Published online Dec 20, 2025. doi: 10.5493/wjem.v15.i4.111542
Revised: July 23, 2025
Accepted: October 27, 2025
Published online: December 20, 2025
Processing time: 170 Days and 1.5 Hours
Sacubitril/valsartan is a first-in-class angiotensin receptor neprilysin inhibitor (ARNI) which combines an angiotensin receptor blocker (valsartan) with sacu
To evaluate the effect of sacubitril/valsartan on N-terminal pro-B-type natriuretic peptide (NT-pro BNP), inflammatory marker [high-sensitivity C-reactive protein (hs-CRP)], and quality of life when given as add-on to standard therapy in pa
Sacubitril/valsartan as add-on therapy for improvement in HF study was a ran
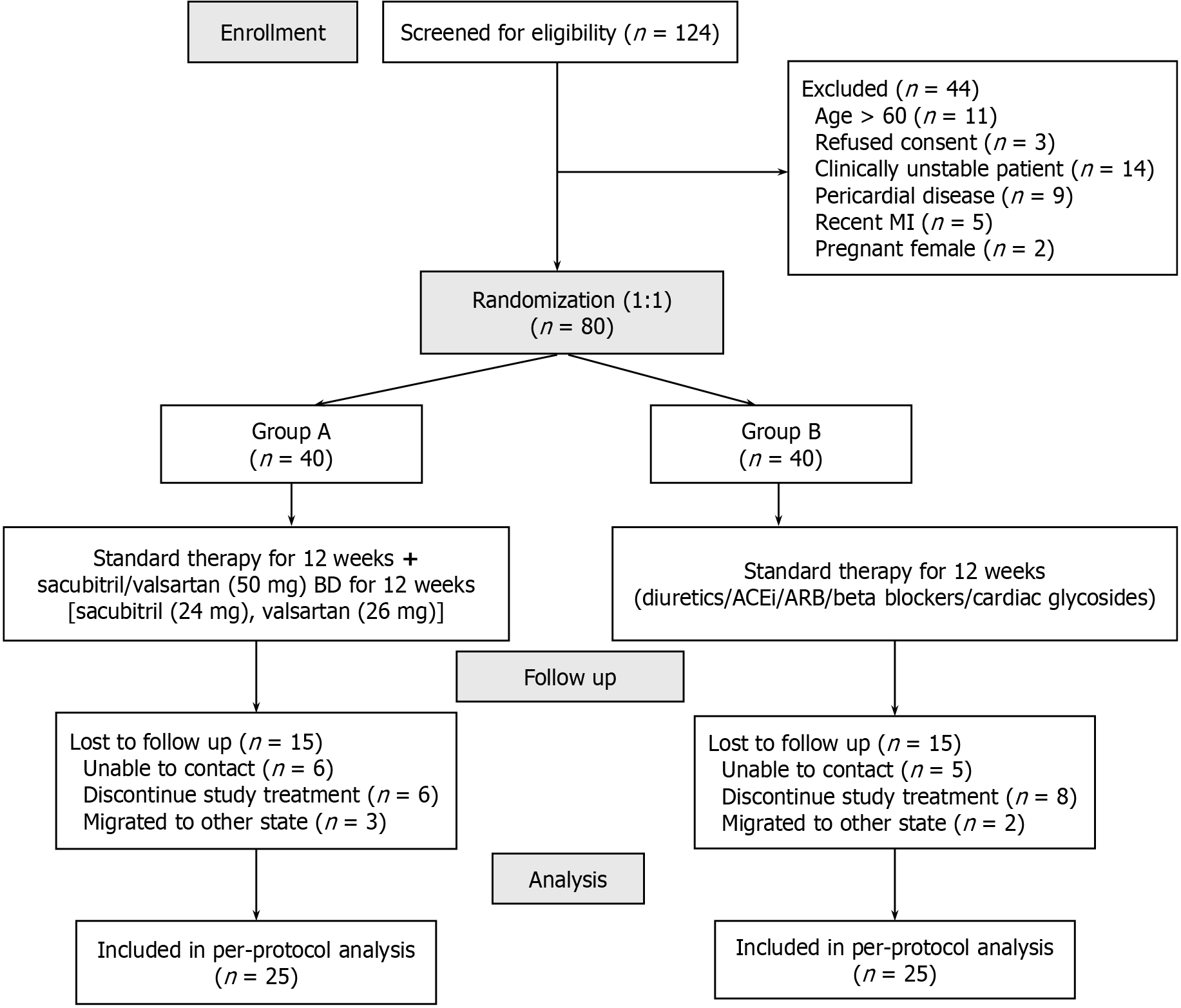
Out of 124 patients screened, 80 were enrolled of which 25 patients in each group were available for per-protocol analysis. At baseline, both the groups were comparable. Sacubitril/valsartan group exhibited a statistically significant greater reduction in NT-pro BNP (-35.91 ± 28.01 vs 24.68 ± 31.86; P < 0.0001) and hs-CRP (-2.87 ± 3.22 vs 0.76 ± 1.60; P < 0.0001) levels at 12 weeks compared to standard treatment group. There was also a greater improvement in quality of life with sacubitril/valsartan group (overall change in WHOQOL-BREF: 13.16 ± 8.73 in sacubitril/valsartan vs -4.12 ± 6.25 in standard treatment arm). No major adverse events were reported in two groups.
Sacubitril/valsartan demonstrated significant improvements in HF biomarkers, inflammation, and quality of life without compromising safety supporting its role as an effective addition to standard HF therapy. Further research is needed to evaluate its long-term benefits on mortality and cardiac remodeling.
Core Tip: This randomized clinical trial evaluated the efficacy and safety of sacubitril/valsartan, a first-in-class angiotensin receptor neprilysin inhibitor, as add-on to standard therapy compared to standard therapy alone in patients with New York Heart Association functional class II-III heart failure (HF). A total of 80 patients were enrolled out of which 25 patients in each group were available for per-protocol analysis. Baseline demographic, clinical and biochemical characteristics in both the groups were comparable. Sacubitril/valsartan demonstrated significant improvements in HF biomarkers, inflammation, and quality of life after 12 weeks without compromising safety supporting its role as an effective addition to standard HF therapy.
- Citation: Dhawan R, Mittal R, Kumar A, Laller KS, Gill PS, Rag A, Mittal N. Sacubitril/valsartan as add-on to standard therapy in patients with heart failure: A randomized controlled trial. World J Exp Med 2025; 15(4): 111542
- URL: https://www.wjgnet.com/2220-315x/full/v15/i4/111542.htm
- DOI: https://dx.doi.org/10.5493/wjem.v15.i4.111542
Heart Failure (HF) is a medical condition characterized by a range of symptoms and signs caused by any structural or functional impairment of the heart's ability to fill or eject blood. HF has emerged as a major global health issue, with an estimated worldwide prevalence of > 37.7 million[1]. The load is fast growing and it is anticipated that by 2030, there will be a 25% increase in the number of HF patients[2]. In patients with HF, there are mainly three treatment goals viz. reduction in mortality; prevention of recurrent hospitalizations due to worsening HF; and improvement in clinical status, functional capacity and quality of life. The mainstay of treatment for HF is pharmacotherapy, which should be used in conjunction with non-pharmacological therapies and before device therapy is considered. Pharmacotherapy consists of angiotensin converting enzyme inhibitors (ACEi), angiotensin receptor blockers (ARBs), sodium glucose co-transporter 2 inhibitors, beta blockers, aldosterone antagonists, diuretics, digoxin, vasodilators, etc.[3,4].
Sacubitril/valsartan, also written as LCZ696, is a first-in-class angiotensin receptor neprilysin inhibitor (ARNI) that comprises the molecular moieties of neprilysin (neutral endopeptidase 24.11) inhibitor prodrug AHU377 and an ARB. Sacubitril inhibits neprilysin, an enzyme that degrades natriuretic peptides, bradykinin, adrenomedullin and other vasoactive peptides, leading to an increase in natriuretic peptides which in turn signal via cGMP and exert potent antimitogenic and vasodilatory effects[5]. Valsartan, an ARB, selectively blocks the angiotensin II receptors, preventing the action of angiotensin II on the cardiovascular system; this leads to vasodilation and decreased secretion of aldosterone which in turn causes a reduction of blood pressure and reduced remodelling of the heart and blood vessels. ARNIs exert dual favourable role in HF, by virtue of increasing the levels of beneficial (natriuretic) peptides while also blocking the effects of angiotensin II. When compared to ACEi/ARB, an ARNI (sacubitril/valsartan) is recommended as a de novo treatment in hospitalized patients with acute HF before discharge because it has proven benefits in terms of health status, prognostic biomarker N-terminal pro-b-type natriuretic peptide (NT-pro BNP) levels and left ventricular (LV) remodeling parameters[6].
Few phase 2 and phase 3 clinical trials such as PARAMOUNT[6], PARAGON-HF[7], PARALLAX[8] and PARADIGM-HF[9] have showcased the role of ARNI in HF. The present study was planned to evaluate the effect of sacubitril/valsartan on the levels of natriuretic peptide (NT-pro BNP) and inflammatory [high-sensitivity C-reactive protein (hs-CRP)] marker; and quality of life when given as add-on to standard therapy in patients with HF.
Sacubitril/valsartan as add-on therapy for improvement in HF study was a randomised controlled trial conducted collaboratively by the departments of Pharmacology and Cardiology at Pt. B. D. Sharma PGIMS, Rohtak, Haryana from July 19, 2023 to November 30, 2024. This study was carried out following the principles of good clinical practice and the Declaration of Helsinki. Prior ethical clearance was obtained from the Biomedical research ethics committee and the trial was registered prospectively with the clinical trials registry of India, No. CTRI/2023/07/055325.
Patients visiting Cardiology Outpatient Department in our tertiary care hospital were screened for potential inclusion in the study. The inclusion criteria were: (1) Age 18-60 years of either gender; (2) Presence of New York Heart Association (NYHA) functional class II-III; (3) Echocardiographic evidence of LV diastolic/systolic dysfunction; and (4) Willing to give written informed consent. Patients having clinically unstable HF patient defined by any change in diuretic dose in the past one month before enrolment; significant valvular heart disease, pericardial disease, hypertrophic or restrictive cardiomyopathy; unstable angina or myocardial infarction within the past 4 weeks; alternative probable cause of patient’s symptoms such as significant pulmonary disease and pregnant or lactating females were excluded.
Eligible patients were randomised in 1:1 ratio to either of the 2 study arms: (1) Group A [tablet sacubitril (24 mg)/valsartan (26 mg) twice daily + standard treatment]; and (2) Group B (standard treatment) for 12 weeks. Randomisation was done using permuted blocks of size 4; allocation concealment was done using sequentially numbered opaque sealed envelopes. Standard treatment comprised of diuretics/ACEi/ARBs/betablockers/cardiac glycosides and was given at the discretion of the treating cardiologist as per the standard treatment guidelines. Available commercial preparations of the drugs were used. The participants were followed up at 4weekly intervals for 12 weeks duration.
Primary outcome was change in NT-pro BNP levels at 12 weeks from baseline. Secondary outcomes included (1) Change in hs-CRP levels; (2) Change in World Health Organisation Quality of Life Questionnaire (WHOQOL-BREF) score; and (3) Incidence of treatment-related adverse events or worsening HF.
Based on the results of PARADIGM-HF trial[10] with mean (SD) reduction in NT-pro BNP levels of 469.7 (375.22) pg/mL in sacubitril/valsartan group vs 80.33 (53) pg/mL in the enalapril group with a desired power of 90% and alpha error of 5%, sample size was calculated as 10 patients in each group. In PARADIGM-HF trial, patients with baseline NT-pro BNP levels ≥ 600 pg/mL (or ≥ 400 pg/mL if hospitalised within previous 12 months) were included while there were no such pre-defined cut-off values in our study; in order to account for the baseline value as a possible covariate for mean change in levels and around 20% dropouts, it was decided to enrol as many patients as possible during the 10 month enrolment period.
Data analysis was carried out using the per-protocol principle. The data was expressed as mean ± SD and numbers (percentages). For NT-pro BNP levels, hs-CRP levels and WHOQOL-BREF, mean change between the two groups was analysed using the student t-test/Mann Whitney U test while within group comparisons were done using paired t-test/Wilcoxon signed rank test. The categorical data was compared using Fisher’s exact test. Statistical Package for the Social Sciences statistics version 30 software was used to analyse the data and P < 0.05 was considered significant.
Out of 124 patients screened, 80 were enrolled of which 25 patients in each group were available for per-protocol analysis (Figure 1). Both the groups had comparable demographic, clinical and biochemical characteristics at baseline (Table 1).
| Characteristic | Group A (n = 25) | Group B (n = 25) | P value |
| Age (years) (mean ± SD) | 49.48 ± 5.83 | 52.12 ± 4.28 | 0.07 |
| Male | 20 (80) | 20 (80) | > 0.99 |
| Body mass index (kg/m2) (mean ± SD) | 25.57 ± 5.59 | 23.86 ± 4.75 | 0.25 |
| Cardiovascular disease risk factors and disease characteristics | |||
| Smoking | 16 (64) | 21(84) | 0.20a |
| Alcohol | 13 (52) | 11 (44) | 0.78 |
| Oedema | 05 (20) | 10 (40) | 0.22 |
| Dyspnea on exertion | 18 (72) | 18 (72) | > 0.99 |
| Orthopnea | 13 (52) | 17 (68) | 0.39 |
| Paroxysmal nocturnal dyspnea | 01 (04) | 08 (32) | 0.02a |
| Medical history | |||
| Diabetes mellitus | 05 (20) | 03 (12) | 0.70 |
| Hypertension | 10 (40) | 11 (44) | > 0.99 |
| Myocardial infarction | 05 (20) | 04 (16) | 0.35 |
| Angina pectoris | 01 (4) | 02 (8) | > 0.99 |
| Stroke | - | 04 (16) | 0.11 |
| Percutaneous coronary intervention | 03 (12) | 03 (12) | > 0.99 |
| Atrial fibrillation | - | 01 (4) | > 0.99 |
| Left ventricular ejection fraction (%) (mean ± SD) | 20 ± 9.35 | 27 ± 20 | 0.6 |
| N-terminal pro-B-type natriuretic peptide (pg/mL) (mean ± SD) | 117.53 ± 38.49 | 97.37 ± 31.18 | 0.05 |
| High-sensitivity C-reactive protein (mg/L) (mean ± SD) | 6.25 ± 4.13 | 5.6436 ± 3.83 | 0.59 |
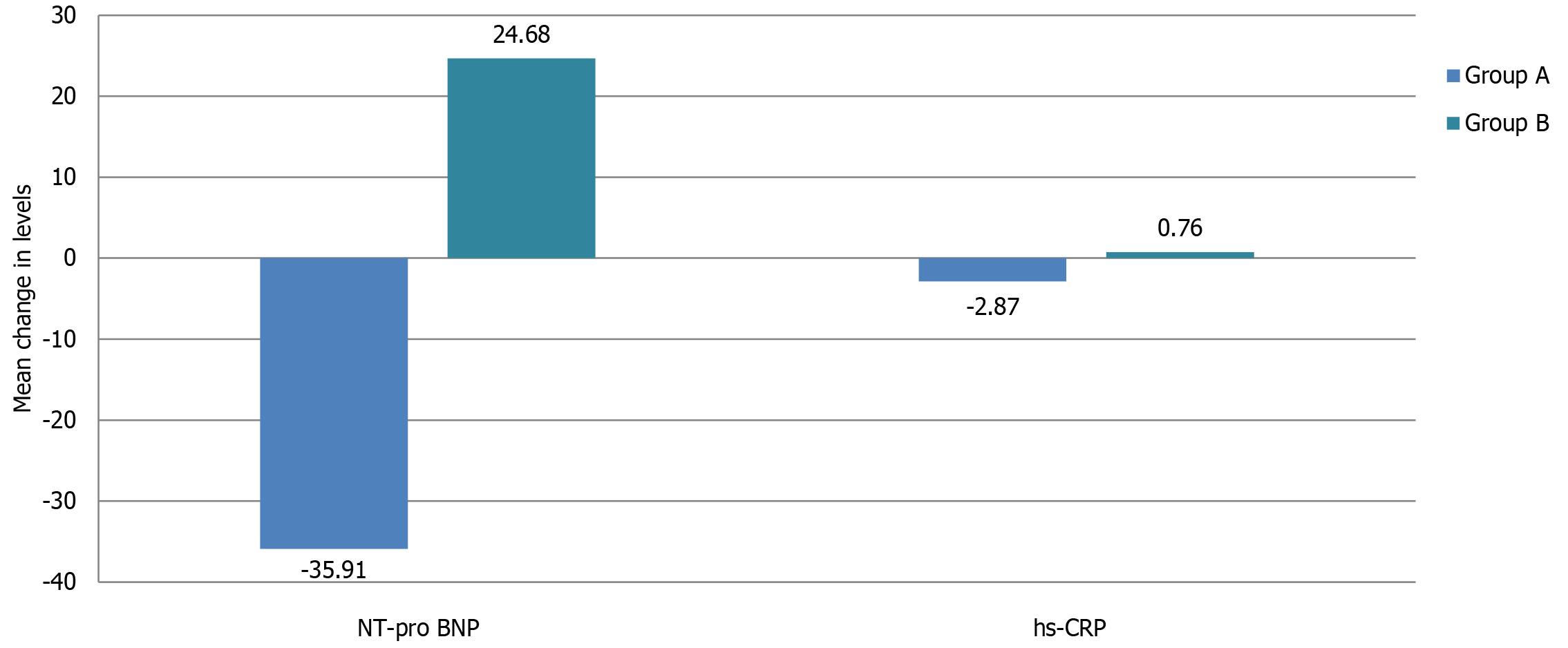
Both the groups demonstrated significant change in NT-pro BNP and hs-CRP levels at 12 weeks compared to baseline (Table 2). However, there was a significant decline in NT-pro BNP levels in sacubitril/valsartan group (-35.91 ± 28.01 pg/mL; -29% ± 17%) in contrast to standard group showing rise in levels (24.68 ± 31.86 pg/mL; 28% ± 35%); this change in NT-pro BNP levels between the two groups was statistically significant (P < 0.0001).
Similarly, the comparison for mean change in hs-CRP levels between two groups (-2.87 ± 3.22 mg/L in group A vs 0.76 ± 1.60 mg/L in group B) was significant statistically (P < 0.0001) (Figure 2).
Table 3 depicts the scores in various domains of WHOQOL-BREF Questionnaire in both groups. Group A demonstrated a significant increase in aggregate score at 12 weeks (70 ± 8.81) compared to baseline (56.84 ± 9.88; P < 0.001) whereas group B had a significant decline in the score (54.72 ± 10.16 at 12 weeks vs 58.84 ± 8.60 at baseline; P = 0.003). The overall mean change in score at 12 weeks from baseline was significantly different between the two groups (13.16 ± 8.73 in group A and -4.12 ± 6.25 in group B; P < 0.001).
| Domain | Group | 0 week | 12 weeks | P value |
| Physical | Group A | 39.44 ± 11.32 | 54.40 ± 11.69 | < 0.0001a |
| Group B | 37.44 ± 11.21 | 33.40 ± 11.87 | 0.0131a | |
| Psychological | Group A | 32.80 ± 11.43 | 47.72 ± 9.34 | < 0.0001a |
| Group B | 33.48 ± 9.99 | 28.04 ± 12.56 | 0.0098a | |
| Social | Group A | 33.16 ± 17.61 | 45.76 ± 14.02 | < 0.0001a |
| Group B | 40.28 ± 14.65 | 36.76 ± 15.81 | 0.075 | |
| Environmental | Group A | 32.6 ± 14.24 | 45.68 ± 13.07 | < 0.0001a |
| Group B | 38.4 ± 13.26 | 33.08 ± 14.67 | 0.004a |
There were 3 and 4 adverse events reported in group A and B, respectively which included nausea, gastritis and vomiting; all were mild in intensity and subsided spontaneously. None of the patient in either group had withdrawal due to treatment-related adverse events or worsening HF.
This prospective randomized open-label controlled clinical trial evaluated the efficacy and safety of sacubitril/valsartan as add-on to standard therapy compared to standard therapy alone when administered to patients with confirmed diagnosis of NYHA II-III HF. A total of 80 patients were enrolled in two treatment groups and followed up for 12 weeks duration. The efficacy end points (NT-pro BNP, hs-CRP and WHOQOL) were assessed at baseline and study completion; primary analysis was done as per per-protocol principle; 25 patients in each group were available for the same. Baseline demographic, clinical and biochemical characteristics in both the groups were comparable.
NT-pro BNP is a cardiac biomarker released in response to increased myocardial wall stress due to elevated intracardiac pressure and volume overload, key features of HF. It aids in diagnosing HF, differentiating cardiac from non-cardiac dyspnea, and predicting adverse outcomes like hospitalization and mortality and the levels correlate with HF severity[11-13]. In the present study, there was a mean 29% reduction in NT-pro BNP levels over 12 weeks in sacubitril/valsartan group compared to the standard therapy group demonstrating mean 28% increase in levels. We did not assess any correlation between change in NT-pro BNP levels with morbidity and mortality rates; however in the landmark PARADIGM-HF trial[9,10], the authors reported that decrease in NT-pro BNP was associated with a lower risk of primary composite end point of cardiovascular death or HF hospitalization [hazard ratio (HR) per halving of NT-pro BNP: 0.81; 95%CI: 0.72-0.90] while increase in NT-pro BNP was linked to greater risk of event (HR per doubling of NT-pro BNP: 1.24; 95%CI: 1.11-1.38). Impressively, in the PARADIGM-HF trial, in 24% participants, significant decline in NT-pro BNP levels were observed as early as 1 month and such reduction was associated with improved clinical outcomes in contrast to participants who did not have fall in levels. PROVE-HF, another study reported that sacubitril/valsartan led to reductions in NT-pro BNP levels along with an improvement in myocardial function and a reduction in the heart’s compensatory mechanisms[14]. Such consistent findings for NT-pro BNP levels in the current and earlier studies underscore the use of NT-pro BNP as a power surrogate biomarker for clinical outcomes in HF especially in clinical trials investigating the role of pharmacological agents which inhibit the degradation of natriuretic peptides.
Systemic inflammation is believed to serve as a direct mediator of the association between diverse comorbidities and compromised cardiac structure and hemodynamic deterioration, hallmark features of HF[15]. Elevated hs-CRP levels in HF reflect systemic inflammation, which contributes to myocardial remodeling, endothelial dysfunction, and progression of ventricular dysfunction[16]; notable correlation between elevated concentrations of hs-CRP and unfavourable prognosis in HF has been demonstrated[17]. Moreover, hs-CRP provides additional prognostic value when integrated with established markers of HF severity, such as B-type natriuretic peptide, offering insights into the inflammatory component of the disease and guiding risk stratification and therapeutic interventions[18,19]. We observed a greater reduction in hs-CRP levels in the sacubitril/valsartan group suggesting an anti-inflammatory benefit beyond its hemodynamic effects, a finding concordant to few earlier studies[18,19].
Shi et al[20], in a preclinical study, explored the underlying molecular mechanisms for the potential therapeutic role of sacubitril/valsartan in HF and suggested that sacubitril/valsartan induced improvement in LV modeling and diastolic dysfunction may be attributable to the drug’s anti-inflammatory, anti-hypertrophic and anti-fibrotic efficacy. Mechanistically, the anti-inflammatory potential of sacubitril/valsartan might be due to down-regulation of expression of intercellular adhesion molecule-1 and vascular endothelial cell adhesion molecule-1. Furthermore, drug induced blockade of phosphorylation of glycogen synthase kinase 3β prevents cardio-myocyte hypertrophy while inhibition of transforming growth factor-beta1/Smads pathway contributes to the drug’s anti-myocardial fibrotic property[20].
Sacubitril/valsartan group demonstrated significant improvement across all domains of WHOQOL-BREF Questionnaire[21], with particularly substantial gains in physical and psychological scores; indicating enhanced physical health, reduced psychological burden, and overall improvement in the quality of life. On the contrary, the standard group exhibited significant decline in the physical, psychological and environmental domains, as evidenced by the negative changes in scores suggesting worsening physical functioning, increased psychological distress (e.g., anxiety, depression), and a diminished perception of environmental factors such as safety, resources, or access to care; overall results reflecting a deteriorating quality of life. These findings in the present study highlight the role of ARNIs in improving functional capacity and patient-reported outcomes.
With respect to safety profile, there were no major adverse events reported during the study period, finding in agreement with the safety data from large-scale trials of sacubitril/valsartan reporting a tolerable adverse event profile, primarily limited to nausea, gastritis, vomiting, etc.
Few limitations of the study need to be considered. The study was conducted among limited number of participants at a single tertiary care center; thereby limiting the generalizability of findings. Also, due to relatively short follow up duration of 3 months, the impact of study treatment on long-term cardiovascular and mortality outcomes could not be ascertained. Despite the limitations, the study's randomized design ensured robust comparisons, and the comprehensive evaluation of biomarkers, quality of life and safety metrics strengthened its clinical relevance.
Sacubitril/valsartan as an add-on to standard HF therapy resulted in significant improvement in HF biomarkers, inflammation, and quality of life as compared to standard therapy alone without compromising safety. These findings support its role as an effective addition to standard HF therapy, with further research needed to evaluate its long-term benefits on mortality and cardiac remodeling.
We express our gratitude to the patients and the laboratory technical staff in the Department of Microbiology at PGIMS, Rohtak.
| 1. | Ziaeian B, Fonarow GC. Epidemiology and aetiology of heart failure. Nat Rev Cardiol. 2016;13:368-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 791] [Cited by in RCA: 1350] [Article Influence: 135.0] [Reference Citation Analysis (1)] |
| 2. | Mazurek JA, Jessup M. Understanding Heart Failure. Heart Fail Clin. 2017;13:1-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Heidenreich PA, Bozkurt B, Aguilar D, Allen LA, Byun JJ, Colvin MM, Deswal A, Drazner MH, Dunlay SM, Evers LR, Fang JC, Fedson SE, Fonarow GC, Hayek SS, Hernandez AF, Khazanie P, Kittleson MM, Lee CS, Link MS, Milano CA, Nnacheta LC, Sandhu AT, Stevenson LW, Vardeny O, Vest AR, Yancy CW; ACC/AHA Joint Committee Members. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2022;145:e895-e1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1618] [Cited by in RCA: 1660] [Article Influence: 415.0] [Reference Citation Analysis (0)] |
| 4. | McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A; ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599-3726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8225] [Cited by in RCA: 8627] [Article Influence: 1725.4] [Reference Citation Analysis (1)] |
| 5. | Wachter R, Shah SJ, Cowie MR, Szecsödy P, Shi V, Ibram G, Zhao Z, Gong J, Klebs S, Pieske B. Angiotensin receptor neprilysin inhibition versus individualized RAAS blockade: design and rationale of the PARALLAX trial. ESC Heart Fail. 2020;7:856-864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 6. | Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E, Shi V, Bransford T, Takeuchi M, Gong J, Lefkowitz M, Packer M, McMurray JJ; Prospective comparison of ARNI with ARB on Management Of heart failUre with preserved ejectioN fracTion (PARAMOUNT) Investigators. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet. 2012;380:1387-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 831] [Cited by in RCA: 915] [Article Influence: 65.4] [Reference Citation Analysis (0)] |
| 7. | Cunningham JW, Vaduganathan M, Claggett BL, Zile MR, Anand IS, Packer M, Zannad F, Lam CSP, Janssens S, Jhund PS, Kober L, Rouleau J, Shah SJ, Chopra VK, Shi VC, Lefkowitz MP, Prescott MF, Pfeffer MA, McMurray JJV, Solomon SD. Effects of Sacubitril/Valsartan on N-Terminal Pro-B-Type Natriuretic Peptide in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2020;8:372-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 8. | Pieske B, Wachter R, Shah SJ, Baldridge A, Szeczoedy P, Ibram G, Shi V, Zhao Z, Cowie MR; PARALLAX Investigators and Committee members. Effect of Sacubitril/Valsartan vs Standard Medical Therapies on Plasma NT-proBNP Concentration and Submaximal Exercise Capacity in Patients With Heart Failure and Preserved Ejection Fraction: The PARALLAX Randomized Clinical Trial. JAMA. 2021;326:1919-1929. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 124] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 9. | McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4078] [Cited by in RCA: 4994] [Article Influence: 416.2] [Reference Citation Analysis (0)] |
| 10. | Zile MR, Claggett BL, Prescott MF, McMurray JJ, Packer M, Rouleau JL, Swedberg K, Desai AS, Gong J, Shi VC, Solomon SD. Prognostic Implications of Changes in N-Terminal Pro-B-Type Natriuretic Peptide in Patients With Heart Failure. J Am Coll Cardiol. 2016;68:2425-2436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 295] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 11. | Li J, Cui Y, Huang A, Li Q, Jia W, Liu K, Qi X. Additional Diagnostic Value of Growth Differentiation Factor-15 (GDF-15) to N-Terminal B-Type Natriuretic Peptide (NT-proBNP) in Patients with Different Stages of Heart Failure. Med Sci Monit. 2018;24:4992-4999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Vergaro G, Gentile F, Meems LMG, Aimo A, Januzzi JL Jr, Richards AM, Lam CSP, Latini R, Staszewsky L, Anand IS, Cohn JN, Ueland T, Gullestad L, Aukrust P, Brunner-La Rocca HP, Bayes-Genis A, Lupón J, Yoshihisa A, Takeishi Y, Egstrup M, Gustafsson I, Gaggin HK, Eggers KM, Huber K, Gamble GD, Ling LH, Leong KTG, Yeo PSD, Ong HY, Jaufeerally F, Ng TP, Troughton R, Doughty RN, Devlin G, Lund M, Giannoni A, Passino C, de Boer RA, Emdin M. NT-proBNP for Risk Prediction in Heart Failure: Identification of Optimal Cutoffs Across Body Mass Index Categories. JACC Heart Fail. 2021;9:653-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 47] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 13. | Booth RA, Hill SA, Don-Wauchope A, Santaguida PL, Oremus M, McKelvie R, Balion C, Brown JA, Ali U, Bustamam A, Sohel N, Raina P. Performance of BNP and NT-proBNP for diagnosis of heart failure in primary care patients: a systematic review. Heart Fail Rev. 2014;19:439-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 14. | Januzzi JL Jr, Prescott MF, Butler J, Felker GM, Maisel AS, McCague K, Camacho A, Piña IL, Rocha RA, Shah AM, Williamson KM, Solomon SD; PROVE-HF Investigators. Association of Change in N-Terminal Pro-B-Type Natriuretic Peptide Following Initiation of Sacubitril-Valsartan Treatment With Cardiac Structure and Function in Patients With Heart Failure With Reduced Ejection Fraction. JAMA. 2019;322:1085-1095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 469] [Cited by in RCA: 465] [Article Influence: 66.4] [Reference Citation Analysis (0)] |
| 15. | Sanders-van Wijk S, Tromp J, Beussink-Nelson L, Hage C, Svedlund S, Saraste A, Swat SA, Sanchez C, Njoroge J, Tan RS, Fermer ML, Gan LM, Lund LH, Lam CSP, Shah SJ. Proteomic Evaluation of the Comorbidity-Inflammation Paradigm in Heart Failure With Preserved Ejection Fraction: Results From the PROMIS-HFpEF Study. Circulation. 2020;142:2029-2044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 195] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 16. | Minami Y, Kajimoto K, Sato N, Hagiwara N, Takano T; ATTEND Study Investigators. C-reactive protein level on admission and time to and cause of death in patients hospitalized for acute heart failure. Eur Heart J Qual Care Clin Outcomes. 2017;3:148-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Schiattarella GG, Rodolico D, Hill JA. Metabolic inflammation in heart failure with preserved ejection fraction. Cardiovasc Res. 2021;117:423-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 195] [Article Influence: 32.5] [Reference Citation Analysis (1)] |
| 18. | El-Sherbeni AA, Khedr NF, Khairat I, Werida RH. Diagnostic and Prognostic Roles of Inflammatory Biomarkers in Patients With Coronary Heart Disease and Heart Failure Treated With Empagliflozin. Clin Ther. 2025;47:e1-e11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 19. | Burger PM, Koudstaal S, Mosterd A, Fiolet ATL, Teraa M, van der Meer MG, Cramer MJ, Visseren FLJ, Ridker PM, Dorresteijn JAN; UCC-SMART study group. C-Reactive Protein and Risk of Incident Heart Failure in Patients With Cardiovascular Disease. J Am Coll Cardiol. 2023;82:414-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 85] [Article Influence: 28.3] [Reference Citation Analysis (0)] |
| 20. | Shi YJ, Yang CG, Qiao WB, Liu YC, Liu SY, Dong GJ. Sacubitril/valsartan attenuates myocardial inflammation, hypertrophy, and fibrosis in rats with heart failure with preserved ejection fraction. Eur J Pharmacol. 2023;961:176170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 21. | World Health Organization. WHO-QOL BREF. Available from: https://www.who.int/tools/whoqol/whoqol-bref. |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/