Published online Nov 25, 2015. doi: 10.5495/wjcid.v5.i4.55
Peer-review started: June 8, 2015
First decision: August 10, 2015
Revised: September 12, 2015
Accepted: October 12, 2015
Article in press: October 13, 2015
Published online: November 25, 2015
Processing time: 176 Days and 22 Hours
Fungal infection is common in critically ill patients. However, this infection is difficult to diagnose, and a large proportion of patients receive empirical antifungal treatment without further confirmation of invasive fungal disease. Whilst prompt appropriate antifungal treatment is associated with better outcome in patients with confirmed infections, this treatment has several drawbacks. In addition, no clear beneficial effect of empirical antifungal treatment was found in patients without confirmed infection. Reducing antifungal treatment in the intensive care unit (ICU) is feasible, and would allow avoiding drawbacks of this treatment without negative impact on outcome. Antifungal stewardship, preemptive antifungal treatment, based on colonization index and fungal biomarkers; and de-escalation of antifungal treatment based on microbiology results and fungal biomarkers could be suggested to reduce antifungal use in the ICU, and are currently under investigation.
Core tip: Prompt appropriate antifungal treatment is associated with better outcome in patients with confirmed infections, this treatment has several drawbacks. Reducing antifungal treatment in the intensive care unit (ICU) is feasible, and would allow avoiding drawbacks of this treatment without negative impact on outcome. Antifungal stewardship, preemptive antifungal treatment, based on colonization index and fungal biomarkers; and de-escalation of antifungal treatment based on microbiology results and fungal biomarkers could be suggested to reduce antifungal use in the ICU, and are currently under investigation.
- Citation: Rouzé A, Jaffal K, Nseir S. How could we reduce antifungal use in the intensive care unit? World J Clin Infect Dis 2015; 5(4): 55-58
- URL: https://www.wjgnet.com/2220-3176/full/v5/i4/55.htm
- DOI: https://dx.doi.org/10.5495/wjcid.v5.i4.55
Fungal infection is common in critically ill patients[1]. Based on the results of the large EPIC II international study, candida spp. represented 17% of all microorganisms isolated in the cohort of 7087 critically ill patients with confirmed infection[2]. The subgroup analysis of patients with bloodstream infection (BSI) related to candida reported that intensive care unit (ICU) mortality was extremely high (43%), compared with that of BSI related to Gram-negative (29%), or to Gram-positive (25%) microorganisms[3]. Further, a recent epidemiological study, performed in Paris area, showed worrisome trends in the incidence of BSI related to candida, and mortality associated with this infection during the last decade[4]. Antifungal treatment could be classified into prophylactic, empirical, pre-emptive, and targeted. Prophylactic treatment is usually given to at high-risk patients without clinical signs of infection. Empirical treatment is prescribed to patients with clinical signs of infection. Antifungal treatment is considered as preemptive, in presence of clinical signs, risk factors for invasive fungal disease, and fungal colonization or high levels of fungal biomarkers. Targeted treatment is defined as a treatment given to patients with documented infection.
In patients with septic shock related to invasive fungal disease, prompt adequate antifungal treatment is the main prognosis factor. In a cohort of 223 patients with septic shock and Candida BSI, Kollef and colleagues identified delayed antifungal treatment, and inadequate source control as the two major independent risk factors for mortality[5]. Other risk factors included metastatic cancer, severe heart failure, red-blood cell transfusion, APACHE II score, and serum albumin level.
Diagnosis of invasive fungal disease is still challenging in critically ill patients, because of the low sensitivity of clinical signs and blood cultures, and the difficulties in performing biopsies or other invasive procedure in these patients. Therefore, empirical antifungal treatment is frequently prescribed in the ICU. A recent one-day cross-sectional cohort study was performed in 169 ICUs[6]. Antifungal treatment was given to 154 patients of the 2047 included patients, including two thirds of patients who received antifungal without any proven invasive fungal disease. Another retrospective study, performed during a 1-year-period, reported a higher rate of empirical antifungal treatment (28% of the 560 included patients)[7]. Chemotherapy, and infection at admission were associated with prolonged empirical antifungal treatment. A recent multicenter study reported that 100 (6.7%) of the 1491 included ICU patients received empirical antifungal treatment. No significant difference was found in mortality rate between patients who received empirical antifungal treatment, and those who did not receive antifungals[8].
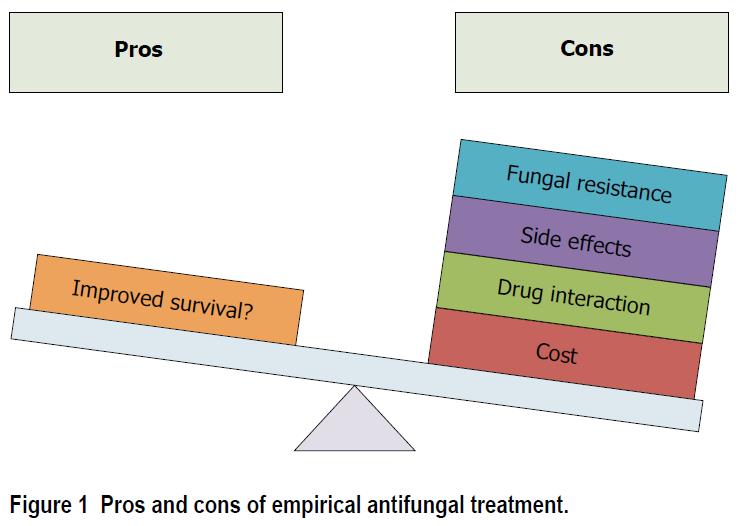
Drawbacks of empirical antifungal treatment include antifungal resistance, side effects, drug interaction, and cost (Figure 1). Whilst fungal resistance is less frequent than bacterial resistance, recent reports suggest an increase in resistance rate among Candida spp[9]. Further, antifungal resistance was also reported to be associated with worse outcome in patients with BSI related to Candida glabrata[10].
Several strategies could be suggested to improve antifungal treatment in critically ill patients, and reduce unnecessary treatment.
Antifungal stewardship could be suggested in patients at higher risk for invasive fungal disease. A recent study performed in hematology department of our hospital during a 10-year-period reported that a decrease of 40% in antifungal consumption was possible using local guidelines, including decision algorithms and preprinted prescriptions allowing only-guideline recommended drugs for a given indication[11]. These local guidelines were based on national and international recommendations on antifungal treatment. The incidence of invasive fungal disease, and mortality rate remained stable during the study period.
A preemptive antifungal treatment could also be suggested to reduce antifungal treatment. Such a strategy could be based on clinical rules, colonization index, and/or fungal biomarkers. Whilst the negative predictive value of clinical rules, and colonization index is very good (72-100), the positive predictive value is low (6%-67%), suggesting that a large proportion of patients receiving empirical or preemptive treatment will be free of invasive fungal disease[12]. A multicenter randomized controlled double-blind study is currently ongoing in France to determine the impact of mycafungin in multi-colonized candida patients with nosocomial sepsis. Its results will be very helpful to determine the impact of such a strategy in critically ill patients[13].
Initial empirical antifungal treatment should be based on local epidemiology and sensitivity patterns, in order to avoid using large-spectrum antifungals in settings where the incidence of resistant fungi is low.
β-D-glucan is a most studied biomarker for invasive fungal disease. As for clinical rule and colonization index, its negative predictive value is very good (77%-98%), but its positive predictive value is low (59%-72%), suggesting that a treatment strategy based on this biomarker would also result in unnecessary use of antifungals[12]. A recent study suggested that combined use of β-D 1, 3-glucan, mannan, mannan-antibody was helpful in improving the predictive value of candidemia[14]. Another recent prospective study performed in 56 patients with candidemia and 200 controls, reported that a combination of β-D-glucan, and mannan had a very good sensitivity (89%), and specificity (85%)[15]. Ostrosky-Zeichner et al[16] performed a randomized controlled double-blind placebo-controlled study to determine the impact caspofungin prophylaxis followed by preemptive treatment on the incidence of invasive fungal disease. Whilst prophylaxis treatment had no impact on the incidence of invasive candidiasis, preemptive treatment based on β-D-glucan results was associated with significantly decreased incidence of invasive candidiasis. However, the impact of preemptive treatment on invasive candidiasis incidence was a secondary objective.
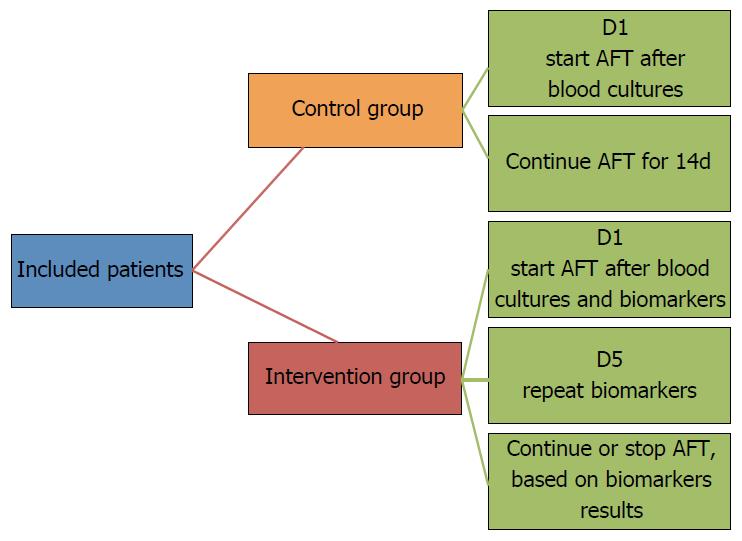
Another strategy to reduce antifungal treatment in critically ill patients is to deescalate antifungal treatment, as soon as results of blood cultures and biomarkers are available. In fact, based on the excellent negative predictive value of the combination of β-D-glucan, mannan, and antimannan, empirical antifungal treatment could be safely stopped in patients with negative blood cultures and negative fungal biomarker results. Our group is conducting a randomized controlled unblind study to determine the impact of a strategy based on fungal biomarker results on the early stop of antifungal treatment in critically ill patients[17]. We plan to include 110 patients in this feasibility study, and to evaluate the safety of such a strategy, including its impact on mortality and recurrence of fungal infection (Figure 2). However, fungal biomarkers are not available in all ICUs, and the cost/effectiveness of such a strategy should be evaluated.
To our knowledge, no study has specifically evaluated the best duration of antifungal treatment in critically ill patients with confirmed fungal infections. However, recent guidelines clearly recommend different durations, based on the site of infection. Therefore, one potential strategy to reduce duration of targeted treatment in these patients is to follow the guidelines, and to evaluate adherence to local guidelines repeatedly.
Antifungal treatment is frequently used in critically ill patients. Prompt adequate antifungal treatment is required in patients with septic shock related to candida infection. However, the beneficial effects of empirical antifungal treatment in patients with suspected invasive fungal infection have not been demonstrated. In addition, fungal resistance, drug interaction, side effects, and cost should be taken into account when starting such a treatment in ICU patients. Further studies should determine the impact of targeted strategies, and de-escalation of empirical treatment on outcome of critically ill patients.
| 1. | Cornely OA, Bassetti M, Calandra T, Garbino J, Kullberg BJ, Lortholary O, Meersseman W, Akova M, Arendrup MC, Arikan-Akdagli S. ESCMID guideline for the diagnosis and management of Candida diseases 2012: non-neutropenic adult patients. Clin Microbiol Infect. 2012;18 Suppl 7:19-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 793] [Cited by in RCA: 915] [Article Influence: 70.4] [Reference Citation Analysis (0)] |
| 2. | Vincent JL, Rello J, Marshall J, Silva E, Anzueto A, Martin CD, Moreno R, Lipman J, Gomersall C, Sakr Y. International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302:2323-2329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2205] [Cited by in RCA: 2411] [Article Influence: 141.8] [Reference Citation Analysis (0)] |
| 3. | Kett DH, Azoulay E, Echeverria PM, Vincent JL. Candida bloodstream infections in intensive care units: analysis of the extended prevalence of infection in intensive care unit study. Crit Care Med. 2011;39:665-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 287] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 4. | Lortholary O, Renaudat C, Sitbon K, Madec Y, Denoeud-Ndam L, Wolff M, Fontanet A, Bretagne S, Dromer F. Worrisome trends in incidence and mortality of candidemia in intensive care units (Paris area, 2002-2010). Intensive Care Med. 2014;40:1303-1312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 220] [Cited by in RCA: 255] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 5. | Kollef M, Micek S, Hampton N, Doherty JA, Kumar A. Septic shock attributed to Candida infection: importance of empiric therapy and source control. Clin Infect Dis. 2012;54:1739-1746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 355] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 6. | Azoulay E, Dupont H, Tabah A, Lortholary O, Stahl J-P, Francais A, Martin C, Guidet B, Timsit J-F. Systemic antifungal therapy in critically ill patients without invasive fungal infection. Crit Care Med. 2012;40:813-822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 110] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 7. | Zein M, Parmentier-Decrucq E, Kalaoun A, Bouton O, Wallyn F, Baranzelli A, Elmanser D, Sendid B, Nseir S. Factors predicting prolonged empirical antifungal treatment in critically ill patients. Ann Clin Microbiol Antimicrob. 2014;13:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Bailly S, Bouadma L, Azoulay E, Orgeas MG, Adrie C, Souweine B, Schwebel C, Maubon D, Hamidfar-Roy R, Darmon M. Failure of empirical systemic antifungal therapy in mechanically ventilated critically ill patients. Am J Respir Crit Care Med. 2015;191:1139-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 9. | Fournier P, Schwebel C, Maubon D, Vesin A, Lebeau B, Foroni L, Hamidfar-Roy R, Cornet M, Timsit JF, Pelloux H. Antifungal use influences Candida species distribution and susceptibility in the intensive care unit. J Antimicrob Chemother. 2011;66:2880-2886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Alexander BD, Johnson MD, Pfeiffer CD, Jiménez-Ortigosa C, Catania J, Booker R, Castanheira M, Messer SA, Perlin DS, Pfaller MA. Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin Infect Dis. 2013;56:1724-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 622] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 11. | Alfandari S, Berthon C, Coiteux V. Antifungal stewardship: implementation in a French teaching hospital. Med Mal Infect. 2014;44:154-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | León C, Ostrosky-Zeichner L, Schuster M. What’s new in the clinical and diagnostic management of invasive candidiasis in critically ill patients. Intensive Care Med. 2014;40:808-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 13. | Timsit JF, Azoulay E, Cornet M, Gangneux JP, Jullien V, Vésin A, Schir E, Wolff M. EMPIRICUS micafungin versus placebo during nosocomial sepsis in Candida multi-colonized ICU patients with multiple organ failures: study protocol for a randomized controlled trial. Trials. 2013;14:399. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Poissy J, Sendid B, Damiens S, Ichi Ishibashi K, François N, Kauv M, Favory R, Mathieu D, Poulain D. Presence of Candida cell wall derived polysaccharides in the sera of intensive care unit patients: relation with candidaemia and Candida colonisation. Crit Care. 2014;18:R135. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Held J, Kohlberger I, Rappold E, Busse Grawitz A, Häcker G. Comparison of (1->3)-β-D-glucan, mannan/anti-mannan antibodies, and Cand-Tec Candida antigen as serum biomarkers for candidemia. J Clin Microbiol. 2013;51:1158-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 83] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 16. | Ostrosky-Zeichner L, Shoham S, Vazquez J, Reboli A, Betts R, Barron MA, Schuster M, Judson MA, Revankar SG, Caeiro JP. MSG-01: A randomized, double-blind, placebo-controlled trial of caspofungin prophylaxis followed by preemptive therapy for invasive candidiasis in high-risk adults in the critical care setting. Clin Infect Dis. 2014;58:1219-1226. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 130] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Geraldine MM, Hideo I, James L, Mohan G
S- Editor: Qiu S L- Editor: A E- Editor: Li D