Published online Mar 20, 2025. doi: 10.5493/wjem.v15.i1.94022
Revised: October 3, 2024
Accepted: October 30, 2024
Published online: March 20, 2025
Processing time: 291 Days and 7.6 Hours
Due to saliva and salivary glands are reservoir to severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2), aerosols and saliva droplets are primary sources of cross-infection and are responsible for the high human–human transmission of SARS-CoV-2. However, there is no evidence about how SARS-CoV-2 interacts with oral structures, particularly resin composites.
To evaluate the interaction of SARS-CoV-2 proteins with monomers present in resin composites using in silico analysis.
Four SARS-CoV-2 proteins [i.e. main protease, 3C-like protease, papain-like protease (PLpro), and glycoprotein spike] were selected along with salivary amylase as the positive control, and their binding affinity with bisphenol-A glycol dimethacrylate, bisphenol-A ethoxylated dimethacrylate, triethylene glycol dimethacrylate, and urethane dimethacrylate was evaluated. Molecular docking was performed using AutoDock Vina and visualised in Chimera UCSF 1.14. The best ligand–protein model was identified based on the binding energy (ΔG–kcal/moL).
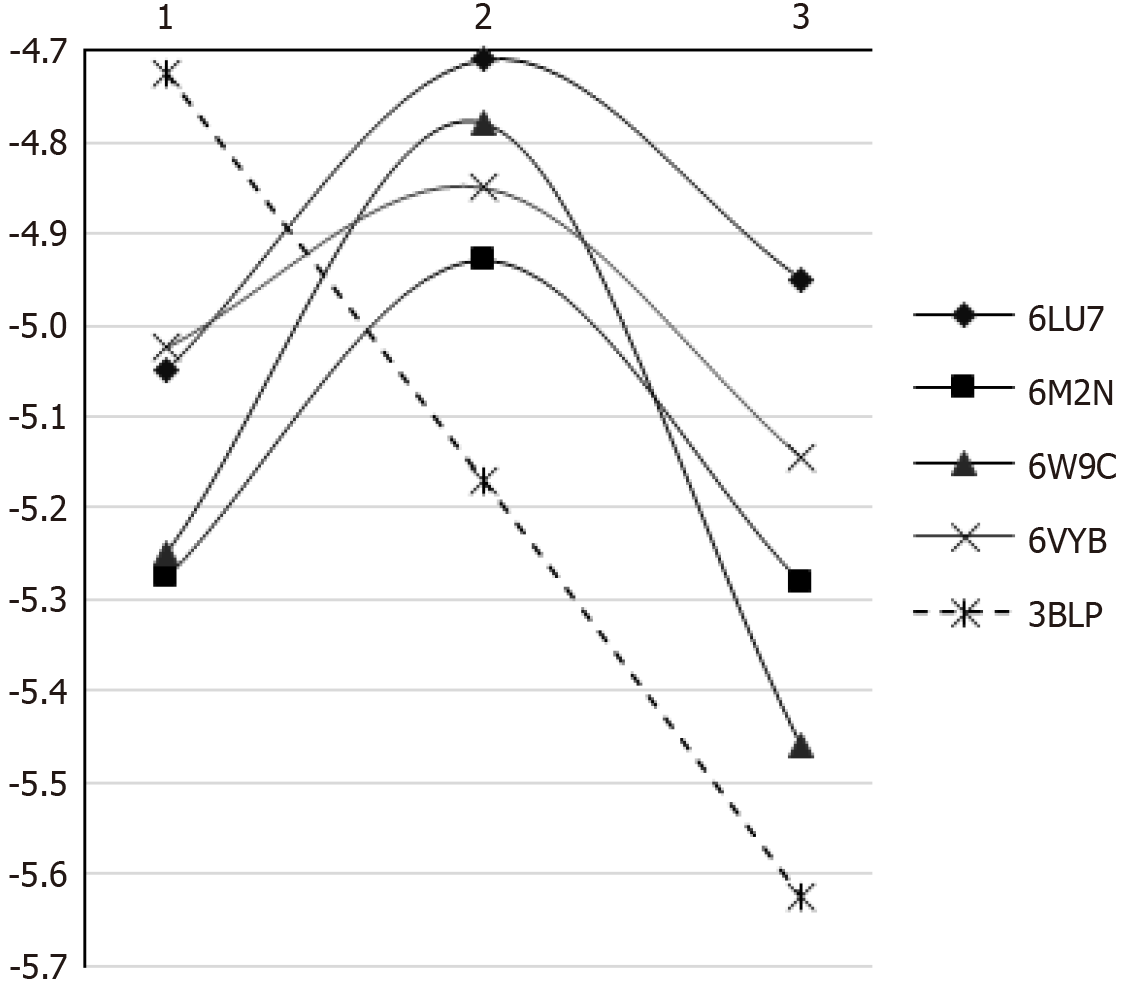
Values for the binding energies ranged from -3.6 kcal/moL to -7.3 kcal/moL. The 3-monomer chain had the lowest binding energy (i.e. highest affinity) to PLpro and the glycoprotein spike. Non-polymerised monomers and polymerised chains interacted with SARS-CoV-2 proteins via hydrogen bonds and hydrophobic interactions. Those findings suggest an interaction between SARS-CoV-2 proteins and resin composites.
SARS-CoV-2 proteins show affinity to non-polymerised and polymerised resin composite chains.
Core Tip: The severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) may interact with monomers of resin composites; triethylene glycol dimethacrylate has the smallest affinity with SARS-CoV-2 among monomers; bisphenol-A glycol dimethacrylate and bisphenol-A ethoxylated dimethacrylate show a remarkable affinity mainly with papain-like protease.
- Citation: Sette-de-Souza PH, Fernandes Costa MJ, Dutra Borges BC. SARS-CoV-2 proteins show great binding affinity to resin composite monomers and polymerized chains. World J Exp Med 2025; 15(1): 94022
- URL: https://www.wjgnet.com/2220-315x/full/v15/i1/94022.htm
- DOI: https://dx.doi.org/10.5493/wjem.v15.i1.94022
Saliva and salivary glands are a significant reservoir for severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2)[1]. Aerosols and saliva droplets are primary sources of cross-infection and are responsible for the high human–human transmission of SARS-CoV-2[2,3]. Once saliva wets oral tissues, tooth structures, and dental restoratives present in the oral cavity, SARS-CoV-2 can bind to them, thereby increasing the permanence of microorganisms in the mouth.
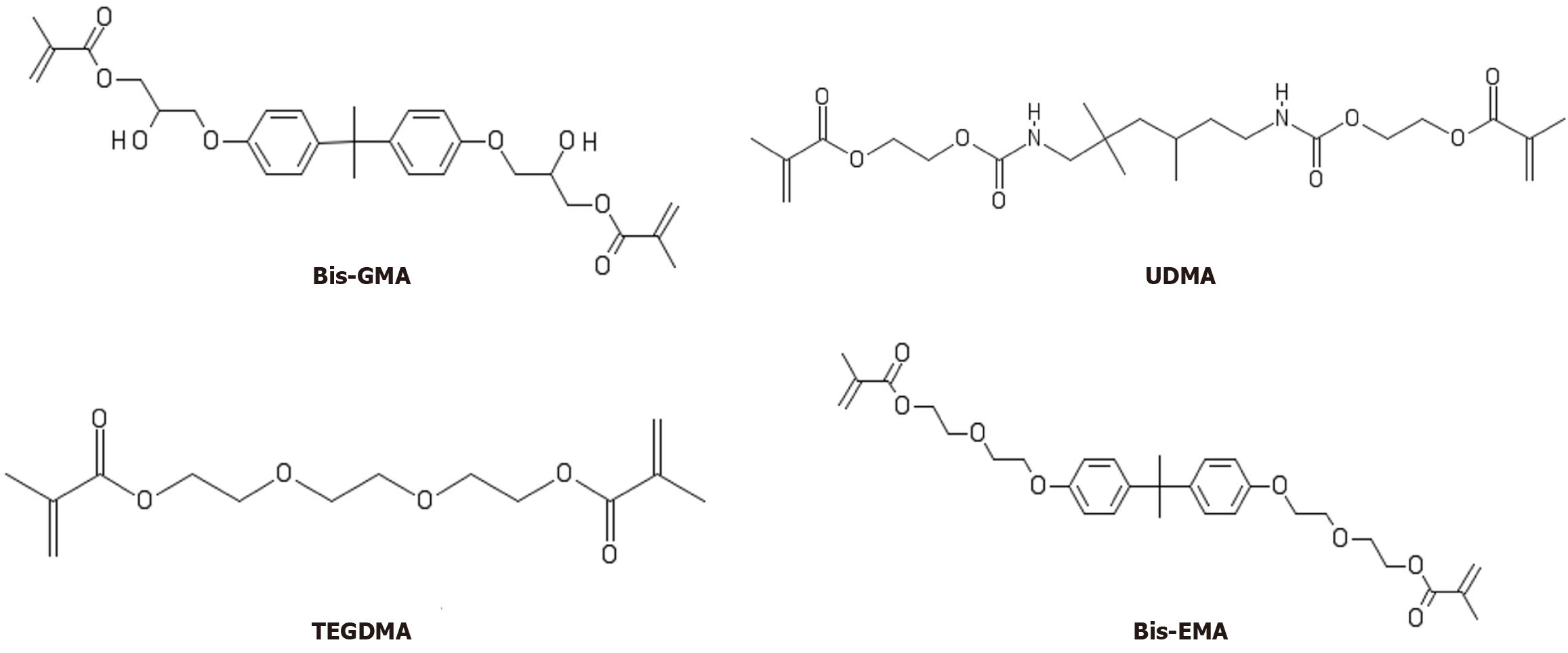
Among dental restoratives, resin composites are widely used to restore decayed teeth[4] due to their aesthetic properties and capacity to preserve healthy tooth tissues. Such materials contain organic monomers such as bisphenol A glycol dimethacrylate [bisphenol-A glycol dimethacrylate (Bis-GMA)], bisphenol A ethoxylated dimethacrylate [bisphenol-A ethoxylated dimethacrylate (Bis-EMA)], triethylene glycol dimethacrylate (TEGDMA), and urethane dimethacrylate (UDMA), along with inorganic filler particles[5]. Those monomers present chemical components such as hydroxyl, oxygen, and nitrogen that affect intermolecular interactions with substrates[6,7]. It has been demonstrated that, aside from taking shelter on dental biofilms, SARS-CoV-2 can interact with oral tissues and tooth structures[8,9]. However, it remains unclear whether SARS-CoV-2 proteins interact with resin composites.
Knowing the sites within the mouth that can harbour SARS-CoV-2 is essential for understanding its spread once saliva is not the only oral harbour for viruses[9]. However, the mechanism by which SARS-CoV-2 colonises dental biofilm remains unclear. At the same time, the acquired pellicle (AP) may form on any exposed surface, including dental materials, through the selective adsorption of proteins[10]. Thus, SARS-CoV-2 proteins may interact with dental materials and collaborate in the formation of AP.
Given the above, in silico analyses play a remarkable role in investigations involving cellular and molecular processes[11,12]. The molecular docking method, which entails searching for probable interactions between microorganisms’ proteins and substrates, has been used worldwide as the first step to understanding probable interactions with SARS-CoV-2[13]. That computational approach is an essential tool due to the urgent need to better understand SARS-CoV-2’s effects on human health.
Against that background, in our study we evaluated the possible interaction of SARS-CoV-2 proteins with monomers and polymers present in resin composite in silico. The null hypothesis tested was that an interaction between the SARS-CoV-2 proteins and the monomers and other proteins would not occur.
SARS-CoV-2 has some proteins involved in biological processes related to coronaviruses[14]. Thus, to simulate a whole new coronavirus, four different SARS-CoV-2 protein groups-the main protease (Mpro) (PDB: 6LU7), 3C-like protease (3CLpro) (PDB: 6M2N), papain-like protease (PLpro) (PDB: 6W9C), and glycoprotein spike (PDB: 6VYB)-were selected in light of previous studies[15–17]. For a positive control, we used salivary amylase (PDB: 3BLP) because it is involved in AP formation on multiple surfaces[10].
The crystal structures of SARS-CoV-2 proteins were obtained from the GenBank National Center for Biotechnology Information (RRID: SCR_002760). The AutoDock (RRID: SCR_012746) was used to delete duplicated chains, heteroatoms, and water molecules, as well as add polar hydrogens atoms and the charge of all atoms in the protein structure. Gasteiger charges were computed, and the structure was saved as a PDBQT file for the docking studies.
The monomers Bis-GMA (C29H36O8, PubChem CID: 15284), TEGDMA (C14H22O6, PubChem CID: 7979), UDMA
After retrieving SMILE codes from the National Center for Biotechnology Information’s chemical structure library (RRID: SCR_004284), we constructed multiple chains through monomer combination using PubChem Draw (RRID: SCR_021249). We also linked the individual chains to simulate the natural polymerised resin composite. Next, we simulated various polymerised chains linking the monomer methacrylate regions during polymerisation, after which we transformed the new SMILE code in a PDB file in Chimera UCSF 1.14 (RRID: SCR_004097).
The rotatable bonds of the ligands were defined using AutoDock, and the structures were saved as PDBQT files for use in the docking studies.
The Autogrid algorithm created the three-dimensional grids to generate the grid parameter files (RRID: SCR_015982). Each grid map was set to the centre of chain A, docking parameters were set according to the protein (Table 1), and all analyses were performed with a/an exhaustiveness value of 8.
| Chain | Binding energy (ΔG–kcal/mol) | ||||
| 6LU71 | 6M2N1 | 6W9C1 | 6VYB1 | 3BLP+ | |
| 3-monomer-chain | -4.95 ± 0.53 | -5.28 ± 0.65 | -5.46 ± 0.83 | -5.15 ± 0.49 | -5.63 ± 0.48 |
| 2-monomer-chain | -4.71 ± 0.72 | -4.93 ± 0.71 | -4.78 ± 0.83 | -4.85 ± 0.56 | -5.17 ± 1.30 |
| Monomers | -5.05 ± 0.66 | -5.28 ± 0.47 | -5.25 ± 1.10 | -5.03 ± 0.53 | -5.05 ± 0.76 |
| Hydroxyapatite | -4.2 | -4.6 | -5.1 | -4.9 | -4.8 |
| PO4 | -3.3 | -3.2 | -3.6 | -3.6 | -3.7 |
Molecular docking was performed using AutoDock Vina (RRID: SCR_011958), and the best ligand–protein model was identified based on the binding energy (ΔG–kcal/moL)[18].
The results obtained through the docking procedure were visualised in Chimera UCSF 1.14 (RRID: SCR_004097). The two-dimensional interactions of the complex protein–ligand structure, including hydrogen bonds and bond lengths, were analysed in LigPlot+ (RRID: SCR_018249) for all interactions[19]. The step-by-step methodological approach that we followed is depicted in Figure 2.
Binding energies ranging from -3.1 kcal/moL to -8.0 kcal/moL were found. The 3-monomer chain had the lowest binding energy (i.e. highest affinity) to PLpro, the glycoprotein spike, and salivary amylase (Table 1). For all tested proteins, the 2-monomer chain demonstrated the highest binding energy.
To observe the specific interactions between monomers and proteins, we used LigPlot+. The central oxygen and nitrogen atoms from monomers were involved in hydrogen bonds with amino acid residues, and some alkene groups of monomers presented hydrophobic interactions with the residues. Those interactions were also observed in the poly
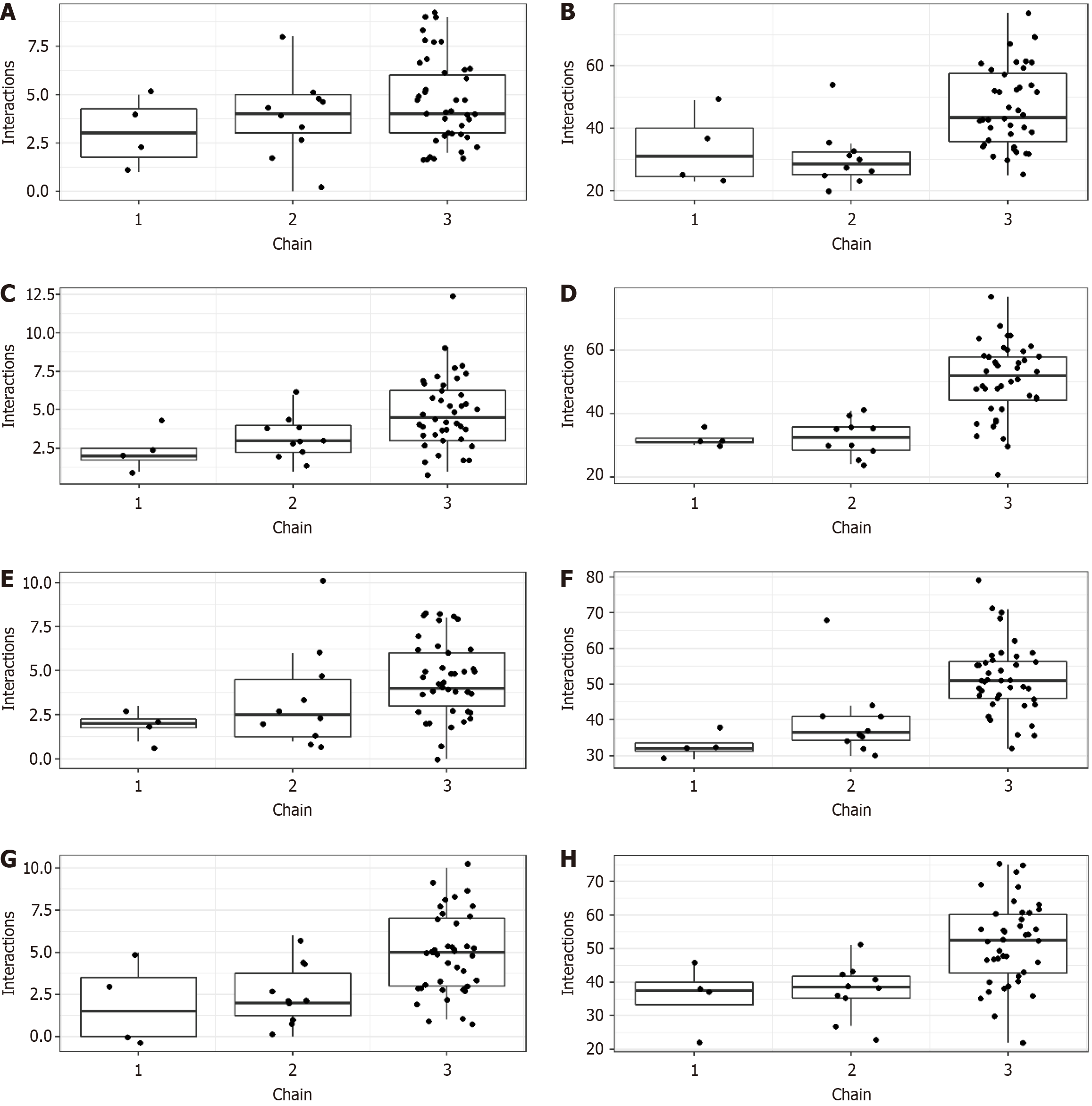
Non-polymerised monomers and polymerised chains interacted with SARS-CoV-2 proteins via hydrogen bonds and hydrophobic interactions (Figure 3). Beyond that, any SARS-CoV-2 protein may have interacted with many non-polymerised and polymerised chains simultaneously (Figure 4).
The null hypothesis tested in our study-that an interaction between the SARS-CoV-2 proteins and the monomers and polymers would not occur-was rejected because the binding affinity between all monomers and polymers and all proteins (i.e. Mpro, 3CLpro, PLpro, and the glycoprotein spike) was observed.
Among other results, Bis-GMA and Bis-EMA showed remarkable binding energy with all tested proteins, with ΔG values equal to or less than -5.0 kcal/moL. Such affinity relates to the number of interactions presented (i.e. hydrogen bonds and hydrophobic interactions) such that hydrogen bonds are more potent than hydrophobic interactions[20]. A higher number of oxygen atoms and a central, highly hydrophobic group present in Bis-GMA and Bis-EMA formed hydrogen bonds with hydroxyl radicals of polar amino acid residues and hydrophobic interactions with nonpolar amino acid residues. The fact that Mpro showed the highest binding energy to UDMA can be due to many interactions, primarily hydrogen bonds. A highly hydrophobic central area of Bis-EMA promoted many hydrophobic interactions with residues of PLpro, which was responsible for promoting the highest binding energy. Meanwhile, the highest binding energy (i.e. lowest affinity) obtained between TEGDMA and all proteins tested related to its having the smallest area of the monomers, which decreased the number of interactions with amino acid residues.
We evaluated binding energy values and interactions between SARS-CoV-2 proteins and non-polymerised methacrylate monomers and polymerised chains of resin composites. The growth of polymeric chains occurs when monomers are linearly connected by converting double C = C bonds into C–C bonds from different terminal methacrylate groups[21]. Monomers, especially Bis-GMA, may also be cross-linked via hydrogen bonds between hydroxyl groups and nitrogen or oxygen[22]. Thus, because hydrogen bonds and hydrophobic interactions between each non-polymerised monomer and SARS-CoV-2 proteins did not involve terminal methacrylate groups or hydroxyl groups in Bis-GMA, it was probable that similar binding between SARS-CoV-2 proteins and a polymerised chain would occur. Our results validate that assumption.
In a tooth preparation restored with resin composite, polymer chains of polymerised monomers and filler particles are likely present[21]. In general, resin composite restorations are polished in clinical conditions in order to achieve adequate smoothness and aesthetic properties and expose filler particles[23]. Thus, further investigations should evaluate the binding affinity of SARS-CoV-2 proteins to different filler particles. If a high binding affinity between them were found, then an increase the number of microorganisms might increase in resin composite restorations, and all implications highlighted by our results might increase. At the same time, another study[10] has shown that salivary amylase interacts with resin composites and collaborates in AP formation in filled resin composites. Thus, given our results, we believe that SARS-CoV-2 may also collaborate in AP formation.
In our computational study of a new microorganism, evidence to compare and corroborate our findings was inadequate. In response, in vitro and in vivo analyses need to be performed to validate our findings. Further research should also be conducted to clarify the mechanisms of interaction observed in our study. Despite those limitations, the chief strength of our work lies in its being the first to provide data about a possible interaction between resin composites and SARS-CoV-2. Besides that, due to concerns about the degradation of the resin–dentin interface[24], further studies could be performed to determine whether the virus will adhere to resin and collaborate in the resin–dentin degradation in dental adhesive systems.
SARS-CoV-2 proteins (i.e. Mpro, 3CLpro, PLpro, and the glycoprotein spike) showed an affinity to non-polymerised and polymerised resin composite chains.
| 1. | Chen L, Zhao J, Peng J, Li X, Deng X, Geng Z, Shen Z, Guo F, Zhang Q, Jin Y, Wang L, Wang S. Detection of SARS-CoV-2 in saliva and characterization of oral symptoms in COVID-19 patients. Cell Prolif. 2020;53:e12923. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 129] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 2. | Adhikari SP, Meng S, Wu YJ, Mao YP, Ye RX, Wang QZ, Sun C, Sylvia S, Rozelle S, Raat H, Zhou H. Epidemiology, causes, clinical manifestation and diagnosis, prevention and control of coronavirus disease (COVID-19) during the early outbreak period: a scoping review. Infect Dis Poverty. 2020;9:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1355] [Cited by in RCA: 1096] [Article Influence: 182.7] [Reference Citation Analysis (0)] |
| 3. | Xu R, Cui B, Duan X, Zhang P, Zhou X, Yuan Q. Saliva: potential diagnostic value and transmission of 2019-nCoV. Int J Oral Sci. 2020;12:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 246] [Cited by in RCA: 226] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 4. | Cheng L, Weir MD, Xu HH, Kraigsley AM, Lin NJ, Lin-Gibson S, Zhou X. Antibacterial and physical properties of calcium-phosphate and calcium-fluoride nanocomposites with chlorhexidine. Dent Mater. 2012;28:573-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 111] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 5. | Alizadehgharib S, Östberg AK, Dahlstrand Rudin A, Dahlgren U, Christenson K. The effects of the dental methacrylates TEGDMA, Bis-GMA, and UDMA on neutrophils in vitro. Clin Exp Dent Res. 2020;6:439-447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 6. | Müller C, Lüders A, Hoth-Hannig W, Hannig M, Ziegler C. Initial bioadhesion on dental materials as a function of contact time, pH, surface wettability, and isoelectric point. Langmuir. 2010;26:4136-4141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 7. | Nguyen S, Adamczak M, Hiorth M, Smistad G, Kopperud HM. Interactions of liposomes with dental restorative materials. Colloids Surf B Biointerfaces. 2015;136:744-751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Sabino-Silva R, Jardim ACG, Siqueira WL. Coronavirus COVID-19 impacts to dentistry and potential salivary diagnosis. Clin Oral Investig. 2020;24:1619-1621. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 342] [Cited by in RCA: 288] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 9. | Gomes SC, Fachin S, da Fonseca JG, Angst PDM, Lamers ML, da Silva ISB, Nunes LN. Dental biofilm of symptomatic COVID-19 patients harbours SARS-CoV-2. J Clin Periodontol. 2021;48:880-885. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 10. | Pelá VT, Prakki A, Wang L, Ventura TMS, de Souza E Silva CM, Cassiano LPS, Brianezzi LFF, Leite AL, Buzalaf MAR. The influence of fillers and protease inhibitors in experimental resins in the protein profile of the acquired pellicle formed in situ on enamel-resin specimens. Arch Oral Biol. 2019;108:104527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Pappalardo F, Russo G, Tshinanu FM, Viceconti M. In silico clinical trials: concepts and early adoptions. Brief Bioinform. 2019;20:1699-1708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 162] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 12. | John JP, Thirunavukkarasu P, Ishizuka K, Parekh P, Sawa A. An in-silico approach for discovery of microRNA-TF regulation of DISC1 interactome mediating neuronal migration. NPJ Syst Biol Appl. 2019;5:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Buonocore M, Marino C, Grimaldi M, Santoro A, Firoznezhad M, Paciello O, Prisco F, D'Ursi AM. New putative animal reservoirs of SARS-CoV-2 in Italian fauna: A bioinformatic approach. Heliyon. 2020;6:e05430. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Tortorici MA, Veesler D. Structural insights into coronavirus entry. Adv Virus Res. 2019;105:93-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 507] [Cited by in RCA: 570] [Article Influence: 81.4] [Reference Citation Analysis (0)] |
| 15. | Ranjbar A, Jamshidi M, Torabi S. Molecular modelling of the antiviral action of Resveratrol derivatives against the activity of two novel SARS CoV-2 and 2019-nCoV receptors. Eur Rev Med Pharmacol Sci. 2020;24:7834-7844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 16. | Sette-DE-Souza PH, Costa MJF, Amaral-Machado L, Araújo FADC, Almeida Filho AT, Lima LRA. Dental workers in front-line of COVID-19: an in silico evaluation targeting their prevention. J Appl Oral Sci. 2021;29:e20200678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Hall DC Jr, Ji HF. A search for medications to treat COVID-19 via in silico molecular docking models of the SARS-CoV-2 spike glycoprotein and 3CL protease. Travel Med Infect Dis. 2020;35:101646. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 218] [Cited by in RCA: 190] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 18. | Trott O, Olson AJ. AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J Comput Chem. 2010;31:455-461. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26714] [Cited by in RCA: 16484] [Article Influence: 1030.3] [Reference Citation Analysis (12)] |
| 19. | Abdel Bar FM, Elsbaey M, Taha N, Elgaml A, Abdel-Fattah GM. Phytochemical, antimicrobial and antiquorum-sensing studies of pulicaria undulata L.: a revision on the structure of 1β,2α,3β,19α,23-pentahydroxy-urs-12-en-28-oic acid. Nat Prod Res. 2020;34:804-809. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Yadav R, Imran M, Dhamija P, Chaurasia DK, Handu S. Virtual screening, ADMET prediction and dynamics simulation of potential compounds targeting the main protease of SARS-CoV-2. J Biomol Struct Dyn. 2021;39:6617-6632. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 21. | Pratap B, Gupta RK, Bhardwaj B, Nag M. Resin based restorative dental materials: characteristics and future perspectives. Jpn Dent Sci Rev. 2019;55:126-138. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 178] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 22. | Lemon MT, Jones MS, Stansbury JW. Hydrogen bonding interactions in methacrylate monomers and polymers. J Biomed Mater Res A. 2007;83:734-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 106] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 23. | de Fátima Alves da Costa G, Melo AMDS, de Assunção IV, Borges BCD. Impact of additional polishing method on physical, micromorphological, and microtopographical properties of conventional composites and bulk fill. Microsc Res Tech. 2020;83:211-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Amin F, Fareed MA, Zafar MS, Khurshid Z, Palma PJ, Kumar N. Degradation and Stabilization of Resin-Dentine Interfaces in Polymeric Dental Adhesives: An Updated Review. Coatings. 2022;12:1094. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/