Published online Mar 20, 2025. doi: 10.5493/wjem.v15.i1.100402
Revised: November 27, 2024
Accepted: December 16, 2024
Published online: March 20, 2025
Processing time: 132 Days and 19.6 Hours
Alcohol use disorder (AUD) is a medical condition that impairs a person's ability to stop or manage their drinking in the face of negative social, occupational, or health consequences. AUD is defined by the National Institute on Alcohol Abuse and Alcoholism as a "severe problem". The central nervous system is the primary target of alcohol's adverse effects. It is crucial to identify various neurological disorders associated with AUD, including alcohol withdrawal syndrome, Wernicke-Korsakoff syndrome, Marchiafava-Bignami disease, dementia, and neuropathy. To gain a better understanding of the neurological environment of alcoholism and to shed light on the role of various neurotransmitters in the phenomenon of alcoholism. A comprehensive search of online databases, including PubMed, EMBASE, Web of Science, and Google Scholar, was conducted to identify relevant articles. Several neurotransmitters (dopamine, gamma-aminobutyric acid, serotonin, and glutamate) have been linked to alcoholism due to a brain imbalance. Alcoholism appears to be a complex genetic disorder, with variations in many genes influencing risk. Some of these genes have been identified, including two alcohol metabolism genes, alcohol dehydrogenase 1B gene and aldehyde dehydrogenase 2 gene, which have the most potent known effects on the risk of alcoholism. Neuronal degeneration and demyelination in people with AUD may be caused by neuronal damage, nutrient deficiencies, and blood brain barrier dysfunction; however, the underlying mechanism is unknown. This review will provide a detailed overview of the neurobiology of alcohol addiction, followed by recent studies published in the genetics of alcohol addiction, molecular mechanism and detailed information on the various acute and chronic neurological manifestations of alcoholism for the Future research.
Core Tip: This review delves into the neurobiology of alcohol use disorder (AUD), highlighting the role of neurotransmitter imbalances, genetic factors like alcohol dehydrogenase 1B gene and aldehyde dehydrogenase 2 gene, and the associated neurological disorders. It explores the complex mechanisms underlying neuronal degeneration and blood brain barrier dysfunction in AUD, offering insights for future research into the acute and chronic neurological effects of alcoholism.
- Citation: Sahu P, Verma HK, Bhaskar L. Alcohol and alcoholism associated neurological disorders: Current updates in a global perspective and recent recommendations. World J Exp Med 2025; 15(1): 100402
- URL: https://www.wjgnet.com/2220-315x/full/v15/i1/100402.htm
- DOI: https://dx.doi.org/10.5493/wjem.v15.i1.100402
Alcohol (ethanol) is an easily accessible, legal, and widely consumed drug in our society. It is used by a large number of people worldwide. Alcohol is a simple two-carbon molecule that rapidly diffuses through almost every biological compartment in our body upon ingestion. In small amounts, alcohol can have some beneficial effects, such as a reduced risk of cardiovascular infections and all-cause mortality among middle-aged and older individuals[1]. However, excessive consumption costs a lot of major issues, including physical, psychological, and social issues[2]. The levels of alcohol in the brain rise within minutes of consumption, and signs of intoxication can be observed shortly after administering a high dose. At low blood concentrations, alcohol functions as a central nervous system (CNS) depressant, leading to reduced anxiety, feelings of euphoria, and behavioral excitation[3]. While at higher blood concentrations it may result in acute intoxication, which can lead to sluggishness, ataxia, slurred speech, stupor and coma. When intake is stopped, blood alcohol levels start to decline. This decline occurs at a consistent rate (zero-order) of roughly 0.016 g/dL/hour for men and 0.018 g/dL/hour for women[4]. When administered the same amount of alcohol per gram of body weight, women tend to experience higher peak blood alcohol levels compared to men[5]. This is because women have larger levels of body fat than men.
Men are more prone than women to regularly consume large quantities of alcohol, a behavior that is linked to substantial risks to their health and safety. Furthermore, these risks escalate in proportion to the amount of alcohol consumed[6]. Further, unsafe alcohol consumption (40-60 g/day of alcohol in females or 60-100 g/day in males) can create clinical changes linked to various diseases[7,8]. The amount and intensity of alcohol consumed distinguish between an alcohol addict and a nonaddict[9]. There is no ideal meaning of alcoholism; however, most judgments require people to drink vigorously throughout an all-encompassing timeframe and have endured numerous significant life issues because of their liquor/alcohol utilization. A subset of alcohol consumers develops problems because of alcohol use disorder (AUD)[10]. Alcoholic cirrhosis, alcoholic pancreatitis, malignancies of the upper gastrointestinal tract and liver, cardiovascular disease, breast cancer, diabetes, and fetal alcohol syndrome are all risk factors for AUD and can exacerbate results (alcohol intake during pregnancy raises the likelihood of congenital defects in the unborn child)[11]. Brain plasticity events contribute to the development of AUD and result in cravings and habitual alcohol-seeking behavior. Furthermore, chronic or high-dose alcohol intake causes adverse or adaptive reactions in the CNS as well as in nearly every organ system[12].
Chronic alcohol exposure induces brain plasticity changes, particularly in the reward system, reinforcing alcohol cravings and compulsive alcohol-seeking behaviors[13]. These neuroadaptive changes involve alterations in neurotransmitter systems such as gamma-aminobutyric acid (GABA), dopamine (DA), and glutamate, impacting brain regions responsible for reward, stress, and executive function[14]. Additionally, alcohol’s neurotoxic effects contribute to structural and functional damage in the CNS, which can impair cognition, decision-making, and emotional regulation, further perpetuating dependence[15]. These findings underline the critical role of CNS adaptations in AUD progression.
The purpose of this review is to demonstrate the various brain manifestations of alcoholism. Alcohol intake is linked to additional inhibitory and excitatory neurotransmitter systems, as well as genes that protect drinkers from future clinical obstacles.
AUDs impact an estimated 76.3 million people worldwide, resulting in nearly 1.8 million deaths each year. A study shows that up to 42% of patients treated to general hospitals and 33% of patients admitted to intensive care units have AUD[16]. Alcohol withdrawal syndrome (AWS) is a well-known condition that occurs in around 8% of hospitalized AUD inpatients following abrupt cessation of excessive or persistent drinking[17]. According to the National Institutes of Health, 28% of persons aged 18 and older consume alcohol on a regular basis at amounts that put them at risk of developing alcoholism, liver disease, and other medical and psychological issues[10].
In 2016, the global average yearly alcohol intake per individual over 15 was 6.4 liters, specifically around 1 liter of wine every week[18]. Alcohol use is responsible for about 5.1 % of the worldwide disease burden and over 3.3 million fatalities per year[19]. AUDs are most frequent in Europe (7.5%) and are least prevalent in the eastern Mediterranean region, which includes Afghanistan, Bahrain, and Egypt. Fifty percent of deaths due to liver cirrhosis, 30% of deaths due to oral and pharyngeal malignancies, 22% of fatalities due to interpersonal violence, 22% of deaths due to self-harm, 15% of deaths due to traffic accidents, 12% of tuberculosis fatalities, and 12% of liver cancer deaths occur globally[19,20].
According to the National Mental Health Survey of India 2015-2016, the prevalence of AUDs in adult men in India was 9%. In India, the alcohol-attributable fraction of all-cause mortality was discovered to be 5.4%. Alcohol was responsible for roughly 62.9% of all fatal liver cirrhosis cases[21].
Alcoholism is an ongoing sickness described by a physical and mental reliance on alcohol. Individuals with alcohol addiction need to drink to work. Signs that might be battling with alcohol dependence include.
In the United Kingdom, this implies a beverage with 8 g of ethanol—for instance, a large portion of 16 ounces of brew or a little (125 mL) glass of wine[22].
It is described as an amount or pattern of alcohol use that puts people at risk for adverse health consequences[23]. It refers to drinking more than 4 units each day for men and 2 units for ladies. These figures are also expressed as the week-by-week aggregates of 21 units each week for men and 14 units for ladies[24].
A chronic disease wherein individuals crave alcohol drinks and can’t handle their drinking. Likewise, an individual with this disease needs to drink more prominent sums to have a similar impact and have withdrawal side effects after stopping alcohol use[25]. Alcohol dependence influences physical and mental health and can cause family, companions, and work issues. Normal heavy alcohol consumption builds the danger of a few kinds of malignancy, like alcohol addiction or Alcoholism[26].
One expects to drink more significant amounts of alcohol to get similar brain-changing impacts. Alcohol tolerance is expanded by ordinary drinking[27]. This diminished affectability to the actual effects of alcohol utilization necessitates that higher amount of liquor be consumed to accomplish similar impacts as before resistance was set up. Reverse tolerance refers to the natural responses to the positive effects of ethanol found in alcoholic beverages. This includes direct tolerance, the rate at which one recovers from intoxication, and the ability to resist or protect against the development of AUD[28].
It happens when the liver is no longer able to produce the necessary enzymes to break down and metabolize alcohol, individuals may experience a condition known as reverse tolerance. This phenomenon is typically observed in individuals with liver damage[29]. Since the liver cannot handle alcohol, it makes people intoxicated more rapidly[30].
Being without alcohol for any timeframe can cause one to feel genuinely physically sick[31]. On the off chance that one drinks alcohol heavily for quite a long time, months, or years, one may have mental and actual issues when he stops or truly cut back on the amount he drinks. This refers to alcohol withdrawal. Side effects can go from gentle to genuine[32].
The individuals who keep on drinking regardless of repetitive social, relational, wellbeing, and legitimate issues because of their alcohol use[33]. It’s a global issue, comprising the seventh driving danger factor for death also, disability. Harmful drinking or alcohol abuse upsets the system[34,35]. It causes hormonal disturbances that may bring about different issues, such as stress intolerance, reproductive dysfunction, thyroid issues, immune abnormalities, and mental and behavioral problems[36].
One experiences serious cravings/yearnings to drink alcohol and gets oneself incapable of quitting drinking in any event, when needed to.
The impacts of alcohol in the CNS are mediated through activities on various Neurotransmitters[39]. There is a complicated interplay between excitatory and inhibitory systems. The numerous neurotransmitters involved in the action of alcohol explain its diverse effects as well as the wide spectrum of pharmacological interactions with both prescribed and illegal medicines (Table 1)[31,40-45]. Alcohol is a powerful substance that affects various neurological pathways and causes major alterations in the brain[46]. Some of the brain pathways impacted by alcohol consumption include the dopaminergic, serotoninergic, aminobutyric acid (GABA), and glutamate pathways[47]. Detailed mechanism depicted in Figure 1
| Name | Primary function | Location and distribution | Receptor | Disease-related | Comments | Ref. |
| Dopamine | Reward pathway; voluntary motions; motor circuit, cognitions | Hypothalamus, ventral tegmental area (mesolimbic area); most regions: Short medium and long axonal projections | D1, D2, D3, D4, D5 | Parkinson’s disease, schizophrenia | Alcohol increases its use in nucleus accumbens, mediating its pleasurable impacts | Adermark et al[40]; Burns et al[41] |
| Serotonin (5-HT) | Mood regulation: Depression, aggression; intestinal movement control appetite; sleep; muscle control | Raphe nuclei in CNS; most regions: Project from pons and brainstem | 5-HT1, 5-HT2A, 5-HT2B, 5-HT2C, 5-HT4, 5-HT6, 5-HT7 | Schizophrenia, depression, anxiety | Alcohol usage stimulation gives nausea, may also be linked to the pleasant effects of drinking | Bauer et al[44] |
| Gamma-Aminobutyric acid | Inhibits CNS | The limbic system, hippocampus, thalamus, basal ganglia; supraspinal interneuron | GABA A, GABA B | Anxiety disorder, seizures, epilepsy | Alcohol potentiates GABA activity, amnesia and sedation | Elholm et al[31]; Alasmari et al[45] |
| Glutamate | Long-term potentiation; learning; memory | CNS, peripheral nervous system; long neuron | NMDA, others | Seizures, schizophrenia | Alcohol blocks excitatory NMDA receptors, restricting it, causing amnesia, depressant impact | Marcinkiewcz[42]; Müller et al[43] |
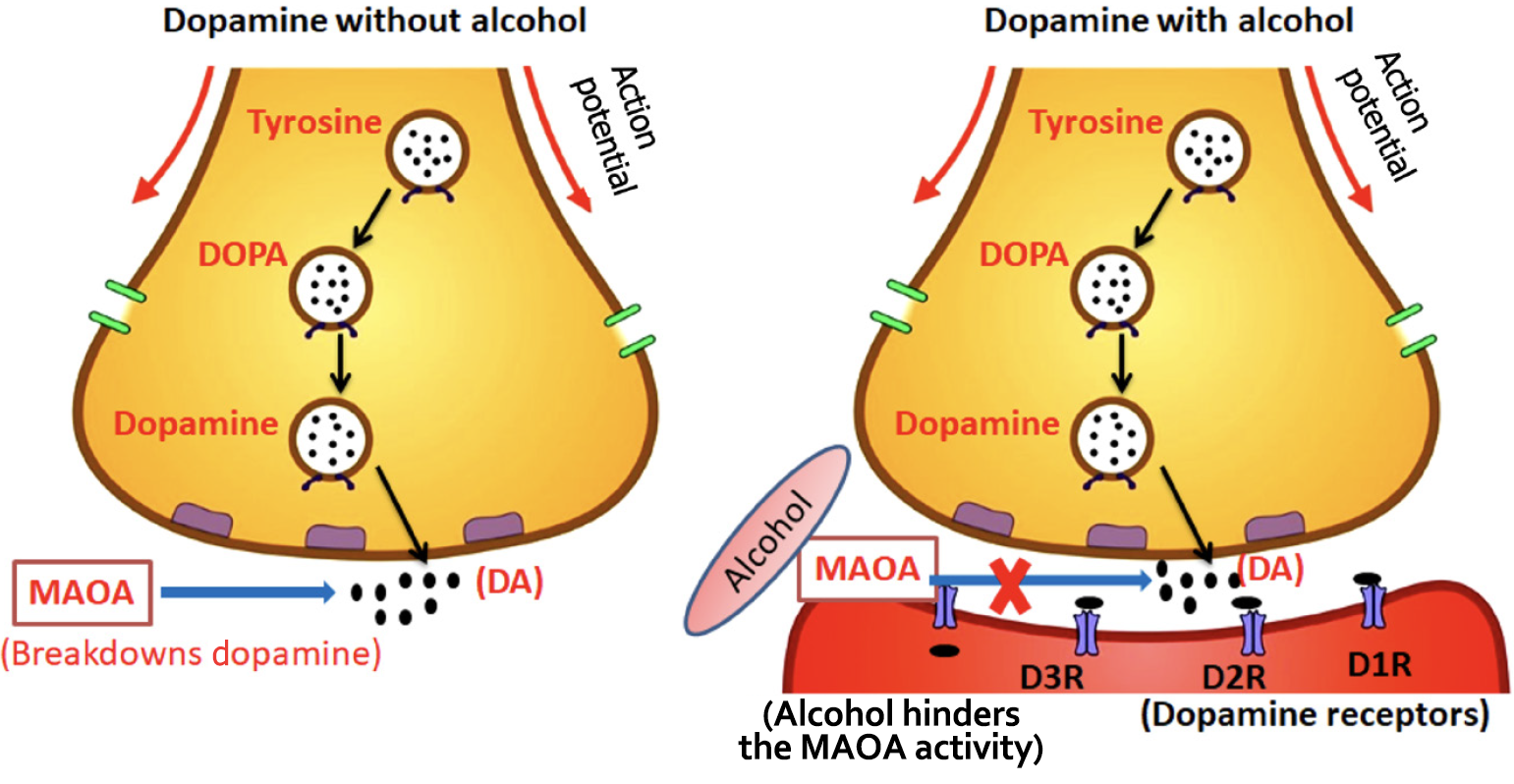
DA is a neurotransmitter primarily involved in a mesolimbic system circuit[48]. It is projected from the brain’s ventral tegmental area to the nucleus accumbens and regulates emotional and motivational behavior via the mesolimbic dopaminergic pathway. According to studies, ethanol injection into the nucleus accumbens causes local DA release in a dose-dependent manner[49]. Ma and Zhu[50] observed a dose-related increase in extracellular DA levels in the amygdala after ethanol injection. They also noted a delayed increase in DA following ethanol injection in the central amygdaloid nucleus, indicating the critical role of the amygdala in the alcohol-induced effects on the brain[50]. Other research has discovered that ethanol can indirectly raise DA levels in the nucleus accumbens by altering GABAergic neurons and opioid receptors[40]. Alcohol appears to enhance the action of endogenous opioid peptides. In the striatum and substantia nigra, opioid agonists efficiently affect DA release, reuptake, and metabolism, lowering DA production[41].
DA synthesis, release, receptor activation, reuptake, and catabolism are all mechanisms involved in the dopaminergic system[51]. Alcohol has the capacity to suppress the function of the protein monoamine oxidase, which is responsible for the breakdown of DA in the synaptic cleft. This inhibition stops DA from being fully digested, resulting in extended activity on the postsynaptic neuron and heightened feelings of pleasure. Individuals may want to continue experiencing the heightened pleasure generated by DA, which can lead to persistent alcohol intake and, eventually, addiction[52]. Because DA is a pleasure chemical, any decrease in its levels causes reward deficit, resulting in aberrant substance-seeking behavior[53]. Detailed mechanism depicted in Figure 2.
Serotonin is an inhibitory neurotransmitter produced by neurons in the raphe nuclei. It is also known as 5-hydroxytryptamine or 5-HT. Reduced serotonin neurotransmission has been linked to higher alcohol use and susceptibility to alcoholism[54-56]. There is an increase in extracellular 5-HT levels after acute alcohol intake. Chronic alcohol consum
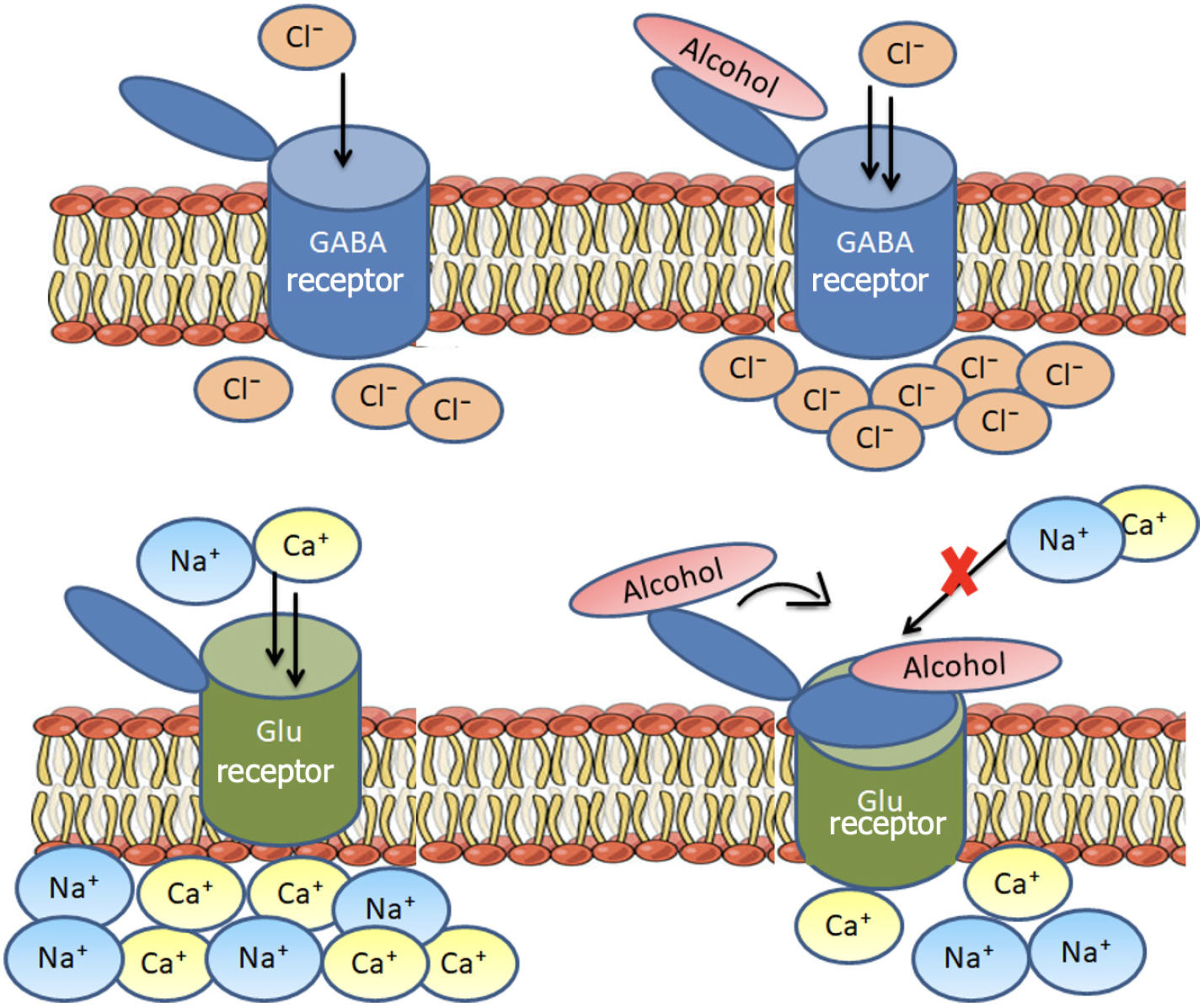
GABA is the brain's primary inhibitory neurotransmitter. When alcohol binds to a GABA receptor on a neuron, it allows the entry of negative chloride ions or the exit of positive ions, resulting in a more negative charge within the cell. This inhibits the neuron's ability to generate an action potential[59]. GABA acts through two receptor subtypes known as GABA A and GABA B[60].
Alcohol affects GABA activity in the brain in two ways. Firstly, it can act on the presynaptic neuron responsible for GABA release, leading to increased GABA release. Secondly, it can act on the postsynaptic neuron, interacting with the GABA A receptor alcohol's effects on GABA transmission are regulated by particles that interfere with GABA A receptor activity (GABA A receptor antagonists) and compounds that stimulate the GABA B receptor (GABA B agonists) in specific brain regions such as the nucleus accumbens, ventral pallidum, bed nucleus of the stria terminalis, and amygdala.
Research has demonstrated that both acute and chronic alcohol exposure increase GABA transmission in these regions[61].
Glutamate is the primary excitatory neurotransmitter in the brain and exerts its effects through several receptor subtypes, including the N-methyl-D-aspartate (NMDA) receptor[44]. It has long been known that the glutamate system is involved in the reinforcing effects of alcohol. By using NMDA receptor antagonists, researchers can mimic the effects of alcohol on an organism[45].
Alcohol suppresses the release of glutamate, which leads to a slowing down of neural activity in the brain[62]. It inhibits glutamate activity in the brain[63]. This can be observed in the reduction of extracellular glutamate levels in the brain's striatum, including the nucleus accumbens and other structures, following acute alcohol exposure. These changes undoubtedly impact glutamate transmission involving both ionotropic (NMDA) receptors and another receptor subtype known as metabotropic glutamate subtype 5 receptors[64]. Maintaining a balance between excitatory glutamate and inhibitory GABA neurotransmitters, by increasing excitatory activity and decreasing inhibitory activity, is crucial for proper brain development and functioning[65-67].
Environmental and genetic factors, as well as biological variables, influence drinking habit. Recent studies in both human and animal models have shown that genes play a role in the development of alcoholism as well as other social or biological reactions to alcohol[10,68]. Polymorphisms in alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH) genes, which alter alcohol metabolism, have been linked to a lower chance of developing alcoholism (Table 2)[69-72].
| Enzyme | Gene name | Allelic variants | Amino acid differences between allele | Chromosomal location | Subunit components or protein name | Class |
| ADH | ADH1A | 4q21-q23 | α1α1 | I | ||
| ADH1B | ADH1B 1 | Arg48, Arg370 (previously Arg47, Arg369) | β1β1 | I | ||
| ADH1B 2 | His48, Arg370 | β2β2 | ||||
| ADH1B 3 | Arg48, Cys370 | β3β3 | ||||
| ADH1C | ADH1C 1 | Arg272, Ile350 | γ1γ1 | I | ||
| ADH1C 2 | Gln272, Val350 | γ2γ2 | ||||
| ADH4 | π π | II | ||||
| ADH5 | χ χ | III | ||||
| ADH6 | μ μ | IV | ||||
| ADH7 | σ σ | V | ||||
| ALDH | ALDH1A1 | 9q21.13 | Cytosolic aldehyde, dehydrogenase 1 | |||
| ALDH2 | ALDH2 1 | 12q24.2 | Mitochondrial aldehyde dehydrogenase |
Although some ethanol metabolism can occur in other organs and produce localized harm, the liver is the principal location for ethanol metabolism[71]. The primary mechanism of ethanol metabolism involves its conversion into acetaldehyde, which is mediated by ADHs. Acetaldehyde is subsequently further oxidized to acetate by ALDH enzymes in a second step[72]. The genes ADH 1B gene (ADH1B) and ALDH 2 gene (ALDH2), particularly mitochondrial ALDH, have the greatest impact on the risk of alcoholism and alcohol intake[73].
Seven closely similar ADHs are found along chromosome 4, which codes for medium-chain ADHs[73]. The ADH enzymes they encode function as dimers, with the active forms consisting of two components. These seven ADH types have been divided into five classes based on similarities in amino acid sequences and kinetic properties[35]. ADH1 genes encode subunits, which join together to create homodimers or heterodimers that account for the majority of the liver's ethanol oxidizing activity[74]. ADH4 generates-ADH, which is required for the oxidation of ethanol at higher doses. ADH5 encodes-ADH, a formaldehyde dehydrogenase with a moderate affinity for ethanol that is extensively expressed. Although ADH6 mRNA is detected in both fetal and adult livers, the enzyme has not been isolated from any tissue. ADH7 produces-ADH, which participates in the oxidation of both ethanol and retinol[75]. In vitro, some studies reveal that the enzymes encoded by ADH1B × 48His and ADH1B × 370Cys metabolize ethanol at 30-40-fold more excellent rates than β1–ADH[76].
Furthermore, research indicates that variations in these genes affect alcohol metabolism rates, influencing acetaldehyde accumulation and contributing to individual differences in alcohol tolerance and dependence[77]. Variants of ADH1B (such as ADH1B × Arg47His) and ALDH2 (particularly ALDH2 × Glu504 Lys) have been shown to significantly reduce alcoholism risk[78], highlighting their substantial protective effects through acetaldehyde-mediated aversive responses. More recent studies of genome-wide association suggest that these genetic differences can modulate susceptibility to alcoholism through interactions with other genetic and environmental factors[79-81].
Acetaldehyde is a toxic intermediate that affects the entire system accumulation, causing an unpleasant sensation of dizziness, nausea, and tachycardia. Two significant ALDH proteins utilize the acetaldehyde created during ethanol oxidation[81-83]. ALDH1, ALDH1A1 is the gene that encodes ALDH2, which is found in the mitochondrial DNA and is encoded by the ALDH2 gene[84]. The mitochondrial ALDH2 is most important in removing acetaldehyde from the body to maintain its low level[85]. The ALDH1A1 gene stretches out over 52 kb on chromosome 9, and ALDH2 reaches out more than 43 kb on chromosome 12[86]. The ALDH2 × 2 allele results in the substitution of lysine for glutamate at position 504. The ALDH2 × 2 SNP rs671 (Glu504 Lys) influences how people metabolize acetaldehyde at a much slower pace. The delayed metabolism of acetaldehyde provides an unpleasant alcohol flushing sensation[87]. When both the ADH and ALDH2 variations are present, they give significant protection against the development of AUD[88]. The exact balance of ethanol and acetaldehyde oxidation rates may be critical in defining acetaldehyde concentrations within cells, and even modest changes in the relative activity of ADH and ALDH can have an effect[89].
Alcohol intoxication (alcohol poisoning): Acute alcohol intoxication is a condition caused by consuming excessive alcohol in a short period[90]. It is the most common of the various alcohol-related diseases affecting both adults and teenaged[91]. In certain circumstances, persons with this disease may have used household goods containing alcohol, like mouthwash, aftershave, vanilla essence, or shampoo by mistake or on purpose[92,93]. In addition to the amount of alcohol consumed, individual body weight, tolerance to alcohol, and the percentage of alcohol in the beverage, the duration of alcohol intake also appears to be particularly relevant in determining the level of acute alcohol intoxication. Alcohol intoxication occurs due to alcohol's inhibitory effect on nerve cells in the brain and spinal cord[7,94]. As alcohol consumption increases, this inhibitory effect spreads to cortical, brain stem, and spinal neurons.
According to the American Psychiatric Association's Diagnostic and Statistical Manual of Mental Disorders (DSM-5) and the World Health Organization's International Classification of Diseases criteria, alcohol poisoning is diagnosed clinically based on the presence of clinical or psychiatric problems accompanied by slurred speech, reduced awareness, and coma with respiratory failure[95,96].
Symptoms are generally linked to the amount of alcohol in one’s blood alcohol concentration (BAC) of more than 300 mg/dL (65.1 mmol/L), which increases the risk of respiratory depression and arrest[97]. A BAC of more than 400-500 mg/dL (108.5 mmol/L) is usually associated with death from acute alcohol intoxication; however, the fatal alcohol dosage might vary[98]. These effects may be decreased in alcohol-dependent people who acquire tolerance to alcohol due to repetitive exposure to ethanol[99]. In this process, compensating variations in excitatory NMDA and inhibitory GABA appear to be involved[97].
The first significant difficulty in alcohol intoxication is transient anterograde amnesia (commonly called “black-out”)[100] when the individual cannot recall a portion of everything that happened during one intoxicated drinking episode[101]. Impairment of judgment and understanding is another typical side effect of alcohol intoxication[102]. The scent of alcohol on the patient’s breath is the first indication of alcohol poisoning[103]. Usually, the diagnosis may be determined through history and physical testing. Information regarding the time of the last drink is essential to avoid and treat withdrawal symptoms, which may emerge 6-8 hours after drinking is stopped[96]. Breath analysis or saliva dipstick can also assess the alcohol level; however, these procedures are less accurate.
The treatment of an alcohol poisoned patient involves support and symptomatic therapy. Management begins with the evaluation of cardiac and respiratory systems and the inspection of the airway. Metadoxine (pyridoxal L-2-pyrrolidone-5-carbohydrate) is thought to speed up ethanol metabolism via increasing acetaldehyde dehydrogenase activity[104]. Dihydromyricetin, a natural flavonoid, is beneficial in combating acute symptoms of alcohol poisoning[105]. Recently, an alternative alcohol-borne antidote and to use biomimetic nano complexes such as oxidase and catalase, which lower blood alcohol levels, as a prophylactic measure have been developed[106].
AWS or abstinence syndrome is a sudden stop to or considerably decreased alcohol consumption in patients with tolerance and dependency on alcohol[107]. AWS can develop intentionally when a person stops drinking freely or unintentionally when abstinence is required due to sickness or injury. Alcohol works primarily through two neural receptors. One way alcohol affects the CNS is by modulating the GABA type A receptor, a neurotransmitter receptor that reduces neuronal excitability. This mechanism helps explain the sedative and hypnotic properties of alcohol. However, alcohol also increases the expression of glutamate NMDA receptors, leading to enhanced glutamate activity and promoting hyperexcitation[108].
Patients in mild withdrawal are always aware and have intact orientation. Symptoms appear 6 hours after cessation or reduction in consumption and can persist up to 48 hours (early withdrawal), like irritability, agitation, anxiety, headache, insomnia, nausea and vomiting, and tremors[10]. Moderate withdrawal symptoms begin after 12-14 hours of cessation and include hallucinations of visual, tactile, or auditory characteristics, as well as illusions experienced when awake. They can persist for up to six days[17]. Seizures from alcohol withdrawal usually start 24–48 hours after stopping drinking[109]. Delirium tremens (DT) (onset 48–72 hours/5 days after removal of drinking) is a severe withdrawal syndrome that can last up to two weeks (late withdrawal)[110]. It is recognized by agitation, disorientation, visual hallucinations, and autonomic symptoms such as hyperventilation, tachycardia, and diaphoresis[111]. It can lead to death due to death respiratory or cardiovascular collapse.
The ideal AWS medication would have a fast onset and extended duration to decrease withdrawal symptoms and a very simple metabolism that is not dependent on liver function[112]. Benzodiazepines (BZDs) are now considered the ‘gold standard’ in AWS treatment[113]. BZDs are the only family of medicines that effectively avoid the development of complex forms of AWS, with an 84% reduction in the incidence of seizures, DT, and the accompanying risk of death[114]. There is more robust evidence for chlordiazepoxide and diazepam, as long-acting medications can produce a smoother withdrawal; propofol potentiates the activity of GABA receptors and can also inhibit NMDA receptors from reducing withdrawal symptoms on multiple receptors[16,115,116].
Wernicke’s encephalopathy (WE) and Korsakoff Syndrome (KS), previously considered distinct diseases, are now recognized as the acute and chronic phases of Wernicke-KS, respectively. WE is an acute neuropsychiatric condition caused by a deficiency of vitamin B1 (thiamine), which serves as a critical coenzyme in carbohydrate metabolism through the Krebs cycle and the pentose phosphate pathways, involving enzymes such as transketolase, α-ketoglutarate dehydrogenase, and pyruvate dehydrogenase[117-119]. A lack of thiamine can cause damage to the brain because these enzymes are known to regulate energy metabolism in the brain, particularly in areas with high metabolic demand, including the thalamic and hypothalamic paraventricular areas, the mammillary bodies, the cerebellar vermis, the floor of the fourth ventricle, and the periaqueductal gray[120]. Other variables that contribute to WE in alcoholics include poor thiamine storage and metabolization in the liver[121]. WE manifests as a slew of symptoms, including ophthalmoparesis (impaired eye movement), altered mental status, gait ataxia (uncoordinated movements), and oculomotor abnormalities[122]. However, only 10% of individuals display all three symptoms, with altered mental status and, in severe cases, coma being the most prevalent clinical findings[15]. Symptoms of WE include reduced attention, memory loss, disorientation, and abulia.
Thiamine blood tests will indicate thiamine serum levels as well as transketolase enzyme activity in peripheral blood. This test, on the other hand, usually takes a long time and is of little use. When it comes to brain imaging exams, magnetic resonance imaging (MRI) is the most important supplemental test for confirming diagnosis. Increased signals in the bilateral medial thalamus, surrounding the third ventricle, and periaqueductal grey matter are shown in T2W and fluid-attenuated inversion recovery imaging in the early phase[123].
The treatment consists of thiamine replacement as soon as possible. Early intravenous thiamine[124] is essential for maintaining an osmotic gradient in the cell membrane, glucose metabolism, and neurotransmitter production[125], and it is usually given before or together with glucose. The average daily thiamine requirement for people is 1.4 mg, or 0.5 mg thiamine should be taken for every 1000 kcal consumed[122]. WE are treated with a high dosage of IV thiamine[126]. Delays in treatment, particularly to pursue diagnostic tests, can be deadly, with a 20% fatality rate[127].
KS, mainly caused by malnutrition in conjunction with prolonged drinking, typically manifests itself in the aftermath of WE[128]. But it can occur in people with no history of WE or with subacute, with unexplained episodes. DSM-5 defines KS as “alcohol-induced major neurocognitive disorder, amnestic confabulatory type”. The 80% of WE patients go on to develop KS[15]. Confabulation, a compensatory response to the inability to recall and retrograde and anterograde amnesia, are all symptoms of KS[129-131]. The confabulatory elements of KS are generally treated symptomatically, but the amnestic results are more challenging to reverse[127]. Clinically, Wernicke-KS is characterized by memory impairment that is disproportionate to other cognitive abilities in a patient who is awake, alert, and responsive.
In most cases, recent memory is more damaged than remote memory[132]. In addition, the KS study has shown that diencephalic regions play a crucial part in the memory function[133], thereby promoting the quest for distinctive and independent brain structure and neuronal circuits underpinning the mnemonic processes[134]. Confusion, lack of muscular coordination, and visual difficulties are other symptoms. The KS occurs slower. Double vision, eyelids may fall, or eyes may be moving fast are some other symptoms[135].
A brain MRI can display changes in brain tissue. But therapy should begin promptly if Wernicke-KS is suspected. The clinical evaluation of those who have KS calls for historical and physical analysis[136]. However, there is no evidence that pharmaceutical treatment is beneficial in KS. Several case reports studies in fluvoxamine, clonidine, reboxetine, or rivastigmine were used to treat KS. These trials did not generate consistent evidence for the effectiveness of any of these interventions. As a result, we can ensure that no effective pharmacological therapy for KS is available[128]. Stopping the usage of alcohol can help to avoid further loss of brain function and nerve damage. A nutritious, well-balanced diet can assist[137].
Marchiafava-Bignami is a neurologic disorder that predominantly affects myelin and is associated with persistent alcohol consumption[138]—originally known as “red wine drinker’s encephalopathy”[10]. Marchiafava E and Bignami A, two Italian pathologists, discovered it in 1903. They described men with an alcohol use disease who died of convulsions and comas, with necrosis of the corpus callosum identified on autopsy[139]. Marchiafava-Bignami Disease (MBD) is a rare disease characterized by demyelination/necrosis of the corpus callosum’s myelinated fiber’s central part (middle lamina) and adjacent subcortical white matter disease[140]. It is a degenerative neurological disorder that most commonly affects middle-aged (45 years) or older alcoholic men[141,142]. MBD illness is hallmarked by corpus callosum demyelination. Demyelination of the corpus callosum, especially the splenium, is the major cause[143]. However, demyelination can affect the optic chiasm and tracts, cerebellar peduncle, subcortical area, adjacent white matter, and, in rare cases, cortical grey matter. An interhemispheric disconnection syndrome develops over time[144], presenting with dementia, limb apraxia, tactile and unilateral agraphia, and hemialexia.
The disease can manifest itself in two primary clinical forms: (1) Acute and chronic; and (2) The latter of which can be fatal[127]. There is no well-defined clinical syndrome; it causes altered mental state, ataxia, mood disorders (depression and mania), and psychotic symptoms (paranoia); also the clinical course varies, some patients will become comatose and die, while others can live with dementia for several years, while others will only recover partially[145].
Brain imaging investigations, particularly MRI, are required to confirm a diagnosis (demyelination, inflammation, or necrosis of corpus callosum)[146]. Marchiafava—Bignami illness has no particular treatment; however, abstinence and vitamin supplements are advised. Some studies have also found a positive response to large dosages of corticosteroids[147]. According to some clinicians, thiamine, folate, vitamin B complexes may be useful in delaying the course of Mar
Cerebellar degeneration is a pathological condition that refers to the progressive accumulation of abnormalities in the cerebellum due to alcohol toxicity[149]. Cerebellar degeneration occurs in both alcoholics deficient in micronutrients and those who are not[127]. When neurons in the cerebellum degenerate and die due to the harmful effects of alcohol, this syndrome arises. The cerebellum is the portion of the brain that is in charge of coordination and balance. Alcoholic cerebellar degeneration (ACD) is characterized by stance and gait ataxia[10]. Persons with cerebellar degeneration can adopt a wide-based gait with short steps, compensating for their balance losses. Other problems may include nystagmus, poor handwriting, upper extremity inconsistency, and moderate dysarthria. Cerebellar ataxia is the clinical manifestation of cerebellar degeneration and can manifest in various ways[150]. Truncal ataxia depicts trunk instability and unbalances that generate corporal oscillations during sitting and causes cerebellar vermis damages[124]. According to some physicians, the length of alcohol consumption is the most critical risk factor for developing clinically severe toxic[151]. It is the most frequent CNS consequence of persistent alcohol consumption, affecting 10% to 25% of alcoholics[152].
While all neuronal cells and the white matter suffer from the injury, Purkinje cells are most affected. Some authors proposed a concept to explain phenomena in which increased gut permeability produced by alcohol-induced intestinal mucosa lesions seen in alcoholic patients might enhance the immune system[153]. After being exposed to harmful antigens (including gliadin peptides), the impairment of the blood-brain barrier caused by chronic alcohol consumption would allow these antibodies to enter the brain via previously unknown pathways, causing the brain to degenerate like gluten-induced cerebellar ataxia[153,154].
Diagnosed clinically, anatomopathological and neuroimaging analyses both indicate degeneration of all microcellular components of the cerebellar cortex, notably Purkinje cells on the anterior and superior vermis surfaces. Cerebellar atrophy is seen on computed tomography and MRI images of the brain[155]. No particular therapy has been established; however, vitamin supplements administration and alcohol abstinence are suggested. Although there is no treatment for these diseases, limited studies indicate that some medicines like Riluzole and physical therapy can help with ataxia symptoms[156].
The phrase “alcohol-related dementia” refers to a type of dementia caused by the direct effects of persistent alcohol use on the brain. Dementia is a clinical condition defined by a gradual decline in cognitive ability and the ability to live and function independently[157]. Dementia impairs memory, reasoning, behavior, and the capacity to do daily tasks[158], and it is a significant cause of impairment in elderly individuals. In observational and imaging investigations, heavy alcohol consumption was linked to structural alterations in the brain and cognitive and executive deficits[159]. The global prevalence of dementia has been estimated to be between 5% and 7% among persons aged 60 and older[160]. According to one research, males who drank ≥ 36 g/day of alcohol had a quicker 10-year decrease in all cognitive areas, with an impact size equivalent to 1.5 to 5.7 additional years of cognitive decline[161]. The CNS shrinkage associated with alcoholic neurodegeneration is produced by myelin breakdown, dendritic connection loss, and neuronal death[15].
Early neuropsychological investigations generally reveal frontal subcortical cognitive impairment, mental slowness, attention deficit, immediate or short-term memory changes, reduced visual-spatial capacity, and decreased management responsibilities, including planning and organization[162]. Imaging studies of simple alcoholics (no nutritional deficit, hepatic failure, or brain damage) have shown structural abnormalities, including alterations to the corpus callosum, pons, and cerebellum[34]. Given that the number of individuals living with dementia is predicted to triple around 2050 and there is currently no treatment, prevention is crucial[163]. The primary mechanism underlying healing from white matter injury is the restoration of myelination and axonal integrity[164]. Abstinence leads to improvements in motor skills and cognition and a reversal of white matter shrinkage. However, if the drinking is restarted, it becomes subject to disturbance once more.
Polyneuropathy, often known as peripheral neuropathy, occurs when numerous peripheral nerves are injured. The most common consequence in alcoholic individuals is chronic polyneuropathy[127], caused by prolonged alcohol use. Paresthesia, pain and ataxia are common symptoms. We don’t know how many people are afflicted by alcohol neuropathy, but studies suggest that at least 66% of chronic alcoholics have neuropathy[165]. It is thought to be the consequence of a multifactorial process mainly driven by direct toxic effects of ethanol or its metabolites impact and regulated by other variables, including genetic susceptibility, malnutrition, thiamine deficiency, and other systemic illnesses[166]. This is a sensory polyneuropathy with distal, symmetric characteristics that is mainly axonal. The longer axons are more prone to be affected initially[165]. The development of symptoms is gradual and symmetric, mostly sensory, manifesting as dysesthesia, burning feeling, and burning pain on the soles of the feet, toes, arm[167], which progresses to cramping in the calves and hands[168]. Muscle weakness and atrophy, particularly in the distal muscles of the upper or lower limbs, are common motor symptoms that appear later. Trophic skin alterations such as glossiness, hair loss, thinning, hyperpigmentation, and reduced sweating are frequent in affected distributions. Compared to males, women have a greater rate of alcoholic polyneuropathy. Chopra and Tiwari[169] showed that alcohol-induced neuro
Diagnosis includes electrodiagnostic testing and physiological findings that reveal typical axonal sensory neuropathy symptoms, with reduced densities of nerve fibers. Except in people with a long history of neuropathic complaints and significant axonal sprouting, the density of tiny myelinated and unmyelinated axons was lower than the density of large myelinated fibers[170].
In some situations, therapies suppress symptoms rather than treating the underlying illness. Alpha-lipoic acid, benfotiamine, acetyl-L-carnitine, and methylcobalamin have all been the subject of extensive investigation. Myo-inositol, vitamin E, topical capsaicin, and N-acetylcysteine are some other botanical or nutritional treatments. The use of current therapy and nutrition can help to reduce morbidity[165,169]. A balanced diet with vitamin supplements, rehabilitation, and alcohol abstinence are all part of the treatment. Recovery, on the other hand, is gradual and frequently incomplete. Drugs like gabapentin and amitriptyline can be used to treat patients with neuropathic pain[171].
When asked about how alcoholism is treated, many people often think of 12-step programs or 28-day inpatient rehab, but they may be unaware of other available options. In reality, there are several therapy options currently accessible. It is important to recognize that there is no one-size-fits-all approach, and what works for one individual may not work for another. Therefore, understanding the various alternatives can be a crucial first step.
Therapies like Cognitive-behavioral therapy with a therapist or in small groups can be carried out alone. The main aim of this type of treatment is the identification of feelings and situations. The objective is to modify the thinking processes leading to alcohol abuse and build the abilities required to face daily situations. Motivational enhancement therapy is carried out over a short period to motivate and enhance drinking behavior. Family and marital counseling involves spouses and other family members in the therapy process and can play a major part in the rehabilitation and develop
Medications like, naltrexone can aid people in drinking heavily. Acamprosate makes abstinence simpler to sustain[173]. Disulfiram inhibits the body’s alcohol breakdown and causes disagreeable sensations, including nausea and skin flushing. People may avoid consuming alcohol while taking disulfiram because of these unpleasant side effects[174]. BZDs, such as diazepam and chlordiazepoxide, are preferable for treating all types of alcohol withdrawal symptoms, including DT, if the liver function test is normal.
Nutrition: During healing, one should consume a diet that balances serotonin (a hormone that aids in relaxing) levels in the brain. This requires consuming carbohydrate-rich meals (grains, fruits, and vegetables), particularly complex carbs found in starchy foods such as legumes (e.g., beans, lentils, and peas), root vegetables (potatoes and carrots), pasta, and bread. Consuming these items in conjunction with protein in daily meals will maintain users at peak performance.
Rediscover hobbies: Many individuals drink to pass the time when they are bored. Pleasurable activities keep one from wanting to drink, but they also help relax, which everyone needs to do.
Most withdrawal symptoms or other alcohol-related issues may be treated well with medicines coupled with proper vitamins, exercise, and sleep[175].
Chronic alcohol abuse can result in various neurological symptoms, including both central and peripheral neurologic problems. Polyneuropathy, cerebellar degeneration, and dementia are the most common, whereas WE, KS, and Marchiafava Bignami are the most dangerous. Because alcohol is highly prevalent, and alcohol is complicated. Due to its significant morbidity and mortality often masked by other medical complexities associated with aging or alcoholicity, it is essential to have a thorough knowledge of this disclosure and quickly recognize its scope. Alcohol primarily interacts with GABA A and NMDA receptors, but it also induces various signaling events within well-defined brain pathways. These events lead to adaptive changes in gene expression, resulting in two main states: (1) Addiction; and (2) Toxicity. A significant biological factor underlying susceptibility to AUD and other neurological consequences of chronic alcohol consumption may involve genetically determined features of myelin structure and alcohol's impact on myelin gene expression. Since alcohol does not selectively affect a single region of the nervous system, it is crucial to identify any cerebellar or motor impairments in individuals with cognitive issues. Early detection and intervention are essential steps that healthcare professionals can take to mitigate the neurological consequences of chronic alcohol abuse. In cases where the condition has already been diagnosed, nutritional supplementation and cessation efforts are important in preventing further harm and may lead to some symptom relief.
| 1. | McGuire S. U.S. Department of Agriculture and U.S. Department of Health and Human Services, Dietary Guidelines for Americans, 2010. 7th Edition, Washington, DC: U.S. Government Printing Office, January 2011. Adv Nutr. 2011;2:293-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 954] [Article Influence: 63.6] [Reference Citation Analysis (0)] |
| 2. | McLellan AT. Substance Misuse and Substance use Disorders: Why do they Matter in Healthcare? Trans Am Clin Climatol Assoc. 2017;128:112-130. [PubMed] |
| 3. | Mukherjee S. Alcoholism and its effects on the central nervous system. Curr Neurovasc Res. 2013;10:256-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 4. | Wilson DF, Matschinsky FM. Ethanol metabolism: The good, the bad, and the ugly. Med Hypotheses. 2020;140:109638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 80] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 5. | Cederbaum AI. Alcohol metabolism. Clin Liver Dis. 2012;16:667-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 767] [Cited by in RCA: 838] [Article Influence: 59.9] [Reference Citation Analysis (0)] |
| 6. | White A, Castle IJ, Chen CM, Shirley M, Roach D, Hingson R. Converging Patterns of Alcohol Use and Related Outcomes Among Females and Males in the United States, 2002 to 2012. Alcohol Clin Exp Res. 2015;39:1712-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 278] [Article Influence: 27.8] [Reference Citation Analysis (0)] |
| 7. | Planas-Ballvé A, Grau-López L, Morillas RM, Planas R. Neurological manifestations of excessive alcohol consumption. Gastroenterol Hepatol. 2017;40:709-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Heather N. A long-standing World Health Organization collaborative project on early identification and brief alcohol intervention in primary health care comes to an end. Addiction. 2007;102:679-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Gardner EL. Addiction and brain reward and antireward pathways. Adv Psychosom Med. 2011;30:22-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 281] [Cited by in RCA: 259] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 10. | Costin BN, Miles MF. Molecular and neurologic responses to chronic alcohol use. Handb Clin Neurol. 2014;125:157-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Rehm J. The risks associated with alcohol use and alcoholism. Alcohol Res Health. 2011;34:135-143. [PubMed] |
| 12. | Crespi C, Galandra C, Manera M, Basso G, Poggi P, Canessa N. Executive Impairment in Alcohol Use Disorder Reflects Structural Changes in Large-Scale Brain Networks: A Joint Independent Component Analysis on Gray-Matter and White-Matter Features. Front Psychol. 2019;10:2479. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 13. | Kuhns L, Kroon E, Lesscher H, Mies G, Cousijn J. Age-related differences in the effect of chronic alcohol on cognition and the brain: a systematic review. Transl Psychiatry. 2022;12:345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 14. | Ryan RM, Ingram SL, Scimemi A. Regulation of Glutamate, GABA and Dopamine Transporter Uptake, Surface Mobility and Expression. Front Cell Neurosci. 2021;15:670346. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 15. | Hammoud N, Jimenez-Shahed J. Chronic Neurologic Effects of Alcohol. Clin Liver Dis. 2019;23:141-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 55] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 16. | Perry EC. Inpatient management of acute alcohol withdrawal syndrome. CNS Drugs. 2014;28:401-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 17. | Jesse S, Bråthen G, Ferrara M, Keindl M, Ben-Menachem E, Tanasescu R, Brodtkorb E, Hillbom M, Leone MA, Ludolph AC. Alcohol withdrawal syndrome: mechanisms, manifestations, and management. Acta Neurol Scand. 2017;135:4-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 146] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 18. | Antai D, Lopez GB, Antai J, Anthony DS. Alcohol drinking patterns and differences in alcohol-related harm: a population-based study of the United States. Biomed Res Int. 2014;2014:853410. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Girish N, Kavita R, Gururaj G, Benegal V. Alcohol use and implications for public health: patterns of use in four communities. Indian J Community Med. 2010;35:238-244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Eashwar VMA, Umadevi R, Gopalakrishnan S. Alcohol consumption in India- An epidemiological review. J Family Med Prim Care. 2020;9:49-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 21. | Vardell E. Global Health Observatory Data Repository. Med Ref Serv Q. 2020;39:67-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 97] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 22. | Britton A, O'Neill D, Bell S. Underestimating the Alcohol Content of a Glass of Wine: The Implications for Estimates of Mortality Risk. Alcohol Alcohol. 2016;51:609-614. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Sachdeva S, Tyagi A, Sachdeva R, Nagar M, Bharti. Alcohol consumption practices amongst adult males in a rural area of Haryana. Med J DY Patil Univ. 2014;7:128. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 24. | Case P, Ng Fat L, Shelton N. Exploring the characteristics of newly defined at-risk drinkers following the change to the UK low risk drinking guidelines: a retrospective analysis using Health Survey for England data. BMC Public Health. 2019;19:902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 25. | Sebold M, Müller CA, Garbusow M, Charlet K, Heinz A. Neurobiology of Alcohol Dependence. In: el-Guebaly N, Carrà G, Galanter M, Baldacchino AM , editor. Textbook of Addiction Treatment. Berlin: Springer, 2021: 9-20. [DOI] [Full Text] |
| 26. | Pilling S, Yesufu-Udechuku A, Taylor C, Drummond C; Guideline Development Group. Diagnosis, assessment, and management of harmful drinking and alcohol dependence: summary of NICE guidance. BMJ. 2011;342:d700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Comley RE, Dry MJ. Acute behavioral tolerance to alcohol. Exp Clin Psychopharmacol. 2020;28:112-129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 28. | Marczinski CA, Stamates AL, Maloney SF. Differential development of acute tolerance may explain heightened rates of impaired driving after consumption of alcohol mixed with energy drinks versus alcohol alone. Exp Clin Psychopharmacol. 2018;26:147-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Verster JC, Slot KA, Arnoldy L, van Lawick van Pabst AE, van de Loo AJAE, Benson S, Scholey A. The Association between Alcohol Hangover Frequency and Severity: Evidence for Reverse Tolerance? J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Hill R, Lyndon A, Withey S, Roberts J, Kershaw Y, MacLachlan J, Lingford-Hughes A, Kelly E, Bailey C, Hickman M, Henderson G. Ethanol Reversal of Tolerance to the Respiratory Depressant Effects of Morphine. Neuropsychopharmacology. 2016;41:762-773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 31. | Elholm B, Larsen K, Hornnes N, Zierau F, Becker U. Alcohol withdrawal syndrome: symptom-triggered versus fixed-schedule treatment in an outpatient setting. Alcohol Alcohol. 2011;46:318-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | Attilia F, Perciballi R, Rotondo C, Capriglione I, Iannuzzi S, Attilia ML, Coriale G, Vitali M, Cereatti F, Fiore M, Ceccanti M; Interdisciplinary Study Group CRARL - SITAC - SIPaD - SITD - SIPDip. Alcohol withdrawal syndrome: diagnostic and therapeutic methods. Riv Psichiatr. 2018;53:118-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 34. | Guilbert JJ. The world health report 2002 - reducing risks, promoting healthy life. Educ Health (Abingdon). 2003;16:230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 1113] [Article Influence: 48.4] [Reference Citation Analysis (0)] |
| 35. | Sanchez-Roige S, Palmer AA, Clarke TK. Recent Efforts to Dissect the Genetic Basis of Alcohol Use and Abuse. Biol Psychiatry. 2020;87:609-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 72] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 36. | Rachdaoui N, Sarkar DK. Pathophysiology of the Effects of Alcohol Abuse on the Endocrine System. Alcohol Res. 2017;38:255-276. [PubMed] |
| 38. | Nestler EJ. Cellular basis of memory for addiction. Dialogues Clin Neurosci. 2013;15:431-443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 164] [Cited by in RCA: 160] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 39. | Banerjee N. Neurotransmitters in alcoholism: A review of neurobiological and genetic studies. Indian J Hum Genet. 2014;20:20-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 40. | Adermark L, Clarke RB, Olsson T, Hansson E, Söderpalm B, Ericson M. Implications for glycine receptors and astrocytes in ethanol-induced elevation of dopamine levels in the nucleus accumbens. Addict Biol. 2011;16:43-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 41. | Burns JA, Kroll DS, Feldman DE, Kure Liu C, Manza P, Wiers CE, Volkow ND, Wang GJ. Molecular Imaging of Opioid and Dopamine Systems: Insights Into the Pharmacogenetics of Opioid Use Disorders. Front Psychiatry. 2019;10:626. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 51] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 42. | Marcinkiewcz CA. Serotonergic Systems in the Pathophysiology of Ethanol Dependence: Relevance to Clinical Alcoholism. ACS Chem Neurosci. 2015;6:1026-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 33] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 43. | Müller CP, Schumann G, Rehm J, Kornhuber J, Lenz B. Self-management with alcohol over lifespan: psychological mechanisms, neurobiological underpinnings, and risk assessment. Mol Psychiatry. 2023;28:2683-2696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 44. | Bauer J, Pedersen A, Scherbaum N, Bening J, Patschke J, Kugel H, Heindel W, Arolt V, Ohrmann P. Craving in alcohol-dependent patients after detoxification is related to glutamatergic dysfunction in the nucleus accumbens and the anterior cingulate cortex. Neuropsychopharmacology. 2013;38:1401-1408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 45. | Alasmari F, Goodwani S, McCullumsmith RE, Sari Y. Role of glutamatergic system and mesocorticolimbic circuits in alcohol dependence. Prog Neurobiol. 2018;171:32-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 64] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 46. | Chvilicek MM, Titos I, Rothenfluh A. The Neurotransmitters Involved in Drosophila Alcohol-Induced Behaviors. Front Behav Neurosci. 2020;14:607700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 47. | Korpi ER, den Hollander B, Farooq U, Vashchinkina E, Rajkumar R, Nutt DJ, Hyytiä P, Dawe GS. Mechanisms of Action and Persistent Neuroplasticity by Drugs of Abuse. Pharmacol Rev. 2015;67:872-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 115] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 48. | Marinelli M, McCutcheon JE. Heterogeneity of dopamine neuron activity across traits and states. Neuroscience. 2014;282:176-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 118] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 49. | Lindgren E, Gray K, Miller G, Tyler R, Wiers CE, Volkow ND, Wang GJ. Food addiction: A common neurobiological mechanism with drug abuse. Front Biosci (Landmark Ed). 2018;23:811-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 98] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 50. | Ma H, Zhu G. The dopamine system and alcohol dependence. Shanghai Arch Psychiatry. 2014;26:61-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 51. | Baik JH. Dopamine signaling in reward-related behaviors. Front Neural Circuits. 2013;7:152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 235] [Cited by in RCA: 355] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 52. | Bhaskar LV, Kumar SA. Polymorphisms in genes encoding dopamine signalling pathway and risk of alcohol dependence: a systematic review. Acta Neuropsychiatr. 2014;26:69-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 53. | Whelan R, Watts R, Orr CA, Althoff RR, Artiges E, Banaschewski T, Barker GJ, Bokde AL, Büchel C, Carvalho FM, Conrod PJ, Flor H, Fauth-Bühler M, Frouin V, Gallinat J, Gan G, Gowland P, Heinz A, Ittermann B, Lawrence C, Mann K, Martinot JL, Nees F, Ortiz N, Paillère-Martinot ML, Paus T, Pausova Z, Rietschel M, Robbins TW, Smolka MN, Ströhle A, Schumann G, Garavan H; IMAGEN Consortium. Neuropsychosocial profiles of current and future adolescent alcohol misusers. Nature. 2014;512:185-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 346] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 54. | Merenäkk L, Mäestu J, Nordquist N, Parik J, Oreland L, Loit HM, Harro J. Effects of the serotonin transporter (5-HTTLPR) and α2A-adrenoceptor (C-1291G) genotypes on substance use in children and adolescents: a longitudinal study. Psychopharmacology (Berl). 2011;215:13-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 55. | Müller CP, Schumann G, Kornhuber J, Kalinichenko LS. The role of serotonin in alcohol use and abuse. In: Müller CP, Cunningham KA, editor. Handbook of Behavioral Neuroscience. Netherlands: Elsevier; 2020: 31: 803-827. [DOI] [Full Text] |
| 56. | Berger M, Gray JA, Roth BL. The expanded biology of serotonin. Annu Rev Med. 2009;60:355-366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1506] [Cited by in RCA: 1493] [Article Influence: 87.8] [Reference Citation Analysis (0)] |
| 57. | Sari Y, Johnson VR, Weedman JM. Role of the serotonergic system in alcohol dependence: from animal models to clinics. Prog Mol Biol Transl Sci. 2011;98:401-443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 58. | Belmer A, Patkar OL, Lanoue V, Bartlett SE. 5-HT1A receptor-dependent modulation of emotional and neurogenic deficits elicited by prolonged consumption of alcohol. Sci Rep. 2018;8:2099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 59. | Ebrahim IO, Shapiro CM, Williams AJ, Fenwick PB. Alcohol and sleep I: effects on normal sleep. Alcohol Clin Exp Res. 2013;37:539-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 246] [Article Influence: 18.9] [Reference Citation Analysis (0)] |
| 60. | Jembrek MJ, Vlainic J. GABA Receptors: Pharmacological Potential and Pitfalls. Curr Pharm Des. 2015;21:4943-4959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 103] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 61. | Tiurenkov IN, Perfilova VN. [Role of GABA receptors in pathological processes]. Eksp Klin Farmakol. 2011;74:47-52. [PubMed] |
| 62. | Rao PS, Bell RL, Engleman EA, Sari Y. Targeting glutamate uptake to treat alcohol use disorders. Front Neurosci. 2015;9:144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 109] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 63. | Thoma R, Mullins P, Ruhl D, Monnig M, Yeo RA, Caprihan A, Bogenschutz M, Lysne P, Tonigan S, Kalyanam R, Gasparovic C. Perturbation of the glutamate-glutamine system in alcohol dependence and remission. Neuropsychopharmacology. 2011;36:1359-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 64. | Suh YH, Chang K, Roche KW. Metabotropic glutamate receptor trafficking. Mol Cell Neurosci. 2018;91:10-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 65. | Tabakoff B, Hoffman PL. The neurobiology of alcohol consumption and alcoholism: an integrative history. Pharmacol Biochem Behav. 2013;113:20-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 94] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 66. | Purkayastha P, Malapati A, Yogeeswari P, Sriram D. A Review on GABA/Glutamate Pathway for Therapeutic Intervention of ASD and ADHD. Curr Med Chem. 2015;22:1850-1859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 67. | Francescangeli J, Karamchandani K, Powell M, Bonavia A. The Serotonin Syndrome: From Molecular Mechanisms to Clinical Practice. Int J Mol Sci. 2019;20:2288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 98] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 68. | Allen MJ, Sabir S, Sharma S. GABA Receptor. 2023 Feb 13. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024. [PubMed] |
| 70. | Park BL, Kim JW, Cheong HS, Kim LH, Lee BC, Seo CH, Kang TC, Nam YW, Kim GB, Shin HD, Choi IG. Extended genetic effects of ADH cluster genes on the risk of alcohol dependence: from GWAS to replication. Hum Genet. 2013;132:657-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 71. | Kołota A. The effect of the products of ethanol metabolism on the liver–a review. AIN. 2018;31:225-242. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 72. | Edenberg HJ. The genetics of alcohol metabolism: role of alcohol dehydrogenase and aldehyde dehydrogenase variants. Alcohol Res Health. 2007;30:5-13. [PubMed] |
| 73. | Edenberg HJ, Foroud T. Genetics and alcoholism. Nat Rev Gastroenterol Hepatol. 2013;10:487-494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 129] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 74. | Gaviria-Calle M, Duque-Jaramillo A, Aranzazu M, Di Filippo D, Montoya M, Roldán I, Palacio N, Jaramillo S, Restrepo JC, Hoyos S, Navas MC. Polymorphisms in alcohol dehydrogenase (ADH1) and cytochrome p450 2E1 (CYP2E1) genes in patients with cirrhosis and/or hepatocellular carcinoma. Biomedica. 2018;38:555-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 75. | Reilly MT, Noronha A, Goldman D, Koob GF. Genetic studies of alcohol dependence in the context of the addiction cycle. Neuropharmacology. 2017;122:3-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 76. | Ferrari P, McKay JD, Jenab M, Brennan P, Canzian F, Vogel U, Tjønneland A, Overvad K, Tolstrup JS, Boutron-Ruault MC, Clavel-Chapelon F, Morois S, Kaaks R, Boeing H, Bergmann M, Trichopoulou A, Katsoulis M, Trichopoulos D, Krogh V, Panico S, Sacerdote C, Palli D, Tumino R, Peeters PH, van Gils CH, Bueno-de-Mesquita B, Vrieling A, Lund E, Hjartåker A, Agudo A, Suarez LR, Arriola L, Chirlaque MD, Ardanaz E, Sánchez MJ, Manjer J, Lindkvist B, Hallmans G, Palmqvist R, Allen N, Key T, Khaw KT, Slimani N, Rinaldi S, Romieu I, Boffetta P, Romaguera D, Norat T, Riboli E. Alcohol dehydrogenase and aldehyde dehydrogenase gene polymorphisms, alcohol intake and the risk of colorectal cancer in the European Prospective Investigation into Cancer and Nutrition study. Eur J Clin Nutr. 2012;66:1303-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 77. | Edenberg HJ. The genetics of alcohol metabolism: role of alcohol dehydrogenase and aldehyde dehydrogenase variants. Alcohol Res Health. 2007;30:5-13. [PubMed] |
| 78. | Li D, Zhao H, Gelernter J. Strong association of the alcohol dehydrogenase 1B gene (ADH1B) with alcohol dependence and alcohol-induced medical diseases. Biol Psychiatry. 2011;70:504-512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 124] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 79. | Gupta I, Dandavate R, Gupta P, Agrawal V, Kapoor M. Recent advances in genetic studies of alcohol use disorders. Curr Genet Med Rep. 2020;8:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 80. | Levchenko A, Malov S, Antonik A, Protsvetkina A, Rybakova KV, Kanapin A, Yakovlev AN, Nenasteva AY, Nikolishin AE, Cherkasov N, Chuprova NA, Blagonravova AS, Sergeeva AV, Zhilyaeva TV, Denisenko MK, Gainetdinov RR, Kibitov AO, Krupitsky EM. A Genome-Wide Association Study Reveals a BDNF-Centered Molecular Network Associated with Alcohol Dependence and Related Clinical Measures. Biomedicines. 2022;10:3007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 81. | Zuo L, Zhang CK, Wang F, Li CS, Zhao H, Lu L, Zhang XY, Lu L, Zhang H, Zhang F, Krystal JH, Luo X. A novel, functional and replicable risk gene region for alcohol dependence identified by genome-wide association study. PLoS One. 2011;6:e26726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 82. | Kuroda A, Hegab AE, Jingtao G, Yamashita S, Hizawa N, Sakamoto T, Yamada H, Suzuki S, Ishii M, Namkoong H, Asakura T, Ozaki M, Yasuda H, Hamamoto J, Kagawa S, Soejima K, Betsuyaku T. Effects of the common polymorphism in the human aldehyde dehydrogenase 2 (ALDH2) gene on the lung. Respir Res. 2017;18:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 83. | Huang YH, Chang KH, Lee YS, Chen CM, Chen YC. Association of alcohol dehydrogenase and aldehyde dehydrogenase Polymorphism with Spontaneous Deep Intracerebral Haemorrhage in the Taiwan population. Sci Rep. 2020;10:3641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 84. | Vaswani M. ADH and ALDH Polymorphisms in Alcoholism and Alcohol Misuse/Dependence. In: Preedy VR, editor. Neuroscience of Alcohol. United States: Academic Press; 2019: 29-38. [DOI] [Full Text] |
| 85. | Tawa EA, Hall SD, Lohoff FW. Overview of the Genetics of Alcohol Use Disorder. Alcohol Alcohol. 2016;51:507-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 110] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 86. | Hubacek JA, Jirsa M, Bobak M, Pelclova D, Zakharov S. Aldehyde dehydrogenase 2 polymorphism affects the outcome of methanol poisoning in exposed humans. Clin Genet. 2018;94:445-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 87. | Matsumura Y, Stiles KM, Reid J, Frenk EZ, Cronin S, Pagovich OE, Crystal RG. Gene Therapy Correction of Aldehyde Dehydrogenase 2 Deficiency. Mol Ther Methods Clin Dev. 2019;15:72-82. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 88. | Kimura M, Yokoyama A, Matsushita S, Higuchi S. Enzymatic Aspects of Alcoholism-ADH and ALDH. In: el-Guebaly N, Carrà G, Galanter M, editor. Textbook of Addiction Treatment: International Perspectives. Berlin: Springer; 2015: 333-342. [DOI] [Full Text] |
| 89. | Zimatkin SM. Acetaldehyde mediates the ethanol effects in developing brain. Front Behav Neurosci. 2013;7:75. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 90. | Mirijello A, Sestito L, Antonelli M, Gasbarrini A, Addolorato G. Identification and management of acute alcohol intoxication. Eur J Intern Med. 2023;108:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 39] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 91. | Jacob A, Wang P. Alcohol Intoxication and Cognition: Implications on Mechanisms and Therapeutic Strategies. Front Neurosci. 2020;14:102. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 92. | Schaper A, Ebbecke M. Intox, detox, antidotes - Evidence based diagnosis and treatment of acute intoxications. Eur J Intern Med. 2017;45:66-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 93. | McMartin K, Jacobsen D, Hovda KE. Antidotes for poisoning by alcohols that form toxic metabolites. Br J Clin Pharmacol. 2016;81:505-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 79] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 94. | Abrahao KP, Salinas AG, Lovinger DM. Alcohol and the Brain: Neuronal Molecular Targets, Synapses, and Circuits. Neuron. 2017;96:1223-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 323] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 95. | First MB. Diagnostic and statistical manual of mental disorders, 5th edition, and clinical utility. J Nerv Ment Dis. 2013;201:727-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 378] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 96. | Jung YC, Namkoong K. Alcohol: intoxication and poisoning - diagnosis and treatment. Handb Clin Neurol. 2014;125:115-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 97. | Wang H, Xu H, Li W, Li B, Shi Q, Ma K, Xiao B, Chen L. Forensic appraisal of death due to acute alcohol poisoning: three case reports and a literature review. Forensic Sci Res. 2019;5:341-347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 98. | Zafonte R, Kurowski B. Blood Alcohol Level. In: Kreutzer JS, DeLuca J, Caplan B, editor. Encyclopedia of Clinical Neuropsychology. Berlin: Springer; 2011: 422-423. [DOI] [Full Text] |
| 99. | Comley RE, Dry MJ. Acute tolerance to alcohol-induced impairment in cognitive performance. Exp Clin Psychopharmacol. 2020;28:659-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 100. | Miller MB, DiBello AM, Meier E, Leavens ELS, Merrill JE, Carey KB, Leffingwell TR. Alcohol-Induced Amnesia and Personalized Drinking Feedback: Blackouts Predict Intervention Response. Behav Ther. 2019;50:25-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 101. | Wetherill RR, Fromme K. Alcohol-Induced Blackouts: A Review of Recent Clinical Research with Practical Implications and Recommendations for Future Studies. Alcohol Clin Exp Res. 2016;40:922-935. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 102. | Camchong J, Endres M, Fein G. Decision making, risky behavior, and alcoholism. Handb Clin Neurol. 2014;125:227-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 103. | Piccioni A, Tarli C, Cardone S, Brigida M, D'Addio S, Covino M, Zanza C, Merra G, Ojetti V, Gasbarrini A, Addolorato G, Franceschi F. Role of first aid in the management of acute alcohol intoxication: a narrative review. Eur Rev Med Pharmacol Sci. 2020;24:9121-9128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 104. | Pianca TG, Sordi AO, Hartmann TC, von Diemen L. Identification and initial management of intoxication by alcohol and other drugs in the pediatric emergency room. J Pediatr (Rio J). 2017;93 Suppl 1:46-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 105. | Shen Y, Lindemeyer AK, Gonzalez C, Shao XM, Spigelman I, Olsen RW, Liang J. Dihydromyricetin as a novel anti-alcohol intoxication medication. J Neurosci. 2012;32:390-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 173] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 106. | Liu Y, Du J, Yan M, Lau MY, Hu J, Han H, Yang OO, Liang S, Wei W, Wang H, Li J, Zhu X, Shi L, Chen W, Ji C, Lu Y. Biomimetic enzyme nanocomplexes and their use as antidotes and preventive measures for alcohol intoxication. Nat Nanotechnol. 2013;8:187-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 249] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 107. | Carlson RW, Kumar NN, Wong-Mckinstry E, Ayyagari S, Puri N, Jackson FK, Shashikumar S. Alcohol withdrawal syndrome. Crit Care Clin. 2012;28:549-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 108. | Becker HC, Mulholland PJ. Neurochemical mechanisms of alcohol withdrawal. Handb Clin Neurol. 2014;125:133-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 109. | Corfee FA. Alcohol withdrawal in the critical care unit. Aust Crit Care. 2011;24:110-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 110. | Schuckit MA. Recognition and management of withdrawal delirium (delirium tremens). N Engl J Med. 2014;371:2109-2113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 187] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 111. | Grover S, Ghosh A. Delirium Tremens: Assessment and Management. J Clin Exp Hepatol. 2018;8:460-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 112. | Long D, Long B, Koyfman A. The emergency medicine management of severe alcohol withdrawal. Am J Emerg Med. 2017;35:1005-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 113. | Mirijello A, D'Angelo C, Ferrulli A, Vassallo G, Antonelli M, Caputo F, Leggio L, Gasbarrini A, Addolorato G. Identification and management of alcohol withdrawal syndrome. Drugs. 2015;75:353-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 114] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 114. | Amato L, Minozzi S, Davoli M. Efficacy and safety of pharmacological interventions for the treatment of the Alcohol Withdrawal Syndrome. Cochrane Database Syst Rev. 2011;2011:CD008537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 115. | Muzyk AJ, Leung JG, Nelson S, Embury ER, Jones SR. The role of diazepam loading for the treatment of alcohol withdrawal syndrome in hospitalized patients. Am J Addict. 2013;22:113-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 116. | Schmidt KJ, Doshi MR, Holzhausen JM, Natavio A, Cadiz M, Winegardner JE. Treatment of Severe Alcohol Withdrawal. Ann Pharmacother. 2016;50:389-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 72] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 117. | Ott M, Werneke U. Wernicke's encephalopathy - from basic science to clinical practice. Part 1: Understanding the role of thiamine. Ther Adv Psychopharmacol. 2020;10:2045125320978106. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 118. | Wijnia JW. A Clinician's View of Wernicke-Korsakoff Syndrome. J Clin Med. 2022;11:6755. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 119. | Becker DA, Balcer LJ, Galetta SL. The Neurological Complications of Nutritional Deficiency following Bariatric Surgery. J Obes. 2012;2012:608534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 90] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 120. | Kareem O, Nisar S, Tanvir M, Muzaffer U, Bader GN. Thiamine deficiency in pregnancy and lactation: implications and present perspectives. Front Nutr. 2023;10:1080611. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 121. | Pacei F, Tesone A, Laudi N, Laudi E, Cretti A, Pnini S, Varesco F, Colombo C. The Relevance of Thiamine Evaluation in a Practical Setting. Nutrients. 2020;12:2810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 122. | Vasan S, Kumar A. Wernicke Encephalopathy. 2023 Aug 14. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024. [PubMed] |
| 123. | Kalidass B, Sunnathkal R, Rangashamanna DV, Paraswani R. Atypical Wernicke's encephalopathy showing involvement of substantia nigra. J Neuroimaging. 2012;22:204-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 124. | Alekseeva N, McGee J, Kelley RE, Maghzi AH, Gonzalez-Toledo E, Minagar A. Toxic-metabolic, nutritional, and medicinal-induced disorders of cerebellum. Neurol Clin. 2014;32:901-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 125. | Sinha S, Kataria A, Kolla BP, Thusius N, Loukianova LL. Wernicke Encephalopathy-Clinical Pearls. Mayo Clin Proc. 2019;94:1065-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 94] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 126. | Ha ND, Weon YC, Jang JC, Kang BS, Choi SH. Spectrum of MR imaging findings in Wernicke encephalopathy: are atypical areas of involvement only present in nonalcoholic patients? AJNR Am J Neuroradiol. 2012;33:1398-1402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 127. | Noble JM, Weimer LH. Neurologic complications of alcoholism. Continuum (Minneap Minn). 2014;20:624-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 128. | Arts NJ, Walvoort SJ, Kessels RP. Korsakoff's syndrome: a critical review. Neuropsychiatr Dis Treat. 2017;13:2875-2890. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 180] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 129. | Covell T, Siddiqui W. Korsakoff Syndrome. 2023 Jan 30. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024. [PubMed] |
| 130. | Akhouri S. Wernicke–Korsakoff Syndrome. In: Lester JN, O'Reilly M, editor. The Palgrave Encyclopedia of Critical Perspectives on Mental Health. United Kingdom: Palgrave Macmillan; 2021. [DOI] [Full Text] |
| 131. | Kopelman MD. What does a comparison of the alcoholic Korsakoff syndrome and thalamic infarction tell us about thalamic amnesia? Neurosci Biobehav Rev. 2015;54:46-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 132. | Ott K, Tubbs RS. Inflammatory and Demyelinating Diseases of the Corpus Callosum. In: Turgut M, Tubbs RS, Turgut AT, Bui CC, editor. The Corpus Callosum. Berlin: Springer, 2023: 201-210. [DOI] [Full Text] |
| 133. | Markowitsch H, Pitel A, Eustache F. Korsakoff's Syndrome☆. Reference Module in Neuroscience and Biobehavioral Psychology. Netherlands: Elsevier; 2017. [DOI] [Full Text] |
| 134. | Fama R, Pitel AL, Sullivan EV. Anterograde episodic memory in Korsakoff syndrome. Neuropsychol Rev. 2012;22:93-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 47] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 135. | Akhouri S, Kuhn J, Newton EJ. Wernicke-Korsakoff Syndrome. 2023 Jun 26. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024. [PubMed] |
| 136. | Thomson AD, Guerrini I, Marshall EJ. The evolution and treatment of Korsakoff's syndrome: out of sight, out of mind? Neuropsychol Rev. 2012;22:81-92. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 111] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 137. | Singh S, Wagh V. Marchiafava Bignami Disease: A Rare Neurological Complication of Long-Term Alcohol Abuse. Cureus. 2022;14:e30863. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 138. | Huang BY. Toxic and Metabolic Brain Disease. In: Naidich TP, Castillo M, Cha S, Smirniotopoulos JG, editor. Imaging of the Brain. Netherlands: Elsevier; 2013. [DOI] [Full Text] |
| 139. | Sönmez D, Hocaoğlu Ç. Marchiafava-Bignami disease presenting with psychiatric symptoms: A case report. Psychiatry Res Case Rep. 2023;2:100174. [DOI] [Full Text] |
| 140. | Namekawa M, Nakamura Y, Nakano I. Cortical involvement in Marchiafava-Bignami disease can be a predictor of a poor prognosis: a case report and review of the literature. Intern Med. 2013;52:811-813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 141. | Garcia-Santibanez R. Marchiafava-Bignami disease presenting as acute dysarthria and ataxia. Alcohol Alcohol. 2015;50:256-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 142. | Khan S, Okuda M, Hasin DS, Secades-Villa R, Keyes K, Lin KH, Grant B, Blanco C. Gender differences in lifetime alcohol dependence: results from the national epidemiologic survey on alcohol and related conditions. Alcohol Clin Exp Res. 2013;37:1696-1705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 143. | Yadala S, Luo JJ. Marchiafava-bignami disease in a nonalcoholic diabetic patient. Case Rep Neurol Med. 2013;2013:979383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 144. | Wenz H, Eisele P, Artemis D, Förster A, Brockmann MA. Acute Marchiafava-Bignami disease with extensive diffusion restriction and early recovery: case report and review of the literature. J Neuroimaging. 2014;24:421-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 145. | Vargas Canas A, Rivas M, Guerrero Torrealba R, Francisca Fajre Caamano M. Marchiafava-Bignami's Disease, as Etiologic Diagnosis of Athetosis. Ann Neurosci. 2017;24:57-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 146. | Hillbom M, Saloheimo P, Fujioka S, Wszolek ZK, Juvela S, Leone MA. Diagnosis and management of Marchiafava-Bignami disease: a review of CT/MRI confirmed cases. J Neurol Neurosurg Psychiatry. 2014;85:168-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 147. | Parmanand H T. Marchiafava-Bignami disease in chronic alcoholic patient. Radiol Case Rep. 2016;11:234-237. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 148. | Latt N, Dore G. Thiamine in the treatment of Wernicke encephalopathy in patients with alcohol use disorders. Intern Med J. 2014;44:911-915. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 104] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 149. | Zeigelboim BS, Albano dos Santos Junior C, Teive H, Spricigo Malisky J, Jose MR, Rodrigues da Rosa M, Lacerda A. Alcoholic cerebellar degeneration: A case report. NCCN. 2019;49:448. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 150. | Del Brutto OH, Mera RM, King NR, Zambrano M, Sullivan LJ. Years of Drinking but Not the Amount of Alcohol Intake Contribute to the Association Between Alcoholic Cerebellar Degeneration and Worse Cognitive Performance. A Population-Based Study. Cerebellum. 2017;16:612-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 151. | Fitzpatrick LE, Jackson M, Crowe SF. Characterization of cerebellar ataxia in chronic alcoholics using the International Cooperative Ataxia Rating Scale (ICARS). Alcohol Clin Exp Res. 2012;36:1942-1951. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 152. | Welch KA. Neurological complications of alcohol and misuse of drugs. Pract Neurol. 2011;11:206-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 153. | Shanmugarajah PD, Hoggard N, Currie S, Aeschlimann DP, Aeschlimann PC, Gleeson DC, Karajeh M, Woodroofe N, Grünewald RA, Hadjivassiliou M. Alcohol-related cerebellar degeneration: not all down to toxicity? Cerebellum Ataxias. 2016;3:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 154. | Currie S, Hoggard N, Clark MJ, Sanders DS, Wilkinson ID, Griffiths PD, Hadjivassiliou M. Alcohol induces sensitization to gluten in genetically susceptible individuals: a case control study. PLoS One. 2013;8:e77638. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 155. | Laureno R. Nutritional cerebellar degeneration, with comments on its relationship to Wernicke disease and alcoholism. Handb Clin Neurol. 2012;103:175-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 156. | Sarva H, Shanker VL. Treatment Options in Degenerative Cerebellar Ataxia: A Systematic Review. Mov Disord Clin Pract. 2014;1:291-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 157. | Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, Ballard C, Banerjee S, Burns A, Cohen-Mansfield J, Cooper C, Fox N, Gitlin LN, Howard R, Kales HC, Larson EB, Ritchie K, Rockwood K, Sampson EL, Samus Q, Schneider LS, Selbæk G, Teri L, Mukadam N. Dementia prevention, intervention, and care. Lancet. 2017;390:2673-2734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3864] [Cited by in RCA: 3994] [Article Influence: 443.8] [Reference Citation Analysis (0)] |
| 158. | Wortmann M. Dementia: a global health priority - highlights from an ADI and World Health Organization report. Alzheimers Res Ther. 2012;4:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 272] [Cited by in RCA: 308] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 159. | Rehm J, Hasan OSM, Black SE, Shield KD, Schwarzinger M. Alcohol use and dementia: a systematic scoping review. Alzheimers Res Ther. 2019;11:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 243] [Cited by in RCA: 273] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 160. | Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimers Dement. 2013;9:63-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2862] [Cited by in RCA: 3301] [Article Influence: 253.9] [Reference Citation Analysis (0)] |
| 161. | Sabia S, Elbaz A, Britton A, Bell S, Dugravot A, Shipley M, Kivimaki M, Singh-Manoux A. Alcohol consumption and cognitive decline in early old age. Neurology. 2014;82:332-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 126] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 162. | Cheng C, Huang CL, Tsai CJ, Chou PH, Lin CC, Chang CK. Alcohol-Related Dementia: A Systemic Review of Epidemiological Studies. Psychosomatics. 2017;58:331-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 163. | Sabia S, Fayosse A, Dumurgier J, Dugravot A, Akbaraly T, Britton A, Kivimäki M, Singh-Manoux A. Alcohol consumption and risk of dementia: 23 year follow-up of Whitehall II cohort study. BMJ. 2018;362:k2927. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 176] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 164. | Sachdeva A, Chandra M, Choudhary M, Dayal P, Anand KS. Alcohol-Related Dementia and Neurocognitive Impairment: A Review Study. Int J High Risk Behav Addict. 2016;5:e27976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 77] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 165. | Dudek I, Hajduga D, Sieńko C, Maani A, Sitarz E, Sitarz M, Forma A. Alcohol-Induced Neuropathy in Chronic Alcoholism: Causes, Pathophysiology, Diagnosis, and Treatment Options. Curr Pathobiol Rep. 2020;8:87-97. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 166. | Julian T, Glascow N, Syeed R, Zis P. Alcohol-related peripheral neuropathy: a systematic review and meta-analysis. J Neurol. 2019;266:2907-2919. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 99] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 167. | Shumway NK, Cole E, Fernandez KH. Neurocutaneous disease: Neurocutaneous dysesthesias. J Am Acad Dermatol. 2016;74: 215-228; quiz 229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 168. | Mellion ML, Silbermann E, Gilchrist JM, Machan JT, Leggio L, de la Monte S. Small-fiber degeneration in alcohol-related peripheral neuropathy. Alcohol Clin Exp Res. 2014;38:1965-1972. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 169. | Chopra K, Tiwari V. Alcoholic neuropathy: possible mechanisms and future treatment possibilities. Br J Clin Pharmacol. 2012;73:348-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 159] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 170. | Hanewinckel R, van Oijen M, Ikram MA, van Doorn PA. The epidemiology and risk factors of chronic polyneuropathy. Eur J Epidemiol. 2016;31:5-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 186] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 171. | Mellion M, Gilchrist JM, de la Monte S. Alcohol-related peripheral neuropathy: nutritional, toxic, or both? Muscle Nerve. 2011;43:309-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 172. | Witkiewitz K, Saville K, Hamreus K. Acamprosate for treatment of alcohol dependence: mechanisms, efficacy, and clinical utility. Ther Clin Risk Manag. 2012;8:45-53. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 44] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 173. | Paulus MP, Stewart JL, Haase L. Treatment approaches for interoceptive dysfunctions in drug addiction. Front Psychiatry. 2013;4:137. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 174. | Airagnes G, Ducoutumany G, Laffy-Beaufils B, Le Faou AL, Limosin F. Alcohol withdrawal syndrome management: Is there anything new? Rev Med Interne. 2019;40:373-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 175. | Arnedt JT, Conroy DA, Brower KJ. Treatment options for sleep disturbances during alcohol recovery. J Addict Dis. 2007;26:41-54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 71] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/