Published online Mar 20, 2025. doi: 10.5493/wjem.v15.i1.99516
Revised: September 17, 2024
Accepted: October 22, 2024
Published online: March 20, 2025
Processing time: 154 Days and 17.9 Hours
Acute myeloid leukemia (AML) is a complicated disease with uncontrolled hematopoietic precursor proliferation induced by various genetic alterations. Runt-related transcription factor-1 (RUNX1) is commonly disrupted by chromo
To characterize RUNX1 gene rearrangements and copy number variations in newly diagnosed adult AML patients, with an emphasis on the impact of clinical and laboratory features on the outcome.
Fluorescence in situ hybridization was used to test RUNX1 gene alterations in 77 newly diagnosed adult AML cases. NPM1, FLT3/ITD, FLT3/TKD, and KIT muta
RUNX1 abnormalities were detected by fluorescence in situ hybridization in 41.6% of patients: 20.8% had translocations, 22.1% had amplification, and 5.2% had deletion. Translocations prevailed in AML-M2 (P = 0.019) with a positive expression of myeloperoxidase (P = 0.031), whereas deletions dominated in M4 and M5 subtypes (P = 0.008) with a positive association with CD64 expression (P = 0.05). The modal chromosomal number was higher in cases having amplifications (P = 0.007) and lower in those with deletions (P = 0.008). RUNX1 abnormalities were associated with complex karyotypes (P < 0.001) and were mutually exclusive of NPM1 mutations. After 44 months of follow-up, RUNX1 abnormalities affected neither patients’ response to treatment nor overall survival.
RUNX1 abnormalities were mutually exclusive of NPM1 mutations. RUNX1 abnormalities affected neither patients’ response to treatment nor overall survival.
Core Tip: In the current study, we characterized the runt-related transcription factor-1 (RUNX1) gene rearrangements and copy number variations in patients with newly diagnosed adult acute myeloid leukemia with an emphasis on the impact of clinical and laboratory features on the outcome. RUNX1 abnormalities were mutually exclusive of NPM1 mutations. RUNX1 abnormalities affected neither patients’ response to treatment nor overall survival.
- Citation: Abd El-Ghany HM, El Ashry MS, Abdellateif MS, Rabea A, Sultan N, Abd El Dayem OY. Prevalence of RUNX1 gene alterations in de novo adult acute myeloid leukemia. World J Exp Med 2025; 15(1): 99516
- URL: https://www.wjgnet.com/2220-315x/full/v15/i1/99516.htm
- DOI: https://dx.doi.org/10.5493/wjem.v15.i1.99516
Acute myeloid leukemia (AML) is a heterogeneous hematologic cancer characterized by the clonal growth of myeloid blasts in the blood, bone marrow (BM), and/or other tissues. It is the most prevalent type of acute leukemia in adults[1]. Over the past few decades, the discovery of recurrent structurally balanced and unbalanced chromosomal abnormalities has significantly influenced the clinical management of patients with AML. These chromosomal abnormalities are the most significant prognostic markers where they can define specific clinicopathologic entities of the disease. The current recommendations of the European Leukemia Network for genetic testing in AML are primarily focused on risk stratification in order to identify an effective therapeutic strategy[2].
Runt-related transcription factor 1 (RUNX1) is the founding member of the mammalian core-binding transcription factor family, which also includes RUNX2, RUNX3, and their non-DNA-binding cofactor CBF[3]. The significance of the transcription factor RUNX1 in the t (8; 21) translocation in AML attracted initial interest. Since its discovery, RUNX1 has been found to play significant roles not only in leukemia but also in the development of the normal hematopoietic system[3]. Additionally, RUNX1 has been associated with epithelial tissue development and carcinogenesis[4]. The RUNX1 gene occupies approximately 261 kb on the long arm of chromosome 21. It controls the expression of genes involved in hematopoietic differentiation, ribosome synthesis, cell cycle regulation, p53, and transforming growth factor signaling pathways via interacting with various proteins through its domains[5].
Four forms of acquired RUNX1 genetic abnormalities have been identified in AML: (1) Translocations involving RUNX1 that result in fusion genes; (2) Molecular mutations; (3) RUNX1 amplifications; and (4) Partial or total deletions of the RUNX1 gene. It has been observed that RUNX1 deletions and gains were most common in patients with unfavorable cytogenetics and that the prognosis differed dramatically, being best for individuals with RUNX1 translocations and worst for those with deletions[6].
There have been reports of the RUNX1 gene fusing with over 40 partner genes encoding structurally diverse proteins. Some RUNX1-fusions are frequent in AML, and their partner genes are known to be implicated in recurrent translocations, including RUNX1 RUNX1T1/t (8; 21) (q22; q22), t (3; 21) (q26.2; q22), t (1; 21) (p36; q22), and t (16; 21) (q24; q22). These translocations have been investigated extensively, and their potential prognostic influence is uncertain. Others have been documented in only a small number of trials, and their potential prognostic influence is uncertain[7].
Genetic testing of patients with newly diagnosed AML with RUNX1 fluorescence in situ hybridization (FISH) probe provides the opportunity to identify more instances with minor rearrangements or new partner genes of the RUNX1 locus. This will improve our understanding of the prognosis for these instances and may ultimately aid in the estab
This study aimed to determine the prevalence of RUNX1 gene changes in patients with newly diagnosed AML and their influence on clinical outcomes. Various prognostic markers and other clinical and laboratory results were examined in relation to the expression of RUNX1 genetic variations.
Among the 263 adult patients who were diagnosed with AML between January 2018 and July 2019, 77 adult patients with de novo AML were included in this study. All patients were presented to the Outpatient Clinic of the Medical Oncology Department of the National Cancer Institute in Cairo, Egypt. Patients with acute promyelocytic leukemia were omitted from the study since their therapy and prognosis differ significantly from other patients with AML. Additionally, those with a history of hematologic disorders were excluded. The diagnosis of AML was based on morphological assessment of peripheral blood (PB) and BM smears, cytochemistry, immunophenotyping by flow cytometry, conventional cytoge
All patients underwent standard induction chemotherapy with the 3 + 7 treatment protocol (doxorubicin as a 3-day brief infusion and cytarabine 100 mg/m2 as a 7-day continuous infusion). Depending on their risk assessment, patients who achieved complete remission (CR) were offered consolidation with high-dose cytarabine and human leukocyte antigen matching, followed by allogeneic BM transplantation. Refractory cases were given a high-dose cytarabine-based regimen for re-induction.
Response to induction therapy was evaluated between days 14 and 28 post-induction. Response was categorized as CR, partial remission, stable disease, relapsed disease, or refractory disease. CR was defined as BM blasts 5%, absence of blasts with Auer rods, lack of extramedullary illness, neutrophil count > 1.0 × 109/L, platelet count > 100 × 109/L, and independence from red cell transfusions[10]. Patients who attained CR were divided into two groups termed normal recovery or delayed recovery based on whether they achieved CR before or after day 35, respectively[11]. Treatment failures were due to either disease resistance or relapse. The resistant disease was defined as the inability to achieve CR after completion of the initial treatment, with evidence of residual leukemia by PB and/or BM examination. Relapse was defined as BM blasts ≥ 5%, recurrence of blasts in PB, or the development of extramedullary illness.
Disease-free survival (DFS) was only defined for patients who attained a CR. It was calculated from the date of CR until the date of relapse or death, regardless of cause, censoring patients who were still alive at the time of the last follow-up. Overall survival (OS) was estimated from the date of protocol inclusion to the date of death or last follow-up/measured from the date of diagnosis to the date of death or last follow-up.
Pretreatment diagnostic conventional karyotyping was applied to BM samples employing G-banded metaphase cells from unstimulated 24-h cultures following the standard techniques. Using the IKAROS imaging system, at least 20 metaphases were analyzed in the majority of cases (Metasystems, Altlussheim, Germany). Through using International System for Human Cytogenetic Nomenclature, the karyotypes were interpreted (ISCN 2016)[12]. FISH was performed according to the manufacturer’s instructions using locus-specific probes XL RUNX1 dual-color Break-Apart probe (MetaSystems) to detect RUNX1 rearrangements, deletions, and amplifications, which together represented total RUNX1 abnormalities.
A minimum of 10 metaphases and 200 interphase nuclei were studied using a fluorescent microscope (AxioImager.Z1 mot; Carl Zeiss Ltd., Hertshire, United Kingdom) with the proper filter settings. The ISIS imaging system was utilized for image capture and processing (Metasystems).
Total RNA was extracted from BM or PB samples using Qiagen RNA Blood Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. RNA was reverse transcribed using a high-capacity complementary DNA reverse transcription Kit (Applied Biosystems, Waltham, MA, United States) for the identification of fusion transcripts t (9; 22) (q34; q11), t (8; 21) (q22; q22), and inv (16) (p13q22) in accordance with the BIOMED-1 guidelines[13]. Mutation analysis of four other significant molecular marker genes, NPM1, FLT3/ITD, FLT3/TKD, and KIT, was carried out using genomic DNA-PCR as directed by the manufacturer.
In all cases, blast cells in bone marrow aspiration samples were immunophenotyped using an EPICS XL Coulter Flow Cytometer (Beckman Coulter, Hialeah, FL, United States). A large panel of myeloid markers [myeloperoxidase (MPO), CD13, CD33, CD117, CD14, and CD15], lymphoid markers (CD10, CD19, CD22, CD79a, CD20, Cyto IgM, Kappa and Lambda for B lymphoid series, and CD3, CD2, 4, 8, 7, and 5 for T lymphoid series), and the stem cell marker CD34, as well as CD56 and human leukocyte antigen-DR, were used to confirm the diagnosis of AML.
Using version 22 of the statistical software program SPSS, data were analyzed (IBM, Armonk, NY, United States). According to the relevant normality test, quantitative data were described as mean ± standard deviation or median and interquartile ranges or as numbers and percentages for qualitative data. The relationship between RUNX-1 anomaly and patient clinical characteristics was evaluated using the χ2 test and/or Fisher exact test, where applicable. Numerical variables from two groups were compared using the Mann-Whitney test. The Kaplan-Meier test was utilized for survival analysis, whilst the log-rank test was utilized to compare survival curves. All tests were run with an alpha level of 0.05 and a confidence interval of 95%.
Every patient gave a written informed consent. The study was conducted in accordance with the Helsinki Declaration of 2011 and was approved by the internal review board of the National Cancer Institute and the Faculty of Medicine Research Ethics Committee at Cairo University (Code: MS-38-2020).
The median age of the 77 Egyptian patients with de novo AML was 42 (range of 18 to 82) years. Males represented 61% (47/77) of patients. Thirty-eight patients (49.4%) were AML-M2, while 22 patients (28.6%) were AML-M4. Based on genetic findings, the patients with AML were classified according to the European LeukemiaNet genetic risk classification into 18 patients (23.4%) with low risk, 42 patients (54.5%) with intermediate risk, and 17 patients (22.1%) with high-risk stratification. By molecular screening, recurrent translocations were identified in 15/77 cases (19.5%); 7 cases (9.1%) with t (8; 21) (q22; q22), 6 cases (7.8%) with inv (16) (p13q22), and 2 cases (2.6%) with t (9; 22) (q34; q11.2). Forty cases (51.9%) achieved CR, while 36 cases (46.8%) died before day 28. The detailed demographic, clinical, and laboratory characteristics of our patients are illustrated in Table 1.
| Parameter | Frequency | Percent | Median (IQR) |
| Sex | |||
| Male | 47 | 61 | |
| Female | 30 | 39 | |
| Age in years | 42 (18-82) | ||
| < 50 | 52 | 67.5 | |
| ≥ 50 | 25 | 32.5 | |
| TLC as × 109/L | 20 (1-377) | ||
| Hb in g/dL | 8.2 ± 2.39 | ||
| Platelets as × 109/L | 32 (1-658) | ||
| PB blast as % | 53 (0-63) | ||
| BM blast as % | 69 (20-97) | ||
| MCN | 45.8 ± 2.62 | ||
| < 46 | 10 | 14.5 | |
| 46 | 47 | 68.1 | |
| > 46 | 12 | 17.4 | |
| BM cellularity | |||
| Hypocellular | 3 | 3.9 | |
| Normocellular | 11 | 14.3 | |
| Hypercellular | 63 | 81.8 | |
| Hepatomegaly | |||
| Absent | 57 | 74 | |
| Present | 20 | 26 | |
| Splenomegaly | |||
| Absent | 60 | 77.9 | |
| Present | 17 | 22.1 | |
| Lymphadenopathy | |||
| Absent | 52 | 67.5 | |
| Present | 25 | 32.5 | |
| FAB classification | |||
| M0 | 4 | 5.2 | |
| M1 | 10 | 13 | |
| M2 | 38 | 49.4 | |
| M4 | 22 | 28.6 | |
| M5a | 2 | 2.6 | |
| M7 | 1 | 1.3 | |
| t (8; 21) | |||
| Absent | 70 | 90.9 | |
| Present | 7 | 9.1 | |
| inv16 | |||
| Absent | 71 | 92.2 | |
| Present | 6 | 7.8 | |
| t (9; 22) | |||
| Absent | 75 | 97.4 | |
| Present | 2 | 2.6 | |
| Genetic risk | |||
| High | 17 | 22.1 | |
| Intermediate | 42 | 54.5 | |
| Low | 18 | 23.4 | |
| FLT3-ITD | |||
| Wild | 69 | 89.6 | |
| Mutant | 8 | 10.4 | |
| FLT3-TKD | |||
| Wild | 75 | 97.4 | |
| Mutant | 2 | 2.6 | |
| C-KIT | |||
| Wild | 76 | 98.7 | |
| Mutant | 1 | 1.3 | |
| NPM | |||
| Wild | 63 | 81.8 | |
| Mutant | 14 | 18.2 | |
| BM blast on day 15 | 0.03 (0-1) | ||
| BM cellularity on day 15 | |||
| Hypocellular | 41 | 68.3 | |
| Normocellular | 11 | 18.3 | |
| Hypercellular | 8 | 13.3 | |
| BM blast on day 28 | 0.03 (0-1) | ||
| BM cellularity on day 28 | |||
| Hypocellular | 6 | 14.6 | |
| Normocellular | 21 | 51.2 | |
| Hypercellular | 14 | 34.1 | |
| CR | |||
| Negative | 37 | 48.1 | |
| Positive | 40 | 51.9 | |
| Delayed CR | |||
| Negative | 70 | 90.9 | |
| Positive | 7 | 9.1 | |
| Resistance | |||
| Negative | 67 | 87 | |
| Positive | 10 | 13 | |
| Relapse | |||
| Negative | 63 | 81.8 | |
| Positive | 14 | 18.2 | |
| Death | |||
| Negative | 20 | 26 | |
| Positive | 57 | 74 | |
| Early death | |||
| Negative | 41 | 53.2 | |
| Positive | 36 | 46.8 | |
Excluding 8 patients who failed to show mitosis, 22 out of 69 patients (31.9%) had normal karyotyping, while 9/69 patients (13.0%) had a complex karyotype including 4 cases with recurrent translocations. The median of the modal chromosome number (MCN) was 45.8 (range: 32-53); 10 patients (14.5%) had hypodiploidy, while 12 cases (17.4%) had a hyperdiploid karyotype (MCN > 46) including 4 cases with concurrent recurrent translocations, 2 cases with inv (16) (p13q22), 1 case with t (8; 21) (q22; q22), and 1 case with t (9; 22) (q34; q11.2).
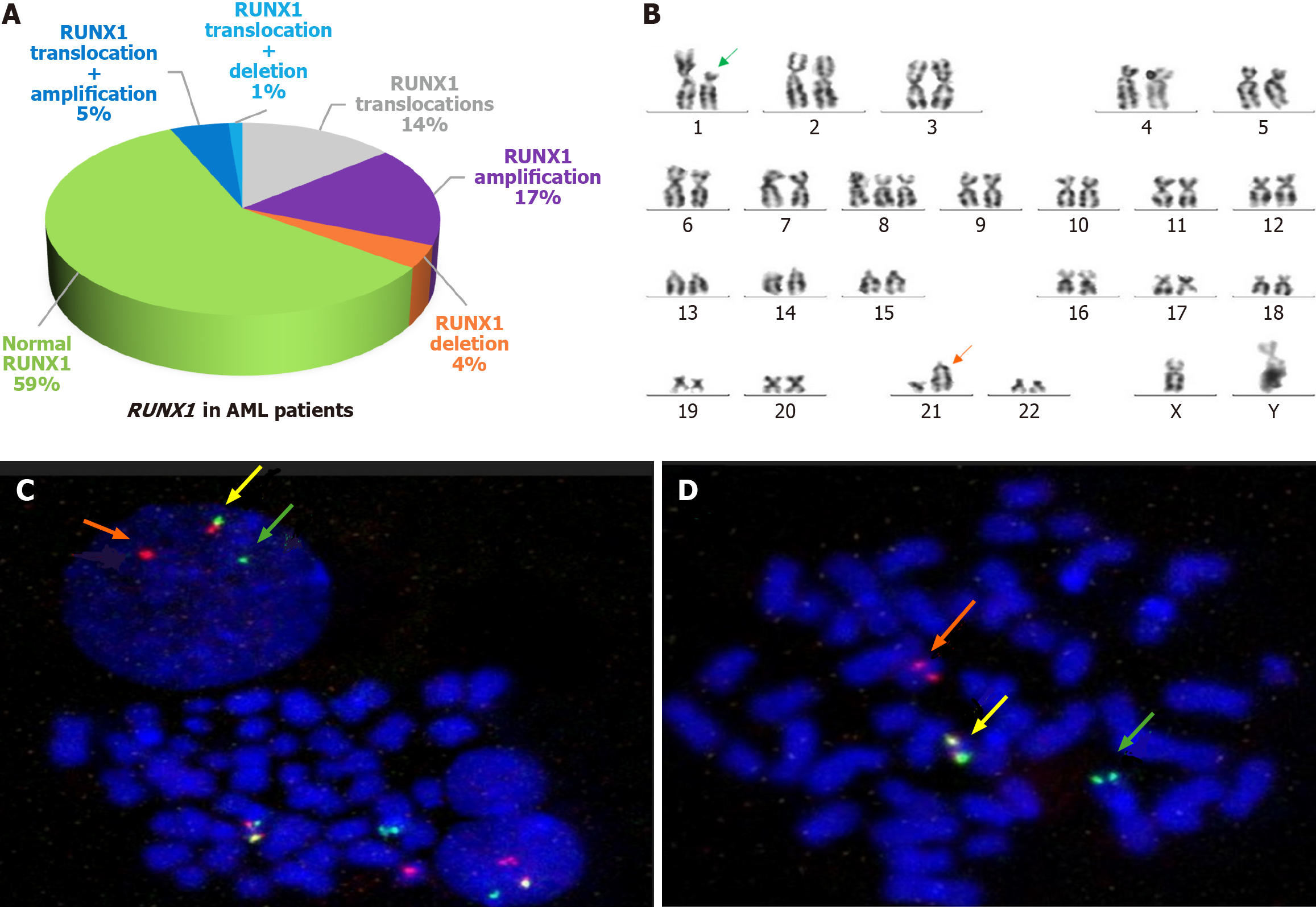
By FISH, RUNX1 gene abnormalities were found in 32/77 patients (41.6%): 16 patients (20.8%) showed RUNX1 rearrangements; 17 patients (22.1%) had RUNX1 amplifications; and 4 patients (5.2%) had RUNX1 deletion encompassing the whole RUNX1 gene (Figure 1A).
Out of 16 cases with RUNX1 translocations, 7 cases (43.8%) had t (8; 21) (q22; q22), 2 cases (12.5%) had t (1; 21) (Figure 1B-D), 1 case (6.3%) had t (16; 21), and 6 cases (37.5%) had unidentified partner chromosome. One or more copies of chromosome 21 in a hyperdiploid karyotype were gained in 4/17 cases (23.5%), while 10/ 17 cases (58.8%) showed RUNX1 duplications including 4 cases with concurrent RUNX1 translocations and 1 case with isochromosome 21. Three cases (17.6%) failed to show mitosis. Therefore, the differentiation could not be done.
Two out of four cases (50%) had -21; 1 case with a hypodiploid karyotype and another case with concurrent inv16 in a complex karyotype. Deletion of RUNX1 was found in 2/4 cases (50%) in a diploid karyotype including 1 case with concurrent RUNX1 translocations. The clinical characteristics of patients with and without different RUNX1 aberrations at diagnosis are presented in Table 1.
Although no statistically significant correlation between RUNX1 abnormalities and hepatomegaly, lymphadenopathy, or splenomegaly was found (P = 0.434, 0.808, and 0.404, respectively), patients with RUNX1 amplification presented with splenomegaly (P = 0.009) and 52.9% of them had lymphadenopathy (P = 0.076).
Hypercellular BM was more frequent in patients with RUNX1 abnormalities (68.8%) and translocations (62.5%) than normocellular and hypocellular marrow (P = 0.042 and 0.09, respectively).
In RUNX1 translocation positive cases, AML-M2 (43.8%) was the most frequent FAB subtype, and M0 and M7 tended to be more frequent among them than in RUNX1 translocations negative cases (75% vs 25% and 100% vs 0%, respectively; P = 0.019). In addition, RUNX1 translocations were positively associated with MPO expression (P = 0.031). Regarding RUNX1 deletion, all cases were of the myeloid with monocytic phenotype (FAB-M4 and M5) (P = 0.008) and were positively associated with the expression of CD64 (P = 0.050). Otherwise, there was no significant difference between RUNX1 positive and negative cases in different clinical characteristics. The comparison of clinical characteristics of patients with and without RUNX1 gene alterations is shown in (Table 2 and Table 3).
| Parameter | RUNX1 abnormalities | P value | RUNX1 translocation | P value | |||
| Negative | Positive | Negative | Positive | ||||
| Age in years, median (IQR) | 43.0 (20-69) | 35.0 (18-78) | 0.472 | 42.5 (20-78) | 30.5 (18-70) | 0.156 | |
| TLC as × 109/L, median (IQR) | 44.7 (1-377) | 20.8 (2-191) | 0.620 | 34.8 (1-377) | 20.1 (2-107) | 0.187 | |
| Hb in g/dL, mean ± SD | 8.38 ± 2.30 | 7.93 ± 2.50 | 0.421 | 8.15 ± 2.20 | 8.37 ± 3.10 | 0.748 | |
| Platelets as × 109/L, median (IQR) | 24 (12-266) | 30 (13-185) | 0.549 | 26 (12-226) | 27 (13-185) | 0.390 | |
| PB blast as %, median (IQR) | 60.0 (0-99) | 54.0 (5-92) | 0.426 | 53.0 (0-99) | 68.5 (33-92) | 0.950 | |
| BM blast as %, median (IQR) | 66 (30-97) | 62 (20-90) | 0.598 | 66 (20-97) | 70 (36-90) | 0.711 | |
| MCN, mean ± SD | 45.90 ± 0.67 | 45.80 ± 4.10 | 0.852 | 46.00 ± 2.50 | 45.30 ± 3.10 | 0.362 | |
| Sex, n (%) | Male | 28 (62.2) | 19 (59.4) | 0.817 | 40 (65.6) | 7 (43.8) | 0.151 |
| Female | 17 (37.8) | 13 (40.6) | 21 (34.4) | 9 (56.3) | |||
| BM cellularity, n (%) | Hypocellular | 1 (2.2 | 2 (6.3) | 0.042 | 3 (4.9) | 0 (0.0) | 0.009 |
| Normocellular | 3 (6.7) | 8 (25.0) | 5 (8.2) | 6 (37.5) | |||
| Hypercellular | 41 (91.1) | 22 (68.8) | 53 (86.9) | 10 (62.5) | |||
| Hepatomegaly, n (%) | Absent | 35 (77.8) | 22 (68.8) | 0.434 | 45 (73.8) | 12 (75.0) | 0.920 |
| Present | 10 (22.2) | 10 (31.3) | 16 (26.2) | 4 (25.0) | |||
| Splenomegaly, n (%) | Absent | 37 (82.2) | 23 (71.9) | 0.404 | 47 (77.0) | 13 (81.3) | 0.718 |
| Present | 8 (17.8) | 9 (28.1) | 14 (23.0) | 3 (18.8) | |||
| Lymphadenopathy, n (%) | Absent | 31 (68.9) | 21 (65.6) | 0.808 | 41 (67.2) | 11 (68.8) | 0.907 |
| Present | 14 (31.1) | 11 (34.4) | 20 (32.8) | 5 (31.3) | |||
| MPO, n (%) | Negative | 3 (6.7) | 5 (15.6) | 0.265 | 4 (6.6) | 4 (25.0) | 0.031 |
| Positive | 42 (93.3) | 27 (84.4) | 57 (93.4) | 12 (75.0) | |||
| CD34, n (%) | Negative | 26 (57.8) | 16 (50.0) | 0.643 | 35 (57.4) | 7 (43.8) | 0.330 |
| Positive | 19 (42.2) | 16 (50.0) | 26 (42.6) | 9 (56.3) | |||
| CD64, n (%) | Negative | 35 (77.8) | 22 (68.8) | 0.434 | 45 (73.8) | 12 (75.0) | 0.920 |
| Positive | 10 (22.2) | 10 (31.3) | 16 (26.2) | 4 (25.0) | |||
| CD14, n (%) | Negative | 39 (86.7) | 29 (90.6) | 0.728 | 53 (86.9) | 15 (93.8) | 0.447 |
| Positive | 6 (13.3) | 3 (9.4) | 8 (13.1) | 1 (6.3) | |||
| FAB, n (%) | M0 | 1 (2.2) | 3 (9.4) | 0.241 | 1 (1.6) | 3 (18.8) | 0.019 |
| M1 | 5 (11.1) | 5 (15.6) | 9 (14.8) | 1 (6.3) | |||
| M2 | 27 (60.0) | 11 (34.4) | 31 (50.8) | 7 (43.8) | |||
| M4 | 11 (24.4) | 11 (34.4) | 19 (31.1) | 3 (18.8) | |||
| M5 | 1 (2.2) | 1 (3.1) | 1 (1.6) | 1 (6.3) | |||
| M7 | 0 (0.0) | 1 (3.1) | 0 (0.0) | 1 (6.3) | |||
| Complex, n (%) | Negative | 40 (100.0) | 20 (69.0) | < 0.001 | 50 (94.3) | 10 (62.5) | 0.001 |
| Positive | 0 (0.0) | 9 (31.0) | 3 (5.7) | 6 (37.5) | |||
| t (8; 21), n (%) | Negative | 45 (100.0) | 25 (78.1) | 0.001 | 61 (100.0) | 9 (56.3) | < 0.001 |
| Positive | 0 (0.0) | 7 (21.9) | 0 (0.0) | 7 (43.8) | |||
| inv16, n (%) | Negative | 41 (91.1) | 30 (93.8) | 0.670 | 55 (90.2) | 16 (100.0) | 0.191 |
| Positive | 4 (8.9) | 2 (6.3) | 6 (9.8) | 0 (0.0) | |||
| t (9; 22), n (%) | Negative | 44 (97.8) | 31 (96.9) | 0.806 | 59 (96.7) | 16 (100.0) | 0.463 |
| Positive | 1 (2.2) | 1 (3.1) | 2 (3.3) | 0 (0.0) | |||
| Cytogenetic risk, n (%) | High | 8 (17.8) | 9 (28.1) | 0.310 | 12 (19.7) | 5 (31.3) | 0.542 |
| Intermediate | 24 (53.3) | 18 (56.3) | 35 (57.4) | 7 (43.8) | |||
| Low | 13 (28.9) | 5 (15.6) | 14 (23.0) | 4 (25.0) | |||
| FLT3-ITD, n (%) | Wildtype | 38 (84.4) | 31 (96.9) | 0.078 | 53 (86.9) | 16 (100.0) | 0.126 |
| Mutant | 7 (15.6) | 1 (3.1) | 8 (13.1) | 0 (0.0) | |||
| C-KIT, n (%) | Wildtype | 45 (100.0) | 31 (96.9) | 0.416 | 61 (100.0) | 15 (93.8) | 0.208 |
| Mutant | 0 (0.0) | 1 (3.1) | 0 (0.0) | 1 (6.3) | |||
| NPM, n (%) | Wildtype | 33 (73.3) | 30 (93.8) | 0.034 | 47 (77.0) | 16 (100.0) | 0.034 |
| Mutant | 12 (26.7) | 2 (6.3) | 14 (23.0) | 0 (0.0) | |||
| BM blast on day 28, n (%) | 0.04 (0-1) | 0.02 (0-1) | 0.082 | 0.03 (0-1) | 0.04 (0-1) | 0.789 | |
| BM cellularity on day 28, | Hypocellular | 4 (19.0) | 2 (10.0) | 0.507 | 5 (16.7) | 1 (6.3) | 0.831 |
| Normocellular | 9 (42.9) | 12 (60.0) | 15 (50.0) | 6 (54.5) | |||
| Hypercellular | 8 (38.1) | 6 (30.0) | 10 (33.3) | 4 (36.4) | |||
| CR, n (%) | Negative | 23 (51.1) | 14 (43.8) | 0.644 | 30 (49.2) | 7 (43.8) | 0.699 |
| Positive | 22 (48.9) | 18 (56.3) | 31 (50.8) | 9 (56.3) | |||
| Delayed CR, n (%) | Negative | 43 (95.6) | 27 (84.4) | 0.093 | 57 (93.4) | 13 (81.3) | 0.131 |
| Positive | 2 (4.4) | 5 (15.6) | 4 (6.6) | 3 (18.8) | |||
| Relapse, n (%) | Negative | 40 (88.9) | 23 (71.9) | 0.075 | 52 (85.2) | 11 (68.8) | 0.128 |
| Positive | 5 (11.1) | 9 (28.1) | 9 (14.8) | 5 (31.3) | |||
| Death, n (%) | Negative | 9 (20.0) | 11 (34.4) | 0.192 | 15 (24.6) | 5 (31.3) | 0.589 |
| Positive | 36 (80.0) | 21 (65.6) | 46 (75.4) | 11 (68.8) | |||
| Early death, n (%) | Negative | 21 (46.7) | 20 (62.5) | 0.247 | 30 (49.2) | 11 (68.8) | 0.163 |
| Positive | 24 (53.3) | 12 (37.5) | 31 (50.8) | 5 (31.3) | |||
| Parameter | RUNX1 amplification | P value | RUNX1 deletion | P value | |||
| Negative | Positive | Negative | Positive | ||||
| Age in years, median (IQR) | 42.0 (18-70) | 36.0 (20-78) | 0.694 | 41.5 (18-78) | 21.5 (21-22) | 0.175 | |
| TLC as × 109/L, median (IQR) | 44.7 (1-377) | 19.2 (2-91) | 0.873 | 29.1 (1-377) | 105.3 (19-191) | 0.630 | |
| Hb in g/dL, mean ± SD | 8.33 ± 2.50 | 7.70 ± 1.70 | 0.359 | 8.20 ± 2.40 | 8.10 ± 1.50 | 0.950 | |
| Platelets as × 109/L, median (IQR) | 24 (12-226) | 45 (21-185) | 0.484 | 26 (12-226) | 24 (18-30) | 0.663 | |
| PB blast (%), median (IQR) | 60 (0-99) | 38 (5-72) | 0.515 | 59 (0-99) | 47 (40-54) | 0.232 | |
| BM blast (%), median (IQR) | 66 (30-97) | 60 (20-90) | 0.35 | 65.5 (20-97) | 74.5 (71-78) | 0.613 | |
| MCN, mean ± SD | 45.4 ± 2.5 | 47.5 ± 2.4 | 0.007 | 46 ± 2.1 | 42.5 ± 7 | 0.008 | |
| Sex, n (%) | Male | 34 (56.7) | 13 (76.5) | 0.168 | 45 (61.6) | 2 (50.0) | 0.641 |
| Female | 26 (43.3) | 4 (23.5) | 28 (38.4) | 2 (50.0) | |||
| BM cellularity, n (%) | Hypocellular | 1 (1.7) | 2 (11.8) | 0.137 | 3 (4.1) | 0 (0.0) | 0.626 |
| Normocellular | 8 (13.3) | 3 (17.6) | 11 (15.1) | 0 (0.0) | |||
| Hypercellular | 51 (85.0) | 12 (70.6) | 59 (80.8) | 4 (100.0) | |||
| Hepatomegaly, n (%) | Absent | 47 (78.3) | 10 (58.8) | 0.125 | 53 (72.6) | 4 (100.0) | 0.568 |
| Present | 13 (21.7) | 7 (41.2) | 20 (27.4) | 0 (0.0) | |||
| Splenomegaly, n (%) | Absent | 51 (85.0) | 9 (52.9) | 0.009 | 56 (76.7) | 4 (100.0) | 0.57 |
| Present | 9 (15.0) | 8 (47.1) | 17 (23.3) | 0 (0.0) | |||
| Lymphadenopathy, n (%) | Absent | 44 (73.3) | 8 (47.1) | 0.076 | 49 (67.1) | 3 (75.0) | 0.743 |
| Present | 16 (26.7) | 9 (52.9) | 24 (32.9) | 1 (25.0) | |||
| MPO, n (%) | Negative | 6 (10.0) | 2 (11.8) | 0.833 | 8 (11.0) | 0 (0.0) | 1 |
| Positive | 54 (90.0) | 15 (88.2) | 65 (89.0) | 4 (100.0) | |||
| CD34, n (%) | Negative | 34 (56.7) | 8 (47.1) | 0.584 | 38 (52.1) | 4 (100.0) | 0.121 |
| Positive | 26 (43.3) | 9 (52.9) | 35 (47.9) | 0 (0.0) | |||
| CD64, n (%) | Negative | 45 (75.0) | 12 (70.6) | 0.758 | 56 (76.7) | 1 (25.0) | 0.052 |
| Positive | 15 (25.0) | 5 (29.4) | 17 (23.3) | 3 (75.0) | |||
| CD14, n (%) | Negative | 51 (85.0) | 17 (100.0) | 0.194 | 66 (90.4) | 2 (50.0) | 0.065 |
| Positive | 9 (15.0) | 0 (0) | 7 (9.6) | 2 (50.0) | |||
| FAB, n (%) | M0 | 2 (3.3) | 2 (11.8) | 0.368 | 4 (5.5) | 0 (0.0) | 0.014 |
| M1 | 6 (10.0) | 4 (23.5) | 10 (13.7) | 0 (0.0) | |||
| M2 | 32 (53.3) | 6 (35.3) | 38 (52.1) | 0 (0.0) | |||
| M4 | 17 (28.3) | 5 (29.4) | 19 (26.0) | 3 (75.0) | |||
| M5 | 2 (3.3) | 0 (0) | 1 (1.4) | 1 (25.0) | |||
| M7 | 1 (1.7) | 0 (0) | 1 (1.4) | 0 (0.0) | |||
| Complex, n (%) | Negative | 52 (94.5) | 8 (47.1) | 0.001 | 57 (87.7) | 3 (75.0) | 0.436 |
| Positive | 3 (5.5) | 6 (35.3) | 8 (12.3) | 1 (25.0) | |||
| t (8; 21), n (%) | Negative | 54 (90.0) | 16 (94.1) | 0.602 | 66 (90.4) | 4 (100.0) | 0.516 |
| Positive | 6 (10.0) | 1 (5.9) | 7 (9.6) | 0 (0.0) | |||
| inv16, n (%) | Negative | 55 (91.7) | 16 (94.1) | 0.739 | 68 (93.2) | 3 (75.0) | 0.282 |
| Positive | 5 (8.3) | 1 (5.9) | 5 (6.8) | 1 (25.0) | |||
| t (9; 22), n (%) | Negative | 59 (98.3) | 16 (94.1) | 0.395 | 71 (97.3) | 4 (100.0) | 0.737 |
| Positive | 1 (1.7) | 1 (5.9) | 2 (2.7) | 0 (0.0) | |||
| Cytogenetic risk, n (%) | High | 12 (20.0) | 5 (29.4) | 0.15 | 15 (20.5) | 2 (50.0) | 0.288 |
| Intermediate | 31 (51.7) | 11 (64.7) | 40 (54.8) | 2 (50.0) | |||
| Low | 17 (28.3) | 1 (5.9) | 18 (24.7) | 0 (0.0) | |||
| FLT3-ITD, n (%) | Wildtype | 52 (86.7) | 17 (100.0) | 0.188 | 66 (90.4) | 3 (75.0) | 0.361 |
| Mutant | 8 (13.3) | 0 (0) | 7 (9.6) | 1 (25.0) | |||
| C-KIT, n (%) | Wildtype | 59 (98.3) | 17 (100.0) | 0.592 | 72 (98.6) | 4 (100.0) | 0.814 |
| Mutant | 1 (1.7) | 0 (0) | 1 (1.4) | 0 (0.0) | |||
| NPM, n (%) | Wildtype | 47 (78.3) | 16 (94.1) | 0.173 | 60 (82.2) | 3 (75.0) | 0.717 |
| Mutant | 13 (21.7) | 1 (5.9) | 13 (17.8) | 1 (25.0) | |||
| BM cellularity on day 28, | Hypocellular | 6 (20.0) | 0 (0) | 0.152 | 5 (12.8) | 1 (25.0) | 0.22 |
| Normocellular | 13 (43.3) | 8 (47.1) | 21 (53.8) | 0 (0.0) | |||
| Hypercellular | 11 (36.7) | 3 (25.0) | 13 (33.3) | 1 (25.0) | |||
| CR, n (%) | Negative | 29 (48.3) | 8 (47.1) | 0.926 | 36 (49.3) | 1 (25.0) | 0.616 |
| Positive | 31 (51.7) | 9 (52.9) | 37 (50.7) | 3 (75.0) | |||
| Delayed CR, n (%) | Negative | 56 (93.30 | 14 | 0.177 | 66 (90.4) | 4 (100.0) | 0.516 |
| Positive | 4 (6.7) | 3 (25.0) | 7 (9.6) | 0 (0.0) | |||
| Relapse, n (%) | Negative | 50 (83.3) | 13 | 0.496 | 61 (83.6) | 2 (50.0) | 0.149 |
| Positive | 10 (16.7) | 4 | 12 (16.4) | 2 (50.0) | |||
| Death, n (%) | Negative | 13 (21.7) | 7 | 0.125 | 19 (26.0) | 1 (25.0) | 0.964 |
| Positive | 47 (78.3) | 10 | 54 (74.0) | 3 (75.0) | |||
| Early death, n (%) | Negative | 30 (50.0) | 11 | 0.41 | 39 (53.4) | 2 (50.0) | 0.894 |
| Positive | 30 (50.0) | 6 | 34 (46.6) | 2 (50.0) | |||
There was a highly statistically significant relation between RUNX1 copy number variations and MCN where positive cases to RUNX1 amplification tended to have higher MCN, while RUNX1 deletion cases tended to have lower MCN (P = 0.007 and 0.008, respectively). Cases positive to RUNX1 abnormalities, translocations, and amplifications tended to have complex karyotypes compared to RUNX1-negative cases (P = 0.000, 0.001, and 0.001, respectively). There was no statistically significant correlation between all types of RUNX1 abnormalities and other different cytogenetic abnormalities (as
To investigate the interaction of gene mutations in the pathogenesis of adult AML, screening of mutational status of four other genes was performed. Among the 32 patients with RUNX1 abnormalities, 4 cases showed additional molecular abnormalities including 2 cases with RUNX1 deletion, of which one had an FLT3-ITD mutation and the other case had concomitant FLT3/TKD and NPM1 mutations, 1 case with t (8; 21) and c-KIT mutation, and 1 case with RUNX1 ampli
FLT3-ITD mutations tended to be more prevalent in RUNX1-negative cases compared to positive cases. Out of 8 patients with AML with FLT3-ITD mutations, 7 patients (87.5%) were negative for RUNX1 abnormalities, while only 1 patient was positive for RUNX1 abnormalities. However, this relation was statistically insignificant (P = 0.078). Similarly, NPM1 mutations were rarely seen in RUNX1 abnormalities and translocations (P = 0.034 and 0.034, respectively). Otherwise, there were no significant differences between RUNX1 positive and negative cases regarding other cytogenetic and molecular abnormalities, as shown in Table 2 and Table 3 (P > 0.05).
RUNX1 translocations, amplifications, and deletions were more frequent in the intermediate risk group (43.8%, 64.7%, and 50.0%) and the high-risk group (31.3%, 29.4%, and 50.0%) than in the low risk group (25.0%, 5.9%, and 0%), but the relationship was not statistically significant (P = 0.542, 0.150, and 0.288, respectively).
The response to treatment at day 28 of starting chemotherapy revealed that 36 of 77 (46.8%) cases died before day 28, and 33 cases (42.9%) achieved CR at day 15, 7 cases (9.1%) achieved delayed CR, 10 cases (13%) were resistant to treatment, and eventually 57 cases (74%) died.
Fourteen cases (18.2%) relapsed after achieving CR, 9/14 cases (64.2%) were positive to RUNX1 abnormalities. One patient had t (9; 22) (q34; q11.2), and another patient had inv(16). The detailed karyotype and analysis of the outcome of those patients are summarized in Supplementary Table 1.
Although positive cases to RUNX1 abnormalities tended to have delayed CR and relapse compared to negative cases (71.4% vs 28.6% P = 0.093 and 64.3% vs 35.7% P = 0.075, respectively), the relationship was not statistically significant. The statistical analysis showed the absence of a relationship between the achievement of CR and RUNX1 abnormalities (P = 0.644), RUNX1 translocation (P = 0.699), RUNX1 amplification (P = 0.926), and RUNX1 deletion (P = 0.616), as shown in Table 2 and Table 3.
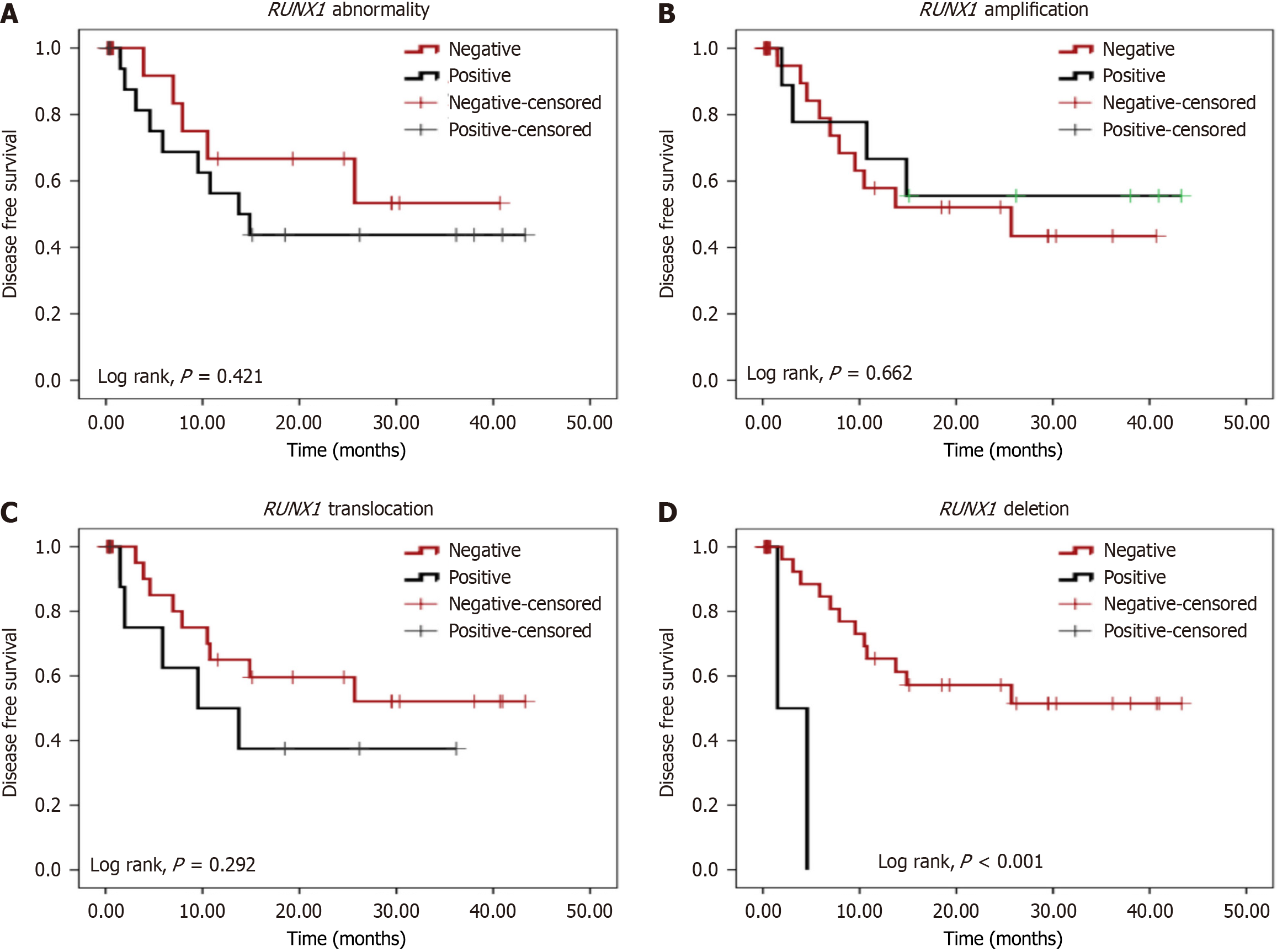
After a follow-up period of 44.2 months, the present study showed that there was no significant difference between positive and negative RUNX1 aberration cases regarding the OS (Figure 2). Patients with RUNX1 deletion had significantly poorer DFS than those without RUNX1 deletion (mean: 3.03 months vs 27.20 months, respectively; P < 0.001). No other significant differences were observed between any other type of RUNX1 alterations and negative cases regarding DFS (Figure 3).
The role of RUNX1 mutations in cytogenetically normal AML had been identified. However, the prognostic impact of RUNX1 translocations other than t (8; 21) (q22; q22), RUNX1 deletions, and amplifications are still unknown. As a result, addressing such interactions is critical for further risk classification and eventually the development of a successful therapeutic plan.
In this study, FISH was used to screen for RUNX1 gene alterations in 77 newly diagnosed adult patients with de novo AML, and the results were compared to clinical characteristics and prognosis. RUNX1 abnormalities were divided into four categories: (1) RUNX1 translocations; (2) RUNX1 amplifications; (3) RUNX1 deletions; and (4) RUNX1 abnormalities, which encompass all three types.
In agreement with Haferlach et al[6], total RUNX1 abnormalities were detected in 41.6% of cases of which RUNX1 amplification was the most common alteration (22.1%) followed by RUNX1 translocations (20.8%) then RUNX1 deletion (5.2%). Baldus et al[14] investigated 12 patients with AML with complicated karyotypes and chromosome 21 anomalies and showed that amplification of two chromosome 21 areas was frequently seen in AML with complex karyotypes using comparative genomic hybridization, supporting the notion that gain of chromosome 21 material appears to be a nonrandom event implicated in AML. Baldus et al[14] reasoned that this might be related to the function of a specific gene or set of genes.
The clinical and genetic characteristics of patients with and without RUNX1 alterations were compared. In agreement with Yamato et al[15], there were no statistically significant differences in any form of RUNX1 abnormalities with respect to age, sex, total leucocytic count, hemoglobin level, platelet count, PB count, or BM blast cell counts at presentation. Tang et al[16] discovered that male patients had a higher rate of RUNX1 alterations than female patients, while Haferlach et al[17] reported that patients with RUNX1 deletion were considerably older than those with two RUNX1 copies and had a lower WBC count. On the other hand, Said et al[18] used reverse transcription-quantitative PCR to explore the role of RUNX1 gene expression in Egyptian patients with AML and found that male patients had significantly higher RUNX1 expression. This discrepancy can be attributed to the difference in the technique used, a difference in the sample size, and variation in inclusion criteria between the two studies, in spite of conducting both studies on the same race.
The current data showed that splenomegaly is common in patients with RUNX1 amplification, and the majority of them had lymphadenopathy. Hypercellular marrow was also more frequent than normocellular and hypocellular marrow in RUNX1-abnormalities and translocations. No published research had associated RUNX1-abnormalities with hepatomegaly, splenomegaly, lymphadenopathy, or BM cellularity at the time of diagnosis, to our knowledge.
Of interest, 43.8% of patients with RUNX1 translocation were FAB AML-M2 and were associated with MPO expression. Also, M0 and M7 were more prevalent in RUNX1 translocation positive cases than in negative instances. All patients with RUNX1 deletion had a myeloid with monocytic phenotype (FAB-M4 and M5) and were favorably related with CD64 expression. These results matched those of Haferlach et al[6], who found that 45.2% of RUNX1 translocation cases were FAB type M1 and M2. However, in contrast to our findings, they found that in cases of RUNX1 deletion, M0 was the most common AML subtype. This discrepancy can be attributed to the racial and sample size variations. While Said et al[18] found that out of 42% of patients with AML classified as M2, translocation t (8; 21) was found in only 6.6% of the cases. The researchers also discovered no link between FAB subtypes and RUNX1 expression.
In keeping with earlier studies, translocations, amplifications, and deletions of RUNX1 were more common in the intermediate and high-risk groups, although not statistically significant.
In addition, as previously reported[17,19,20], positive cases of RUNX1 amplification have a higher MCN, whereas RUNX1 deletion cases have a lower MCN. This could be explained by the fact that chromosomal gain is nonrandom in patients with AML with hyperdiploid karyotypes, and chromosome 21 is one of the most frequently gained chro
The present data showed that RUNX1 abnormalities, translocations, and amplifications are associated with more complex karyotypes than RUNX1-negative instances, which supports prior research[6,14,17]. It was concluded that chromosome 21 amplifications are common in people with complicated karyotypes.
In terms of other molecular mutations, 8 cases tested positive for the FLT3-ITD mutation. RUNX1 translocations and amplifications were all negative, but RUNX1 deletion was positive in 1 patient (12.5%). These relationships, however, did not exhibit statistical significance. According to Said et al[18], patients with higher RUNX1 expression were more likely to have FLT3-ITD mutation than patients with lower RUNX1 expression. This suggested that RUNX1 could operate as an oncogene that causes leukemogenesis and as a surrogate marker for other mutations, particularly FLT3-ITD, when expressed at high levels.
Furthermore, NPM1 mutations were mutually exclusive of RUNX1 abnormalities and RUNX1 translocations. This suggests that RUNX1 mutation shares a similar genetic pathway role with NPM1 mutations in leukemia development. This was supported by the study of Zuo et al[21], who stated that NPM1 mutant interacts with PU.1/CEB-PA/RUNX1 transcription factor complexes to block myeloid differentiation. Additionally, NPM1 mutations were found at a lower frequency in RUNX1 copy number variation-positive patients than in negative cases, but the difference was not statistically significant. These RUNX1 mutations have genetic characteristics that are similar to those previously described in patients with AML[6,15,17].
There was no statistically significant association between any form of RUNX1 modification and the achievement of CR or OS in terms of clinical outcome. Although the median DFS differed significantly amongst the three types of RUNX1 changes (9.5, 25.7, and 1.5 months, respectively), RUNX1 amplifications had the best prognosis. Only those with RUNX1 deletion exhibited a considerably worse outcome than cases without the mutation. Consistently, previous research has found that OS varies dramatically between the different forms of RUNX1 alterations, with RUNX1 deletions having the worst outcome[6].
On the contrary, other studies[6,22] found that the outcome differed considerably across RUNX1 alterations and was better in individuals with RUNX1 translocations. Discrepancies in the results could be related to the fact that most research looked at RUNX1 mutations in combination with cytogenetic abnormalities, whereas just a few looked at the impact of RUNX1 translocations, amplifications, and deletions in AML cases from different risk groups. Furthermore, it is thought that the different sorts of techniques used in the studies are a key source of heterogeneity. Said et al[18] found no significant impact of RUNX1 expression on OS or DFS rates, but Chen et al[23] found that RUNX1 mutation was linked to a lower risk-free survival rate.
Nine out of seventeen (53%) RUNX1 amplification positive cases had RUNX1 duplications. Four cases (29.4%) had RUNX1 translocations at the same time, and five cases (29.4%) obtained one or more copies of chromosome 21, four of which were in a hyperdiploid karyotype. All of these are favorable prognostic markers that may help patients with positive RUNX1 amplification live longer. This could explain the disparity in clinical outcomes between our data and that of others.
Furthermore, when inv16 and t (9; 22) cases with favorable prognosis were excluded from statistical analysis, the mean DFS in positive RUNX1 deletion cases was 3.033 months compared to 27.231 months in negative RUNX1 deletion cases. This suggests that RUNX1 deletion has the worst prognosis, even if other strong prognostic markers like inv16 and t (9; 22) are present.
Our data presented a pilot study for RUNX1 gene alterations in a cohort of patients with de novo AML. RUNX1 abnormalities were detected in 41.6% of patients. RUNX1 translocations occurred predominantly in FAB M2, M0, and M7 while RUNX1 deletions were of myeloid with monocytic phenotype (FAB-M4 and M5). Cases positive for RUNX1 abnormalities, translocations, and amplifications tended to have complex karyotypes. RUNX1 abnormalities were mutually exclusive of NPM1 mutations. RUNX1 deletion was an independent adverse parameter for DFS. Further trials with larger numbers of RUNX1 abnormal cases are warranted to further highlight the prognostic features and the predictive significance of this abnormality.
The authors would like to thank all the patients who were included in this study.
| 1. | Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13300] [Cited by in RCA: 15621] [Article Influence: 2231.6] [Reference Citation Analysis (11)] |
| 2. | Mack EKM, Marquardt A, Langer D, Ross P, Ultsch A, Kiehl MG, Mack HID, Haferlach T, Neubauer A, Brendel C. Comprehensive genetic diagnosis of acute myeloid leukemia by next-generation sequencing. Haematologica. 2019;104:277-287. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 3. | Mevel R, Draper JE, Lie-A-Ling M, Kouskoff V, Lacaud G. RUNX transcription factors: orchestrators of development. Development. 2019;146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 164] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 4. | Hong D, Fritz AJ, Gordon JA, Tye CE, Boyd JR, Tracy KM, Frietze SE, Carr FE, Nickerson JA, Van Wijnen AJ, Imbalzano AN, Zaidi SK, Lian JB, Stein JL, Stein GS. RUNX1-dependent mechanisms in biological control and dysregulation in cancer. J Cell Physiol. 2019;234:8597-8609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 5. | Yenamandra A. Unique RUNX1 Gene Rearrangements in Acute Myeloid Leukemia Identified (AML). Int Clin Pathol J. 2017;5:202-203. [DOI] [Full Text] |
| 6. | Haferlach C, Nadarajah N, Kern W, Schnittger S, Haferlach T. The RUNX1 Gene Is Altered in 26% of AML Patients Either By Translocation, Mutation, Gain or Deletion. Blood. 2014;124:123. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Panagopoulos I, Gorunova L, Jacobsen EM, Andersen K, Micci F, Heim S. RUNX1-PDCD6 fusion resulting from a novel t(5;21)(p15;q22) chromosome translocation in myelodysplastic syndrome secondary to chronic lymphocytic leukemia. PLoS One. 2018;13:e0196181. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (2)] |
| 8. | Newell LF, Cook RJ. Advances in acute myeloid leukemia. BMJ. 2021;375:n2026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 265] [Article Influence: 53.0] [Reference Citation Analysis (0)] |
| 9. | Hwang SM. Classification of acute myeloid leukemia. Blood Res. 2020;55:S1-S4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 10. | Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, Dombret H, Ebert BL, Fenaux P, Larson RA, Levine RL, Lo-Coco F, Naoe T, Niederwieser D, Ossenkoppele GJ, Sanz M, Sierra J, Tallman MS, Tien HF, Wei AH, Löwenberg B, Bloomfield CD. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129:424-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3010] [Cited by in RCA: 4454] [Article Influence: 445.4] [Reference Citation Analysis (0)] |
| 11. | Murphy T, Zou J, Daher-reyes GS, Gupta V, Mcnamara CJ, Minden MD, Schimmer AD, Sibai H, Yee KW, Stockley TL, Kamel-reid S, Maze D, Bratman S, Schuh A, Chan SM. Delayed Hematologic Recovery in AML Patients after Induction Chemotherapy Is Associated with Inferior Relapse-Free Survival and Persistence of Preleukemic Mutations. Blood. 2018;132 Suppl 1:992. [RCA] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 12. | McGowan-Jordan J, Simons A and Schmid M. ISCN 2016: An International System for Human Cytogenomic Nomenclature (2016). Basel: Karger, 2020. [DOI] [Full Text] |
| 13. | van Dongen JJ, Macintyre EA, Gabert JA, Delabesse E, Rossi V, Saglio G, Gottardi E, Rambaldi A, Dotti G, Griesinger F, Parreira A, Gameiro P, Diáz MG, Malec M, Langerak AW, San Miguel JF, Biondi A. Standardized RT-PCR analysis of fusion gene transcripts from chromosome aberrations in acute leukemia for detection of minimal residual disease. Report of the BIOMED-1 Concerted Action: investigation of minimal residual disease in acute leukemia. Leukemia. 1999;13:1901-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 828] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 14. | Baldus CD, Liyanarachchi S, Mrózek K, Auer H, Tanner SM, Guimond M, Ruppert AS, Mohamed N, Davuluri RV, Caligiuri MA, Bloomfield CD, de la Chapelle A. Acute myeloid leukemia with complex karyotypes and abnormal chromosome 21: Amplification discloses overexpression of APP, ETS2, and ERG genes. Proc Natl Acad Sci U S A. 2004;101:3915-3920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 140] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 15. | Yamato G, Shiba N, Yoshida K, Hara Y, Shiraishi Y, Ohki K, Okubo J, Park MJ, Sotomatsu M, Arakawa H, Kiyokawa N, Tomizawa D, Adachi S, Taga T, Horibe K, Miyano S, Ogawa S, Hayashi Y. RUNX1 mutations in pediatric acute myeloid leukemia are associated with distinct genetic features and an inferior prognosis. Blood. 2018;131:2266-2270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (1)] |
| 16. | Tang JL, Hou HA, Chen CY, Liu CY, Chou WC, Tseng MH, Huang CF, Lee FY, Liu MC, Yao M, Huang SY, Ko BS, Hsu SC, Wu SJ, Tsay W, Chen YC, Lin LI, Tien HF. AML1/RUNX1 mutations in 470 adult patients with de novo acute myeloid leukemia: prognostic implication and interaction with other gene alterations. Blood. 2009;114:5352-5361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 273] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 17. | Haferlach C, Grossmann V, Zenger M, Kern W, Haferlach T, Kohlmann A, Schnittger S. RUNX1 Deletions Are a Novel Mechanism of Loss of Function in AML and Are Associated with Adverse Cytogenetics. Blood. 2012;120:2517. [RCA] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 18. | Said F, Shafik RE, Hassan NM. RUNX1 gene expression in Egyptian acute myeloid leukemia patients: may it have therapeutic implications? Egypt J Med Hum Genet. 2021;58. [RCA] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 19. | Sandahl JD, Kjeldsen E, Abrahamsson J, Ha SY, Heldrup J, Jahnukainen K, Jónsson OG, Lausen B, Palle J, Zeller B, Forestier E, Hasle H. Ploidy and clinical characteristics of childhood acute myeloid leukemia: A NOPHO-AML study. Genes Chromosomes Cancer. 2014;53:667-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Abaza Y, Cortes J, Ravandi F, Kadia T, Garcia-Manero G, Pemmaraju N, Shetty A, Pierce S, Qiao W, Kantarjian HM, Borthakur G. Prognostic significance of hyperdiploidy in adult acute myeloid leukemia. Am J Hematol. 2018;93:E357-E360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Zuo Z, Medeiros LJ, Yin CC. Acute myeloid leukemia with concurrent NPM1 and RUNX1 mutations. Leuk Res Rep. 2023;20:100385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Nguyen D, Li Y, Safah H, Brown TC. RUNX1 deletion/amplification in therapy-related acute myeloid leukemia: A case report and review of the literature. Cancer Genet. 2019;238:37-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Chen X, Zhu H, Qiao C, Zhao S, Liu L, Wang Y, Jin H, Qian S, Wu Y. Next-generation sequencing reveals gene mutations landscape and clonal evolution in patients with acute myeloid leukemia. Hematology. 2021;26:111-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/