Published online Dec 20, 2024. doi: 10.5493/wjem.v14.i4.99239
Revised: August 22, 2024
Accepted: August 27, 2024
Published online: December 20, 2024
Processing time: 104 Days and 16.9 Hours
Obesity is increasingly prevalent worldwide, with genetic factors contributing to its development. The hypothalamic leptin-melanocortin pathway is central to the regulation of appetite and weight; leptin activates the proopiomelanocortin neurons, leading to the production of melanocortin peptides; these in turn act on melanocortin 4 receptors (MC4R) which suppress appetite and increase energy expenditure. MC4R mutations are responsible for syndromic and non-syndromic obesity. These mutations are classified based on their impact on the receptor's life cycle: i.e. null mutations, intracellular retention, binding defects, signaling defects, and variants of unknown function. Clinical manifestations of MC4R mutations include early-onset obesity, hyperphagia, and metabolic abnormalities such as hyperinsulinemia and dyslipidemia. Management strategies for obesity due to MC4R mutations have evolved with the development of targeted therapies such as Setmelanotide, an MC4R agonist which can reduce weight and manage symptoms without adverse cardiovascular effects. Future research directions must include expansion of population studies to better understand the epide
Core Tip: The leptin-melanocortin pathway regulates energy balance and body weight. Melanocortin-4 receptor (MC4R) plays a key role in this pathway by reducing hunger, inducing satiety and increasing energy expenditure. Mutations of MC4R result in obesity and hyperphagia in childhood. Setmelanotide is an MC4R agonist approved for use in obesity caused by leptin-melanocortin pathway dysfunction.
- Citation: Sridhar GR, Gumpeny L. Melanocortin 4 receptor mutation in obesity. World J Exp Med 2024; 14(4): 99239
- URL: https://www.wjgnet.com/2220-315x/full/v14/i4/99239.htm
- DOI: https://dx.doi.org/10.5493/wjem.v14.i4.99239
Body weight is maintained by a balance between energy intake and expenditure through the central hypothalamic leptin-melanocortin pathway. Among the hormones involved, leptin acts by activating the release by proopiomelanocortin (POMC) which is further cleaved into melanocortin ligands a and b; the b- melanocyte-stimulating hormone (MSH) binds and activates the melanocortin-4 receptor (MC4R) which results in reduced hunger, induction of satiety and increased energy expenditure. Variants of MC4R are associated with rare forms of recalcitrant obesity, usually manifesting in infancy or early childhood. A number of other mutations by MC4R and its proximate and downstream signals have been identified to cause syndromic obesity. Less serious disruptions by the pathway are responsible for intermediate degrees of obesity.
While syndromic or genetic causes of obesity are rare, the prevalence of obesity has been rising. A 2021 global report from the World Health Organization reported that obesity nearly tripled since 1975. As of 2016, more than 1.9 billion adults were overweight[1]. Adverse outcomes of obesity are dependent on race and ethnicity[2], which are projected to peak between 2026 and 2054, first in the United States, followed by European nations[3]. Similar findings were reported from the United Kingdom[4], South East Asia[5] and Africa[6].
A trend towards increasing obesity and overweight rates is observed even in childhood[7]. A 2017 Lancet report[8] on worldwide trends in body-mass index (BMI) showed that the rate of excess body weight was increasing in Asia. The increase in overweight outpaced the lowered prevalence of underweight[9]. The obesity pandemic in children was observed even in regions where obesity rates had plateaued before the coronavirus disease 2019 pandemic[10].
Gao et al[11] published a comprehensive global analysis on spatial and temporal trends in childhood overweight and obesity from 191 countries. Although genetics and environmental factors have a role in the pathogenesis, the rapid increase suggests a greater contribution by environmental factors. Since childhood obesity is the precursor of obesity in adulthood, prevention is essential[11]. The first step is to recognize the underlying lifestyle factors, which can then be addressed.
One of the proposed theories for the burgeoning rates of obesity in low and middle-income countries is the ‘moderni
These trends were attributed to global economic development, and cultural differences as well as intergeneration effects by malnutrition in early life. Countries with the most rapid growth shared rapid economic development, social and cultural changes leading to consumption of unhealthy ultra-processed foods. Reversal or stabilization of trends occurred due to interventions by governments[13].
Weight and appetite are principally regulated by the ventromedial nucleus in the hypothalamus. Other brain areas include the arcuate nucleus, paraventricular nucleus and lateral hypothalamic area, where MC4R regulates energy metabolism by suppressing food intake and increasing energy expenditure. Identification of monogenic or non-syndromic obesity disorders revealed a complex interplay among different hormones and neurotransmitters, among which MC4R plays a central role. Appetite suppressing hormones (anorexigenic) and appetite stimulating hormones (orexigenic) communicate between the peripheral tissues and the hypothalamus[14].
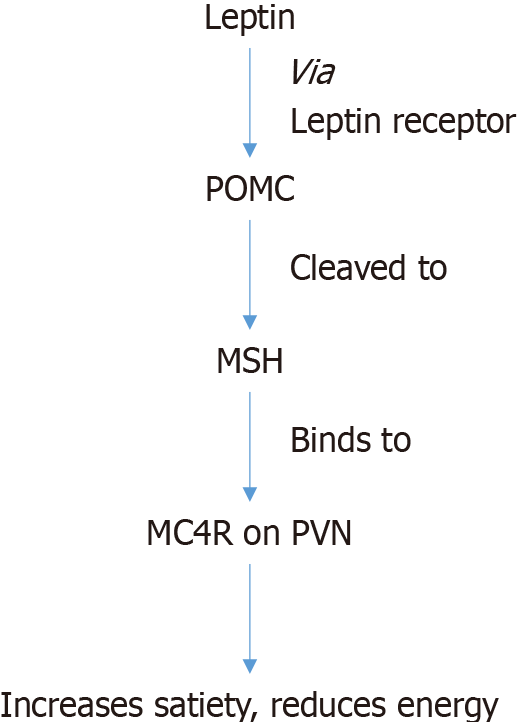
Leptin, a hormone secreted by the adipose tissue binds to its receptor expressed on POMC neurons located in the arcuate nucleus of the hypothalamus. This leads to the formation of propeptide POMC, which is then cleaved to melanocortin ligands and MSH. MSH in turn activates MC4R, expressed in the paraventricular nucleus, leading to its effects on metabolism[15] (Figure 1).
POMC is an ancient gene, having been in existence for over 700 million years[16,17]. The melanocortin system has three components: Pro-peptide POMC, the melanocortin peptides and endogenous antagonists by these receptors [agouti and agouti-related protein (AgRP)][18].
Among the many genes related to obesity, MC4R is by far the most significant. It is localized in chromosome 18q22 and codes a 332 amino acid transmembrane protein[19]. The gene does not contain introns and has the highest homology with melanocortin 3 receptor (MC3R), another member of the MCR family[20]. Evolutionary analysis by MC4R showed that it underwent purifying selection, resulting in low levels of silent polymorphisms in humans[21].
Five melanocortin receptors (MCRs) are responsible for diverse actions[22] (Table 1) which are named 1-5 based on the sequence by their cloning. MC1R receptor, expressed in the skin and hair follicles regulates skin pigmentation. MC2R in the adrenal cortex regulates adrenal steroidogenesis. MCR3 and MCR4 are referred to as neural MCRs as they are principally expressed in the central nervous system. MC5R has a wide tissue expression, particularly the exocrine glands.
| Name | Tissue expression | Principal actions |
| MC1R | Skin, hair follicles | Regulates pigmentation |
| MC2R | Adrenal cortex | Regulates adrenal steroidogenesis |
| MC3R | Central nervous system | Regulates energy homeostasis |
| MC4R | Central nervous system | Regulates energy homeostasis |
| MC5R | Exocrine glands; wide expression | Regulates exocrine gland secretion |
Research studies of MCR4 showed it was chiefly expressed in brain regions such as thalamus, hypothalamus and hippocampus. mRNA of MCR4 was identified in dentate gyrus, cortex and amygdala and in astrocytes. MC4R mRNA was first expressed on embryonic day 14, followed by other tissues by day 19[22]. MC3R and MC4R are chiefly expressed in the brain and MC5R in the peripheral tissues[18].
Upon binding by α-MSH to MC4R, adenylate cyclase is activated via G protein (guanine nucleotide-binding protein). Cyclic AMP (cAMP) is increased intracellularly, followed by activation of protein kinase A, exchange protein, extracellular regulated kinases 1 and 2 and cAMP response element binding protein. In addition, there is increased transcription by the proto-oncogene c-FOS with a simultaneous reduction of 5' AMP-activated protein kinase (AMPK)[23].
In addition to this pathway, different ligands induce different signals on binding, which are not mutually exclusive. In case of MC4R, food intake is controlled via biased signaling controls involving the Kir7.1, the inward rectifier potassium channel[24].
The understanding of G-protein-coupled receptor (GPCR) signaling, of which MC4R is a member, has expanded in recent years. Initially, GPCR was believed to act as a lock which was opened by the ligand, functioning as a key[25]. As the complexity of GPCR was revealed, other models were proposed, such as ternary-complex model, in which signaling was initiated by three principal components: Ligand, receptor and transducer such as GPCRs[26]. The ternary-complex model was further expanded where the receptor exists in two equilibrated states: The inactive state that cannot signal and the active state that can recruit transducers to render them functional. Finally the cubic-ternary complex model was proposed in which G-proteins and ligands were considered to belong to a common pool accessible to each receptor[27]. Metzger et al[28] recently suggested downstream MC4R signaling via β-restin recruitment and activation by MAPK. MC4R signaling was also shown to occur through MC4R/Gq/11 pathway[28].
MC4R, through its regulatory role in body weight homeostasis may influence the course of metabolic syndrome and multiple sclerosis via its anti-inflammatory and neuroprotective effects[29]. It also plays a role the regulation of glucose homeostasis, erectile function and cardiovascular tone[30].
The melanocortin peptides act through central MC4R to regulate appetite, body weight and energy expenditure[30]. Despite early evidence that intracerebrovascular administration of α-MSH and adrenocorticotropic hormone reduced food intake in rats, the critical importance of MC4R in regulation of energy homeostasis was not fully recognized until the mid 1990s[22]. Hypothalamic melanocortinergic neurons exert a tonic inhibitory action on feeding. Disruption of this pathway led to changes in food intake, suggesting that MC4R which is highly expressed in PVC is the primary mediator of melanocortin regulation[31], playing a pivotal role in the complex neural regulation of appetite[32]. The role of MC4R in energy homeostasis has been identified from mice knock out models which showed a gene dosage effect[33]. Energy balance is a result of reduced food intake (responsible for 60% of the effect) while 40% is due to changes in the expenditure of energy. Thermogenesis is regulated via activation of sympathetic nervous system-BAT-uncoupling protein 1 axis and hypothalamic-pituitary-thyroid axis[34].
To summarize, in states of starvation, leptin levels are low, POMC neuronal activity is reduced and AgRP neuronal activity is increased resulting in reduced MC4R signaling. In the fed state, POMC neurons are activated along with inhibition of AgRP neurons and increased MC4R signaling; all these resulting in diminished food intake and increased expenditure of energy[22].
MC4R regulates energy homeostasis in other mammals and in lower vertebrates as well, including chickens and rainbow trout[22].
In a Chinese study, significant interactions were observed between variants near MC4R gene and obesity-related phenotypes (rs12970134), which was modified by physical activity[35-37].
Spontaneous and genetically induced variations of the melanocortin system showed the importance of this pathway in the regulation of body weight[38]. The first gene to be deleted in the mouse was the MC4R, which led to obesity. MC4R
Identification of a variant MC4R in an obese individual does not necessarily imply that obesity is caused by the mutation[22]. Additional supporting information must be obtained such as familial co-segregation of the mutation and obesity; in vitro functional characterization by the mutant receptor confirms its causal role[41].
Classification of MC4R mutations is based on the receptor life cycle[22]: (1) Class I: Null mutations: impaired protein synthesis and/or enhanced protein degradation leads to low levels of protein (e.g. nonsense mutations); (2) Class II: Mutant receptors are produced in the cell but are misfolded and retained in the endoplasmic reticulum. This forms the largest group of mutations; (3) Class III: Binding defective mutants are expressed on the cellular surface but cannot bind with the ligands due to impaired binding capacity and/or affinity. Therefore signaling is impaired; (4) Class IV: Signaling-defective mutants properly reach the cell surface and bind the ligand, but transmit the signal with lower efficacy or not at all; and (5) Class V: Variants of unknown defect do not fit into any of the above.
More recently, Courbage et al[42] showed that MC4R variants can impact functions in three ways: (1) High: Nonsense, frameshift and splice variants, missense variants and rare variants with conclusive functional tests; (2) Moderate: Missense variants that are predicted as ‘damaging’ by at least four of the seven prediction tools; and (3) Low: Missense variants predicted as ‘less likely damaging’ by at least four of the seven prediction tools. The classification was used for the analysis of 6467 subjects to assess the significance of heterozygous variants on the leptin-melanocortin system among the severely obese[42].
To assess the prevalence and phenotypic effects of MC4R mutants, 20537 electronic medical records and genomics (eMERGE) participants with MC4R coding region sequencing data were studied; in addition 77454 independent persons with genome-wide genotyping data at this locus were also studied. The authors identified 125 coding variants (n: 1839 eMERGE participants), including 30 variants that were unreported earlier[43]. MC4R associated obesity was the most common form of monogenic obesity spectrum among 170 rare genetic variants associated with hyperphagia and early onset obesity[44,45].
The genotype-phenotype association of heterogeneous variants in leptin-melanocortin pathway was studied in 6467 subjects (6347 probands and 120 relatives)[42]. Specifically the MC4R gene was sequenced for 1165 subjects of the 1486 probands. To assess the combined effect of heterozygous variants on phenotype, subjects were chosen who were sequenced on the five genes, viz LEP, LEPR, POMC, PCSK1 and MC4R. BMI of subjects with combined heterozygous variants was higher than in those with a single heterozygous variant (BMI65.2+/-13.2 kg/m2vs 49.0 +/-9.1 kg/m2, P < 0.01)[42].
Aggregated data from 200 MC4R genetic mutations among nearly 1000 patients[46], (homozygous or compound heterozygous) showed early-onset severe obesity.
In heterozygous carriers, obesity was variable: Children carrying heterozygous mutation had similar BMI to wild-type children with obesity. In contrast, adults with heterozygous carriers had higher BMI compared to wild type patients with obesity. Gender differences were also observed; BMI was higher in middle aged women compared to middle aged men.
Where information on age of obesity was available (n = 104) mean age of onset of obesity was at 1.2 years old in homozygous carriers and 3.8 years old in those with heterozygous mutation. Children had an initial accelerated height, although the final height was lower than average.
Hyperphagia was common in patients with MC4R mutations. When recorded (n = 175), hyperphagia was observed in 95% (100% in homozygous patients and 95.1% in heterozygous patients).
There was no specific change in the course of puberty and ultimate fertility in patients with MC4R mutations. Data on cognitive function was available in 30 cases; nine had mild disability: Speech delay, motor retardation, and mild mental retardation. Acanthosis nigricans was observed in 31%-41% of subjects.
Hyperinsulinemia was observed more often in children (55%) than in adults (20%). Type 2 diabetes mellitus was reported in 16.9% of the cohort (n = 148). Dyslipidemia was present in 33% of mutation carriers (n = 20/60: 32% children and 34.8% adults). Advanced bone age and greater bone density were also reported.
In Qatar two subjects with MC4R mutation identified reduced expression of MC4R on the cell surface, intracellular retention by the MC4R protein, and failure to activate downstream signaling of the MC4R[47].
The obesity phenotype can be modified by the interaction of other genetic factors which may either be protective or deleterious, as well as by environmental factors[48].
Currently there are no established guidelines for newborn genetic screening. However with the availability of drugs to treat subjects with MC4R mutations (setmelanotide)[49,50], a case is made for childhood genetic screening in obesity. In childhood addition, genetic analysis by MC4R mutations could help predict responsiveness to drug treatment[51,52].
An understanding of the MC4R receptor, its expression and downstream signaling enabled the identification of agonists which mimic its physiological actions. Initially, analogues were hampered by MC4R activation of sympathetic nervous system, thereby affecting blood pressure and heart rate[53]; sexual arousal was another serious side effect. Development of a number of molecules was stalled early due to adverse effects including tachycardia, hypertension, sexual arousal and skin pigmentation driven by MC1R activation[53]. These were largely due to the complex intracellular signaling by MC4R. It now seems likely that internalization of agonist induced MC4R could be the key element in the control of energy homeostasis.
Setmelanotide, an MC4R agonist has been approved for use in the treatment of obesity due to MC4R mutations. It showed no adverse effects on heart and blood pressure in humans, while having a substantial weight loss effect, with a favorable therapeutic index[53].
Animal studies published in 2016 showed that setmelanotide had the potential to be used as a replacement therapy for rare syndromic forms of obesity due to impaired POMC neuronal function[54]. Coulter et al[55] proposed that setme
A number of case series showed that setmelanotide was effective in genetic and syndromic forms of obesity[49,50]. The Anti-Obesity Medications and Investigational Agents: An Obesity Medicine Association Clinical Practice Statement 2022 published guidelines for the use of setmelanotide along with other anti-obesity agents[56].
Setmelanotide is indicated for chronic weight management in adult and children aged 6 years or older when obesity is due to POMC, PCSK1, or LEPR deficiency. The diagnosis must be based on genetic testing which shows ‘variants in POMC, PCSK1, or LEPR genes that are interpreted as pathogenic, likely pathogenic, or by variant of uncertain significance’. The drug is discontinued if, after 12–16 weeks of treatment, there is no weight loss by 5% from baseline or 5% baseline BMI for children with continued growth potential.
Setmelanotide is started at a dose of 2 mg subcutaneously once daily for 2 weeks with monitoring for gastrointestinal (GI) adverse reactions. If this dose cannot be tolerated, it can be reduced to 1 mg once daily. If the lower dose is tolerated, uptitration to 2 mg can be attempted for additional weight reduction, with a further increase to 3 mg a day; however the lowest tolerable dose must be used for maintenance treatment.
In children between the ages of six and 12, the drug is begun at a dose of 1 mg injected subcutaneously once daily for 2 weeks, while monitoring for adverse GI events. If the dose is not tolerated, it must be reduced to 0.5 mg once daily. If it is tolerated and for additional weight reduction, the dose can be increased to 1 mg once daily. It can be further increased to 2 mg once daily. In case the higher dose is not tolerated, it can be reduced to 1 mg once daily. The highest dose that can be given is limited to 3 mg once daily.
Common side effects, seen in about 25% of subjects consist of injection site reactions, skin hyperpigmentation, nausea, headache, GI effects, depression, upper respiratory tract infection, and spontaneous penile erection.
In addition to MC4R agonists and antagonists, inverse agonists are potential areas for investigation; these include AgRP and its mimics for MC4R[27] (Table 2).
| Drug | Analogue |
| Setmelanotide | Analogue of α-melanocyte melanocyte-stimulating hormone |
| Metreleptin | Human leptin analogue |
| Liraglutide | Glucagon-like peptide-1 analogue |
| Semaglutide | Long-acting glucagon-like peptide-1 analogue |
| Others |
Identification of monogenic obesity syndromes due to dysfunction of MC4R clarified the role of leptin-melanocortin pathway in regulating energy balance[57]. Impaired activity of MC4R led to rare monogenic forms of obesity whereas gene polymorphisms were related to weight gain and metabolic syndrome. Other MC4R gene polymorphisms were protective against obesity. MC4R could also have anti-inflammatory and neuroprotective effects[57]. Understanding the expression and downstream effects of MC4R resulted in the development of agonists such as setmelanotide which is approved for treatment of obesity due to POMC disorders[56]. In addition to MC4R agonists and antagonists, inverse agonists could be developed such as AgRP and its mimics for MC4R[27]. Early identification of genetic forms of obesity helps in tailoring management from a young age, which can prevent progressive metabolic abnormalities and improve long term prognosis[52]. Future research on MC4R and its role in obesity should focus on studying larger and more diverse population groups. This enhances knowledge of the MC4R signaling pathways, and aids in the development of personalized and effective therapies. Finally, interdisciplinary collaboration combining genetics, endocrinology, and pharmacology is necessary for translation into clinical applications and better patient outcomes.
We thank Mr Venkat Yarabati and Prof PV Nageswararao for their assistance in the preparation of this manuscript.
| 1. | Abawi O, Wahab RJ, Kleinendorst L, Blankers LA, Brandsma AE, van Rossum EFC, van der Voorn B, van Haelst MM, Gaillard R, van den Akker ELT. Genetic Obesity Disorders: Body Mass Index Trajectories and Age of Onset of Obesity Compared with Children with Obesity from the General Population. J Pediatr. 2023;262:113619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 2. | Zhang Y, Chen GC, Sotres-Alvarez D, Perreira KM, Daviglus ML, Pirzada A, Gallo LC, Llabre MM, Cai J, Xue X, Isasi CR, Kaplan R, Qi Q. General or Central Obesity and Mortality Among US Hispanic and Latino Adults. JAMA Netw Open. 2024;7:e2351070. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 20] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 3. | Janssen F, Bardoutsos A, Vidra N. Obesity Prevalence in the Long-Term Future in 18 European Countries and in the USA. Obes Facts. 2020;13:514-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 144] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 4. | Keaver L, Xu B, Jaccard A, Webber L. Morbid obesity in the UK: A modelling projection study to 2035. Scand J Public Health. 2020;48:422-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 5. | Tham KW, Abdul Ghani R, Cua SC, Deerochanawong C, Fojas M, Hocking S, Lee J, Nam TQ, Pathan F, Saboo B, Soegondo S, Somasundaram N, Yong AML, Ashkenas J, Webster N, Oldfield B. Obesity in South and Southeast Asia-A new consensus on care and management. Obes Rev. 2023;24:e13520. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 92] [Reference Citation Analysis (0)] |
| 6. | NCD Risk Factor Collaboration (NCD-RisC) – Africa Working Group. Trends in obesity and diabetes across Africa from 1980 to 2014: an analysis of pooled population-based studies. Int J Epidemiol. 2017;46:1421-1432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 182] [Article Influence: 20.2] [Reference Citation Analysis (1)] |
| 7. | Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, Abraham JP, Abu-Rmeileh NM, Achoki T, AlBuhairan FS, Alemu ZA, Alfonso R, Ali MK, Ali R, Guzman NA, Ammar W, Anwari P, Banerjee A, Barquera S, Basu S, Bennett DA, Bhutta Z, Blore J, Cabral N, Nonato IC, Chang JC, Chowdhury R, Courville KJ, Criqui MH, Cundiff DK, Dabhadkar KC, Dandona L, Davis A, Dayama A, Dharmaratne SD, Ding EL, Durrani AM, Esteghamati A, Farzadfar F, Fay DF, Feigin VL, Flaxman A, Forouzanfar MH, Goto A, Green MA, Gupta R, Hafezi-Nejad N, Hankey GJ, Harewood HC, Havmoeller R, Hay S, Hernandez L, Husseini A, Idrisov BT, Ikeda N, Islami F, Jahangir E, Jassal SK, Jee SH, Jeffreys M, Jonas JB, Kabagambe EK, Khalifa SE, Kengne AP, Khader YS, Khang YH, Kim D, Kimokoti RW, Kinge JM, Kokubo Y, Kosen S, Kwan G, Lai T, Leinsalu M, Li Y, Liang X, Liu S, Logroscino G, Lotufo PA, Lu Y, Ma J, Mainoo NK, Mensah GA, Merriman TR, Mokdad AH, Moschandreas J, Naghavi M, Naheed A, Nand D, Narayan KM, Nelson EL, Neuhouser ML, Nisar MI, Ohkubo T, Oti SO, Pedroza A, Prabhakaran D, Roy N, Sampson U, Seo H, Sepanlou SG, Shibuya K, Shiri R, Shiue I, Singh GM, Singh JA, Skirbekk V, Stapelberg NJ, Sturua L, Sykes BL, Tobias M, Tran BX, Trasande L, Toyoshima H, van de Vijver S, Vasankari TJ, Veerman JL, Velasquez-Melendez G, Vlassov VV, Vollset SE, Vos T, Wang C, Wang X, Weiderpass E, Werdecker A, Wright JL, Yang YC, Yatsuya H, Yoon J, Yoon SJ, Zhao Y, Zhou M, Zhu S, Lopez AD, Murray CJ, Gakidou E. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766-781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7951] [Cited by in RCA: 8165] [Article Influence: 680.4] [Reference Citation Analysis (0)] |
| 8. | NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390:2627-2642. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5072] [Cited by in RCA: 4754] [Article Influence: 528.2] [Reference Citation Analysis (2)] |
| 9. | Yanovski JA. Obesity: Trends in underweight and obesity - scale of the problem. Nat Rev Endocrinol. 2018;14:5-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 10. | Jebeile H, Kelly AS, O'Malley G, Baur LA. Obesity in children and adolescents: epidemiology, causes, assessment, and management. Lancet Diabetes Endocrinol. 2022;10:351-365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 612] [Article Influence: 153.0] [Reference Citation Analysis (0)] |
| 11. | Gao L, Peng W, Xue H, Wu Y, Zhou H, Jia P, Wang Y. Spatial-temporal trends in global childhood overweight and obesity from 1975 to 2030: a weight mean center and projection analysis of 191 countries. Global Health. 2023;19:53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 12. | Fox A, Feng W, Asal V. What is driving global obesity trends? Global Health. 2019;15:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 121] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 13. | Krue S, Coolidge J. The prevalence of overweight and obesity among Danish school children. Obes Rev. 2010;11:489-491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Gan HW, Cerbone M, Dattani MT. Appetite- and Weight-Regulating Neuroendocrine Circuitry in Hypothalamic Obesity. Endocr Rev. 2024;45:309-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 37] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 15. | Wabitsch M, Farooqi S, Flück CE, Bratina N, Mallya UG, Stewart M, Garrison J, van den Akker E, Kühnen P. Natural History of Obesity Due to POMC, PCSK1, and LEPR Deficiency and the Impact of Setmelanotide. J Endocr Soc. 2022;6:bvac057. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 43] [Reference Citation Analysis (0)] |
| 16. | Heinig JA, Keeley FW, Robson P, Sower SA, Youson JH. The appearance of proopiomelanocortin early in vertebrate evolution: cloning and sequencing of POMC from a Lamprey pituitary cDNA library. Gen Comp Endocrinol. 1995;99:137-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 66] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Dores RM. Observations on the evolution of the melanocortin receptor gene family: distinctive features of the melanocortin-2 receptor. Front Neurosci. 2013;7:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 18. | Yeo GSH, Chao DHM, Siegert AM, Koerperich ZM, Ericson MD, Simonds SE, Larson CM, Luquet S, Clarke I, Sharma S, Clément K, Cowley MA, Haskell-Luevano C, Van Der Ploeg L, Adan RAH. The melanocortin pathway and energy homeostasis: From discovery to obesity therapy. Mol Metab. 2021;48:101206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 156] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 19. | Magenis RE, Smith L, Nadeau JH, Johnson KR, Mountjoy KG, Cone RD. Mapping of the ACTH, MSH, and neural (MC3 and MC4) melanocortin receptors in the mouse and human. Mamm Genome. 1994;5:503-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 48] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Gantz I, Miwa H, Konda Y, Shimoto Y, Tashiro T, Watson SJ, DelValle J, Yamada T. Molecular cloning, expression, and gene localization of a fourth melanocortin receptor. J Biol Chem. 1993;268:15174-15179. [PubMed] |
| 21. | Hughes DA, Hinney A, Brumm H, Wermter AK, Biebermann H, Hebebrand J, Stoneking M. Increased constraints on MC4R during primate and human evolution. Hum Genet. 2009;124:633-647. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 22. | Tao YX. The melanocortin-4 receptor: physiology, pharmacology, and pathophysiology. Endocr Rev. 2010;31:506-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 418] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 23. | Baldini G, Phelan KD. The melanocortin pathway and control of appetite-progress and therapeutic implications. J Endocrinol. 2019;241:R1-R33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 182] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 24. | Ghamari-Langroudi M, Digby GJ, Sebag JA, Millhauser GL, Palomino R, Matthews R, Gillyard T, Panaro BL, Tough IR, Cox HM, Denton JS, Cone RD. G-protein-independent coupling of MC4R to Kir7.1 in hypothalamic neurons. Nature. 2015;520:94-98. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 148] [Cited by in RCA: 156] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 25. | Rang HP. The receptor concept: pharmacology's big idea. Br J Pharmacol. 2006;147 Suppl 1:S9-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 105] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 26. | De Lean A, Stadel JM, Lefkowitz RJ. A ternary complex model explains the agonist-specific binding properties of the adenylate cyclase-coupled beta-adrenergic receptor. J Biol Chem. 1980;255:7108-7117. [PubMed] |
| 27. | Liu Z, Hruby VJ. MC4R biased signalling and the conformational basis of biological function selections. J Cell Mol Med. 2022;26:4125-4136. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 28. | Metzger PJ, Zhang A, Carlson BA, Sun H, Cui Z, Li Y, Jahnke MT, Layton DR, Gupta MB, Liu N, Kostenis E, Gavrilova O, Chen M, Weinstein LS. A human obesity-associated MC4R mutation with defective Gq/11α signaling leads to hyperphagia in mice. J Clin Invest. 2024;134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 19] [Reference Citation Analysis (0)] |
| 29. | Hainer V, Aldhoon Hainerová I, Kunešová M, Taxová Braunerová R, Zamrazilová H, Bendlová B. Melanocortin pathways: suppressed and stimulated melanocortin-4 receptor (MC4R). Physiol Res. 2020;69:S245-S254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 30. | Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571-578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1080] [Cited by in RCA: 1166] [Article Influence: 55.5] [Reference Citation Analysis (0)] |
| 31. | Giraudo SQ, Billington CJ, Levine AS. Feeding effects of hypothalamic injection of melanocortin 4 receptor ligands. Brain Res. 1998;809:302-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 146] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 32. | Andermann ML, Lowell BB. Toward a Wiring Diagram Understanding of Appetite Control. Neuron. 2017;95:757-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 422] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 33. | Huang J, Wang C, Zhang HB, Zheng H, Huang T, Di JZ. Neuroimaging and neuroendocrine insights into food cravings and appetite interventions in obesity. Psychoradiology. 2023;3:kkad023. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 34. | Fan W, Voss-Andreae A, Cao WH, Morrison SF. Regulation of thermogenesis by the central melanocortin system. Peptides. 2005;26:1800-1813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 35. | Song JY, Song QY, Wang S, Ma J, Wang HJ. Physical Activity and Sedentary Behaviors Modify the Association between Melanocortin 4 Receptor Gene Variant and Obesity in Chinese Children and Adolescents. PLoS One. 2017;12:e0170062. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 36. | Chermon D, Birk R. Predisposition of the Common MC4R rs17782313 Female Carriers to Elevated Obesity and Interaction with Eating Habits. Genes (Basel). 2023;14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 37. | Sull JW, Kim G, Jee SH. Association of MC4R (rs17782313) with diabetes and cardiovascular disease in Korean men and women. BMC Med Genet. 2020;21:160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | Adan RA, Tiesjema B, Hillebrand JJ, la Fleur SE, Kas MJ, de Krom M. The MC4 receptor and control of appetite. Br J Pharmacol. 2006;149:815-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 188] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 39. | Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell. 1997;88:131-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2235] [Cited by in RCA: 2226] [Article Influence: 76.8] [Reference Citation Analysis (0)] |
| 40. | Ste Marie L, Miura GI, Marsh DJ, Yagaloff K, Palmiter RD. A metabolic defect promotes obesity in mice lacking melanocortin-4 receptors. Proc Natl Acad Sci U S A. 2000;97:12339-12344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 292] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 41. | Farooqi IS, Yeo GS, O'Rahilly S. Binge eating as a phenotype of melanocortin 4 receptor gene mutations. N Engl J Med. 2003;349:606-9; author reply 606. [PubMed] |
| 42. | Courbage S, Poitou C, Le Beyec-Le Bihan J, Karsenty A, Lemale J, Pelloux V, Lacorte JM, Carel JC, Lecomte N, Storey C, De Filippo G, Coupaye M, Oppert JM, Tounian P, Clément K, Dubern B. Implication of Heterozygous Variants in Genes of the Leptin-Melanocortin Pathway in Severe Obesity. J Clin Endocrinol Metab. 2021;106:2991-3006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 35] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 43. | Namjou B, Stanaway IB, Lingren T, Mentch FD, Benoit B, Dikilitas O, Niu X, Shang N, Shoemaker AH, Carey DJ, Mirshahi T, Singh R, Nestor JG, Hakonarson H, Denny JC, Crosslin DR, Jarvik GP, Kullo IJ, Williams MS; eMERGE Network, Harley JB. Evaluation of the MC4R gene across eMERGE network identifies many unreported obesity-associated variants. Int J Obes (Lond). 2021;45:155-169. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 44. | Oswal A, Yeo GS. The leptin melanocortin pathway and the control of body weight: lessons from human and murine genetics. Obes Rev. 2007;8:293-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 45. | Farooqi IS, Keogh JM, Yeo GS, Lank EJ, Cheetham T, O'Rahilly S. Clinical spectrum of obesity and mutations in the melanocortin 4 receptor gene. N Engl J Med. 2003;348:1085-1095. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1184] [Cited by in RCA: 1200] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 46. | Renard E, Thevenard-Berger A, Meyre D. Medical semiology of patients with monogenic obesity: A systematic review. Obes Rev. 2024;e13797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 47. | Mohammed I, Selvaraj S, Ahmed WS, Al-Barazenji T, Hammad AS, Dauleh H, Saraiva LR, Al-Shafai M, Hussain K. Functional Characterization of Novel MC4R Variants Identified in Two Unrelated Patients with Morbid Obesity in Qatar. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 48. | Moore BS, Mirshahi T. Genetic variants help define the role of the MC4R C-terminus in signaling and cell surface stability. Sci Rep. 2018;8:10397. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 49. | Ferraz Barbosa B, Aquino de Moraes FC, Bordignon Barbosa C, Palavicini Santos PTK, Pereira da Silva I, Araujo Alves da Silva B, Cristine Marques Barros J, Rodríguez Burbano RM, Pereira Carneiro Dos Santos N, Rodrigues Fernandes M. Efficacy and Safety of Setmelanotide, a Melanocortin-4 Receptor Agonist, for Obese Patients: A Systematic Review and Meta-Analysis. J Pers Med. 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 50. | Trapp CM, Censani M. Setmelanotide: a promising advancement for pediatric patients with rare forms of genetic obesity. Curr Opin Endocrinol Diabetes Obes. 2023;30:136-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 51. | Ayers KL, Glicksberg BS, Garfield AS, Longerich S, White JA, Yang P, Du L, Chittenden TW, Gulcher JR, Roy S, Fiedorek F, Gottesdiener K, Cohen S, North KE, Schadt EE, Li SD, Chen R, Van der Ploeg LHT. Melanocortin 4 Receptor Pathway Dysfunction in Obesity: Patient Stratification Aimed at MC4R Agonist Treatment. J Clin Endocrinol Metab. 2018;103:2601-2612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 63] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 52. | Mainieri F, La Bella S, Rinaldi M, Chiarelli F. Rare genetic forms of obesity in childhood and adolescence: A narrative review of the main treatment options with a focus on innovative pharmacological therapies. Eur J Pediatr. 2024;183:1499-1508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 53. | Sharma S, Garfield AS, Shah B, Kleyn P, Ichetovkin I, Moeller IH, Mowrey WR, Van der Ploeg LHT. Current Mechanistic and Pharmacodynamic Understanding of Melanocortin-4 Receptor Activation. Molecules. 2019;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 54. | Bischof JM, Van Der Ploeg LH, Colmers WF, Wevrick R. Magel2-null mice are hyper-responsive to setmelanotide, a melanocortin 4 receptor agonist. Br J Pharmacol. 2016;173:2614-2621. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 55. | Coulter AA, Rebello CJ, Greenway FL. Centrally Acting Agents for Obesity: Past, Present, and Future. Drugs. 2018;78:1113-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 95] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 56. | Bays HE, Fitch A, Christensen S, Burridge K, Tondt J. Anti-Obesity Medications and Investigational Agents: An Obesity Medicine Association (OMA) Clinical Practice Statement (CPS) 2022. Obes Pillars. 2022;2:100018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 30] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 57. | Kamermans A, Verhoeven T, van Het Hof B, Koning JJ, Borghuis L, Witte M, van Horssen J, de Vries HE, Rijnsburger M. Setmelanotide, a Novel, Selective Melanocortin Receptor-4 Agonist Exerts Anti-inflammatory Actions in Astrocytes and Promotes an Anti-inflammatory Macrophage Phenotype. Front Immunol. 2019;10:2312. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/