, Abiru S, Komori A, Nagaoka S, Saeki A, Hashimoto S, Sasaki R, Bekki S, Kugiyama Y. Elevated serum levels of Wisteria floribunda agglutinin-positive human Mac-2 binding protein predict the development of hepatocellular carcinoma in hepatitis C patients. Hepatology. 2014;60:1563-1570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 212] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 82. | Beneduce L, Castaldi F, Marino M, Quarta S, Ruvoletto M, Benvegnù L, Calabrese F, Gatta A, Pontisso P, Fassina G. Squamous cell carcinoma antigen-immunoglobulin M complexes as novel biomarkers for hepatocellular carcinoma. Cancer. 2005;103:2558-2565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 100] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 83. | Biasiolo A, Chemello L, Quarta S, Cavalletto L, Bortolotti F, Caberlotto C, Beneduce L, Bernardinello E, Tono N, Fassina G. Monitoring SCCA-IgM complexes in serum predicts liver disease progression in patients with chronic hepatitis. J Viral Hepat. 2008;15:246-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 84. | Giannini EG, Basso M, Bazzica M, Contini P, Marenco S, Savarino V, Picciotto A. Successful antiviral therapy determines a significant decrease in squamous cell carcinoma antigen-associated (SCCA) variants’ serum levels in anti-HCV positive cirrhotic patients. J Viral Hepat. 2010;17:563-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 85. | Fransvea E, Trerotoli P, Sacco R, Bernabucci V, Milella M, Napoli N, Mazzocca A, Renna E, Quaranta M, Angarano G. SCCA-IC serum levels are predictive of clinical response in HCV chronic hepatitis to antiviral therapy: a multicentric prospective study. J Viral Hepat. 2012;19:704-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 86. | Martini A, Fattovich G, Guido M, Bugianesi E, Biasiolo A, Ieluzzi D, Gallotta A, Fassina G, Merkel C, Gatta A. HCV genotype 3 and squamous cell carcinoma antigen (SCCA)-IgM are independently associated with histological features of NASH in HCV-infected patients. J Viral Hepat. 2015;22:800-808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 87. | Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005-2023. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2413] [Cited by in RCA: 2647] [Article Influence: 189.1] [Reference Citation Analysis (3)] |
| 88. | Pontisso P, Quarta S, Caberlotto C, Beneduce L, Marino M, Bernardinello E, Tono N, Fassina G, Cavalletto L, Gatta A. Progressive increase of SCCA-IgM immune complexes in cirrhotic patients is associated with development of hepatocellular carcinoma. Int J Cancer. 2006;119:735-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 89. | Buccione D, Fatti G, Gallotta A, Loggi E, Di Donato R, Testa L, Saitta C, Santi V, Di Micoli A, Erroi V. Serum Scca-IgM as a predictor of hepatocellular carcinoma in patients with liver cirrhosis. OJGas. 2012;2:56-61. [RCA] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 90. | Jang JY, Shao RX, Lin W, Weinberg E, Chung WJ, Tsai WL, Zhao H, Goto K, Zhang L, Mendez-Navarro J. HIV infection increases HCV-induced hepatocyte apoptosis. J Hepatol. 2011;54:612-620. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 91. | Welsch C, Jesudian A, Zeuzem S, Jacobson I. New direct-acting antiviral agents for the treatment of hepatitis C virus infection and perspectives. Gut. 2012;61 Suppl 1:i36-i46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 146] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 92. | Lutchman G, Kim WR. A glass half full: Implications of screening for hepatitis C virus in the era of highly effective antiviral therapy. Hepatology. 2015;61:1455-1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Guan YS, Valenti L
S- Editor: Ji FF L- Editor: A E- Editor: Li D