Published online Dec 20, 2024. doi: 10.5493/wjem.v14.i4.96422
Revised: August 27, 2024
Accepted: September 19, 2024
Published online: December 20, 2024
Processing time: 177 Days and 17.5 Hours
As identified in 1936 by Hans Selye, stress is shaping diseases through the induc
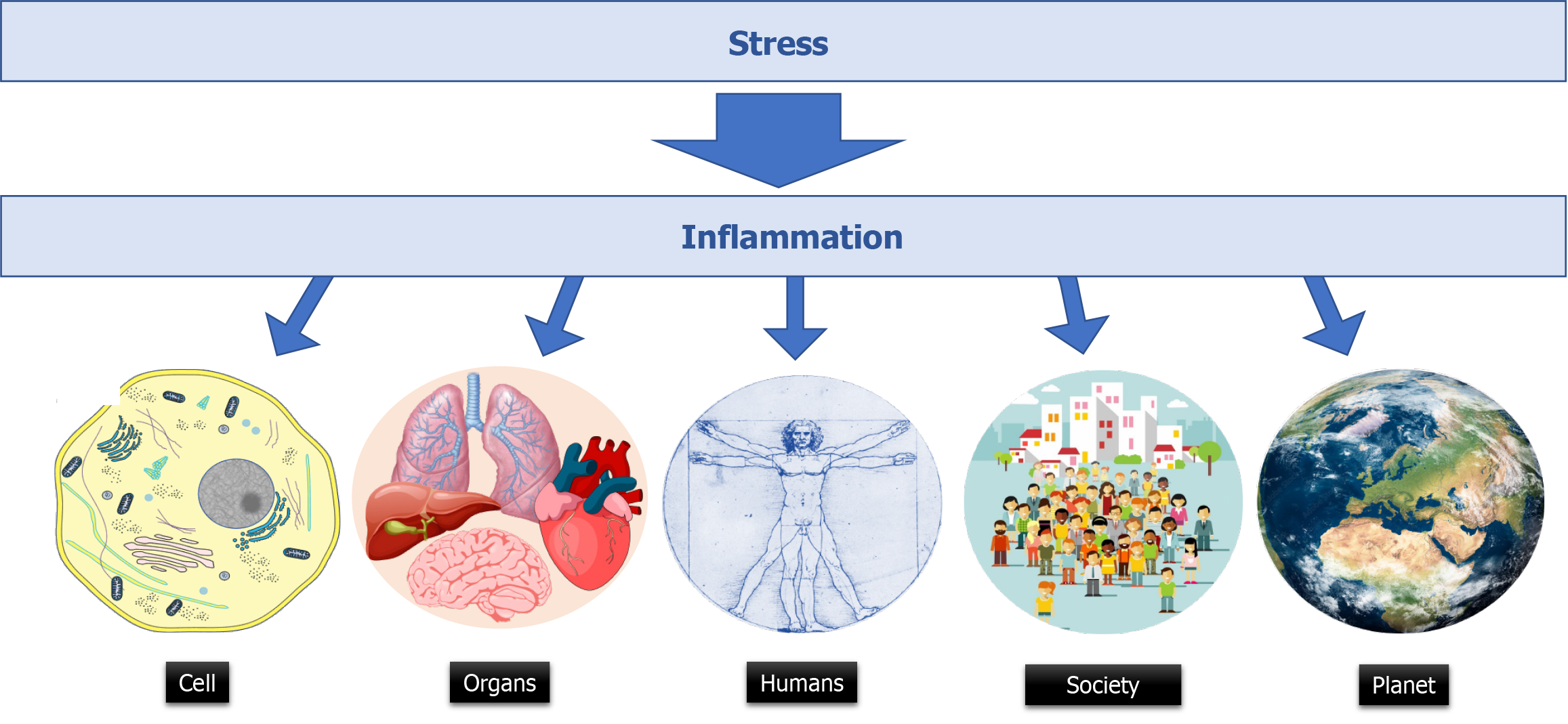
Core Tip: Global health is dependent on healthy cells, healthy organs, healthy indi
- Citation: Cavaillon JM, Chaudry IH. Facing stress and inflammation: From the cell to the planet. World J Exp Med 2024; 14(4): 96422
- URL: https://www.wjgnet.com/2220-315x/full/v14/i4/96422.htm
- DOI: https://dx.doi.org/10.5493/wjem.v14.i4.96422
Recently, a fascinating lead article and two accompanying papers[1-3] offered a contemporary reflection on the globaliza
Inflammation has accompanied humans since their first ancestors appeared on Earth. Aulus Cornelius Celsus [25 before Christ (BC) to 50 Anno Domini (AD)], a Roman encyclopedist, offered a still valid statement about inflammation: “Notae vero inflammationis sunt quatuor: Rubor et tumor cum calore et dolore”, defining the four cardinal signs of inflammation as redness and swelling with heat and pain. A 5th element, the loss of organ function was later added. While it was claimed to have been conceived by Galen (129 AD to 216 AD), a careful analysis of his texts led to the conclusion that it was not the case[4]. Thus, either Thomas Sydenham (1624-1689), an English physician or Rudolf Virchow (1821-1902), a German physician could have proposed the concept. While inflammation has long been considered as a morbid phe
Two more recent contributions were mentioned by Ghezzi[2]: (1) Rothman[7] who bridged causes and diseases, and most importantly evoked the concept of synergy, an event that is particularly relevant to the innate immune response and the inflammatory process; and (2) Goh et al[8] who analyzed the “diseasome” (a word coined by Barabási[9]), defined as a network of all known diseases associated with a network of gene mutations. Later in this review we will address the important contribution of genetic components in the sensitivity to stress and in the nature of the inflammatory response. Among the diseases with a genetic component[10] and an inflammatory link[11] is obesity of which a recent local pandemic has been recognized[12]. Obesity has been recognized as a comorbidity factor that contributed to higher risk of mortality during the corona virus disease 2019 (COVID-19) crisis[13].
Hans Selye (1907-1982), a Hungarian-Canadian medical endocrinologist, was born in Vienna. He got his degree in medicine in Prague and worked at John Hopkins University (Baltimore) before joining McGill University (Montréal). He pioneered the field of stress research in biology with his first article published in 1936 making a link between stress and disorders[14]. He also published numerous books including: “The physiology and pathology of exposure to stress-A treatise based on the concept of general-adaptation syndrome and the diseases of adaptation” (1950) and “The Stress of Life” (1956). In 1974, he introduced the terms distress and eustress to distinguish between bad and good stress. His famous statement “It’s not stress that kills us, it is our reaction to it” remains fully relevant. This sentence can be related to that attributed to William Osler (1849-1919) a Canadian physician who had also worked at McGill University before joining the University of Pennsylvania in Philadelphia, and the John Hopkins hospital. About sepsis, William Osler would have claimed: “Except on few occasions, the patient appears to die from the body’s response to infection rather than from it”. But this statement is absent from his writings! Hans Selye precised: “What is the cause of our illness, the microbe or the stress? I think both are and equally so. In most instance diseases is due neither to the germ as such, nor to our adaptive reactions as such, but to the inadequacy of our reaction against the germ”. To illustrate how Selye was a predecessor, let us quote Selye (from his book “The stress of life”, 1956-1978): “If we compare inflammation with the defensive reactions of a whole human being, or even of a whole nation, we find striking similarities in the over-all pattern everywhere. By recognizing these, we may gain more insight into the mechanism, and even philosophy, of defense in general, insight which penetrates far beyond the confines of medicine. In all these examples of reaction, whether we deal with the problems of a few cells, with those of a whole man, or of an entire nation-defense may bring salvation, or it may bring self-inflicted injuries” (Figure 1).
Among other pioneers, Androulakis[3] also appropriately cites the works of Walter Bradford Cannon (1871-1945), a physiologist from Harvard Medical School. Cannon further developed the idea of “milieu intérieur”, initially introduced by Claude Bernard (1813-1878), as a pillar of homeostasis. Interestingly, he coined the term “fight or flight response” to which Vodovotz et al[1] are regularly referring to. Indeed, dealing with stress requires adjustments of homeostatic set points and adaptation of the milieu intérieur concept.
In most severe cases of inflammation such as sepsis, the overwhelmed immune system may end to organ failure and death of the patient. The inflammatory cascade initiated by an infectious process, the release of pathogen associated molecular patterns and damage associated molecular patterns may end to cell death[15]. Many types of cell death have been recognized, such as netosis, a kind of suicide of neutrophils aimed to prevent bacterial infection. Pyroptosis, is a macrophage cell death following gasdermin activation being associated with the inflammasome activation aimed to release, interleukin (IL)-1β and IL-18, two key players in the fight against the microbial pathogens. Apoptosis of immune cells is a hallmark of sepsis and is responsible for lymphopenia and altered immune status. Finally, necrosis occurs in tissue like during necrotizing fasciitis after the action of cytotoxic toxins. It is accompanied by the release the cytoplasmic content of the cells into extracellular space causing inflammatory reactions in the surrounding tissue. In a recent investigation in a murine model of severe influenza A virus infection, it was elegantly demonstrated that preventing alveolar epithelial cell death by an inhibitor of receptor interacting protein kinase 3 prevented the lung injury and protected the animal from death[16].
An estimated 14.83 million excess deaths have been attributed to the COVID-19 pandemic between 2020 and 2021[17]. Fortunately, the vaccines against the severe acute respiratory syndrome coronavirus-2 (SARS-Cov-2) virus developed in record time are estimated to have averted 19.8 million additional deaths[18]. An estimation performed at the European region ended with more modest values[19]: COVID-19 vaccines ended to 1.6 million lives saved in those aged 25 years or older: 96% of lives saved were aged 60 years or older and 52% were aged 80 years or older. But pandemics have always accompanied humanity. The first known pandemic ended to the death of 70% of those who were living in Tel El-Amarna around 1350 BC in Egypt, and was due to malaria. The plague of Athens killed around 100000 people in 430 BC, probably because of typhus. The Justinian plague took the life of 25 million people around the Mediterranean Sea (541 AC). It was due to bubonic plague which had its greatest score in 1331-1353 with around 75-200 million victims during the black plague over Europe, Asia and Africa. Plague was regularly taking the life of inhabitants in major cities such as London (1664-1666), Vienna (1679), Marseille (1720), and Hong Kong (1894). Cholera also contributed to the deaths of many citizens in London (1849-1854), Paris (1876), Alexandria (1883), Calcutta (1884), Constantinople (1893), Constantine (1906). In 1918-1919, the Spanish flu affected 33% of the world population ending to the deaths of 25 to 100 million people, including the grand-father of the former president of United States, Donald Trump. Finally, acquired immunodeficiency syndrome pandemic which started in 1981 has been responsible for the death of 40.4 million people according to World Health Organization, more than half being African heterosexuals[20].
A complex interplay exists between natural events occurring on Earth and humans. An association between phases of climate change and episodes of acute health crisis has been recently proposed to explain the occurrence of the major pandemic events of Roman antiquity. The Antonine plague [onset approximately 165 common era (CE)], and the plague of cyprian (onset approximately 251 CE), are strongly associated with pronounced climate change. The Antonine Plague occurred during the cold pulse between approximately 160 and approximately 180 CE that followed several decades of trends toward cooling and aridity. The plague of cyprian coincides with a second phase of severe cooling, with even more arid conditions, after the brief warmer period between approximately 215 and approximately 245 CE. The pronounced cold phases are associated with pandemic disease, suggesting that climate stress interacted with social and biological variables[21]. Eruptions of Lipari volcanoes could have been the culprits of these cooling periods. This illustrates the inter connection between environmental stress and biological stress.
In ten years, the Earth lost 82 million hectares of forests in fires. Globally, boreal forests have the highest proportion of forest loss due to fire (69%-73%), followed by subtropical (19%-22%), temperate (17%-21%), and tropical forests (6%-9%)[22]. The death of all these trees has most severe potential impacts on biodiversity and animal survival. For example, in Amazonia, fires have comprised numerous species, including 83-85 bird species, 53-55 mammal species, 5-9 reptile species and 95-107 amphibian species[23]. Drought, storms and extreme temperatures also have major consequences on human health and are responsible for numerous deaths among the human population[24]. Most remarkably, human pathogenic diseases and transmission pathways are aggravated by climatic hazards. As pointed out by Mora et al[25], exposure to life-threatening conditions such as floods and hurricanes, extraneous conditions during heatwaves and depression from lost livelihood due to drought are a few examples in which climatic hazards are inducive to stress. Asso
The immune system is often explained to children as an army of specialized soldiers aimed to defend our body, entering in war against pathogens[28]. Interesting auto-immunity has been compared to a civil war[29]. Wars have always accom
Homeostatic life is associated with a bit of “physiological” stress. Eustress is beneficial for health through an optimization of homeostasis. Therefore, an ideal stress level is essential for building biological mechanisms needed for normal life processes[35]. Interestingly, wild mice have higher corticosterone levels than laboratory ones[36]. Indeed, laboratory mice having food ad libitum and not practicing any physical exercise are poorly replicating normal animals and are metabolically morbid[37]. Stress has existed with humans since the beginning of their presence on Earth. What evolution has selected to maintain life in an appropriate balance? The prehistoric humans were stressed by wild animals, by natural events, by the need to find food, to keep the fire on, to defend their cave against their neighbors, to deal with wounds, to fight infectious bugs... Are the stresses associated with our contemporary way of life (see below) need or would generate other mediators, other pathways, other regulatory mechanisms than those initially selected by evolution? This is quite unlikely. On Earth, environmental stress could have shaped human traits. Drought, high or low temperatures are stressful situations for which adaptation could have been required for survival. Accordingly, stress-responsive mecha
Many conditions including hypoxia, starvation, infections lead to stress on animal physiology, on tissues and on cells (Table 1). Within the cells, the endoplasmic reticulum (ER) stress is a disturbance of the protein folding process. It is also associated with an up-regulation of death receptor expression that mediates cell death[39]. ER stress has recently been linked to inflammation in a variety of human pathologies including autoimmune, infectious, neurodegenerative, and metabolic disorders. In the cell, ER stress and inflammation share signaling regulators and effectors. These two signaling pathways have been shown to form a vicious cycle in exacerbating cellularity function and causing apoptosis in many cells and tissues[40]. Cell death and particularly apoptosis is accompanied by an increase of mitochondrial membrane permeability and the release into the cytosol of pro-apoptotic factors associated with their production of reactive oxygen species (ROS)[41]. The excessive production of ROS induces mitophagy to remove damaged mitochondria. The mito
| Target | Inflammatory stress |
| Cell | PAMPs; DAMPs; Toxins; Inflammatory cytokines; Lipid mediators; Free radicals; Microplastics; Radiation |
| Organ | Inflammatory mediators; Ischemia; Hypoxia |
| Individuals | Physical stress: trauma; Burns; Surgery; Pathogens; Heat; Cold; Chemicals; Pollutants; UV; Allergens. |
| Psychological stress: Harassment; Wars; Family disruption; Loneliness… | |
| Society | Low or high incomes countries; Political regime |
| Planet | Loss of ecologic balance; Global warming; Natural disasters; Overpopulation |
Within organs, through short or long-distance mediators-mediated cross-talks, individual cells of different natures display a highly interactive network that has been compared to a multi-layered social network[43]. This social network revealed by quantitative proteomics provides a framework for the orchestration of cellular interplay, and is a reference for altered communication associated with pathology[44]. The immune system is profoundly affected by its stressful environment, particularly because immune cells have specific receptors for stress hormones that shape the immune response.
Different experimental approaches have demonstrated the profound perturbation of the immune system following stressful events. For example, a cold-water stress augments the production of IL-1β, tumor necrosis factor (TNF), and IL-6 by lipopolysaccharide (LPS)-activated murine peritoneal macrophages[45]. With the use of specific inhibitors and antagonists, the authors identified substance P as the neuro mediator responsible for the effects in this model. Of note, epinephrine and norepinephrine display opposite effects on in vivo induction of TNF by LPS in mice[46]. Impressively, stress applied to pregnant rhesus monkeys diminishes the cytokine response of leukocytes to LPS stimulation in two-years old rhesus monkeys[47]. Stress also alters the interferon production in mice infected with viruses, impairing the ability of the host to control viral replication prolonging the infectious period[48]. Similarly, the severity of a cutaneous infection due to an intradermal injection of Streptococcus pyogenes is significantly increased by stress[49]. The authors showed that it was consecutive of the inhibitory effects of glucocorticoids on the expression of cutaneous antimicrobial peptides. In human chronic stress, it is a functional resistance to glucocorticoids in monocytes that has been observed, enabling activation of pro-inflammatory transcription control pathways[50]. Even moderate stress can modify the production of cytokines as observed in students after a major written exam[51]. Exposure to stress and violence is associated with altered immune status as illustrated by the higher frequency of asthma morbidity observed in children who had been exposed to physical or sexual abuse[52].
Immune cells (e.g., macrophages, neutrophils, natural killer (NK) cells, and T-lymphocytes), as well as endothelial cells, differ from one compartment to another and contribute to specific organ responses to sterile and microbial insults[53]. Furthermore, tissue-specific microbiota influences the local and systemic response. At homeostasis gene expression and surface markers are different among spleen, lung and peritoneal, and microglial cells[54]. Furthermore, in tissue-resident macrophages environment in different organs governs diversity in the chromatin state of tissue-resident macrophages and epigenetic status[55]. Upon stimulation, cytokine production differs between blood monocytes and intestinal macro
It was nicely demonstrated in models associating sepsis and trauma/hemorrhage that the ex vivo TNF production in response to LPS was reduced in peripheral blood mononuclear cells (PBMC) and spleen macrophages, while it was enhanced in alveolar macrophages and Kupffer cells[68]. Also, polymicrobial sepsis does not affect the function of skin cluster of differentiation (CD)8+ T-cells in terms of interferon-gamma (IFN-γ) production[69] while the capacity of brain microglial cells to produce TNF was enhanced[70]. A similar discrepancy was observed in terms of macrophage reprogramming after LPS injection which was observed in peritoneal macrophages and blood monocytes but not in alveolar macrophages[71]. Most interestingly, a comparison of differentially expressed gene transcripts in different organs (lungs, heart, kidneys, liver, and spleen) from deceased meningococcal septic shock patients revealed a great number of modulated genes specific to each organ[72]. A similar study including transcriptomic analysis of the cortex and hippocampus of patients who died of septic shock, revealed that the brain also displays a specific pattern of activated and repressed genes[73].
Of course, higher education is associated with better professional positions and thus higher income. Furthermore, high-income countries ensure a high level of employment, better access to health system. Of course, the need for money, and monetary inflation are not affecting only low- and middle-income countries and the stress to offer enough financial support for oneself and one’s family is universal. Most interestingly, a study revealed that among many potential confounders, high economical stress was associated with increased inflammatory activity, illustrated by higher IL-6, C-reactive protein (CRP) and IL-1 receptor antagonist levels in women with stable coronary heart disease[74].
Watching negative news on TV is an obvious contemporary threat when violence, drug traffic, natural disasters, wars (…) enter the home[75]. During the COVID-19 pandemic, during the lockdown, when people were spending more time in front of their TV set, the French health director was announcing the number of deaths every day. Such news are common stressors. However, the results of a recent study (performed before the war in Ukraine and in Gaza) came as a surprise[76]. The study included half a million news headlines across 16 country over three weeks. These results demonstrate a dominance of positive news headlines (70.5%) over the negative ones (29.5%). Among the countries with the highest percent of positive news (> 85%) were some of the countries with the lowest press freedom index (PFI) (e.g., Russia, China). However, there was no direct correlation between this index and the frequency of positive or negative bad news. Among the countries with the lowest percent of positive news (52%), Egypt and Lebanon have a significantly different PFI. Of course, different negative events such as the war in Ukraine would not be addressed the same way in different countries. While the Europeans are concerned, people living in China or in Kenya will not face so much the news about this war on European ground.
Fake news on social media are bad for society, and could be bad for health. During the COVID-19 pandemic, fake news have been associated with psychological disorders and panic, fear, depression, and fatigue[77]. Misinformation also resulted in vaccine hesitancy which was itself associated with an increased number of deaths due to COVID-19. An estimation of at least 232000 deaths could have been prevented among unvaccinated adults in the United States[78]. Misinformation concerning health has particularly severe consequences with regard to people’s quality of life and even their risk of mortality[79].
Disinformation and propaganda are weapons in hybrid warfare. Historical revisionism-based information and influencing campaigns conducted by Russia is an example of how a country acts to influence the democratic countries and aims to alters their societies[80], while brain washing its own population with propaganda via its TV channels.
Physical health is closely linked to mental health[81], and mental health is influenced by the numerous parameters mentioned in this chapter. Social and professional recognition is a key element for human welfare while unemployment or fear of unemployment can alter the well-being. The professional environment is a micro-society which is under the influence of its human components. Professional harassment can end in depression and even suicides. It has been reported that healthcare workers with workplace violence, especially emotional abuse, threat and verbal sexual harassment, are more likely to experience burn-out[82]. Of course harassment also exists in the academic world and scientists have been unprecedently attacked during the covid-19 crisis[83]. Stress-induced chronic low-grade inflammation and a decline in immune surveillance are both implicated in cancer development and progression[84]. A meta-analysis revealed significant associations between work stress and the risk of colorectal, lung, and esophagus cancers were found. A statistically significant effect of work stress on colorectal cancer risk was observed in North America, but not significant in Europe. By contrast, a significant association between work stress and esophagus cancer was found in Europe, but not in North America. In contrast there was no association between work stress and the risk of prostate, breast, or ovarian cancers[85]. Moral harassment also exists at school with similar consequences on children than those observed in the adult professional environment. Born in 2006, the hashtag me too was created by African-American Tarana Burke (born 1973) to help women victims of sexual violence. But sexual harassment has been revealed to the public with the Weinstein affair in 2017. Since, in many countries new laws have been voted to better protect the rights of women. On March 8th, 2024, France was the very first country in the world to modify its constitution to guarantee freedom of women to resort to voluntary termination of pregnancy.
In 2022, the number of women and girls killed globally was nearly 89000, and around 48800 women and girls worldwide were killed by their intimate partners or other family members[85,86]. This means that, on average, more than 133 women or girls were killed every day by someone in their own family. The rates of femicide differ depending on the specific country[87]. Among those with the highest rate are Central African Republic, Jamaica and South Africa. In contrast, Singapore, Japan and Belgium are countries with the lowest rate.
The COVID-19 pandemic has been responsible for an increased frequency of domestic violence: The home confinement led to constant contact between perpetrators and victims, resulting in increased violence and decreased reports[88].
Less violent but still with consequences on the mental health of parents and children is the family disruption. The impact of family structure on the health and well-being of children demonstrates that children living with their married, biological parents consistently have better physical, emotional, and academic well-being while there are clearly negative long-term consequences of divorce on children, parents, and society[89].
Famine is closely linked to climatic conditions and poverty while access to safe water is also key for the health of planet Earth inhabitants. The consequences of starvation of new born and young children on their physical and intellectual developments are very severe and sometimes lead to irreversible consequences in adulthood[90-92]. Similarly, the immune system is profoundly affected by under-nutrition. Of note, limited access to food is not restricted to low income countries. In rich countries such as France, the non-governmental organization “Les resto du coeur” served 8.5 million meals in 1985, while in 2022-2023, 171 million meals were served to 1.3 million people, including 126000 children under 3 years old[93].
In 1994, Matzinger[94] proposed a new definition of the immune system and the nature of its triggering signal. Then, emerged the concept of danger and its associated damage associated molecular patterns released by damaged cells and injured tissues that initiate the immune response. The vision of Matzinger[94] completed that of Janeway[95] proposed five years earlier who defined innate Immunity system as able to recognize conserved patterns of pathogens using specific receptors. Indeed, similar receptors (the pattern recognition receptors) can sense the signal of sterile danger occurring after trauma (car crashes, surgery, intensive cares) or burns as they do for pathogen associated molecular patterns released during an infectious process[96]. Similar downstream signaling are initiated within the immune cells, and similar cytokines orchestrate the immune response and the whole-body information, the production of acute phase proteins by the liver, and the control of the response by the neuro-endocrine system. The discoveries of the toll molecule in drosophila, and its homologs in the animal kingdom (Toll-like receptors) offer an evolution perspective unifying through the different species the mechanism of defense against sterile and infectious insult[97].
The incidence patterns of immune-mediated inflammatory diseases such as asthma, inflammatory bowel disease, multiple sclerosis, rheumatoid arthritis, psoriasis, and atopic dermatitis varies across the world[98]. Chronic inflammation favors the occurrence of these diseases as well as exposure to stress. Certain social, environmental and lifestyle factors can promote low-grade systemic chronic inflammation. It includes chronic infections, physical inactivity, obesity, intestinal dysbiosis, diet, social isolation, psychological stress, disturbed sleep and disrupted circadian rhythm, and exposure to xenobiotics such as air pollutants, hazardous waste products, industrial chemicals and tobacco smoking. These settings in turn, lead to several diseases that collectively represent the leading causes of disability and mortality worldwide, such as cardiovascular disease, cancer, type 2 diabetes, chronic kidney disease, non-alcoholic fatty liver disease and autoimmune and neurodegenerative disorders, sarcopenia, or osteoporosis[99].
A very interesting experiment was performed in 276 healthy adult volunteers who were challenged with two rhinoviruses, and followed for 5 days with nasal washes for viral isolation and assessment of signs/symptoms of a common cold. Those with recent exposure to a long-term threatening stressful experience demonstrated glucocorticoid receptor resistance, and were at higher risk of subsequently developing a cold. Greater glucocorticoid receptor resistance predicted the production of more local proinflammatory cytokines among infected subjects[100].
Post trauma distress syndrome (PTSD) is an altered mental health condition in certain people who have experienced or witnessed a traumatic event such as war[101], act of terrorism[102], natural disasters[103], rape[104], but it can also occur after hospitalization in intensive care unit[105]. Interestingly, PTSD was also observed among the healthcare professionals after the COVID-19 crisis[106]. People with PTSD may also experience physical symptoms, such as increased blood pressure and heart rate, fatigue, muscle tension, nausea, joint pain, headaches, back pain or other types of pain.
While PTSD is diagnosed based on classic psychological and behavioral symptoms, a link exists between this disorder and alterations in the immune and inflammatory systems. Compared to healthy controls, individuals with PTSD exhibit significantly elevated levels of proinflammatory cytokines, and CRP[107]. Glucocorticoids and adrenergic agents cause both immediate and late sequelae[108]. Traumatic stress disorder and immune disease share a common genetic basis at the gene expression level[109]. PTSD is associated with many epigenetic modifications including DNA methylation, histone modification and downregulation of certain miRNAs while an elevated expressions of IFN-γ and IL-12 in PBMC have been observed[110].
The planetary insults consecutive to human activities have consequences on human health. Exposure to atmospheric pollutants and exposure to contaminating plastics have direct negative effects on the human and animal health. Global warming is modifying the geographic distribution of insects and as a consequence the exposures of herds and humans to vector borne diseases[111]. Of note, some of these new exposures may have already existed in the past. The name malaria is derived from mal aria (“bad air” in medieval Italian) and Giovanni Maria Lancisi (1654-1720), the physician of three consecutive popes, established a correlation between the presence of mosquitoes and the prevalence of malaria in certain areas of Italy.
Climate change is exacerbating mental disorders, which already affect almost one billion people and are among the world’s biggest causes of ill health[112]. The climate anxiety (eco-anxiety) around the world particularly affects the youngest and in country as diverse as Philippines, India, Brazil, Portugal, France, and Australia, more than 50% of the people aged 16-25 years say they are extremely worried about it. Only around 3% of people with depression receive adequate treatment in low- and lower-middle-income countries, and 23% in high-income countries[112].
Activities of human societies contribute to an alteration of their environment. Air pollution is by far the most dominant environmental risk factor for health in general, and is responsible for over 9 million annual deaths globally, most of which are due to cardiovascular causes. The major sources of modern-day air pollution in high-income countries include fossil fuel combustion, while other sources such as wildfires, volcanic ashes and wars play a major role in air pollution. The association between air pollution and metabolic and cardiovascular disorders has been recognized, including diabetes[113]. At the organ (e.g. lungs) and cell level, oxidative stress, and ER stress play an important role[114].
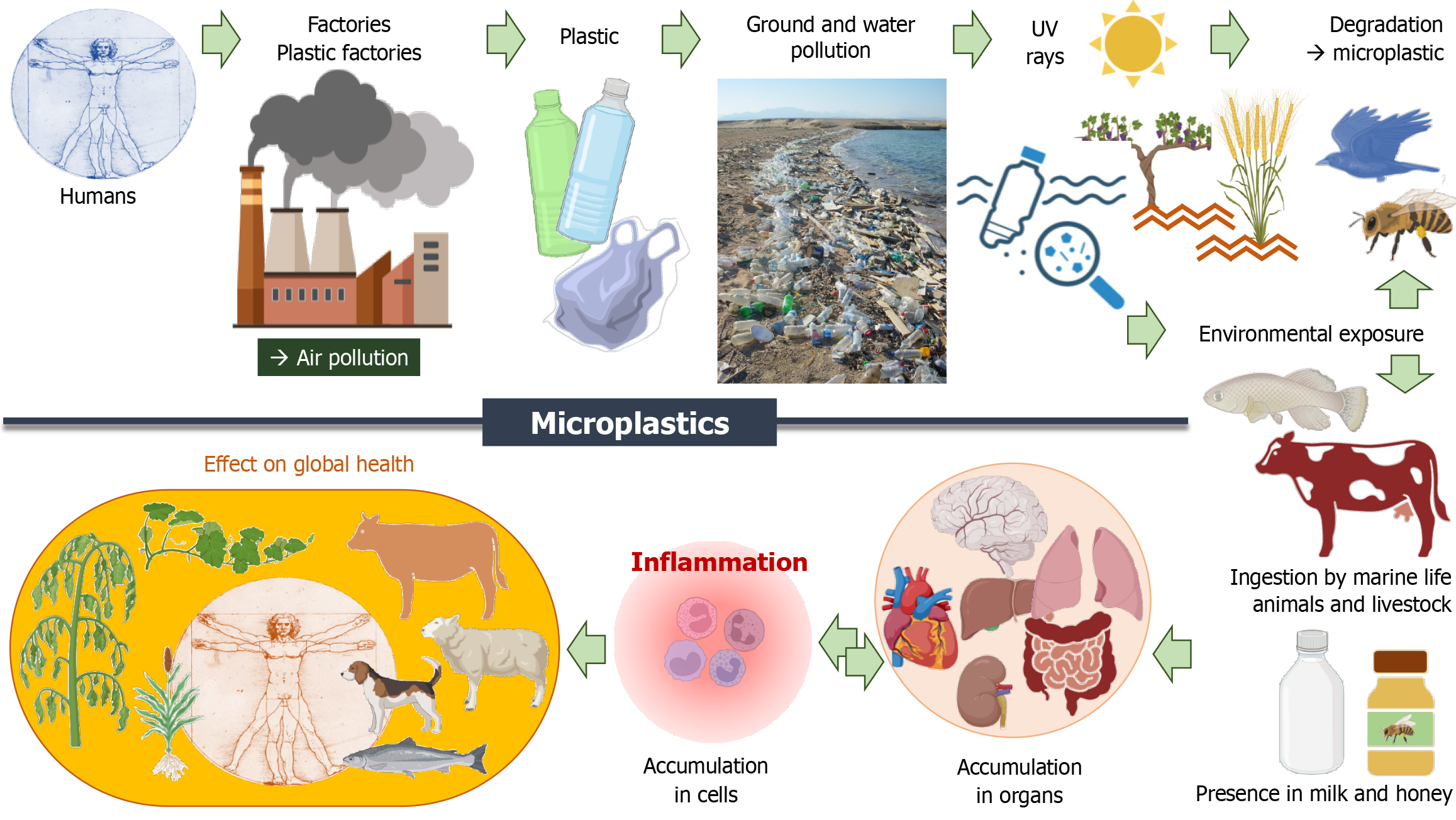
Since 1950, plastic has revolutionized the way humans do shopping and their food containers. But, we are now facing a huge plastic waste crisis with major environmental consequences. Their degradation leads to microplastics (MPs) and nano plastics present in air and water. They have been detected in many marine species and organisms, but also in drinking water and in numerous foods, such as honey and salt (Figure 2). For instance, the MPs concentration in table salt in Indonesia, is around 100 times higher than that in the United States. The per capita daily MP dietary and inhalation uptake rates is particularly important in South Asian countries such as Indonesia, Malaysia, the Philippines, and Vietnam, with more than 50% by aquatic sources via fish consumption[115]. Once absorbed in the lungs after inhalation, or in the gut barrier after ingestion, plastic micro- and nanoparticles can distribute to the liver, spleen, heart, thymus, reproductive organs, kidneys and even cross the blood-brain barrier[116]. The remarkable increase during the COVID-19 pandemic in use of face masks, which mainly contain polypropylene, and poor waste management have led to worsening MPs pollution. Ultra-violet (UV) light and wind break down and modify MPs in the environment into smaller particles which display increased toxicity[117]. MPs can be taken up by cells, altering the immune homeostasis and finally causing damage to tissues and organs. Once attached to the plasma membrane, MPs can be taken up through endocytosis disrupting the intracellular signaling pathways. MPs can induce intracellular ROS and oxidative stress by affecting the mitochondria function. The MPs could be released out from cells after cell death[118]. When human gingival fibroblasts are in vitro exposed to MPs, their inflammatory response is initiated ending to the up-regulation of gene and protein expression of NF-κB, MyD88 and NLRP3[119]. These results illustrate that the inflammation process is stimulated by MPs at the cellular level. In vivo short-term exposure of mice to MPs in drinking water led to the detection of MPs in liver, kidney, gastrointestinal tract, lung, spleen, heart, and brain, and an increase in TNF mRNA expression was observed in the liver. Finally, the investigators noted behavioral changes in terms of exploratory comportment and spontaneous locomotion. These changes differed depending on age, indicating a possible age-dependent effect[120].
In human patients who underwent carotid endarterectomy for asymptomatic carotid artery disease, polyethylene was detected in plaques of 58.4% (n = 150) of them. In this study, patients with carotid artery plaque in which MPs were detected had a higher risk of a composite of myocardial infarction, stroke, or death from any cause at 34 months of follow-up than those in whom MPs were not detected[121].
Societies used to a democratic leadership are not immune to totalitarianism. The fear of totalitarianism is like a homeo
There are very few humans who are concerned by the stress associated with spaceflight. But who knows whether in the future more people will undertake a long travel to the moon or to Mars. Astronauts are exposed to various stressors including radiation, dietary restrictions, microgravity, isolation, confinement, noise, circadian rhythm disturbance. The complicated space environment does not only affect their physical functions but may also induce psychological problems, such as anxiety, depression, and cognitive decline[124]. The human body is intrinsically adapted to Earth’s gravity, thus exposure to conditions of microgravity can lead to a plethora of complications in normal body functions. Particularly, bone loss has been regularly observed. Osteoclasts have been demonstrated to display an increased resorptive activity in response to microgravity[125]. Since IL-1 was initially identified as the “osteoclast activating factor”[126], because the production of IL-1 is enhanced by spaceflight[127], one could hypothesize that IL-1 is an inflammatory cytokine that activates the osteoclasts ending to an alteration of the bone status during spaceflight. Various immune parameters such as the maturation of the immune cells, and the distribution of leukocytes are altered. Neutrophils are elevated and eosinophils are reduced in the peripheral blood of astronauts. Moreover, in vitro activation of T-cells is significantly reduced, granulocyte and monocyte function, and NK cell function are also modified[128]. Accordingly, acquired immune responses are disturbed by gravitational fluctuation, stressors, and space radiation both directly and in a stress hormone-dependent manner.
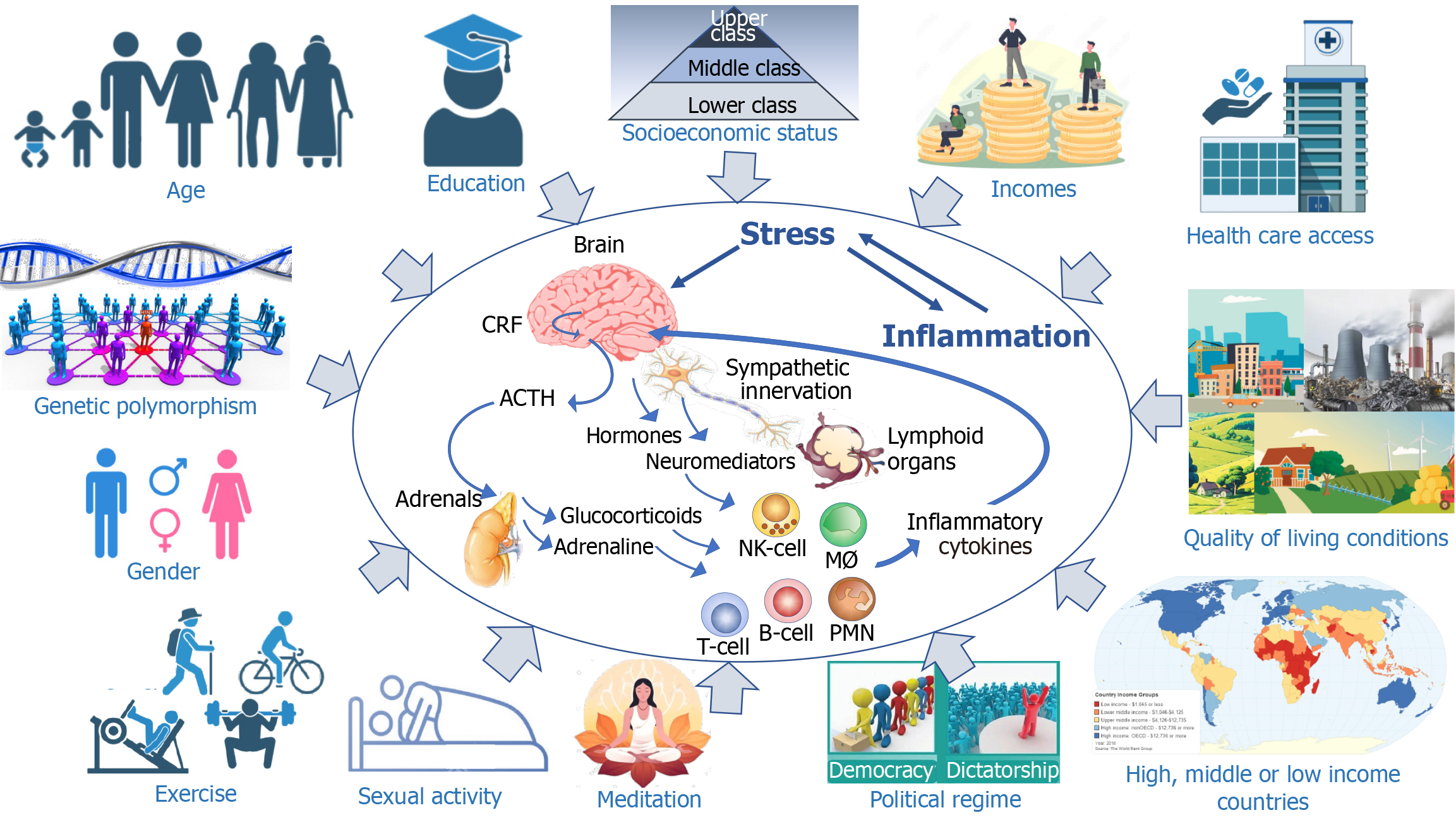
To illustrate the heterogeneity of humans in terms of response to stress and inflammation, let us quote the bright medical investigator, Antoine Béchamp (1816-1908). He was the unfortunate competitor of Louis Pasteur, having understood before him the process of fermentation and the infectious origin of the diseases of silkworms[129]. His statement adapted from his view “the pathogen is nothing, the terrain is everything” has been regularly erroneously attributed to either Louis Pasteur or Claude Bernard. In this chapter we will review the different parameters which influence the response to stress (Figure 3).
The immune system has to be educated not to attack one’s own tissues and organs to prevent autoimmune disease. The behavior, function and life of immune cells are fully dependent upon their education associated with early life microbial exposure imprinting long-lasting fate of the immune system[130]. Among immune cells, T-lymphocytes follow a specific educational program within the thymus during which both non-functional and self-reactive T cell clones are eliminated by means of positive and negative selection[131,132]. Another educational program is needed during pregnancy which presents a unique challenge, since the fetus expresses proteins genetically distinct from the mother[133].
In a study on social determinants of human health it was revealed that length of life expectancy was associated with education levels[134]. Adults with higher educational attainment have better health and lifespans compared to their less-educated peers[135]. A study of a cohort of adults born 1906-1915 revealed that one additional year of education was associated with approximately 0.4 more years of life[136].
Socioeconomic status mentioned earlier as a stress factor can indirectly affect the health of the individuals. This was particularly well illustrated during the lockdown consecutive to the COVID-19 pandemic which has disproportionately impacted the most vulnerable and widened the health disparity gap in both physical and mental well-being. The impact of the COVID-19 pandemic on unhealthy lifestyle patterns encompasses reduced physical activity, increased sedentary behavior, augmented screentime, disturbed work and sleep schedules, more smoking and alcohol consumption. These altered behaviors were associated with mental health disorders, such as anxiety and depression[137]. Individuals with lower social resources, lower economic resources, and greater exposure to stressors (e.g., job loss) reported a greater burden of depression symptoms[138]. Parent-reported mental health problems were more likely to affect children with low socioeconomic status, with complex chronic disease and those whose parents screened positive for depression[139]. Individuals of racial minority groups and lower socio-economic status experienced the worst economic outcomes of employment losses[140]. Altogether, the lockdown was not egalitarian: the costs fell on the most economically disad
Aging is responsible for a great disparity among individuals and among societies. The percent of the population younger than 20 represent more than 50% in the African countries. The older populations are found in Japan (30% of the population is over 65 years old), and in the European countries (around 20%). Major modifications of the immune system occur with aging ending to the so-called immunosenescence which combines decreases in innate and adaptive immune responses in addition to the exacerbated production of inflammatory cytokines[141]. COVID-19 has been particularly deadly among the oldest population. At the beginning of the pandemic, according to the Chinese Centre for Disease Control and prevention, the mortality rate was 14.8% among those older than 80 years, 8% among those 70%-79%, and less that 0.5% among the younger than 49[142]. In Italy, the case-fatality rate increased from 4.2% to 14.0% between March 9 and June 30, 2020, and more than 90% of the change was due to increasing age specific case-fatality rates[143]. Although there was a certain discrepancy in terms of mortality from one country to another, the influence of age remains a common feature of mortality[144]. In 2021, the highest COVID-19 mortality rates continued to be observed at ages 75 +, despite vaccinations having specifically targeted those ages[145]. A coordinated CD4+ T cell, CD8+ T cell, and antibody responses are protective, but uncoordinated responses frequently fail to control disease, with a connection between aging and impaired adaptive immune responses to SARS-CoV-2[146]. Trauma is another setting which illustrates the altered responsiveness among aged people. After trauma, the discharge to home or rehabilitation (59% vs 30%), the discharge to long term care facilities (28% vs 47%) and the 28 days mortality (9% vs 18%) was significantly different among people aged below or above 55[147]. An experimental study performed in young and aged mice combining polytrauma and pneumonia ended to a greater mortality among old mice, and, most interestingly analysis of bone marrow gene expre
Despite the constitutions of many countries claim the equality between male and female for their rights, and despite the fact that Women’s Lib has been fighting to obtain the societal equality, many biological parameters differ between genders. Associated with this heterogeneity, differences exist in the male and female response to sterile and infectious stress, not only at the physiological level but also at the psychological one. For example, gender is one factor among many that affected the frequency of suicides associated with the stress generated by the COVID-19 crisis and its associated lockdown. It has also been shown to vary as a function of age, economic level of countries, and political regime. In most countries there was evidence of lower-than-expected numbers of suicide during the COVID-19 crisis[153]. However, a greater level of COVID-19 associated suicides has been reported among women in Japan[154], in United Kingdom[155], in Mexico[156], and in South Korea[157]. Furthermore, the confinement has led to an increase in the number of domestic violence, particularly against women[88].
Regarding the influence of gender on the sensitivity to infection, there are numerous reports in animal models that exemplified it[158]. Of note, depending on the pathogen, male or female are the most sensitive[159]. A fascinating study revealed that upon Coxiella burnetii infection in mice (Q fever), 1857 genes were modulated in the liver of male animals vs 1290 genes in females with only 398 in common. Upon castration of both genders, the number of modulated genes fell to 774 in males and 912 in females, with 396 in common[160]. Differences also exist in humans. For example, the early acquisition of mucoid Pseudomonas aeruginosa contribute to a poorer survival of female cystic fibrosis patients as compared to male[161]. In contrast, male gender is associated with an increased risk for postinjury pneumonia as revealed in a study on 20288 trauma patients[162]. Regarding sepsis, females appear less susceptible to sepsis and seem to recover more effectively than males; however, a great disparity exists between studies in terms of incidence and mortality[163]. In murine model of sepsis post-hemorrhage proestrus female survived better than male[164]. The key role played by female sexual hormones was demonstrated with the disappearance of the protective effect of gender in ovariectomized mice[165]. In male, sterile and infectious stressful insults result in an alteration of the immune status as illustrated by the reduced IL-2 release by ex vivo activated splenocytes[165,166]. Such an alteration disappears after male castration and is evident in ovariectomized mice. In the later as well as in male mice, in vivo 17 β-estradiol administration prevents the alteration. In normal female mice, the treatment with 5 α-dihydrotestosterone will allow the observation of an altered immune status after trauma-hemorrhage[166,167]. Altogether, a number of studies have reported gender dimorphism in terms of response to trauma, hemorrhage, shock and sepsis. The advantageous outcome in females in the proestrus stage is due to the prevailing hormonal milieu, i.e., high estrogen levels. In this respect, various experimental and clinical studies have demonstrated beneficial effects of estrogen for the central nervous system, the cardiopulmonary system, the liver, the kidneys, the immune system, and for the overall survival of the host[168]. Estrogen have been regularly reported to dampen the inflammatory response. For example, 17 α-ethinylestradiol sulfate, which acts through the estrogen receptor can blunt multiple harmful outcomes arising from hypoxia and hypovolemia. In a rat model of hemorrhagic shock, ethinyl estradiol sulfate dramatically reduces damage by apoptosis, proinflammatory activity, and nitric oxide production and improved heart performance even in the absence of any fluid resuscitation following hemorrhage[169]. In a swine model of combined traumatic brain injury and hemorrhagic shock, this estrogen was efficacious in promoting survival and more rapidly restoring cardiovascular homeostasis following polytraumatic injuries in pre-hospital environments (i.e., rural and military) in the absence of standard therapies[170]. In another swine model of multiple injuries and hemorrhagic shock, the administration of this estrogen, even in the absence of fluid resuscitation reduced mortality and improved cardiac inotropy[171]. The role of sexual hormones may, however, vary depending on the inflammatory settings. In experimental acute cholangitis, testosterone was shown to be protective against liver inflammation[172].
Julius Strasburger (1871-1934) claimed in 1902 that the human gut was hosting 1.28 × 1014 bacteria[173]. One century later, the number has only been slightly adjusted to 3 × 1013, very close to the number of human cells[174]. A strong symbiosis exists between the bacterial flora and its host[175]. Not only the gut microbiota, but also the skin microbiota shapes the immune system[176]. Gut dysbiosis has adverse health effects on the human body that lead to a variety of chronic diseases[177]. This is supported by studies which showed that diet has a profound impact on the gut microbiota[178]. Among the many nutritional elements which favorably modify the quality of the immune and inflammatory response, let us mention vitamin D[179], fish oils[180] and probiotics[181]. For example, dietary supplementation with long-chain n-3 fatty acids in human volunteers suppressed the ex vivo production of inflammatory cytokines such as IL-1α, IL-1β and TNF[182] and inhibits tachycardia and attenuated maximal increases in oral temperature and metabolic rate following typhoid vaccine[183]. After listening Stamen Grigorov (1878-1945) who discovered the Lactobacillus bulgaricus, Elie Metchnikoff suggested the use of probiotic to limit degeneration associated with aging and to prevent the occurrence of various inflammatory disorders such as atherosclerosis consecutive to bacterial gut metabolites[184]. One century later Metchnikoff’s view was confirmed[184]. Interestingly, in 1910, the treatment of patients with melancholia (depression) with lactic acid bacillus allowed eleven out of eighteen to recover from their depression and accompanying delusions[185,186]. It was recently confirmed that gut bacteria influence behavior, and both depression and anxiety symptoms are directly associated with alterations in the microbiota, while psychobiotics, are defined as probiotics that confer mental health benefits to the host[187].
The epidemic of obesity has not affected the world similarly. The prevalence of obesity in 2015 was above 25% in Turkey, Mexico, South Africa, Iraq, United States, and Egypt and below 2% in Vietnam, Bangladesh, China, Ethiopia, Indonesia, Myanmar, Nigeria, India, Japan, and South Korea[188]. Nowadays, only Viet Nam remains below 2%. Obesity is asso
Many genetic elements govern the individual inflammatory response. Genetic predisposal to produce high or low levels of corticotropin releasing factor (CRF), adrenocorticotropic hormone (ACTH), cortisol and to respond to these mediators via genetic diversity of the respective receptors govern the individual response to inflammation and thus the threshold of resilience. The genetic heterogeneity of the human population, the genetic predisposal to diseases and the genetic diversity of the inflammatory response govern the great disparity of the response to any stress and insult. For example, cortisol or corticosterone levels and cortico sensitivity are under genetic influence[193,194]. One given genotype is associated with greater cortisol reactivity to social threats[195]. A polymorphism within the promoter of the serotonin transporter gene has been associated with differential psychological sensitivity to stressful experiences. Furthermore, variants have been found in the genes of the mineralocorticoid and glucocorticoid receptors that operate in balance and coordinate behavioral, autonomic, and neuroendocrine response patterns involved in homeostasis and health[196]. These variants contribute to individual differences in resilience and vulnerability to stressors. Similarly, the hypothalamic-pituitary-adrenocortical axis is influenced by gene polymorphism[197] and levels of CRF, CRF binding protein and ACTH can vary from individuals to individuals. Good examples of these genetic heterogeneity to sense stress and to respond to it, are the genetic polymorphisms associated with the PTSD which results in many epigenetic modifications[198]. PTSD does not affect similarly all individuals exposed to a stressful situation. Using a genome-wide association study, eight distinct significant regions were identified in patients with PTSD[199]. The glucocorticoid receptor gene (NR3C1) single nucleotide polymorphism (SNP)[200], a SNP in Tolloid-Like 1 gene[201], the retinoid-related orphan receptor alpha gene[202], the genes INTS8 and TP53INP1[203], and genetic variability in the CRP gene[204] are associated with PTSD. Polymorphism of genes, such as those of catechol-O-methyltransferase, serotonin transporter, and neuropeptide Y[205] have an impact on resilience. Finally, genetic factors also influence the occurrence of depression following exposure to stress. Such association has been particularly reported for genes coding for galanin receptor 2, brain-derived neurotrophic factor, purinergic receptor P2X7, and 5-hydroxytryptamine (serotonin) receptor 1A[206].
The global population was 1 billion in 1800, estimated to be about 8 billion in 2020 and anticipated to be 10.4 billion in 2100. This growth is expected to be concentrated in sub-Saharan Africa where there are already resource problems. This overpopulation is associated with global stress for humans and for the planet. Overpopulation is associated with a rise in food demands which has an impact on water consumption by agriculture. Humans exceeded the natural carrying capacity of the planet. “Earth overshoot day” is the calculated illustrative calendar date on which humanity’s resource consumption for the year exceeds Earth’s capacity to regenerate those resources that year. In 2023, the non-governmental organization “Global Footprint Network” considered that it happened on August 2nd. Overpopulation is responsible for a precarious situation on Earth[207]. All forms of natural resources are consumed for human needs and in turn producing waste, pollution, depletion of natural resources such as petroleum and metals, loss of biodiversity and ecosystems, environmental degradation, emission of carbon dioxide produced from burning fossil fuels and greenhouse gases causing global warming and climate change. In turns these modifications affect human physical and mental health. Population control measures are needed despite religious and cultural taboos, and confusion about rights. They should not be coercive and always voluntary and would then be highly beneficial for women’s health and rights, for economic better
However, on other continents than Africa, societies will have to deal with another stressful situation: The net repro
Hans Selye himself approached the yin yang aspect of stress and inflammation. He showed in a rat air pouch model that a restraint stress induced after a weak irritant (diluted croton oil) has been added earlier in the pouch, ends to cure of the inflammatory reaction. In contrast, the restraint stress following the addition of a strong irritant in the pouch (more concentrated croton oil) ended in a strong inflammatory reaction and to damaged tissues[212]. Nowadays, similar demonstration can be achieved with the two-hit model. For example, in a murine model, a mild systemic inflammatory response (a pancreatitis induced by cerulein injection) fully protected against a subsequent peritonitis whereas a severe systemic inflammatory reaction (induced by cerulein + injection of LPS) ended to a more severe response to sepsis (up to 100% mortality)[213].
The concept of yin yang also illustrates the detrimental and beneficial effects of different infectious disease–influencing alleles in the human genome. Certain mutations serve as a fortress against one infection while conferring susceptibility to another[214]. The yin yang phenomenon is particularly significant in the immune response to infectious insult. For example, the same cytokines can be either beneficial or deleterious during sepsis and severe infections depending upon the investigations. This was shown for IL-17[215,216], IL-33[217,218], Granulocyte/macrophage-colony-stimulating factor[219,220]. Similarly, Treg[221,222] and apoptotic cells can protect or be detrimental[223,224]. Indeed, a given cytokine may behave as a pro- as well as an anti-inflammatory cytokine. The cytokine amount, the nature of the target cell, the nature of the action and even the experimental model are parameters which influence cytokine properties[225]. Similarly, prostaglandin E2 regulates the immune response in opposite fashion depending on the target cells[226].
Stress can simultaneously favor both fear and the induction of analgesia because of a double-edged sword of stress hormones. It was observed that exposure of mice and rats to male but not female experimenters produces pain inhibition. Male-related stimuli (androstenone and androstadienone pheromones) induced a robust physiological stress response that results in stress-induced analgesia. Experimenter gender can thus affect apparent baseline responses in behavioral testing[227].
“Saturday night fever” was a movie in which the characters take part in gang fights as well as racist and sexist behavior, and there is a disturbing gang rape scene in the back of a car. So that fever in the city was clearly one with negative effects. However, good stress (eustress) vs bad one (distress) is illustrated by the occurrence of fever in humans, one of the cardinal signs of the response to an inflammatory insult. There are good and bad fevers which may be different depending on whether the inflammatory insult is microbial or not. Intermediate fever is associated with better outcome in patients with sepsis whereas high fever is associated with poorer outcome in both septic patients and non-septic patients[228,229].
The most appropriate definition of fever was proposed by Welch[230] (1850-1934), one of the founding professors at the Johns Hopkins Hospital. He wrote: “The real enemy in most fevers is the noxious substance which invades the body, and there is nothing to prevent us from believing that fever is a weapon employed by nature to combat assaults of this enemy. According to this view, the fever-producing agents light the fire which consumes them. It is not incompatible with this conception of fever to suppose that the fire may prove injurious also to the patients and may require the controlling hand of the physician”.
Exercise is given as offering a protective effect vs sedentary lifestyle. However, while this is the case of moderate exercise, heavy exertion favors inflammation and increases the risk of infection[231]. Heavy exercisers reported higher mental health issues, and more stress, but also higher mental toughness scores and less sleep disturbances. As such, the asso
In a very convincing report, it was demonstrated in animal model that regular exercise reduces the risk of cancer and disease recurrence. In this study, tumor-bearing mice randomized to voluntary wheel running showed over 60% reduction in tumor incidence. Training induces upregulation of pathways associated with NK cell immune function. Infiltration was significantly increased in tumors from running mice. NK cells were mobilized by epinephrine, which induces a selective mobilization of IL-6-sensitive NK cells, controlling tumor growth[234]. Insufficient physical activity increases the risk of non-communicable diseases, poor physical and cognitive function, weight gain, and mental ill-health. If one draws a relationship between all-cause mortality and incident cardiovascular diseases as a function of exercise (inactive, optimal active, most active), one gets a reverse J-curve suggesting that to improve life expectancy, a regular regimen of moderate activity is adequate[235].
An estimation of the prevalence of insufficient physical activity for 163 countries from 2000 to 2022 was recently published. The global age-standardised prevalence of insufficient physical activity was 31.3% in 2022, an increase from 23.4% in 2000 and 26.4% in 2010. Prevalence was higher among female (33.8%) than male (28.7%) individuals. Insufficient physical activity increased in people aged 60 years and older in all regions and both sexes[236].
An appropriate response to an inflammatory insult associates a pro-inflammatory response aimed to control the aggression, and an anti-inflammatory response aimed to control the pro-inflammatory arm and to allow a return to homeostasis. There are evidences that inflammation is normally compartmentalized within the body[237,238]. Host defenses are acting at sites of injury or microbial invasion while a systemic inflammatory response can accompany the process. Importantly, the intensity of both responses should be proportional allowing the body’s normal responses to stress to prevent systemic inflammation[239]. However, despite appropriate balance, the anti-inflammatory response [compensatory anti-inflammatory response (CARS)] can lead to an alteration of the immune status[240]. We postulate that SIRS and CARS should not be opposed, but most probably occur concomitantly in different compartments: SIRS predominates within the inflamed tissues while blood leukocytes show hyperactivity.
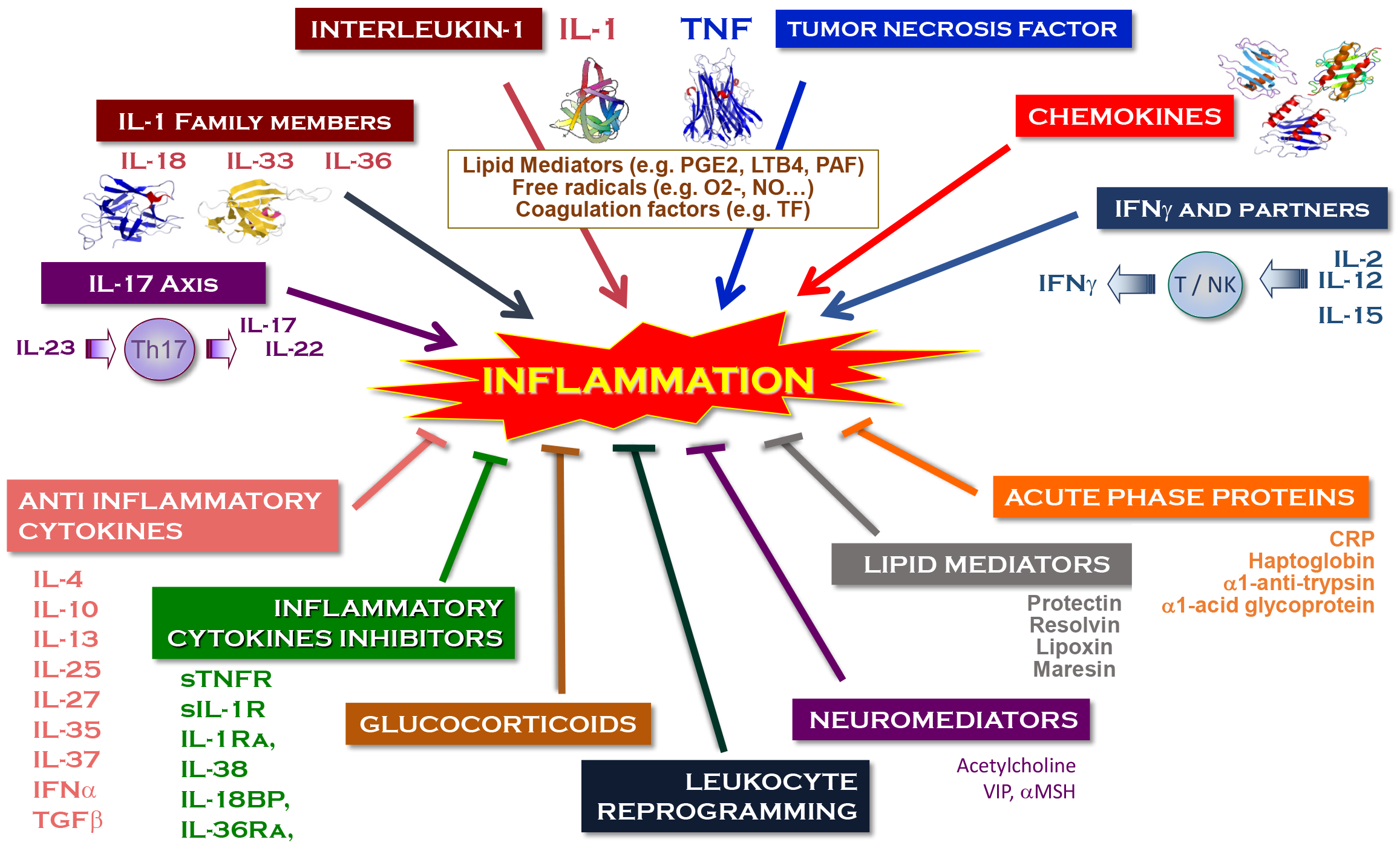
As mentioned above, the anti-inflammatory players enter into action concomitantly with the pro-inflammatory mediators in response to the same stressful insult. There are numerous cytokines that display anti-inflammatory properties (Figure 4) while the two conductors of the orchestra (namely IL-1 and TNF) have their own specific inhibitors. Many investigators have shown in sepsis that there was a correlation between the levels of IL-10, a key anti-inflammatory cytokine, and the pro-inflammatory cytokines[241-243]. Indeed, as with any pro-inflammatory cytokines, IL-10 is a marker of severity in sepsis[244] or in sterile SIRS such as in patients resuscitated after cardiac arrest[245] and can be predictive of outcome. Among the anti-inflammatory mediators let us mention the resolving lipidic mediators[246] and the acute phase proteins[247],the latest being induced by the inflammatory cytokines, particularly IL-6 within a regu
| Target | Anti-inflammatory stress processes |
| Cell | Anti-inflammatory cytokines; Resolution lipid mediators; Acetylcholine |
| Organ | Anti-inflammatory drugs; Glucocorticoids |
| Individuals | Yoga; Meditation; Psychologist; Anti-depressant drugs |
| Society | United Nations; NATO; Non-governmental organizations; OECD |
| Planet | Climate change conferences (COP) |
Hans Selye has been a pioneer in our understanding of how stress activates the hypothalamus-pituitary-adrenal axis, which results in the development of the “general adaptation syndrome”. CRF produced by the hypothalamus was identified in 1981 by Vale et al[248]. It acts on the pituitary gland that in turn produce the ACTH which was characterized in 1933 by Collip et al[249]. ACTH acts on the adrenals ending the production of glucocorticoids. They were discovered in 1936-1941 by three scientists [Philip Showalter Hench (1896-1965), Edward Calvin Kendall (1886-1972), and Tadeusz Reichstein (1897-1996)] who were awarded the Nobel prize in 1950 for their discoveries related to the hormones of the adrenal cortex, their purification, their structure, and their biological effects.
When added to epithelial cells, some 2766 different genes are modulated (1436 up-regulated; 1330 down-regulated). Most interestingly, the addition of glucocorticoid to TNF activated cells further induced or repressed additional genes[250]. The glucocorticoid receptors are within the nucleus of the responding cells. On the cell surface many other receptors and cell surface molecules contribute to the negative signaling (e.g., anti-inflammatory receptors, α-7 nicotinic receptor, resolving receptor, CD39, programmed death 1/programmed cell death-ligand 1). Within the cell, many negative signaling molecules (e.g., myeloid differentiation factor 88, IL-1 receptor associated kinase, suppressor of cytokine-1, tumor necrosis factor alpha-induced protein 3…), miRNA, NF-κB inhibitors and epigenetic modification participate in the negative regulation[251].
The team of Kevin Tracey has brilliantly demonstrated how the vagus nerve was controlling the inflammatory process. At the site of insult, cytokines trigger the afferent vagus nerve till the brain. Then the efferent vagus nerve induce the release of acetylcholine, which induces in the spleen the release of norepinephrine by adrenergic splenic neurons. Locally, T-cells bearing a β-2 adrenergic receptor release on their turn acetylcholine that shuts down the production of inflammatory cytokines by macrophages[252,253]. An implantable vagus nerve-stimulating device was shown to inhibit peripheral blood production of TNF, IL-1β, and IL-6. Vagus nerve stimulation in rheumatoid arthritis patients significantly improved the clinical scores and reduced disease severity[254]. A vagus nerve stimulator implanted in 16 biologic drug-refractory patients with moderately to severely active Crohn’s disease, led to clinically meaningful reduction of the disease activity[255]. Noninvasive transcutaneous auricular vagus nerve stimulation in patients with mild to moderate pediatric-onset Crohn’s disease or ulcerative colitis (UC), led to clinical remission in 3/6 (50%) with Crohn’s disease and 2/6 (33%) with UC at week 16[256].
No stress suffered by the cell or the individual leaves them unharmed. Whether the first encounter with a given insult will end to a tolerance/desensitization status or a priming effect depends on the nature of the inflammatory insult. Endotoxin tolerance was first reported as a decreased fever to a subsequent injection of endotoxin following a former one[257]. The phenomenon is associated with a reduction of certain inflammatory cytokines but not all[258]. It protects against the inflammatory consequences of trauma and hemorrhagic shock[259] and of ischemia/reperfusion[260]. Furthermore, endotoxin tolerance protects against fungal[261], bacterial[262], polymicrobial[263], viral[264] and parasitic[265] infections. Proposed in 1993[266], the term reprogramming has been associated with the clinical status of patients after sepsis[267] and has been considered as an appropriate terminology to define as well trained innate immunity[268]. While memory was only assigned to adaptive immunity, it becomes clear that innate immunity displays some kind of memory as well[269]. Among the primary signals that end to innate memory, β-glucan and bacillus Calmette-Guérin (BCG) have been widely studied. Indeed, it was shown a long time ago that BCG could lead to non-specific protection against bacteria! infection[270] and cancer[271].
Stress can induce depression and modify immune status in some individuals, illustrating that some external insults can alter the mental well-being and the health of individuals. It makes sense that in some individuals the reverse could be true. In other words, psychological therapy, yoga and meditation could have beneficial effects on mental and physical status and immunological defense mechanisms.
For example, a review of literature identified 16 studies among 124 trials which met rigorous criteria to establish the beneficial effect of yoga in treating depression and sleep complaints, and having adjunctive value in schizophrenia and attention-deficit hyperactivity disorder[272]. Ten trial totaling 840 patients with rheumatoid arthritis included in a meta-analysis revealed a statistically significant overall effect in favor of yoga for physical function, disease activity and grip strength but no effects were found for pain, tender joints, swollen joints count or inflammatory cytokines[273]. A review of 28 randomized controlled trials involving 1789 participants with inflammatory bowel disease was indicative of beneficial effects on generic inflammation as well as disease-specific biomarkers (fecal calprotectin and CRP)[274]. In adults with chronic inflammatory–related disorders, the most common biomarkers measured have been IL-6 and CRP. Most studies (n = 11/15) reported positive effects on inflammatory biomarkers from baseline to post-yoga intervention[275]. Other approaches such as brain education-based meditation, a modernized method of traditional mind-body training in Korea performed in patients with type-2 diabetes/hypertension led to reduce low density lipoproteins cholesterol and inflammatory gene expression, and improved some elements of the investigated physical and mental health of the patients[276]. Exposure to natural environmental stimuli to induce physiological relaxation, potentially enhancing immune functions and aiding in disease prevention is also proposed. However, the current body of evidence offers limited support for advocating nature immersion therapies as a primary approach to reducing stress, depression, and anxiety[277].
Not surprisingly higher self-reported stress in daily life of women was associated with lower levels of sexual activity and a decrease in relationship satisfaction[278]. Despite there are few studies to consider whether sexual activity is a player of eustress, there are few examples to suggest it. Sexual intercourse relieved stress for both men and women in satisfying relationships, but not in unsatisfying relationships[279]. A study on 425 German women (mean age 26.6 years) revealed that 92% of them indicated having masturbated in the past 12 months, once or several times a day (10%), once to two or three times a week (53.1%). For many women, masturbation does not represent “a partner substitute” to seek sexual pleasure, but rather is a stress coping and relaxation strategy[280]. During the COVID-19 pandemic, pornography consumption and solo sex activities offered an alternative to conventional sexual behavior during a highly stressful period and were found to have positive effects of relieving psychosocial stress otherwise induced by the pandemic. Those who maintained an active sexual life experienced less anxiety and depression, and greater relational health than those who were not sexually active[281]. Results from cross-lagged models suggest that high frequency of sex is positively related to later risk of cardiovascular events for men but not women, whereas good sexual quality seems to protect women but not men from cardiovascular risk in later life[282]. The demonstration that in mole rats sexual activity is favoring longevity, through the putative release of dehydroepiandrosterone, an anti-aging molecule, remains to be transposed to humans[283]. Sexual activity triggers the release of dopamine, endorphins, and oxytocin, which help promote relaxation and well-being and reduce the stress hormone cortisol. Oxytocin can impact stress, anxiety, and the processing of negative emotional stimuli[284]. It has been shown that oxytocin maintains homeostasis, shifts the set point for adaptation to a changing environment (allostasis) and contributes to recovery from the shifted set point by inducing active coping responses to stressful stimuli (resilience)[285]. Indeed, oxytocin has a beneficial therapeutic effect for the treatment of stress-related neuropsychiatric disorders (anxiety, depression and post-traumatic stress disorder)[286].
An interesting study performed in male on the effect of orgasm on the immune system failed to demonstrate any effects on T cell and B cell or on the production of IL-6 and TNF. In contrast, sexual arousal and orgasm increased the absolute number of leukocytes, in particular NK cells (CD3-, CD16+, CD56+), in the peripheral blood[287]. Whether orgasm would boost the immunity against sexually transmissible infection remains to be fully established.
Steroids and nonsteroidal anti-inflammatory drugs are medications aimed to reduce or eliminate symptoms related to an inflammatory disorder. They have direct inhibitory properties in the inflammatory process. Of note, anti-depressant drugs have also different actions on immune cells and inflammation[288,289].
Most chemotherapeutic drugs employed to treat cancer patients display side effects such as nausea, vomiting and depressed food intake[290]. Indeed, cancer cachexia is responsible for up to 30% of cancer deaths. Recently, it has been reported that a liver stress pathway ends to an increased hepatic expression and circulating levels of growth differentiation factor 15, which plays a crucial role in regulating body weight in response to cisplatin and doxorubicin. Its increase follows a selective activation by chemotherapy of inositol-requiring transmembrane kinase endoribonuclease-1α, an ER stress sensor within the liver[291].
Some foods or some excesses (red meat, salt, sugars, lipids) can favor inflammatory disorders. For example, the consumption of ultra-processed foods was associated with a 5% increased risk of cardiovascular diseases and a 12% higher mortality[292]. The inflammatory status can be modulated by the intake of food containing anti-inflammatory constituents such as omega-3 fatty acid in fish oil, vitamin D, or probiotics (see above). Other natural constituents can be taken on purpose as additive such as red ginseng very popular in South Korean[293], or resveratrol, a molecule found in red wine, which, like red ginseng, inhibits the inflammasome activation[294].
Just like bad air mentioned above, there is also good air, a healthy air that helps diseased humans to regain their health. During the 19th century, Madeira was a place to be when one was suffering tuberculosis[295]. However, this was not an absolute remedy. Princess Maria Amélia (1831-1853), the daughter of the Brazilian Emperor Dom Pedro I, developed a persistent cough, the onset of tuberculosis. The princess traveled to the island of Madeira where she passed away at the age of 21. Thomas Wakley (1795-1862) an English surgeon, and the founding editor of The Lancet had been in declining health for about ten years because of tuberculosis. He joined Madeira where he died in May 1862. Two famous Pasteurians lost their wife in Madeira. It was the case of Rose A. Shedlock who married Emile Roux and who passed away in Funchal on October 10th, 1879. She was 30 years old[296]. Few years earlier, Élie Metchnikoff accompanied his first wife in Madeira. Ludmila Vassilievna Feodorovna passed away on April 20th, 1873. She was 26 years old. Paul Langerhans (1947-1988), the discover of the Langerhans cells of the skin, and the islets of Langerhans in the pancreas, after he contracted tuberculosis in Freiburg, and after the failure of several treatments, left Germany for the island of Madeira in the hope of finding a climate there conducive to curing his illness. Unfortunately, he passed away in Funchal on July 20th, 1888. He was 41 years old.
Hermann Brehmer (1826-1889) when he was a student was diagnosed with tuberculosis. He went to the Himalayas, and came back cured. Back in Germany he authored a dissertation titled “Tuberculosis is a Curable Disease”. In 1854, he founded the first sanatorium in Görbersdorf/Sokołowsko, offering to his patients fresh air, and good nutrition. In 1876, John Harvey Kellogg (1852-1943), in his sanitarium, introduced vegetarianism and invented for his patients the famous cornflakes. Kellogg strongly discouraged sexual relations which he condemned. Even more than sexual relations, it was onanism that Dr. Kellogg abhorred. To combat what he considered absolute evil in humans, Kellogg even recommended various forms of genital mutilation!
In addition to the quality of environmental air, and a healthy food, the quality of water does also play a role on health. Two epidemiological studies conducted in France and Quebec established an increased frequency of Alzheimer cases among the population living in areas where water is prepared by aluminum precipitation[297,298].
The sun is another environmental parameter which illustrates the yin yang concept while influencing our lives. On one hand, it contributes to the skin production of the beneficial vitamin D, and a limited exposure to sun like in the Inuit population is associated with a prevalence of vitamin D insufficiency[299]. On another hand, exposure to sunlight and spending leisure time in greenspaces have a positive impact on people’s mental health, including depression, anxiety, and stress states[300]. This is also illustrated by the regional distribution of suicides and the relationship with sunlight duration[301]. The rate of production of serotonin by the brain is directly related to the prevailing duration of bright sunlight. Serotonin is a neurotransmitter involved not only in mood, but also in cognition, regulation of feeding behavior, anxiety, aggression, pain, sexual activity, and sleep[302]. There are substantial amounts of evidence linking variants of genes coding for tryptophan hydroxylase-1 (the enzyme involved in the synthesis of serotonin), the serotonin transporter and the serotonin receptor 2A gene with suicidal behavior[303]. In addition, serotonin has immunomodulatory properties via the serotonin receptor 2A expressed by human PBMCs, including inhibition of TNF[304]. On the other hand, the beneficial effects of sun exposure are opposed to the increased frequency of skin cancer upon exposure to UV[305] in genetically predisposed people[306].
While Nature has set up efficient mechanisms to prevent propagation of inflammation at the level of the cells, the organs and the individuals, unfortunately no efficient mechanism exists at the societal level. After the horrors of world word II, with the idea of “never again” in mind, on 25 June 1945, fifty nations adopted the charter of United Nations (UN) with aim to maintain international peace, to protect the human rights, and to deliver humanitarian aid. The most recent events (i.e., war in Ukraine, war in Gaza, war in Ethiopia) are a sad illustration of the failure of a regulatory mechanism. In 2022, fatalities from organized violence increased by staggering 97%, compared to the previous year, from 120000 in 2021 to 237000 in 2022, making 2022 the deadliest year since the genocide in 1994 during which some 800000 people were slaughtered in Rwanda by ethnic Hutu extremists[307]. But deaths are not the only consequence of wars. The number of people displaced by war, persecution, violence and human rights violations worldwide is likely to have exceeded 114 million by the end of September 2023, according to the UN[308].
Planet Earth has its own regulatory process to avoid perpetuating the insults made to the fragile balance of its climate: The UN climate change conferences (Conference of the parties) meet every year since 1995. Thirty years of awareness that the planet is suffering from natural and human driven outrages. Global warming has never been so obvious and 2023 has been the warmest year in centuries. Despite an ambitious and utopist goal limiting warming to 1.5 °C by the reduction of greenhouse gas emissions, and the transition from energy fossils to renewable energy, the Intergovernmental Panel on climate change expects the 20-year average global temperature to exceed + 1.5 °C in the early 2030s[309].
The effects of climate change on societies is a source of chronic environmental stress. Climate change makes people uncertain and stressed, with a sense of powerlessness. Mental health outcomes of climate change range from minimal stress and distress symptoms to clinical disorders, ranging from anxiety and sleep disturbances to depression, post-traumatic stress, and suicidal thoughts[310]. A large number of people exposed to climate or weather-related natural disasters experience distress and serious mental health consequences. In late December 2022, more than 190 parties adopted the 30 × 30 target, i.e. to protect at least 30% of the world’s lands, oceans, and inland waters by 2030[311]. Because climate change is contributing to an increase in immune-mediated diseases, such as asthma and other allergic diseases, autoimmune diseases, and cancer, there are urgently needs to adapt to and mitigate the effects of climate change. To favor planetary health and human health, key actions include reducing emissions and improving air quality (through reduced fossil fuel use), providing safe housing (e.g., improving weatherization), improving diets (i.e., quality and diversity) and agricultural practices, and increasing environmental biodiversity and green spaces[312].
Psychological resilience is the ability to cope with adversity and to adapt to stressful life events. It varies widely from person to person and depends on environmental as well as personal factors[313]. We are not born resilient, we become resilient. But not surprising, genetic markers are associated with the low or high reactivity to environmental stress[314]. In the case of a disability linked to a life accident, the implementation of adaptive strategies first allows one to survive, in society, with one's new body. Once this objective is achieved, we are then equipped to embark on another path, that of resilience. The athletes with disabilities are an example of individuals whose strength to fight against adversity has a positive impact on their levels of well-being, personal development, quality of life and integration into society[315]. The concept of psychological resilience applies to both the individual and the society. Depending upon the country, the population may not sense similarly a stressful situation. In other words, the perception of a similar stressful situation can be fairly different from countries to countries. Brain washing, lies and propaganda, can modify the perception of reality by a society, and the use of certain words (e.g., special military operation) instead of others (e.g., war) has boosted the resilience of the Russian populations as it happened during the first years of the war Russia initiated in invading Ukraine. Different is the resilience of the Ukrainian population which is directly facing the horrors perpetuated by the Russian army on its territory and the threat for loss of independence and sovereignty. The Ukrainian population under risk, despite a lower sense of wellbeing and higher levels of distress, sense of danger, and perceived threats has developed an enhanced societal resilience and hope[316]. A recent study investigating 30000 years of human history established that resistance and resilience to disturbances was key in terms of adaptation[317].
Lifespan exhibits a significant, positive correlation with resistance to stress. Genetic pathways and molecular mechanisms which enhance resistance to exogenous stressors participate in extending longevity[318,319].
Insults such as severe infection, trauma or ischemia-reperfusion can result in multiple organ dysfunction and death. A lack of sufficient adenosine triphosphate provision to fuel normal metabolic processes drives downregulation of metabolism, and thus cellular functionality. In turn, a decrease in metabolism will provide negative feedback to the mitochondria, inducing a bioenergetic shutdown. These processes may offer protection against a prolonged inflammatory hit by sparing the cell from initiation of death pathways, allowing a later recovery phase. This adaptive process has been called ”hibernation” by Singer[320]. Immune resilience is defined as the capacity to preserve and/or rapidly restore immune functions that promote disease resistance (immunocompetence) and control inflammation in infectious diseases as well as other causes of inflammatory stress[321]. Immune resilience despite inflammatory stress promotes longevity and favorable health.
Most interestingly resilience can also be observed within the vegetal world. Forests exhibit complex drought responses, indicating both resilience (photosynthetic greening) and vulnerability (browning and tree mortality). Notably, the resilience of shallow-water-table forest weakened as drought lengthened. By contrast, lower-fertility northern Amazonia, with slower-growing but hardier trees (or, alternatively, tall forests, with deep-rooted water access), supported more-drought-resilient forests independent of water-table depth[322].
Vertebrates vary in resistance and resilience to infectious diseases. For example, mice are 105 more resistant to LPS than humans[323]. Variability in the sensitivity of species to the induction of damaging inflammation in response to equivalent pathogen loads complicates the use of animal models that reflect human disease. It has been reported that the induction of proinflammatory cytokines from macrophages in response to inflammatory stimuli in vitro is regulated by proteins in the sera of species in inverse proportion to their in vivo resilience to lethal doses of bacterial LPS over a range of 10000-fold. This finding suggests that proteins in serum rather than intrinsic cellular differences may play a role in regulating variations in resilience to microbe-associated molecular patterns between species[324]. Zoonosis is illustrating how animals can host pathogens which remain healthy while transferring the infectious disease to humans. The human deficiency virus (HIV) has been spreading since a hunter in Kinshasa in 1920 was bitten by a monkey, itself infected with a simian virus (SIV) close to HIV[325]. In striking contrast to HIV infection, natural SIV infection of African nonhuman primates is asymptomatic and usually does not induce significant CD4+ T cell depletion despite high levels of virus replication[326]. Migratory birds can also be zoonotic reservoirs and can contribute to the dispersion of microorganisms as biological carriers transmitted initially to domestic birds. Avian species do not always have symptomatic influenza infection. It has been suggested that the recognition and elimination of invading pathogens (resistance) or the control of the infection associated tissue damage (tolerance) may explain asymptomatic or minimally symptomatic infections[327].
Bats occupy all regions of the planet, except the Arctic and Antarctic poles. Bats harbor many viruses and share human and domestic animal environments. They have a long-life expectancy and their diversity and winged locomotion favors the emergence and dispersal of new viral species. It has been estimated that at least 3200 different coronaviruses exist in all the different bats[328]. Bats display an altered inflammasome, leading to a reduced ability to produce IL-1. They have dampened transcriptional priming, reduced protein function, loss of one player (AIM2), and reduced caspase-1 activity which leads to an overall reduction in inflammation[329,330].
Of course, resilience is also a mechanism displayed by bacteria to address soil environmental changes and exposure to antibiotics. Climate change is responsible for longer period of drought that select bacterial community more resistant and resilient to drought[331]. The ability of bacteria to recover after being perturbed by an antibiotic corresponds to resilience[332]. For example, a resilience regulator has been identified in Mycobacterium tuberculosis of which mutants shorten the post-antibiotic effect, meaning that they enable the bacteria to resume growth after drug exposure faster than wild-type strains[333]. Interestingly, in Pseudomonas aeruginosa, pyocyanin, a self-produced antibiotic activates defenses that confer collateral tolerance specifically to structurally similar synthetic clinical antibiotics[334].
Disease tolerance is a mechanism that allow the host to maintain tissue integrity and to limit organ damage[335]. During inflammation, following microbial or sterile insult, different physiological mechanisms are turned on allowing a return to homeostasis. We previously mentioned the mitochondrial metabolism shutdown that allows a further recovery of the organs during systemic inflammation[320]. Interestingly, during this process, the same players can display a yin yang behavior. For example, IL-6 is a paradigm of ambiguity[225]. It can protect the lungs during a viral infection[336], but it favors coagulation[337]; IL-6 can promote the regeneration of tracheal epithelium[338], but it can also contribute to cachexia[339]. Furthermore, IL-6 is the dominant cytokine inducible upon acute stress and is a key regulator of the acute psychological response to stress. Stress-inducible IL-6 is produced from brown adipocytes in a beta-3-adrenergic-receptor-dependent fashion. During stress, endocrine IL-6 is the required instructive signal for mediating hyperglycemia through hepatic gluconeogenesis, which is necessary for anticipating and fueling “fight or flight” responses[340].
Hans Selye was the first one to propose a very simplistic and preliminary mathematical model: “Yet the visible degree of stress in a man is proportionate to the sum of everything that is going on him at the time. How could the body calculate the sum of, say, that much contraction plus that vision plus that much inflammation?” Many authors have offered more sophisticated models[1,341-345]. It is not sure that any of the mathematical models can integrate all the elements listed in this review that affect individual response to stress and inflammatory processes. Particularly, the genetic diversity and individual responsiveness to stress could complexify the mathematical models. Ghezzi[2] challenged the fact that theoretical and mathematical models need to be validated in a relevant population. Indeed, it is not sure that the mathematical models could easily fit globally knowing the genetic heterogeneity of the human population, the genetic predis
Personalized medicine (or precision medicine) will revolutionize the 21st century medicine. Whether mathematical modeling will offer the ground for personalized medicine remains an open question. Of note, personalized medicine may in part require individual’s genetic profiling to guide decisions made regarding the prevention, diagnosis, and treatment of diseases[346].
The aim of precision medicine is to design and improve diagnosis, therapeutics and prognostication through the use of large complex datasets that incorporate individual genes, function, and environmental variations. There is also a large heterogeneity among the patient with psychiatric disorders with respect to symptoms, treatment responses, causal role of immune mechanisms in the pathogenesis of depression and the nature of the psychological or environmental stressors such as childhood maltreatment. Personalizing clinical phenotyping and definition of subgroups of patients become a challenge in order to offer the most appropriate treatment[347,348]. Artificial intelligence (AI) is expected to interact constructively with precision medicine. Genetic, epigenetic, metabolomic, microbiotic and proteomic analyses are generating a huge amount of information that AI will consider for further individual stratification[349]. The complex pathophysiology involving multiple proinflammatory pathways and molecular dysregulation across different inflammatory disorders can be modeled by AI and used to design drug candidates in silico, and predict drug efficacy in virtual patients[350].
As pointed out by Androulakis[3]: “Inflammatory stress-related non-communicable conditions are a major challenge for 21st century medicine”. As we tried to illustrate in this review, the cell, the organ, the individual, the society, and the planet share many stressors of which the consequences are extremely interconnected ending to the domino effect and the butterfly effect[351-353]. These effects are exemplified by the consequences of climate change on wine production[354], or on decline of bees[355], both events having an economic impact. In addition, increased melting of ice in Greenland and Antarctica has decreased the angular velocity of Earth more rapidly than before, with effect on Coordinated Universal time which closely follows the rotation of Earth[356]. Similarly, external distress suffered by an individual will lead to the release of mediators that will affect its mood, its organs and its cells. Nature has set up a complex mechanism aimed to counteract the inflammatory response to stress, and the brightness of scientists can further help nature to address the inflammatory insults and to favor global health. However, those stressful events which depend on the human activity (war, climate change …) remain weakly controlled, with an increased alteration by societies and the planet. To conclude, let us quote Dr Tedros Adhanom Ghebreyesus, World Health Organization director-general: “If our planet was a patient, it would be admitted to intensive care. Its vital signs are alarming. It is running a fever, with each of the last nine months the hottest on record, as we hurtle towards the 1.5 °C threshold. Its lung capacity is compromised, with the destruction of forests that absorb carbon dioxide and produce oxygen. And many of the earth’s water sources - its lifeblood - are contaminated. Most concerning of all, its condition is deteriorating rapidly. Is it any wonder, then, that human health is suffering, when the health of the planet on which we depend is in peril? The health of humans, animals and our environment are woven together in a bond that is inextricable, yet fragile. We belong to the same unique, finely balanced ecosystem. This is not a new realization. Hippocrates, the father of medicine, wrote in the 5th century BC that, ‘The physician treats, but nature heals’[357]”.
| 1. | Vodovotz Y, Arciero J, Verschure PFMJ, Katz DL. A multiscale inflammatory map: linking individual stress to societal dysfunction. Front Sci. 2024;1. [RCA] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 2. | Ghezzi P. Societal factors in network medicine and causation. Front Sci. 2024;2. [DOI] [Full Text] |
| 3. | Androulakis IP. From cells to society: untangling the web of stress, inflammation, and social determinants of health. Front Sci. 2024;2. [DOI] [Full Text] |
| 4. | Rather LJ. Disturbance of function (functio laesa): the legendary fifth cardinal sign of inflammation, added by Galen to the four cardinal signs of Celsus. Bull N Y Acad Med. 1971;47:303-322. [PubMed] |
| 5. | Cavaillon JM. Once upon a time, inflammation. J Venom Anim Toxins Incl Trop Dis. 2021;27:e20200147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 6. | Cavaillon JM, Legout S. Centenary of the death of Elie Metchnikoff: a visionary and an outstanding team leader. Microbes Infect. 2016;18:577-594. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 34] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 7. | Rothman KJ. Causes. Am J Epidemiol. 1976;104:587-592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 566] [Cited by in RCA: 524] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 8. | Goh KI, Cusick ME, Valle D, Childs B, Vidal M, Barabási AL. The human disease network. Proc Natl Acad Sci U S A. 2007;104:8685-8690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2340] [Cited by in RCA: 2139] [Article Influence: 112.6] [Reference Citation Analysis (0)] |
| 9. | Barabási AL. Network medicine--from obesity to the "diseasome". N Engl J Med. 2007;357:404-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 319] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 10. | Hinney A, Körner A, Fischer-Posovszky P. The promise of new anti-obesity therapies arising from knowledge of genetic obesity traits. Nat Rev Endocrinol. 2022;18:623-637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 57] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 11. | Rohm TV, Meier DT, Olefsky JM, Donath MY. Inflammation in obesity, diabetes, and related disorders. Immunity. 2022;55:31-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 1257] [Article Influence: 314.3] [Reference Citation Analysis (0)] |
| 12. | Kimm SY, Obarzanek E. Childhood obesity: a new pandemic of the new millennium. Pediatrics. 2002;110:1003-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 136] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | Wolińska IA, Kraik K, Poręba R, Gać P, Poręba M. Environmental factors of obesity before and after COVID-19 pandemic: a review. Front Public Health. 2023;11:1213033. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 14. | Selye H. A syndrome produced by diverse nocuous agents. 1936. J Neuropsychiatry Clin Neurosci. 1998;10:230-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 402] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 15. | Nedeva C. Inflammation and Cell Death of the Innate and Adaptive Immune System during Sepsis. Biomolecules. 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 173] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 16. | Gautam A, Boyd DF, Nikhar S, Zhang T, Siokas I, Van de Velde LA, Gaevert J, Meliopoulos V, Thapa B, Rodriguez DA, Cai KQ, Yin C, Schnepf D, Beer J, DeAntoneo C, Williams RM, Shubina M, Livingston B, Zhang D, Andrake MD, Lee S, Boda R, Duddupudi AL, Crawford JC, Vogel P, Loch C, Schwemmle M, Fritz LC, Schultz-Cherry S, Green DR, Cuny GD, Thomas PG, Degterev A, Balachandran S. Necroptosis blockade prevents lung injury in severe influenza. Nature. 2024;628:835-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 75] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 17. | Msemburi W, Karlinsky A, Knutson V, Aleshin-Guendel S, Chatterji S, Wakefield J. The WHO estimates of excess mortality associated with the COVID-19 pandemic. Nature. 2023;613:130-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 533] [Article Influence: 177.7] [Reference Citation Analysis (0)] |
| 18. | Watson OJ, Barnsley G, Toor J, Hogan AB, Winskill P, Ghani AC. Global impact of the first year of COVID-19 vaccination: a mathematical modelling study. Lancet Infect Dis. 2022;22:1293-1302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 457] [Cited by in RCA: 1053] [Article Influence: 263.3] [Reference Citation Analysis (0)] |
| 19. | Meslé MMI, Brown J, Mook P, Katz MA, Hagan J, Pastore R, Benka B, Redlberger-Fritz M, Bossuyt N, Stouten V, Vernemmen C, Constantinou E, Maly M, Kynčl J, Sanca O, Krause TG, Vestergaard LS, Leino T, Poukka E, Gkolfinopoulou K, Mellou K, Tsintziloni M, Molnár Z, Aspelund G, Thordardottir M, Domegan L, Kelly E, O'Donell J, Urdiales AM, Riccardo F, Sacco C, Bumšteinas V, Liausediene R, Mossong J, Vergison A, Borg ML, Melillo T, Kocinski D, Pollozhani E, Meijerink H, Costa D, Gomes JP, Leite PP, Druc A, Gutu V, Mita V, Lazar M, Popescu R, Popovici O, Musilová M, Mrzel M, Socan M, Učakar V, Limia A, Mazagatos C, Olmedo C, Dabrera G, Kall M, Sinnathamby M, McGowan G, McMenamin J, Morrison K, Nitzan D, Widdowson MA, Smallwood C, Pebody R; WHO European Respiratory Surveillance Network. Estimated number of lives directly saved by COVID-19 vaccination programmes in the WHO European Region from December, 2020, to March, 2023: a retrospective surveillance study. Lancet Respir Med. 2024;12:714-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 20. | WHO. HIV data and statistics. [cited 18 September 2024]. Available from: https://www.who.int/data/gho/data/themes/hiv-aids. |
| 21. | Zonneveld KAF, Harper K, Klügel A, Chen L, De Lange G, Versteegh GJM. Climate change, society, and pandemic disease in Roman Italy between 200 BCE and 600 CE. Sci Adv. 2024;10:eadk1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Reference Citation Analysis (0)] |
| 22. | Tyukavina A, Potapov P, Hansen MC, Pickens AH, Stehman SV, Turubanova S, Parker D, Zalles V, Lima A, Kommareddy I, Song X, Wang L, Harris N. Global Trends of Forest Loss Due to Fire From 2001 to 2019. Front Remote Sens. 2022;3. [DOI] [Full Text] |
| 23. | Feng X, Merow C, Liu Z, Park DS, Roehrdanz PR, Maitner B, Newman EA, Boyle BL, Lien A, Burger JR, Pires MM, Brando PM, Bush MB, McMichael CNH, Neves DM, Nikolopoulos EI, Saleska SR, Hannah L, Breshears DD, Evans TP, Soto JR, Ernst KC, Enquist BJ. How deregulation, drought and increasing fire impact Amazonian biodiversity. Nature. 2021;597:516-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 42] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 24. | Ebi KL, Vanos J, Baldwin JW, Bell JE, Hondula DM, Errett NA, Hayes K, Reid CE, Saha S, Spector J, Berry P. Extreme Weather and Climate Change: Population Health and Health System Implications. Annu Rev Public Health. 2021;42:293-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 372] [Article Influence: 74.4] [Reference Citation Analysis (0)] |
| 25. | Mora C, McKenzie T, Gaw IM, Dean JM, von Hammerstein H, Knudson TA, Setter RO, Smith CZ, Webster KM, Patz JA, Franklin EC. Over half of known human pathogenic diseases can be aggravated by climate change. Nat Clim Chang. 2022;12:869-875. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 409] [Article Influence: 102.3] [Reference Citation Analysis (1)] |
| 26. | van Daalen KR, Tonne C, Semenza JC, Rocklöv J, Markandya A, Dasandi N, Jankin S, Achebak H, Ballester J, Bechara H, Beck TM, Callaghan MW, Carvalho BM, Chambers J, Pradas MC, Courtenay O, Dasgupta S, Eckelman MJ, Farooq Z, Fransson P, Gallo E, Gasparyan O, Gonzalez-Reviriego N, Hamilton I, Hänninen R, Hatfield C, He K, Kazmierczak A, Kendrovski V, Kennard H, Kiesewetter G, Kouznetsov R, Kriit HK, Llabrés-Brustenga A, Lloyd SJ, Batista ML, Maia C, Martinez-Urtaza J, Mi Z, Milà C, Minx JC, Nieuwenhuijsen M, Palamarchuk J, Pantera DK, Quijal-Zamorano M, Rafaj P, Robinson EJZ, Sánchez-Valdivia N, Scamman D, Schmoll O, Sewe MO, Sherman JD, Singh P, Sirotkina E, Sjödin H, Sofiev M, Solaraju-Murali B, Springmann M, Treskova M, Triñanes J, Vanuytrecht E, Wagner F, Walawender M, Warnecke L, Zhang R, Romanello M, Antó JM, Nilsson M, Lowe R. The 2024 Europe report of the Lancet Countdown on health and climate change: unprecedented warming demands unprecedented action. Lancet Public Health. 2024;9:e495-e522. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 104] [Article Influence: 52.0] [Reference Citation Analysis (0)] |
| 27. | Mahon MB, Sack A, Aleuy OA, Barbera C, Brown E, Buelow H, Civitello DJ, Cohen JM, de Wit LA, Forstchen M, Halliday FW, Heffernan P, Knutie SA, Korotasz A, Larson JG, Rumschlag SL, Selland E, Shepack A, Vincent N, Rohr JR. A meta-analysis on global change drivers and the risk of infectious disease. Nature. 2024;629:830-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 62] [Article Influence: 31.0] [Reference Citation Analysis (0)] |
| 28. | Liu WJ, Xu J. The Arms Race in the War Between Virus and Host: Implications for Anti-Infection Immunity. Infect Dis Immun. 2022;2:129-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 29. | Wells WA. Civil war in the immune system. J Cell Biol. 2003;163:928-929. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 30. | Samara M, Hammuda S, Vostanis P, El-Khodary B, Al-Dewik N. Children's prolonged exposure to the toxic stress of war trauma in the Middle East. BMJ. 2020;371:m3155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 31. | Singhal S. Early life shocks and mental health: The long-term effect of war in Vietnam. J Dev Econ. 2019;141:102244. [DOI] [Full Text] |
| 32. | Hazer L, Gredebäck G. The effects of war, displacement, and trauma on child development. Humanit Soc Sci Commun. 2023;10:909. [DOI] [Full Text] |
| 33. | Alastalo H, von Bonsdorff MB, Räikkönen K, Pesonen AK, Osmond C, Barker DJ, Heinonen K, Kajantie E, Eriksson JG. Early life stress and physical and psychosocial functioning in late adulthood. PLoS One. 2013;8:e69011. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 34. | Todd N, Valleron AJ, Bougnères P. Prenatal loss of father during World War One is predictive of a reduced lifespan in adulthood. Proc Natl Acad Sci U S A. 2017;114:4201-4206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 35. | Lu S, Wei F, Li G. The evolution of the concept of stress and the framework of the stress system. Cell Stress. 2021;5:76-85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 99] [Article Influence: 19.8] [Reference Citation Analysis (2)] |
| 36. | Carrilho M, Monarca RI, Aparício G, Mathias MDL, Tapisso JT, von Merten S. Physiological and behavioural adjustment of a wild rodent to laboratory conditions. Physiol Behav. 2024;273:114385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 37. | Martin B, Ji S, Maudsley S, Mattson MP. "Control" laboratory rodents are metabolically morbid: why it matters. Proc Natl Acad Sci U S A. 2010;107:6127-6133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 287] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 38. | Levis NA, Pfennig DW. Phenotypic plasticity, canalization, and the origins of novelty: Evidence and mechanisms from amphibians. Semin Cell Dev Biol. 2019;88:80-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 39. | Iurlaro R, Muñoz-Pinedo C. Cell death induced by endoplasmic reticulum stress. FEBS J. 2016;283:2640-2652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 540] [Cited by in RCA: 841] [Article Influence: 76.5] [Reference Citation Analysis (0)] |
| 40. | Cao SS, Luo KL, Shi L. Endoplasmic Reticulum Stress Interacts With Inflammation in Human Diseases. J Cell Physiol. 2016;231:288-294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 41. | Fleury C, Mignotte B, Vayssière JL. Mitochondrial reactive oxygen species in cell death signaling. Biochimie. 2002;84:131-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 742] [Cited by in RCA: 796] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 42. | Yuan Q, Zeng ZL, Yang S, Li A, Zu X, Liu J. Mitochondrial Stress in Metabolic Inflammation: Modest Benefits and Full Losses. Oxid Med Cell Longev. 2022;2022:8803404. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 24] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 43. | Bergthaler A, Menche J. The immune system as a social network. Nat Immunol. 2017;18:481-482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 44. | Rieckmann JC, Geiger R, Hornburg D, Wolf T, Kveler K, Jarrossay D, Sallusto F, Shen-Orr SS, Lanzavecchia A, Mann M, Meissner F. Social network architecture of human immune cells unveiled by quantitative proteomics. Nat Immunol. 2017;18:583-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 284] [Article Influence: 31.6] [Reference Citation Analysis (0)] |
| 45. | Zhu GF, Chancellor-Freeland C, Berman AS, Kage R, Leeman SE, Beller DI, Black PH. Endogenous substance P mediates cold water stress-induced increase in interleukin-6 secretion from peritoneal macrophages. J Neurosci. 1996;16:3745-3752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 46. | Elenkov IJ, Haskó G, Kovács KJ, Vizi ES. Modulation of lipopolysaccharide-induced tumor necrosis factor-alpha production by selective alpha- and beta-adrenergic drugs in mice. J Neuroimmunol. 1995;61:123-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 149] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 47. | Coe CL, Kramer M, Kirschbaum C, Netter P, Fuchs E. Prenatal stress diminishes the cytokine response of leukocytes to endotoxin stimulation in juvenile rhesus monkeys. J Clin Endocrinol Metab. 2002;87:675-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 56] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 48. | Ortiz GC, Sheridan JF, Marucha PT. Stress-induced changes in pathophysiology and interferon gene expression during primary HSV-1 infection. Brain Behav Immun. 2003;17:329-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 49. | Aberg KM, Radek KA, Choi EH, Kim DK, Demerjian M, Hupe M, Kerbleski J, Gallo RL, Ganz T, Mauro T, Feingold KR, Elias PM. Psychological stress downregulates epidermal antimicrobial peptide expression and increases severity of cutaneous infections in mice. J Clin Invest. 2007;117:3339-3349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 169] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 50. | Miller GE, Chen E, Sze J, Marin T, Arevalo JM, Doll R, Ma R, Cole SW. A functional genomic fingerprint of chronic stress in humans: blunted glucocorticoid and increased NF-kappaB signaling. Biol Psychiatry. 2008;64:266-272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 448] [Cited by in RCA: 434] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 51. | Dobbin JP, Harth M, McCain GA, Martin RA, Cousin K. Cytokine production and lymphocyte transformation during stress. Brain Behav Immun. 1991;5:339-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 92] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 52. | Cohen RT, Canino GJ, Bird HR, Celedón JC. Violence, abuse, and asthma in Puerto Rican children. Am J Respir Crit Care Med. 2008;178:453-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 53. | Cavaillon JM, Chousterman BG, Skirecki T. Compartmentalization of the inflammatory response during bacterial sepsis and severe COVID-19. J Intensive Med. 2024;4:326-340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 54. | Gautier EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, Helft J, Chow A, Elpek KG, Gordonov S, Mazloom AR, Ma'ayan A, Chua WJ, Hansen TH, Turley SJ, Merad M, Randolph GJ; Immunological Genome Consortium. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol. 2012;13:1118-1128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1402] [Cited by in RCA: 1622] [Article Influence: 115.9] [Reference Citation Analysis (0)] |
| 55. | Lavin Y, Winter D, Blecher-Gonen R, David E, Keren-Shaul H, Merad M, Jung S, Amit I. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell. 2014;159:1312-1326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1374] [Cited by in RCA: 1710] [Article Influence: 155.5] [Reference Citation Analysis (0)] |
| 56. | Smythies LE, Sellers M, Clements RH, Mosteller-Barnum M, Meng G, Benjamin WH, Orenstein JM, Smith PD. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest. 2005;115:66-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 372] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 57. | Blanchet C, Jouvion G, Fitting C, Cavaillon JM, Adib-Conquy M. Protective or deleterious role of scavenger receptors SR-A and CD36 on host resistance to Staphylococcus aureus depends on the site of infection. PLoS One. 2014;9:e87927. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 58. | Rasid O, Ciulean IS, Fitting C, Doyen N, Cavaillon JM. Local Microenvironment Controls the Compartmentalization of NK Cell Responses during Systemic Inflammation in Mice. J Immunol. 2016;197:2444-2454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 59. | Hacham M, Cristal N, White RM, Segal S, Apte RN. Complementary organ expression of IL-1 vs. IL-6 and CSF-1 activities in normal and LPS-injected mice. Cytokine. 1996;8:21-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 60. | Hviid CV, Erdem JS, Kunke D, Ahmed SM, Kjeldsen SF, Wang YY, Attramadal H, Aasen AO. The matri-cellular proteins 'cysteine-rich, angiogenic-inducer, 61' and 'connective tissue growth factor' are regulated in experimentally-induced sepsis with multiple organ dysfunction. Innate Immun. 2012;18:717-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 61. | Collange O, Charles AL, Lavaux T, Noll E, Bouitbir J, Zoll J, Chakfé N, Mertes M, Geny B. Compartmentalization of Inflammatory Response Following Gut Ischemia Reperfusion. Eur J Vasc Endovasc Surg. 2015;49:60-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 62. | Mirsoian A, Bouchlaka MN, Sckisel GD, Chen M, Pai CC, Maverakis E, Spencer RG, Fishbein KW, Siddiqui S, Monjazeb AM, Martin B, Maudsley S, Hesdorffer C, Ferrucci L, Longo DL, Blazar BR, Wiltrout RH, Taub DD, Murphy WJ. Adiposity induces lethal cytokine storm after systemic administration of stimulatory immunotherapy regimens in aged mice. J Exp Med. 2014;211:2373-2383. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 113] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 63. | Annane D, Sanquer S, Sébille V, Faye A, Djuranovic D, Raphaël JC, Gajdos P, Bellissant E. Compartmentalised inducible nitric-oxide synthase activity in septic shock. Lancet. 2000;355:1143-1148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 109] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 64. | Brownstein BH, Logvinenko T, Lederer JA, Cobb JP, Hubbard WJ, Chaudry IH, Remick DG, Baker HV, Xiao W, Mannick JA. Commonality and differences in leukocyte gene expression patterns among three models of inflammation and injury. Physiol Genomics. 2006;24:298-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 65. | White LE, Hassoun HT. Inflammatory Mechanisms of Organ Crosstalk during Ischemic Acute Kidney Injury. Int J Nephrol. 2012;2012:505197. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 66. | Rasid O, Cavaillon JM. Compartment diversity in innate immune reprogramming. Microbes Infect. 2018;20:156-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 67. | Miyamoto Y, Kikuta J, Matsui T, Hasegawa T, Fujii K, Okuzaki D, Liu YC, Yoshioka T, Seno S, Motooka D, Uchida Y, Yamashita E, Kobayashi S, Eguchi H, Morii E, Tryggvason K, Shichita T, Kayama H, Atarashi K, Kunisawa J, Honda K, Takeda K, Ishii M. Periportal macrophages protect against commensal-driven liver inflammation. Nature. 2024;629:901-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 66] [Article Influence: 33.0] [Reference Citation Analysis (0)] |
| 68. | Suzuki T, Shimizu T, Szalay L, Choudhry MA, Rue LW 3rd, Bland KI, Chaudry IH. Androstenediol ameliorates alterations in immune cells cytokine production capacity in a two-hit model of trauma-hemorrhage and sepsis. Cytokine. 2006;34:76-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 69. | Danahy DB, Anthony SM, Jensen IJ, Hartwig SM, Shan Q, Xue HH, Harty JT, Griffith TS, Badovinac VP. Polymicrobial sepsis impairs bystander recruitment of effector cells to infected skin despite optimal sensing and alarming function of skin resident memory CD8 T cells. PLoS Pathog. 2017;13:e1006569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 70. | Singer BH, Dickson RP, Denstaedt SJ, Newstead MW, Kim K, Falkowski NR, Erb-Downward JR, Schmidt TM, Huffnagle GB, Standiford TJ. Bacterial Dissemination to the Brain in Sepsis. Am J Respir Crit Care Med. 2018;197:747-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 71. | Philippart F, Fitting C, Cavaillon JM. Lung microenvironment contributes to the resistance of alveolar macrophages to develop tolerance to endotoxin*. Crit Care Med. 2012;40:2987-2996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 72. | Brusletto BS, Løberg EM, Hellerud BC, Goverud IL, Berg JP, Olstad OK, Gopinathan U, Brandtzaeg P, Øvstebø R. Extensive Changes in Transcriptomic "Fingerprints" and Immunological Cells in the Large Organs of Patients Dying of Acute Septic Shock and Multiple Organ Failure Caused by Neisseria meningitidis. Front Cell Infect Microbiol. 2020;10:42. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 73. | Pinheiro da Silva F, Gonçalves ANA, Duarte-Neto AN, Dias TL, Barbeiro HV, Breda CNS, Breda LCD, Câmara NOS, Nakaya HI. Transcriptome analysis of six tissues obtained post-mortem from sepsis patients. J Cell Mol Med. 2023;27:3157-3167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 74. | Gémes K, Ahnve S, Janszky I. Inflammation a possible link between economical stress and coronary heart disease. Eur J Epidemiol. 2008;23:95-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 75. | Pfefferbaum B, Newman E, Nelson SD, Nitiéma P, Pfefferbaum RL, Rahman A. Disaster media coverage and psychological outcomes: descriptive findings in the extant research. Curr Psychiatry Rep. 2014;16:464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 76. | Melnyk I, Melnyk A, Pryshliak Y, Melnyk O, Soroka A, Churevych V, El-ibiary R, Ertanowska D, Sun H. Good News – Bad News. Proportion between Positive and Negative Headlines in the Global News Feed (Based on the Google News Aggregator). SMC. 2023;11:244. [DOI] [Full Text] |
| 77. | Rocha YM, de Moura GA, Desidério GA, de Oliveira CH, Lourenço FD, de Figueiredo Nicolete LD. The impact of fake news on social media and its influence on health during the COVID-19 pandemic: a systematic review. Z Gesundh Wiss. 2021;1-10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 101] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 78. | Jia KM, Hanage WP, Lipsitch M, Johnson AG, Amin AB, Ali AR, Scobie HM, Swerdlow DL. Estimated preventable COVID-19-associated deaths due to non-vaccination in the United States. Eur J Epidemiol. 2023;38:1125-1128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 25] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 79. | Swire-Thompson B, Lazer D. Public Health and Online Misinformation: Challenges and Recommendations. Annu Rev Public Health. 2020;41:433-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 459] [Article Influence: 65.6] [Reference Citation Analysis (0)] |
| 80. | Arribas CM, Arcos R, Gértrudix M, Mikulski K, Hernández-Escayola P, Teodor M, Novăcescu E, Surdu I, Stoian V, García-Jiménez A. Information manipulation and historical revisionism: Russian disinformation and foreign interference through manipulated history-based narratives. Open Res Eur. 2023;3:121. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 81. | Prince M, Patel V, Saxena S, Maj M, Maselko J, Phillips MR, Rahman A. No health without mental health. Lancet. 2007;370:859-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2094] [Cited by in RCA: 1993] [Article Influence: 104.9] [Reference Citation Analysis (0)] |
| 82. | Chen Z, Peng K, Liu X, Yang J, Long L, Liu Y, Li Y, Tian Y. Association between high burn-out and workplace violence among healthcare workers in China: a WeChat-based survey. BMJ Open. 2022;12:e064729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 83. | Nogrady B. Harassment of scientists is surging - institutions aren't sure how to help. Nature. 2024;629:748-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 84. | Vignjević Petrinović S, Milošević MS, Marković D, Momčilović S. Interplay between stress and cancer-A focus on inflammation. Front Physiol. 2023;14:1119095. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 30] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 85. | Yang T, Qiao Y, Xiang S, Li W, Gan Y, Chen Y. Work stress and the risk of cancer: A meta-analysis of observational studies. Int J Cancer. 2019;144:2390-2400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 79] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 86. | UNODC. Gender-related killings of women and girls (femicide/feminicide): Global estimates of female intimate partner/family-related homicides in 2022. [cited 18 September 2024]. Available from: https://www.unwomen.org/en/digital-library/publications/2023/11/gender-related-killings-of-women-and-girls-femicide-feminicide-global-estimates-2022. |
| 87. | World Population Review. [cited 18 September 2024]. Available from: https://worldpopulationreview.com/country-rankings/femicide-rates-by-country. |
| 88. | Kourti A, Stavridou A, Panagouli E, Psaltopoulou T, Spiliopoulou C, Tsolia M, Sergentanis TN, Tsitsika A. Domestic Violence During the COVID-19 Pandemic: A Systematic Review. Trauma Violence Abuse. 2023;24:719-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 178] [Article Influence: 59.3] [Reference Citation Analysis (0)] |
| 89. | Anderson J. The impact of family structure on the health of children: Effects of divorce. Linacre Q. 2014;81:378-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 90. | De Sanctis V, Soliman A, Alaaraj N, Ahmed S, Alyafei F, Hamed N. Early and Long-term Consequences of Nutritional Stunting: From Childhood to Adulthood. Acta Biomed. 2021;92:e2021168. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 99] [Reference Citation Analysis (0)] |
| 91. | Roger K, Vannasing P, Tremblay J, Bringas Vega ML, Bryce CP, Rabinowitz A, Valdes-Sosa PA, Galler JR, Gallagher A. Early childhood malnutrition impairs adult resting brain function using near-infrared spectroscopy. Front Hum Neurosci. 2023;17:1287488. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 92. | Tharumakunarajah R, Lee A, Hawcutt DB, Harman NL, Sinha IP. The Impact of Malnutrition on the Developing Lung and Long-Term Lung Health: A Narrative Review of Global Literature. Pulm Ther. 2024;10:155-170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 93. | Papport Annuel. Les restaurants du Coeur-Rapport annuel 2022-2023. [cited 18 September 2024]. Available from: https://www.restosducoeur.org/wp-content/uploads/2023/11/v17-ra_2022_2023_reduit_01-presentation-1.pdf. |
| 94. | Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3387] [Cited by in RCA: 3131] [Article Influence: 97.8] [Reference Citation Analysis (0)] |
| 95. | Janeway CA Jr. Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54 Pt 1:1-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2085] [Cited by in RCA: 2148] [Article Influence: 58.1] [Reference Citation Analysis (0)] |
| 96. | Gong T, Liu L, Jiang W, Zhou R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat Rev Immunol. 2020;20:95-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 508] [Cited by in RCA: 1223] [Article Influence: 174.7] [Reference Citation Analysis (0)] |
| 97. | Leulier F, Lemaitre B. Toll-like receptors--taking an evolutionary approach. Nat Rev Genet. 2008;9:165-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 383] [Cited by in RCA: 437] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 98. | GBD 2019 IMID Collaborators. Global, regional, and national incidence of six major immune-mediated inflammatory diseases: findings from the global burden of disease study 2019. EClinicalMedicine. 2023;64:102193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 84] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 99. | Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, Ferrucci L, Gilroy DW, Fasano A, Miller GW, Miller AH, Mantovani A, Weyand CM, Barzilai N, Goronzy JJ, Rando TA, Effros RB, Lucia A, Kleinstreuer N, Slavich GM. Chronic inflammation in the etiology of disease across the life span. Nat Med. 2019;25:1822-1832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1084] [Cited by in RCA: 3160] [Article Influence: 451.4] [Reference Citation Analysis (0)] |
| 100. | Cohen S, Janicki-Deverts D, Doyle WJ, Miller GE, Frank E, Rabin BS, Turner RB. Chronic stress, glucocorticoid receptor resistance, inflammation, and disease risk. Proc Natl Acad Sci U S A. 2012;109:5995-5999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 739] [Cited by in RCA: 887] [Article Influence: 63.4] [Reference Citation Analysis (0)] |
| 101. | Raza Z, Hussain SF, Foster VS, Wall J, Coffey PJ, Martin JF, Gomes RSM. Exposure to war and conflict: The individual and inherited epigenetic effects on health, with a focus on post-traumatic stress disorder. Front Epidemiol. 2023;3:1066158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 102. | Durodié B, Wainwright D. Terrorism and post-traumatic stress disorder: a historical review. Lancet Psychiatry. 2019;6:61-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 103. | Güzel A, Samancı Tekin Ç, Uçan Yamaç S. Exploring the impacts of perceived locus of control on post-traumatic stress disorder among disaster survivors: A systematic review. J Psychiatr Ment Health Nurs. 2024;31:776-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 104. | van der Walt L, Suliman S, Martin L, Lammers K, Seedat S. Resilience and post-traumatic stress disorder in the acute aftermath of rape: a comparative analysis of adolescents versus adults. J Child Adolesc Ment Health. 2014;26:239-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 105. | Hatch R, Young D, Barber VS, Griffiths J, Harrison DA, Watkinson PJ. Anxiety, depression and post-traumatic stress disorder management after critical illness: a UK multi-centre prospective cohort study. Crit Care. 2020;24:633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 106. | Deltour V, Poujol AL, Laurent A. Post-traumatic stress disorder among ICU healthcare professionals before and after the Covid-19 health crisis: a narrative review. Ann Intensive Care. 2023;13:66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 107. | Hori H, Kim Y. Inflammation and post-traumatic stress disorder. Psychiatry Clin Neurosci. 2019;73:143-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 246] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 108. | Griffin GD, Charron D, Al-Daccak R. Post-traumatic stress disorder: revisiting adrenergics, glucocorticoids, immune system effects and homeostasis. Clin Transl Immunology. 2014;3:e27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 109. | Sun Y, Qu Y, Zhu J. The Relationship Between Inflammation and Post-traumatic Stress Disorder. Front Psychiatry. 2021;12:707543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 32] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 110. | Bam M, Yang X, Zhou J, Ginsberg JP, Leyden Q, Nagarkatti PS, Nagarkatti M. Evidence for Epigenetic Regulation of Pro-Inflammatory Cytokines, Interleukin-12 and Interferon Gamma, in Peripheral Blood Mononuclear Cells from PTSD Patients. J Neuroimmune Pharmacol. 2016;11:168-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 111. | Thomson MC, Stanberry LR. Climate Change and Vectorborne Diseases. N Engl J Med. 2022;387:1969-1978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 100] [Article Influence: 25.0] [Reference Citation Analysis (0)] |
| 112. | Pearson H. The rise of eco-anxiety: scientists wake up to the mental-health toll of climate change. Nature. 2024;628:256-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 17] [Reference Citation Analysis (0)] |
| 113. | Rajagopalan S, Brook RD, Salerno PRVO, Bourges-Sevenier B, Landrigan P, Nieuwenhuijsen MJ, Munzel T, Deo SV, Al-Kindi S. Air pollution exposure and cardiometabolic risk. Lancet Diabetes Endocrinol. 2024;12:196-208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 80] [Article Influence: 40.0] [Reference Citation Analysis (0)] |
| 114. | Kelly FJ, Fussell JC. Role of oxidative stress in cardiovascular disease outcomes following exposure to ambient air pollution. Free Radic Biol Med. 2017;110:345-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 102] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 115. | Zhao X, You F. Microplastic Human Dietary Uptake from 1990 to 2018 Grew across 109 Major Developing and Industrialized Countries but Can Be Halved by Plastic Debris Removal. Environ Sci Technol. 2024;58:8709-8723. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 33] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 116. | Ziani K, Ioniță-Mîndrican CB, Mititelu M, Neacșu SM, Negrei C, Moroșan E, Drăgănescu D, Preda OT. Microplastics: A Real Global Threat for Environment and Food Safety: A State of the Art Review. Nutrients. 2023;15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 203] [Article Influence: 67.7] [Reference Citation Analysis (0)] |
| 117. | Kim HY, Ashim J, Park S, Kim W, Ji S, Lee SW, Jung YR, Jeong SW, Lee SG, Kim HC, Lee YJ, Kwon MK, Hwang JS, Shin JM, Lee SJ, Yu W, Park JK, Choi SK. A preliminary study about the potential risks of the UV-weathered microplastic: The proteome-level changes in the brain in response to polystyrene derived weathered microplastics. Environ Res. 2023;233:116411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 24] [Reference Citation Analysis (0)] |
| 118. | Yang W, Jannatun N, Zeng Y, Liu T, Zhang G, Chen C, Li Y. Impacts of microplastics on immunity. Front Toxicol. 2022;4:956885. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 119. | Caputi S, Diomede F, Lanuti P, Marconi GD, Di Carlo P, Sinjari B, Trubiani O. Microplastics Affect the Inflammation Pathway in Human Gingival Fibroblasts: A Study in the Adriatic Sea. Int J Environ Res Public Health. 2022;19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 32] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 120. | Gaspar L, Bartman S, Coppotelli G, Ross JM. Acute Exposure to Microplastics Induced Changes in Behavior and Inflammation in Young and Old Mice. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 47] [Reference Citation Analysis (0)] |
| 121. | Marfella R, Prattichizzo F, Sardu C, Fulgenzi G, Graciotti L, Spadoni T, D'Onofrio N, Scisciola L, La Grotta R, Frigé C, Pellegrini V, Municinò M, Siniscalchi M, Spinetti F, Vigliotti G, Vecchione C, Carrizzo A, Accarino G, Squillante A, Spaziano G, Mirra D, Esposito R, Altieri S, Falco G, Fenti A, Galoppo S, Canzano S, Sasso FC, Matacchione G, Olivieri F, Ferraraccio F, Panarese I, Paolisso P, Barbato E, Lubritto C, Balestrieri ML, Mauro C, Caballero AE, Rajagopalan S, Ceriello A, D'Agostino B, Iovino P, Paolisso G. Microplastics and Nanoplastics in Atheromas and Cardiovascular Events. N Engl J Med. 2024;390:900-910. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 486] [Cited by in RCA: 539] [Article Influence: 269.5] [Reference Citation Analysis (0)] |
| 122. | Solovev K, Pröllochs N. Moralized language predicts hate speech on social media. PNAS Nexus. 2023;2:pgac281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 123. | Schuster R, Rockel JS, Kapoor M, Hinz B. The inflammatory speech of fibroblasts. Immunol Rev. 2021;302:126-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 127] [Article Influence: 25.4] [Reference Citation Analysis (0)] |
| 124. | Yin Y, Liu J, Fan Q, Zhao S, Wu X, Wang J, Liu Y, Li Y, Lu W. Long-term spaceflight composite stress induces depression and cognitive impairment in astronauts-insights from neuroplasticity. Transl Psychiatry. 2023;13:342. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 125. | Man J, Graham T, Squires-Donelly G, Laslett AL. The effects of microgravity on bone structure and function. NPJ Microgravity. 2022;8:9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 76] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 126. | Luben RA, Mundy GR, Trummel CL, Raisz LG. Partial purification of osteoclast-activating factor from phytohemagglutinin-stimulated human leukocytes. J Clin Invest. 1974;53:1473-1480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 74] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 127. | Chapes SK, Morrison DR, Guikema JA, Lewis ML, Spooner BS. Cytokine secretion by immune cells in space. J Leukoc Biol. 1992;52:104-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 56] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 128. | Akiyama T, Horie K, Hinoi E, Hiraiwa M, Kato A, Maekawa Y, Takahashi A, Furukawa S. How does spaceflight affect the acquired immune system? NPJ Microgravity. 2020;6:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 129. | Cavaillon JM, Legout S. Louis Pasteur: Between Myth and Reality. Biomolecules. 2022;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 130. | Jain N. The early life education of the immune system: Moms, microbes and (missed) opportunities. Gut Microbes. 2020;12:1824564. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 131. | Klein L, Kyewski B, Allen PM, Hogquist KA. Positive and negative selection of the T cell repertoire: what thymocytes see (and don't see). Nat Rev Immunol. 2014;14:377-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1005] [Cited by in RCA: 1006] [Article Influence: 83.8] [Reference Citation Analysis (0)] |
| 132. | Givony T, Leshkowitz D, Del Castillo D, Nevo S, Kadouri N, Dassa B, Gruper Y, Khalaila R, Ben-Nun O, Gome T, Dobeš J, Ben-Dor S, Kedmi M, Keren-Shaul H, Heffner-Krausz R, Porat Z, Golani O, Addadi Y, Brenner O, Lo DD, Goldfarb Y, Abramson J. Thymic mimetic cells function beyond self-tolerance. Nature. 2023;622:164-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 51] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 133. | Gillis-Buck E, Miller H, Sirota M, Sanders SJ, Ntranos V, Anderson MS, Gardner JM, MacKenzie TC. Extrathymic Aire-expressing cells support maternal-fetal tolerance. Sci Immunol. 2021;6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 134. | Marmot M, Allen J, Bell R, Bloomer E, Goldblatt P; Consortium for the European Review of Social Determinants of Health and the Health Divide. WHO European review of social determinants of health and the health divide. Lancet. 2012;380:1011-1029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 830] [Cited by in RCA: 883] [Article Influence: 63.1] [Reference Citation Analysis (0)] |
| 135. | Raghupathi V, Raghupathi W. The influence of education on health: an empirical assessment of OECD countries for the period 1995-2015. Arch Public Health. 2020;78:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 455] [Article Influence: 75.8] [Reference Citation Analysis (0)] |
| 136. | Lleras-Muney A, Price J, Yue D. The association between educational attainment and longevity using individual-level data from the 1940 census. J Health Econ. 2022;84:102649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 137. | Zhao Z, Li L, Sang Y. The COVID-19 pandemic increased poor lifestyles and worsen mental health: a systematic review. Am J Transl Res. 2023;15:3060-3066. [PubMed] |
| 138. | Ettman CK, Abdalla SM, Cohen GH, Sampson L, Vivier PM, Galea S. Prevalence of Depression Symptoms in US Adults Before and During the COVID-19 Pandemic. JAMA Netw Open. 2020;3:e2019686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1144] [Cited by in RCA: 1483] [Article Influence: 247.2] [Reference Citation Analysis (0)] |
| 139. | Geweniger A, Barth M, Haddad AD, Högl H, Insan S, Mund A, Langer T. Impact of the COVID-19 Pandemic on Mental Health Outcomes of Healthy Children, Children With Special Health Care Needs and Their Caregivers-Results of a Cross-Sectional Study. Front Pediatr. 2022;10:759066. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 140. | Geranios K, Kagabo R, Kim J. Impact of COVID-19 and Socioeconomic Status on Delayed Care and Unemployment. Health Equity. 2022;6:91-97. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 25] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 141. | Horiguchi H, Loftus TJ, Hawkins RB, Raymond SL, Stortz JA, Hollen MK, Weiss BP, Miller ES, Bihorac A, Larson SD, Mohr AM, Brakenridge SC, Tsujimoto H, Ueno H, Moore FA, Moldawer LL, Efron PA; Sepsis and Critical Illness Research Center Investigators. Innate Immunity in the Persistent Inflammation, Immunosuppression, and Catabolism Syndrome and Its Implications for Therapy. Front Immunol. 2018;9:595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 125] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 142. | Liang H, Zheng L, Xia H, Tang J. SARS-CoV-2 infection in China-Before the pandemic. PLoS Negl Trop Dis. 2020;14:e0008472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 143. | Dudel C, Riffe T, Acosta E, van Raalte A, Strozza C, Myrskylä M. Monitoring trends and differences in COVID-19 case-fatality rates using decomposition methods: Contributions of age structure and age-specific fatality. PLoS One. 2020;15:e0238904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 80] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 144. | Bauer P, Brugger J, König F, Posch M. An international comparison of age and sex dependency of COVID-19 deaths in 2020: a descriptive analysis. Sci Rep. 2021;11:19143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 145. | Torres C, García J, Meslé F, Barbieri M, Bonnet F, Camarda CG, Cambois E, Caporali A, Couppié É, Poniakina S, Robine JM. Identifying age- and sex-specific COVID-19 mortality trends over time in six countries. Int J Infect Dis. 2023;128:32-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 146. | Rydyznski Moderbacher C, Ramirez SI, Dan JM, Grifoni A, Hastie KM, Weiskopf D, Belanger S, Abbott RK, Kim C, Choi J, Kato Y, Crotty EG, Kim C, Rawlings SA, Mateus J, Tse LPV, Frazier A, Baric R, Peters B, Greenbaum J, Ollmann Saphire E, Smith DM, Sette A, Crotty S. Antigen-Specific Adaptive Immunity to SARS-CoV-2 in Acute COVID-19 and Associations with Age and Disease Severity. Cell. 2020;183:996-1012.e19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1670] [Cited by in RCA: 1458] [Article Influence: 243.0] [Reference Citation Analysis (0)] |
| 147. | Nacionales DC, Szpila B, Ungaro R, Lopez MC, Zhang J, Gentile LF, Cuenca AL, Vanzant E, Mathias B, Jyot J, Westerveld D, Bihorac A, Joseph A, Mohr A, Duckworth LV, Moore FA, Baker HV, Leeuwenburgh C, Moldawer LL, Brakenridge S, Efron PA. A Detailed Characterization of the Dysfunctional Immunity and Abnormal Myelopoiesis Induced by Severe Shock and Trauma in the Aged. J Immunol. 2015;195:2396-2407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 148. | Fang M, Roscoe F, Sigal LJ. Age-dependent susceptibility to a viral disease due to decreased natural killer cell numbers and trafficking. J Exp Med. 2010;207:2369-2381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 80] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 149. | Saito H, Sherwood ER, Varma TK, Evers BM. Effects of aging on mortality, hypothermia, and cytokine induction in mice with endotoxemia or sepsis. Mech Ageing Dev. 2003;124:1047-1058. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 141] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 150. | Starr ME, Evers BM, Saito H. Age-associated increase in cytokine production during systemic inflammation: adipose tissue as a major source of IL-6. J Gerontol A Biol Sci Med Sci. 2009;64:723-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 127] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 151. | Motegi A, Kinoshita M, Sato K, Shinomiya N, Ono S, Nonoyama S, Hiraide H, Seki S. An in vitro Shwartzman reaction-like response is augmented age-dependently in human peripheral blood mononuclear cells. J Leukoc Biol. 2006;79:463-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 152. | Cao Y, Fan Y, Li F, Hao Y, Kong Y, Chen C, Hao X, Han D, Li G, Wang Z, Song C, Han J, Zeng H. Phenotypic and functional alterations of monocyte subsets with aging. Immun Ageing. 2022;19:63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 47] [Reference Citation Analysis (0)] |
| 153. | Pirkis J, Gunnell D, Shin S, Del Pozo-Banos M, Arya V, Aguilar PA, Appleby L, Arafat SMY, Arensman E, Ayuso-Mateos JL, Balhara YPS, Bantjes J, Baran A, Behera C, Bertolote J, Borges G, Bray M, Brečić P, Caine E, Calati R, Carli V, Castelpietra G, Chan LF, Chang SS, Colchester D, Coss-Guzmán M, Crompton D, Ćurković M, Dandona R, De Jaegere E, De Leo D, Deisenhammer EA, Dwyer J, Erlangsen A, Faust JS, Fornaro M, Fortune S, Garrett A, Gentile G, Gerstner R, Gilissen R, Gould M, Gupta SK, Hawton K, Holz F, Kamenshchikov I, Kapur N, Kasal A, Khan M, Kirtley OJ, Knipe D, Kõlves K, Kölzer SC, Krivda H, Leske S, Madeddu F, Marshall A, Memon A, Mittendorfer-Rutz E, Nestadt P, Neznanov N, Niederkrotenthaler T, Nielsen E, Nordentoft M, Oberlerchner H, O'Connor RC, Papsdorf R, Partonen T, Phillips MR, Platt S, Portzky G, Psota G, Qin P, Radeloff D, Reif A, Reif-Leonhard C, Rezaeian M, Román-Vázquez N, Roskar S, Rozanov V, Sara G, Scavacini K, Schneider B, Semenova N, Sinyor M, Tambuzzi S, Townsend E, Ueda M, Wasserman D, Webb RT, Winkler P, Yip PSF, Zalsman G, Zoja R, John A, Spittal MJ. Suicide numbers during the first 9-15 months of the COVID-19 pandemic compared with pre-existing trends: An interrupted time series analysis in 33 countries. EClinicalMedicine. 2022;51:101573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 142] [Article Influence: 35.5] [Reference Citation Analysis (1)] |
| 154. | Yoshioka E, Hanley SJB, Sato Y, Saijo Y. Impact of the COVID-19 pandemic on suicide rates in Japan through December 2021: An interrupted time series analysis. Lancet Reg Health West Pac. 2022;24:100480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 37] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 155. | O'Connor RC, Wetherall K, Cleare S, McClelland H, Melson AJ, Niedzwiedz CL, O'Carroll RE, O'Connor DB, Platt S, Scowcroft E, Watson B, Zortea T, Ferguson E, Robb KA. Mental health and well-being during the COVID-19 pandemic: longitudinal analyses of adults in the UK COVID-19 Mental Health & Wellbeing study. Br J Psychiatry. 2021;218:326-333. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 627] [Cited by in RCA: 662] [Article Influence: 132.4] [Reference Citation Analysis (0)] |
| 156. | Valdez-Santiago R, Villalobos A, Arenas-Monreal L, González-Forteza C, Hermosillo-de-la-Torre AE, Benjet C, Wagner FA. Comparison of suicide attempts among nationally representative samples of Mexican adolescents 12 months before and after the outbreak of the Covid-19 pandemic. J Affect Disord. 2022;298:65-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 157. | Ryu S, Nam HJ, Jhon M, Lee JY, Kim JM, Kim SW. Trends in suicide deaths before and after the COVID-19 outbreak in Korea. PLoS One. 2022;17:e0273637. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 34] [Reference Citation Analysis (0)] |
| 158. | Gomes CK, Guedes M, Potula HH, Dellagostin OA, Gomes-Solecki M. Sex Matters: Male Hamsters Are More Susceptible to Lethal Infection with Lower Doses of Pathogenic Leptospira than Female Hamsters. Infect Immun. 2018;86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 159. | Pasche B, Kalaydjiev S, Franz TJ, Kremmer E, Gailus-Durner V, Fuchs H, Hrabé de Angelis M, Lengeling A, Busch DH. Sex-dependent susceptibility to Listeria monocytogenes infection is mediated by differential interleukin-10 production. Infect Immun. 2005;73:5952-5960. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 54] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 160. | Textoris J, Ban LH, Capo C, Raoult D, Leone M, Mege JL. Sex-related differences in gene expression following Coxiella burnetii infection in mice: potential role of circadian rhythm. PLoS One. 2010;5:e12190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 161. | Demko CA, Byard PJ, Davis PB. Gender differences in cystic fibrosis: Pseudomonas aeruginosa infection. J Clin Epidemiol. 1995;48:1041-1049. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 211] [Cited by in RCA: 206] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 162. | Gannon CJ, Pasquale M, Tracy JK, McCarter RJ, Napolitano LM. Male gender is associated with increased risk for postinjury pneumonia. Shock. 2004;21:410-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 146] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 163. | Lakbar I, Einav S, Lalevée N, Martin-Loeches I, Pastene B, Leone M. Interactions between Gender and Sepsis-Implications for the Future. Microorganisms. 2023;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 45] [Reference Citation Analysis (0)] |
| 164. | Zellweger R, Wichmann MW, Ayala A, Stein S, DeMaso CM, Chaudry IH. Females in proestrus state maintain splenic immune functions and tolerate sepsis better than males. Crit Care Med. 1997;25:106-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 299] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 165. | Knöferl MW, Angele MK, Diodato MD, Schwacha MG, Ayala A, Cioffi WG, Bland KI, Chaudry IH. Female sex hormones regulate macrophage function after trauma-hemorrhage and prevent increased death rate from subsequent sepsis. Ann Surg. 2002;235:105-112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 157] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 166. | Wichmann MW, Zellweger R, DeMaso CM, Ayala A, Chaudry IH. Mechanism of immunosuppression in males following trauma-hemorrhage. Critical role of testosterone. Arch Surg. 1996;131:1186-91; discussion 1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 132] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 167. | Angele MK, Ayala A, Cioffi WG, Bland KI, Chaudry IH. Testosterone: the culprit for producing splenocyte immune depression after trauma hemorrhage. Am J Physiol. 1998;274:C1530-C1536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 94] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 168. | Bösch F, Angele MK, Chaudry IH. Gender differences in trauma, shock and sepsis. Mil Med Res. 2018;5:35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 78] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 169. | Hubbard WJ, Yang S, Chaudry IH. Ethinyl estradiol sulfate acts without fluid resuscitation through estrogen receptors to rapidly protect the cardiovascular system from severe hemorrhage. J Trauma Acute Care Surg. 2021;90:353-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 170. | Mayer AR, Dodd AB, Rannou-Latella JG, Stephenson DD, Dodd RJ, Ling JM, Mehos CJ, Robertson-Benta CR, Pabbathi Reddy S, Kinsler RE, Vermillion MS, Gigliotti AP, Sicard V, Lloyd AL, Erhardt EB, Gill JM, Lai C, Guedes VA, Chaudry IH. 17α-Ethinyl estradiol-3-sulfate increases survival and hemodynamic functioning in a large animal model of combined traumatic brain injury and hemorrhagic shock: a randomized control trial. Crit Care. 2021;25:428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 171. | Abdou H, Morrison JJ, Edwards J, Patel N, Lang E, Richmond MJ, Elansary N, Gopalakrishnan M, Berman J, Hubbard WJ, Scalea TM, Chaudry IH. An estrogen (17α-ethinyl estradiol-3-sulfate) reduces mortality in a swine model of multiple injuries and hemorrhagic shock. J Trauma Acute Care Surg. 2022;92:57-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 172. | Schwinge D, Carambia A, Quaas A, Krech T, Wegscheid C, Tiegs G, Prinz I, Lohse AW, Herkel J, Schramm C. Testosterone suppresses hepatic inflammation by the downregulation of IL-17, CXCL-9, and CXCL-10 in a mouse model of experimental acute cholangitis. J Immunol. 2015;194:2522-2530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 59] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 173. | Strasburger J. Untersuchungen über die Bakterienmenge in menschlichen Fäces. Zeitschrift für Klinische Medicin. 1902;48:413-444. |
| 174. | Sender R, Fuchs S, Milo R. Are We Really Vastly Outnumbered? Revisiting the Ratio of Bacterial to Host Cells in Humans. Cell. 2016;164:337-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1022] [Cited by in RCA: 1345] [Article Influence: 134.5] [Reference Citation Analysis (0)] |
| 175. | Belkaid Y, Hand TW. Role of the microbiota in immunity and inflammation. Cell. 2014;157:121-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2488] [Cited by in RCA: 3677] [Article Influence: 306.4] [Reference Citation Analysis (1)] |
| 176. | Chen YE, Fischbach MA, Belkaid Y. Skin microbiota-host interactions. Nature. 2018;553:427-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 493] [Article Influence: 61.6] [Reference Citation Analysis (0)] |
| 177. | Chen Y, Zhou J, Wang L. Role and Mechanism of Gut Microbiota in Human Disease. Front Cell Infect Microbiol. 2021;11:625913. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 354] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
| 178. | De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010;107:14691-14696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3584] [Cited by in RCA: 4131] [Article Influence: 258.2] [Reference Citation Analysis (0)] |
| 179. | Aranow C. Vitamin D and the immune system. J Investig Med. 2011;59:881-886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 851] [Cited by in RCA: 788] [Article Influence: 52.5] [Reference Citation Analysis (0)] |
| 180. | Gutiérrez S, Svahn SL, Johansson ME. Effects of Omega-3 Fatty Acids on Immune Cells. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 188] [Cited by in RCA: 361] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 181. | Gill HS, Rutherfurd KJ, Cross ML, Gopal PK. Enhancement of immunity in the elderly by dietary supplementation with the probiotic Bifidobacterium lactis HN019. Am J Clin Nutr. 2001;74:833-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 291] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 182. | Endres S, Ghorbani R, Kelley VE, Georgilis K, Lonnemann G, van der Meer JW, Cannon JG, Rogers TS, Klempner MS, Weber PC. The effect of dietary supplementation with n-3 polyunsaturated fatty acids on the synthesis of interleukin-1 and tumor necrosis factor by mononuclear cells. N Engl J Med. 1989;320:265-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1482] [Cited by in RCA: 1372] [Article Influence: 37.1] [Reference Citation Analysis (1)] |
| 183. | Cooper AL, Gibbons L, Horan MA, Little RA, Rothwell NJ. Effect of dietary fish oil supplementation on fever and cytokine production in human volunteers. Clin Nutr. 1993;12:321-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 184. | Metchnikoff É. Études sur la flore intestinale. Poisons intestinaux et scléroses. Ann nst. Pasteur. 1910;24:755-770. |
| 185. | Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, Britt EB, Fu X, Wu Y, Li L, Smith JD, DiDonato JA, Chen J, Li H, Wu GD, Lewis JD, Warrier M, Brown JM, Krauss RM, Tang WH, Bushman FD, Lusis AJ, Hazen SL. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. 2013;19:576-585. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2783] [Cited by in RCA: 3295] [Article Influence: 253.5] [Reference Citation Analysis (0)] |
| 186. | Phillips JGP. The Treatment of Melancholia by the Lactic Acid Bacillus. J ment sci. 1910;56:422-430. [RCA] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 187. | Del Toro-Barbosa M, Hurtado-Romero A, Garcia-Amezquita LE, García-Cayuela T. Psychobiotics: Mechanisms of Action, Evaluation Methods and Effectiveness in Applications with Food Products. Nutrients. 2020;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 92] [Article Influence: 15.3] [Reference Citation Analysis (1)] |
| 188. | Chooi YC, Ding C, Magkos F. The epidemiology of obesity. Metabolism. 2019;92:6-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1143] [Cited by in RCA: 1852] [Article Influence: 264.6] [Reference Citation Analysis (0)] |
| 189. | Ouchi N, Parker JL, Lugus JJ, Walsh K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol. 2011;11:85-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3103] [Cited by in RCA: 3310] [Article Influence: 220.7] [Reference Citation Analysis (1)] |
| 190. | Yin Z, Deng T, Peterson LE, Yu R, Lin J, Hamilton DJ, Reardon PR, Sherman V, Winnier GE, Zhan M, Lyon CJ, Wong ST, Hsueh WA. Transcriptome analysis of human adipocytes implicates the NOD-like receptor pathway in obesity-induced adipose inflammation. Mol Cell Endocrinol. 2014;394:80-87. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 191. | López-Reyes A, Martinez-Armenta C, Espinosa-Velázquez R, Vázquez-Cárdenas P, Cruz-Ramos M, Palacios-Gonzalez B, Gomez-Quiroz LE, Martínez-Nava GA. NLRP3 Inflammasome: The Stormy Link Between Obesity and COVID-19. Front Immunol. 2020;11:570251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 192. | Rodrigues TS, de Sá KSG, Ishimoto AY, Becerra A, Oliveira S, Almeida L, Gonçalves AV, Perucello DB, Andrade WA, Castro R, Veras FP, Toller-Kawahisa JE, Nascimento DC, de Lima MHF, Silva CMS, Caetite DB, Martins RB, Castro IA, Pontelli MC, de Barros FC, do Amaral NB, Giannini MC, Bonjorno LP, Lopes MIF, Santana RC, Vilar FC, Auxiliadora-Martins M, Luppino-Assad R, de Almeida SCL, de Oliveira FR, Batah SS, Siyuan L, Benatti MN, Cunha TM, Alves-Filho JC, Cunha FQ, Cunha LD, Frantz FG, Kohlsdorf T, Fabro AT, Arruda E, de Oliveira RDR, Louzada-Junior P, Zamboni DS. Inflammasomes are activated in response to SARS-CoV-2 infection and are associated with COVID-19 severity in patients. J Exp Med. 2021;218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 315] [Cited by in RCA: 605] [Article Influence: 100.8] [Reference Citation Analysis (0)] |
| 193. | Bolton JL, Hayward C, Direk N, Lewis JG, Hammond GL, Hill LA, Anderson A, Huffman J, Wilson JF, Campbell H, Rudan I, Wright A, Hastie N, Wild SH, Velders FP, Hofman A, Uitterlinden AG, Lahti J, Räikkönen K, Kajantie E, Widen E, Palotie A, Eriksson JG, Kaakinen M, Järvelin MR, Timpson NJ, Davey Smith G, Ring SM, Evans DM, St Pourcain B, Tanaka T, Milaneschi Y, Bandinelli S, Ferrucci L, van der Harst P, Rosmalen JG, Bakker SJ, Verweij N, Dullaart RP, Mahajan A, Lindgren CM, Morris A, Lind L, Ingelsson E, Anderson LN, Pennell CE, Lye SJ, Matthews SG, Eriksson J, Mellstrom D, Ohlsson C, Price JF, Strachan MW, Reynolds RM, Tiemeier H, Walker BR; CORtisol NETwork (CORNET) Consortium. Genome wide association identifies common variants at the SERPINA6/SERPINA1 locus influencing plasma cortisol and corticosteroid binding globulin. PLoS Genet. 2014;10:e1004474. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 116] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 194. | Harizi H, Homo-Delarche F, Amrani A, Coulaud J, Mormède P. Marked genetic differences in the regulation of blood glucose under immune and restraint stress in mice reveals a wide range of corticosensitivity. J Neuroimmunol. 2007;189:59-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 195. | Way BM, Taylor SE. The serotonin transporter promoter polymorphism is associated with cortisol response to psychosocial stress. Biol Psychiatry. 2010;67:487-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 125] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 196. | DeRijk R, de Kloet ER. Corticosteroid receptor genetic polymorphisms and stress responsivity. Endocrine. 2005;28:263-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 70] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 197. | Baghai TC, Schule C, Zwanzger P, Minov C, Zill P, Ella R, Eser D, Oezer S, Bondy B, Rupprecht R. Hypothalamic-pituitary-adrenocortical axis dysregulation in patients with major depression is influenced by the insertion/deletion polymorphism in the angiotensin I-converting enzyme gene. Neurosci Lett. 2002;328:299-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 58] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 198. | Howie H, Rijal CM, Ressler KJ. A review of epigenetic contributions to post-traumatic stress disorder . Dialogues Clin Neurosci. 2019;21:417-428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 199. | Gelernter J, Sun N, Polimanti R, Pietrzak R, Levey DF, Bryois J, Lu Q, Hu Y, Li B, Radhakrishnan K, Aslan M, Cheung KH, Li Y, Rajeevan N, Sayward F, Harrington K, Chen Q, Cho K, Pyarajan S, Sullivan PF, Quaden R, Shi Y, Hunter-Zinck H, Gaziano JM, Concato J, Zhao H, Stein MB; Department of Veterans Affairs Cooperative Studies Program (#575B) and Million Veteran Program. Genome-wide association study of post-traumatic stress disorder reexperiencing symptoms in >165,000 US veterans. Nat Neurosci. 2019;22:1394-1401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 155] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 200. | Castro-Vale I, Durães C, van Rossum EFC, Staufenbiel SM, Severo M, Lemos MC, Carvalho D. The Glucocorticoid Receptor Gene (NR3C1) 9β SNP Is Associated with Posttraumatic Stress Disorder. Healthcare (Basel). 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 201. | Xie P, Kranzler HR, Yang C, Zhao H, Farrer LA, Gelernter J. Genome-wide association study identifies new susceptibility loci for posttraumatic stress disorder. Biol Psychiatry. 2013;74:656-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 202. | Logue MW, Baldwin C, Guffanti G, Melista E, Wolf EJ, Reardon AF, Uddin M, Wildman D, Galea S, Koenen KC, Miller MW. A genome-wide association study of post-traumatic stress disorder identifies the retinoid-related orphan receptor alpha (RORA) gene as a significant risk locus. Mol Psychiatry. 2013;18:937-942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 177] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 203. | Schijven D, Geuze E, Vinkers CH, Pulit SL, Schür RR, Malgaz M, Bekema E, Medic J, van der Kust KE, Veldink JH, Boks MP, Vermetten E, Luykx JJ. Multivariate genome-wide analysis of stress-related quantitative phenotypes. Eur Neuropsychopharmacol. 2019;29:1354-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 204. | Michopoulos V, Rothbaum AO, Jovanovic T, Almli LM, Bradley B, Rothbaum BO, Gillespie CF, Ressler KJ. Association of CRP genetic variation and CRP level with elevated PTSD symptoms and physiological responses in a civilian population with high levels of trauma. Am J Psychiatry. 2015;172:353-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 158] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 205. | Maul S, Giegling I, Fabbri C, Corponi F, Serretti A, Rujescu D. Genetics of resilience: Implications from genome-wide association studies and candidate genes of the stress response system in posttraumatic stress disorder and depression. Am J Med Genet B Neuropsychiatr Genet. 2020;183:77-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 65] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 206. | Gonda X, Hullam G, Antal P, Eszlari N, Petschner P, Hökfelt TG, Anderson IM, Deakin JFW, Juhasz G, Bagdy G. Significance of risk polymorphisms for depression depends on stress exposure. Sci Rep. 2018;8:3946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 207. | Global Foot Print Network. Earth Overshoot Day. [cited 18 September 2024]. Available from: https://www.footprintnetwork.org/our-work/earth-overshoot-day/. |
| 208. | Washington H, Kopnina H. Discussing the Silence and Denial around Population Growth and Its Environmental Impact. How Do We Find Ways Forward? World. 2022;3:1009-1027. [DOI] [Full Text] |
| 209. | Saraswati CM, Judge MA, Weeda LJZ, Bassat Q, Prata N, Le Souëf PN, Bradshaw CJA. Net benefit of smaller human populations to environmental integrity and individual health and wellbeing. Front Public Health. 2024;12:1339933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 210. | Vollset SE, Goren E, Yuan CW, Cao J, Smith AE, Hsiao T, Bisignano C, Azhar GS, Castro E, Chalek J, Dolgert AJ, Frank T, Fukutaki K, Hay SI, Lozano R, Mokdad AH, Nandakumar V, Pierce M, Pletcher M, Robalik T, Steuben KM, Wunrow HY, Zlavog BS, Murray CJL. Fertility, mortality, migration, and population scenarios for 195 countries and territories from 2017 to 2100: a forecasting analysis for the Global Burden of Disease Study. Lancet. 2020;396:1285-1306. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 404] [Cited by in RCA: 603] [Article Influence: 100.5] [Reference Citation Analysis (1)] |
| 211. | GBD 2021 Fertility and Forecasting Collaborators. Global fertility in 204 countries and territories, 1950-2021, with forecasts to 2100: a comprehensive demographic analysis for the Global Burden of Disease Study 2021. Lancet. 2024;403:2057-2099. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 270] [Cited by in RCA: 271] [Article Influence: 135.5] [Reference Citation Analysis (0)] |
| 212. | Selye H. Interactions between systemic and local stress. Br Med J. 1954;1:1167-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 213. | Takahashi H, Tsuda Y, Takeuchi D, Kobayashi M, Herndon DN, Suzuki F. Influence of systemic inflammatory response syndrome on host resistance against bacterial infections. Crit Care Med. 2004;32:1879-1885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 214. | Ahuja SK, He W. Double-edged genetic swords and immunity: lesson from CCR5 and beyond. J Infect Dis. 2010;201:171-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 215. | Freitas A, Alves-Filho JC, Victoni T, Secher T, Lemos HP, Sônego F, Cunha FQ, Ryffel B. IL-17 receptor signaling is required to control polymicrobial sepsis. J Immunol. 2009;182:7846-7854. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 216. | Flierl MA, Rittirsch D, Gao H, Hoesel LM, Nadeau BA, Day DE, Zetoune FS, Sarma JV, Huber-Lang MS, Ferrara JL, Ward PA. Adverse functions of IL-17A in experimental sepsis. FASEB J. 2008;22:2198-2205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 163] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 217. | Alves-Filho JC, Sônego F, Souto FO, Freitas A, Verri WA Jr, Auxiliadora-Martins M, Basile-Filho A, McKenzie AN, Xu D, Cunha FQ, Liew FY. Interleukin-33 attenuates sepsis by enhancing neutrophil influx to the site of infection. Nat Med. 2010;16:708-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 393] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 218. | Yagami A, Orihara K, Morita H, Futamura K, Hashimoto N, Matsumoto K, Saito H, Matsuda A. IL-33 mediates inflammatory responses in human lung tissue cells. J Immunol. 2010;185:5743-5750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 195] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 219. | Spight D, Trapnell B, Zhao B, Berclaz P, Shanley TP. Granulocyte-macrophage-colony-stimulating factor-dependent peritoneal macrophage responses determine survival in experimentally induced peritonitis and sepsis in mice. Shock. 2008;30:434-442. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 220. | Bozinovski S, Jones JE, Vlahos R, Hamilton JA, Anderson GP. Granulocyte/macrophage-colony-stimulating factor (GM-CSF) regulates lung innate immunity to lipopolysaccharide through Akt/Erk activation of NFkappa B and AP-1 in vivo. J Biol Chem. 2002;277:42808-42814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 135] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 221. | Heuer JG, Zhang T, Zhao J, Ding C, Cramer M, Justen KL, Vonderfecht SL, Na S. Adoptive transfer of in vitro-stimulated CD4+CD25+ regulatory T cells increases bacterial clearance and improves survival in polymicrobial sepsis. J Immunol. 2005;174:7141-7146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 97] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 222. | Hiraki S, Ono S, Tsujimoto H, Kinoshita M, Takahata R, Miyazaki H, Saitoh D, Hase K. Neutralization of interleukin-10 or transforming growth factor-β decreases the percentages of CD4+ CD25+ Foxp3+ regulatory T cells in septic mice, thereby leading to an improved survival. Surgery. 2012;151:313-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 223. | Ren Y, Xie Y, Jiang G, Fan J, Yeung J, Li W, Tam PK, Savill J. Apoptotic cells protect mice against lipopolysaccharide-induced shock. J Immunol. 2008;180:4978-4985. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 120] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 224. | Weaver JG, Rouse MS, Steckelberg JM, Badley AD. Improved survival in experimental sepsis with an orally administered inhibitor of apoptosis. FASEB J. 2004;18:1185-1191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 62] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 225. | Cavaillon JM. Pro- versus anti-inflammatory cytokines: myth or reality. Cell Mol Biol (Noisy-le-grand). 2001;47:695-702. [PubMed] |
| 226. | Kalinski P. Regulation of immune responses by prostaglandin E2. J Immunol. 2012;188:21-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 967] [Cited by in RCA: 1367] [Article Influence: 97.6] [Reference Citation Analysis (0)] |
| 227. | Sorge RE, Martin LJ, Isbester KA, Sotocinal SG, Rosen S, Tuttle AH, Wieskopf JS, Acland EL, Dokova A, Kadoura B, Leger P, Mapplebeck JC, McPhail M, Delaney A, Wigerblad G, Schumann AP, Quinn T, Frasnelli J, Svensson CI, Sternberg WF, Mogil JS. Olfactory exposure to males, including men, causes stress and related analgesia in rodents. Nat Methods. 2014;11:629-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 626] [Article Influence: 52.2] [Reference Citation Analysis (0)] |
| 228. | Lee BH, Inui D, Suh GY, Kim JY, Kwon JY, Park J, Tada K, Tanaka K, Ietsugu K, Uehara K, Dote K, Tajimi K, Morita K, Matsuo K, Hoshino K, Hosokawa K, Lee KH, Lee KM, Takatori M, Nishimura M, Sanui M, Ito M, Egi M, Honda N, Okayama N, Shime N, Tsuruta R, Nogami S, Yoon SH, Fujitani S, Koh SO, Takeda S, Saito S, Hong SJ, Yamamoto T, Yokoyama T, Yamaguchi T, Nishiyama T, Igarashi T, Kakihana Y, Koh Y; Fever and Antipyretic in Critically ill patients Evaluation (FACE) Study Group. Association of body temperature and antipyretic treatments with mortality of critically ill patients with and without sepsis: multi-centered prospective observational study. Crit Care. 2012;16:R33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 127] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 229. | Cavaillon JM. Good and bad fever. Crit Care. 2012;16:119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 230. | Welch WH. The General Pathology of Fever. Boston Medical Surg J. 1888;118:361-368. [DOI] [Full Text] |
| 231. | Nieman DC. Is infection risk linked to exercise workload? Med Sci Sports Exerc. 2000;32:S406-S411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 130] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 232. | Golshani S, Najafpour A, Hashemian SS, Goudarzi N, Shahmari F, Golshani S, Babaei M, Firoozabadi K, Dürsteler KM, Brühl AB, Shakeri J, Brand S, Sadeghi-Bahmani D. When Much Is Too Much-Compared to Light Exercisers, Heavy Exercisers Report More Mental Health Issues and Stress, but Less Sleep Complaints. Healthcare (Basel). 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 233. | Duggal NA, Pollock RD, Lazarus NR, Harridge S, Lord JM. Major features of immunesenescence, including reduced thymic output, are ameliorated by high levels of physical activity in adulthood. Aging Cell. 2018;17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 188] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 234. | Pedersen L, Idorn M, Olofsson GH, Lauenborg B, Nookaew I, Hansen RH, Johannesen HH, Becker JC, Pedersen KS, Dethlefsen C, Nielsen J, Gehl J, Pedersen BK, Thor Straten P, Hojman P. Voluntary Running Suppresses Tumor Growth through Epinephrine- and IL-6-Dependent NK Cell Mobilization and Redistribution. Cell Metab. 2016;23:554-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 626] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 235. | O'Keefe EL, Torres-Acosta N, O'Keefe JH, Lavie CJ. Training for Longevity: The Reverse J-Curve for Exercise. Mo Med. 2020;117:355-361. [PubMed] |
| 236. | Strain T, Flaxman S, Guthold R, Semenova E, Cowan M, Riley LM, Bull FC, Stevens GA; Country Data Author Group. National, regional, and global trends in insufficient physical activity among adults from 2000 to 2022: a pooled analysis of 507 population-based surveys with 5·7 million participants. Lancet Glob Health. 2024;12:e1232-e1243. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 398] [Article Influence: 199.0] [Reference Citation Analysis (0)] |
| 237. | Munford RS, Pugin J. Normal responses to injury prevent systemic inflammation and can be immunosuppressive. Am J Respir Crit Care Med. 2001;163:316-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 372] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 238. | Cavaillon JM, Adib-Conquy M, Cloëz-Tayarani I, Fitting C. Immunodepression in sepsis and SIRS assessed by ex vivo cytokine production is not a generalized phenomenon: a review. J Endotoxin Res. 2001;7:85-93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 239. | Cavaillon JM. During Sepsis and COVID-19, the Pro-Inflammatory and Anti-Inflammatory Responses Are Concomitant. Clin Rev Allergy Immunol. 2023;65:183-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 240. | Adib-Conquy M, Cavaillon JM. Compensatory anti-inflammatory response syndrome. Thromb Haemost. 2009;101:36-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 232] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 241. | van Deuren M, van der Ven-Jongekrijg J, Bartelink AK, van Dalen R, Sauerwein RW, van der Meer JW. Correlation between proinflammatory cytokines and antiinflammatory mediators and the severity of disease in meningococcal infections. J Infect Dis. 1995;172:433-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 159] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 242. | Gómez-Jiménez J, Martín MC, Sauri R, Segura RM, Esteban F, Ruiz JC, Nuvials X, Bóveda JL, Peracaula R, Salgado A. Interleukin-10 and the monocyte/macrophage-induced inflammatory response in septic shock. J Infect Dis. 1995;171:472-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 60] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 243. | Cavaillon JM, Adib-Conquy M, Fitting C, Adrie C, Payen D. Cytokine cascade in sepsis. Scand J Infect Dis. 2003;35:535-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 217] [Cited by in RCA: 230] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 244. | Kellum JA, Kong L, Fink MP, Weissfeld LA, Yealy DM, Pinsky MR, Fine J, Krichevsky A, Delude RL, Angus DC; GenIMS Investigators. Understanding the inflammatory cytokine response in pneumonia and sepsis: results of the Genetic and Inflammatory Markers of Sepsis (GenIMS) Study. Arch Intern Med. 2007;167:1655-1663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 510] [Cited by in RCA: 615] [Article Influence: 32.4] [Reference Citation Analysis (0)] |
| 245. | Adrie C, Adib-Conquy M, Laurent I, Monchi M, Vinsonneau C, Fitting C, Fraisse F, Dinh-Xuan AT, Carli P, Spaulding C, Dhainaut JF, Cavaillon JM. Successful cardiopulmonary resuscitation after cardiac arrest as a "sepsis-like" syndrome. Circulation. 2002;106:562-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 707] [Cited by in RCA: 757] [Article Influence: 31.5] [Reference Citation Analysis (0)] |
| 246. | Serhan CN, Levy BD. Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J Clin Invest. 2018;128:2657-2669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 613] [Cited by in RCA: 1002] [Article Influence: 125.3] [Reference Citation Analysis (0)] |
| 247. | Mantovani A, Garlanda C. Humoral Innate Immunity and Acute-Phase Proteins. N Engl J Med. 2023;388:439-452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 191] [Article Influence: 63.7] [Reference Citation Analysis (0)] |
| 248. | Vale W, Spiess J, Rivier C, Rivier J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science. 1981;213:1394-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3371] [Cited by in RCA: 3210] [Article Influence: 71.3] [Reference Citation Analysis (16)] |
| 249. | Collip J, Anderson E, Thomson D. The adrenotropic hormone of the anterior pituitary lobe. The Lancet. 1933;222:347-348. [DOI] [Full Text] |
| 250. | Lannan EA, Galliher-Beckley AJ, Scoltock AB, Cidlowski JA. Proinflammatory actions of glucocorticoids: glucocorticoids and TNFα coregulate gene expression in vitro and in vivo. Endocrinology. 2012;153:3701-3712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 76] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 251. | Cavaillon JM, Giamarellos-Bourboulis EJ. Immunosuppression is Inappropriately Qualifying the Immune Status of Septic and SIRS Patients. Shock. 2019;52:307-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 252. | Rosas-Ballina M, Olofsson PS, Ochani M, Valdés-Ferrer SI, Levine YA, Reardon C, Tusche MW, Pavlov VA, Andersson U, Chavan S, Mak TW, Tracey KJ. Acetylcholine-synthesizing T cells relay neural signals in a vagus nerve circuit. Science. 2011;334:98-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1074] [Cited by in RCA: 1177] [Article Influence: 78.5] [Reference Citation Analysis (0)] |
| 253. | Andersson U, Tracey KJ. Neural reflexes in inflammation and immunity. J Exp Med. 2012;209:1057-1068. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 277] [Cited by in RCA: 298] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 254. | Koopman FA, Chavan SS, Miljko S, Grazio S, Sokolovic S, Schuurman PR, Mehta AD, Levine YA, Faltys M, Zitnik R, Tracey KJ, Tak PP. Vagus nerve stimulation inhibits cytokine production and attenuates disease severity in rheumatoid arthritis. Proc Natl Acad Sci U S A. 2016;113:8284-8289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 561] [Cited by in RCA: 744] [Article Influence: 74.4] [Reference Citation Analysis (0)] |
| 255. | D'Haens G, Eberhardson M, Cabrijan Z, Danese S, van den Berg R, Löwenberg M, Fiorino G, Schuurman PR, Lind G, Almqvist P, Olofsson PS, Tracey KJ, Hanauer SB, Zitnik R, Chernoff D, Levine YA. Neuroimmune Modulation Through Vagus Nerve Stimulation Reduces Inflammatory Activity in Crohn's Disease Patients: A Prospective Open-label Study. J Crohns Colitis. 2023;17:1897-1909. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 256. | Sahn B, Pascuma K, Kohn N, Tracey KJ, Markowitz JF. Transcutaneous auricular vagus nerve stimulation attenuates inflammatory bowel disease in children: a proof-of-concept clinical trial. Bioelectron Med. 2023;9:23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 257. | Beeson PB. Development of tolerance to typhoid bacterial pyrogen and its abolition by reticulo-endothelial blockade. Proc Soc Exp Biol Med. 1946;61:248-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 83] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 258. | Rayhane N, Fitting C, Cavaillon J. Dissociation of IFN-γ from IL-12 and IL-18 production during endotoxin tolerance. J Endotoxin Res. 1999;5:319-324. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 259. | Zweifach BW, THOMAS L. The relationship between the vascular manifestations of shock produced by endotoxin, trauma, and hemorrhage. I. Certain similarities between the reactions in normal and endotoxin-tolerant rats. J Exp Med. 1957;106:385-401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 80] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 260. | Meng X, Brown JM, Ao L, Rowland RT, Nordeen SK, Banerjee A, Harken AH. Myocardial gene reprogramming associated with a cardiac cross-resistant state induced by LPS preconditioning. Am J Physiol. 1998;275:C475-C483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 261. | Rayhane N, Fitting C, Lortholary O, Dromer F, Cavaillon JM. Administration of endotoxin associated with lipopolysaccharide tolerance protects mice against fungal infection. Infect Immun. 2000;68:3748-3753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 262. | Lehner MD, Ittner J, Bundschuh DS, van Rooijen N, Wendel A, Hartung T. Improved innate immunity of endotoxin-tolerant mice increases resistance to Salmonella enterica serovar typhimurium infection despite attenuated cytokine response. Infect Immun. 2001;69:463-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 90] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 263. | Echtenacher B, Männel DN. Requirement of TNF and TNF receptor type 2 for LPS-induced protection from lethal septic peritonitis. J Endotoxin Res. 2002;8:365-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 264. | Shinya K, Ito M, Makino A, Tanaka M, Miyake K, Eisfeld AJ, Kawaoka Y. The TLR4-TRIF pathway protects against H5N1 influenza virus infection. J Virol. 2012;86:19-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 265. | Canavese M, Dottorini T, Crisanti A. VEGF and LPS synergistically silence inflammatory response to Plasmodium berghei infection and protect against cerebral malaria. Pathog Glob Health. 2015;109:255-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 266. | Zhang X, Morrison DC. Lipopolysaccharide structure-function relationship in activation versus reprogramming of mouse peritoneal macrophages. J Leukoc Biol. 1993;54:444-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 267. | Cavaillon JM, Adib-Conquy M. Bench-to-bedside review: endotoxin tolerance as a model of leukocyte reprogramming in sepsis. Crit Care. 2006;10:233. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 352] [Cited by in RCA: 402] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 268. | Boraschi D, Italiani P. Innate Immune Memory: Time for Adopting a Correct Terminology. Front Immunol. 2018;9:799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 73] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 269. | Netea MG, Quintin J, van der Meer JW. Trained immunity: a memory for innate host defense. Cell Host Microbe. 2011;9:355-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 850] [Cited by in RCA: 1167] [Article Influence: 77.8] [Reference Citation Analysis (0)] |
| 270. | Williams CA Jr, DUBOS RJ. Studies on fractions of methanol extracts of tubercle bacilli. I. Fractions which increase resistance to infection. J Exp Med. 1959;110:981-1004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 271. | Sylvester RJ, van der MEIJDEN AP, Lamm DL. Intravesical bacillus Calmette-Guerin reduces the risk of progression in patients with superficial bladder cancer: a meta-analysis of the published results of randomized clinical trials. J Urol. 2002;168:1964-1970. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 844] [Cited by in RCA: 825] [Article Influence: 34.4] [Reference Citation Analysis (0)] |
| 272. | Balasubramaniam M, Telles S, Doraiswamy PM. Yoga on our minds: a systematic review of yoga for neuropsychiatric disorders. Front Psychiatry. 2012;3:117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 123] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 273. | Ye X, Chen Z, Shen Z, Chen G, Xu X. Yoga for Treating Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Front Med (Lausanne). 2020;7:586665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 31] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 274. | Seaton N, Hudson J, Harding S, Norton S, Mondelli V, Jones ASK, Moss-Morris R. Do interventions for mood improve inflammatory biomarkers in inflammatory bowel disease?: a systematic review and meta-analysis. EBioMedicine. 2024;100:104910. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 275. | Djalilova DM, Schulz PS, Berger AM, Case AJ, Kupzyk KA, Ross AC. Impact of Yoga on Inflammatory Biomarkers: A Systematic Review. Biol Res Nurs. 2019;21:198-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 45] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 276. | Lee SH, Hwang SM, Kang DH, Yang HJ. Brain education-based meditation for patients with hypertension and/or type 2 diabetes: A pilot randomized controlled trial. Medicine (Baltimore). 2019;98:e15574. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 277. | von Werthern M, Robjant K, Chui Z, Schon R, Ottisova L, Mason C, Katona C. The impact of immigration detention on mental health: a systematic review. BMC Psychiatry. 2018;18:382. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 123] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 278. | Bodenmann G, Atkins DC, Schär M, Poffet V. The association between daily stress and sexual activity. J Fam Psychol. 2010;24:271-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 99] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 279. | Ein-dor T, Hirschberger G. Sexual healing. J Soc Pers Relat. 2012;29:126-139. [DOI] [Full Text] |
| 280. | Burri A, Carvalheira A. Masturbatory Behavior in a Population Sample of German Women. J Sex Med. 2019;16:963-974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 281. | Fotinos K, Sansone A, Greifenberger A, Katzman MA, Jannini TB, Reisman Y, Limoncin E, Jannini EA. Pornography and sexual function in the post-pandemic period: a narrative review from psychological, psychiatric, and sexological perspectives. Int J Impot Res. 2024;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 282. | Liu H, Waite LJ, Shen S, Wang DH. Is Sex Good for Your Health? A National Study on Partnered Sexuality and Cardiovascular Risk among Older Men and Women. J Health Soc Behav. 2016;57:276-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 62] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 283. | Sahm A, Platzer M, Koch P, Henning Y, Bens M, Groth M, Burda H, Begall S, Ting S, Goetz M, Van Daele P, Staniszewska M, Klose JM, Costa PF, Hoffmann S, Szafranski K, Dammann P. Increased longevity due to sexual activity in mole-rats is associated with transcriptional changes in the HPA stress axis. Elife. 2021;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 284. | Love TM. The impact of oxytocin on stress: the role of sex. Curr Opin Behav Sci. 2018;23:136-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 285. | Takayanagi Y, Onaka T. Roles of Oxytocin in Stress Responses, Allostasis and Resilience. Int J Mol Sci. 2021;23. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 86] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 286. | Zhang S, Zhang YD, Shi DD, Wang Z. Therapeutic uses of oxytocin in stress-related neuropsychiatric disorders. Cell Biosci. 2023;13:216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 287. | Haake P, Krueger TH, Goebel MU, Heberling KM, Hartmann U, Schedlowski M. Effects of sexual arousal on lymphocyte subset circulation and cytokine production in man. Neuroimmunomodulation. 2004;11:293-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 288. | Tynan RJ, Weidenhofer J, Hinwood M, Cairns MJ, Day TA, Walker FR. A comparative examination of the anti-inflammatory effects of SSRI and SNRI antidepressants on LPS stimulated microglia. Brain Behav Immun. 2012;26:469-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 284] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 289. | Szałach ŁP, Lisowska KA, Cubała WJ. The Influence of Antidepressants on the Immune System. Arch Immunol Ther Exp (Warsz). 2019;67:143-151. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 78] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 290. | Gupta K, Walton R, Kataria SP. Chemotherapy-Induced Nausea and Vomiting: Pathogenesis, Recommendations, and New Trends. Cancer Treat Res Commun. 2021;26:100278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 103] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 291. | Tang Y, Yao T, Tian X, Xia X, Huang X, Qin Z, Shen Z, Zhao L, Zhao Y, Diao B, Ping Y, Zheng X, Xu Y, Chen H, Qian T, Ma T, Zhou B, Xu S, Zhou Q, Liu Y, Shao M, Chen W, Shan B, Wu Y. Hepatic IRE1α-XBP1 signaling promotes GDF15-mediated anorexia and body weight loss in chemotherapy. J Exp Med. 2024;221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 292. | Rauber F, Laura da Costa Louzada M, Chang K, Huybrechts I, Gunter MJ, Monteiro CA, Vamos EP, Levy RB. Implications of food ultra-processing on cardiovascular risk considering plant origin foods: an analysis of the UK Biobank cohort. Lancet Reg Health Eur. 2024;43:100948. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 25] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 293. | Cho HJ, Kim E, Yi YS. Korean Red Ginseng Saponins Play an Anti-Inflammatory Role by Targeting Caspase-11 Non-Canonical Inflammasome in Macrophages. Int J Mol Sci. 2023;24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 294. | Chang YP, Ka SM, Hsu WH, Chen A, Chao LK, Lin CC, Hsieh CC, Chen MC, Chiu HW, Ho CL, Chiu YC, Liu ML, Hua KF. Resveratrol inhibits NLRP3 inflammasome activation by preserving mitochondrial integrity and augmenting autophagy. J Cell Physiol. 2015;230:1567-1579. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 173] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 296. | McIntyre N. The marriage (1878) of Emile Roux (1853-1933) and Rose Anna Shedlock (b. c. 1850). J Med Biogr. 2008;16:175-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 297. | Rondeau V, Commenges D, Jacqmin-Gadda H, Dartigues JF. Relation between aluminum concentrations in drinking water and Alzheimer's disease: an 8-year follow-up study. Am J Epidemiol. 2000;152:59-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 185] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 298. | Van Dyke N, Yenugadhati N, Birkett NJ, Lindsay J, Turner MC, Willhite CC, Krewski D. Association between aluminum in drinking water and incident Alzheimer's disease in the Canadian Study of Health and Aging cohort. Neurotoxicology. 2021;83:157-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 299. | El Hayek J, Egeland G, Weiler H. Vitamin D status of Inuit preschoolers reflects season and vitamin D intake. J Nutr. 2010;140:1839-1845. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 300. | Taniguchi K, Takano M, Tobari Y, Hayano M, Nakajima S, Mimura M, Tsubota K, Noda Y. Influence of External Natural Environment Including Sunshine Exposure on Public Mental Health: A Systematic Review. Psychiatry Int. 2022;3:91-113. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 301. | Souêtre E, Wehr TA, Douillet P, Darcourt G. Influence of environmental factors on suicidal behavior. Psychiatry Res. 1990;32:253-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 302. | Lambert GW, Reid C, Kaye DM, Jennings GL, Esler MD. Effect of sunlight and season on serotonin turnover in the brain. Lancet. 2002;360:1840-1842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 326] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 303. | Antypa N, Serretti A, Rujescu D. Serotonergic genes and suicide: a systematic review. Eur Neuropsychopharmacol. 2013;23:1125-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 304. | Cloëz-Tayarani I, Petit-Bertron AF, Venters HD, Cavaillon JM. Differential effect of serotonin on cytokine production in lipopolysaccharide-stimulated human peripheral blood mononuclear cells: involvement of 5-hydroxytryptamine2A receptors. Int Immunol. 2003;15:233-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 190] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 305. | Armstrong BK, Kricker A. The epidemiology of UV induced skin cancer. J Photochem Photobiol B. 2001;63:8-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1231] [Cited by in RCA: 1160] [Article Influence: 46.4] [Reference Citation Analysis (0)] |
| 306. | Shraim R, Farran MZ, He G, Marunica Karsaj J, Zgaga L, McManus R. Systematic review on gene-sun exposure interactions in skin cancer. Mol Genet Genomic Med. 2023;11:e2259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 307. | Davies S, Pettersson T, Öberg M. Organized violence 1989–2022, and the return of conflict between states. J Peace Res. 2023;60:691-708. [DOI] [Full Text] |
| 308. | United Nations. Over 114 million displaced by war, violence worldwide. [cited 18 September 2024]. Available from: https://news.un.org/en/story/2023/10/1142827. |
| 309. | IPCC. Summary for Policymakers. [cited 18 September 2024]. Available from: https://www.ipcc.ch/sr15/chapter/spm/. |
| 310. | Cianconi P, Betrò S, Janiri L. The Impact of Climate Change on Mental Health: A Systematic Descriptive Review. Front Psychiatry. 2020;11:74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 421] [Cited by in RCA: 438] [Article Influence: 73.0] [Reference Citation Analysis (0)] |
| 311. | Dinerstein E, Joshi AR, Hahn NR, Lee ATL, Vynne C, Burkart K, Asner GP, Beckham C, Ceballos G, Cuthbert R, Dirzo R, Fankem O, Hertel S, Li BV, Mellin H, Pharand-deschênes F, Olson D, Pandav B, Peres CA, Putra R, Rosenthal A, Verwer C, Wikramanayake E, Zolli A. Conservation Imperatives: securing the last unprotected terrestrial sites harboring irreplaceable biodiversity. Front Sci. 2024;2. [DOI] [Full Text] |
| 312. | Agache I, Akdis C, Akdis M, Al-hemoud A, Annesi-maesano I, Balmes J, Cecchi L, Damialis A, Haahtela T, Haber AL, Hart JE, Jutel M, Mitamura Y, Mmbaga BT, Oh J, Ostadtaghizadeh A, Pawankar R, Prunicki M, Renz H, Rice MB, Filho NAR, Sampath V, Skevaki C, Thien F, Traidl-hoffmann C, Wong GWK, Nadeau KC. Immune-mediated disease caused by climate change-associated environmental hazards: mitigation and adaptation. Front Sci. 2024;2. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 313. | Afek A, Ben-Avraham R, Davidov A, Berezin Cohen N, Ben Yehuda A, Gilboa Y, Nahum M. Psychological Resilience, Mental Health, and Inhibitory Control Among Youth and Young Adults Under Stress. Front Psychiatry. 2020;11:608588. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 314. | Rende R. Behavioral resilience in the post-genomic era: emerging models linking genes with environment. Front Hum Neurosci. 2012;6:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 315. | Mira T, Costa AM, Jacinto M, Diz S, Monteiro D, Rodrigues F, Matos R, Antunes R. Well-Being, Resilience and Social Support of Athletes with Disabilities: A Systematic Review. Behav Sci (Basel). 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 316. | Kimhi S, Eshel Y, Marciano H, Adini B. Impact of the war in Ukraine on resilience, protective, and vulnerability factors. Front Public Health. 2023;11:1053940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 317. | Riris P, Silva F, Crema E, Palmisano A, Robinson E, Siegel PE, French JC, Jørgensen EK, Maezumi SY, Solheim S, Bates J, Davies B, Oh Y, Ren X. Frequent disturbances enhanced the resilience of past human populations. Nature. 2024;629:837-842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 318. | Thorogood R, Mustonen V, Aleixo A, Aphalo PJ, Asiegbu FO, Cabeza M, Cairns J, Candolin U, Cardoso P, Eronen JT, Hällfors M, Hovatta I, Juslén A, Kovalchuk A, Kulmuni J, Kuula L, Mäkipää R, Ovaskainen O, Pesonen AK, Primmer CR, Saastamoinen M, Schulman AH, Schulman L, Strona G, Vanhatalo J. Understanding and applying biological resilience, from genes to ecosystems. NPJ Biodivers. 2023;2:16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 23] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 319. | Soo SK, Rudich ZD, Ko B, Moldakozhayev A, AlOkda A, Van Raamsdonk JM. Biological resilience and aging: Activation of stress response pathways contributes to lifespan extension. Ageing Res Rev. 2023;88:101941. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 320. | Singer M. Critical illness and flat batteries. Crit Care. 2017;21:309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 64] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 321. | Ahuja SK, Manoharan MS, Lee GC, McKinnon LR, Meunier JA, Steri M, Harper N, Fiorillo E, Smith AM, Restrepo MI, Branum AP, Bottomley MJ, Orrù V, Jimenez F, Carrillo A, Pandranki L, Winter CA, Winter LA, Gaitan AA, Moreira AG, Walter EA, Silvestri G, King CL, Zheng YT, Zheng HY, Kimani J, Blake Ball T, Plummer FA, Fowke KR, Harden PN, Wood KJ, Ferris MT, Lund JM, Heise MT, Garrett N, Canady KR, Abdool Karim SS, Little SJ, Gianella S, Smith DM, Letendre S, Richman DD, Cucca F, Trinh H, Sanchez-Reilly S, Hecht JM, Cadena Zuluaga JA, Anzueto A, Pugh JA; South Texas Veterans Health Care System COVID-19 team, Agan BK, Root-Bernstein R, Clark RA, Okulicz JF, He W. Immune resilience despite inflammatory stress promotes longevity and favorable health outcomes including resistance to infection. Nat Commun. 2023;14:3286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 48] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 322. | Chen S, Stark SC, Nobre AD, Cuartas LA, de Jesus Amore D, Restrepo-Coupe N, Smith MN, Chitra-Tarak R, Ko H, Nelson BW, Saleska SR. Amazon forest biogeography predicts resilience and vulnerability to drought. Nature. 2024;631:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 323. | Warren HS, Fitting C, Hoff E, Adib-Conquy M, Beasley-Topliffe L, Tesini B, Liang X, Valentine C, Hellman J, Hayden D, Cavaillon JM. Resilience to bacterial infection: difference between species could be due to proteins in serum. J Infect Dis. 2010;201:223-232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 205] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 324. | Faria NR, Rambaut A, Suchard MA, Baele G, Bedford T, Ward MJ, Tatem AJ, Sousa JD, Arinaminpathy N, Pépin J, Posada D, Peeters M, Pybus OG, Lemey P. HIV epidemiology. The early spread and epidemic ignition of HIV-1 in human populations. Science. 2014;346:56-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 412] [Article Influence: 34.3] [Reference Citation Analysis (0)] |
| 325. | Silvestri G, Paiardini M, Pandrea I, Lederman MM, Sodora DL. Understanding the benign nature of SIV infection in natural hosts. J Clin Invest. 2007;117:3148-3154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 161] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 326. | Liovat AS, Jacquelin B, Ploquin MJ, Barré-Sinoussi F, Müller-Trutwin MC. African non human primates infected by SIV - why don't they get sick? Lessons from studies on the early phase of non-pathogenic SIV infection. Curr HIV Res. 2009;7:39-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 327. | Pereira PDC, Diniz DG, da Costa ER, Magalhães NGM, da Silva AJF, Leite JGS, Almeida NIP, Cunha KN, de Melo MAD, Vasconcelos PFDC, Diniz JAP, Brites D, Anthony DC, Diniz CWP, Guerreiro-Diniz C. Genes, inflammatory response, tolerance, and resistance to virus infections in migratory birds, bats, and rodents. Front Immunol. 2023;14:1239572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 328. | Anthony SJ, Johnson CK, Greig DJ, Kramer S, Che X, Wells H, Hicks AL, Joly DO, Wolfe ND, Daszak P, Karesh W, Lipkin WI, Morse SS; PREDICT Consortium, Mazet JAK, Goldstein T. Global patterns in coronavirus diversity. Virus Evol. 2017;3:vex012. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 234] [Cited by in RCA: 277] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 329. | Ahn M, Anderson DE, Zhang Q, Tan CW, Lim BL, Luko K, Wen M, Chia WN, Mani S, Wang LC, Ng JHJ, Sobota RM, Dutertre CA, Ginhoux F, Shi ZL, Irving AT, Wang LF. Dampened NLRP3-mediated inflammation in bats and implications for a special viral reservoir host. Nat Microbiol. 2019;4:789-799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 249] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 330. | Irving AT, Ahn M, Goh G, Anderson DE, Wang LF. Lessons from the host defences of bats, a unique viral reservoir. Nature. 2021;589:363-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 276] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 331. | Tang Y, Winterfeldt S, Brangarí AC, Hicks LC, Rousk J. Higher resistance and resilience of bacterial growth to drought in grasslands with historically lower precipitation. Soil Biol Biochem. 2023;177:108889. [DOI] [Full Text] |
| 332. | Meredith HR, Andreani V, Ma HR, Lopatkin AJ, Lee AJ, Anderson DJ, Batt G, You L. Applying ecological resistance and resilience to dissect bacterial antibiotic responses. Sci Adv. 2018;4:eaau1873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 333. | Liu Q, Zhu J, Dulberger CL, Stanley S, Wilson S, Chung ES, Wang X, Culviner P, Liu YJ, Hicks ND, Babunovic GH, Giffen SR, Aldridge BB, Garner EC, Rubin EJ, Chao MC, Fortune SM. Tuberculosis treatment failure associated with evolution of antibiotic resilience. Science. 2022;378:1111-1118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 72] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 334. | Meirelles LA, Perry EK, Bergkessel M, Newman DK. Bacterial defenses against a natural antibiotic promote collateral resilience to clinical antibiotics. PLoS Biol. 2021;19:e3001093. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 335. | Soares MP, Teixeira L, Moita LF. Disease tolerance and immunity in host protection against infection. Nat Rev Immunol. 2017;17:83-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 252] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 336. | Dienz O, Rud JG, Eaton SM, Lanthier PA, Burg E, Drew A, Bunn J, Suratt BT, Haynes L, Rincon M. Essential role of IL-6 in protection against H1N1 influenza virus by promoting neutrophil survival in the lung. Mucosal Immunol. 2012;5:258-266. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 189] [Cited by in RCA: 236] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 337. | van der Poll T, Levi M, Hack CE, ten Cate H, van Deventer SJ, Eerenberg AJ, de Groot ER, Jansen J, Gallati H, Büller HR. Elimination of interleukin 6 attenuates coagulation activation in experimental endotoxemia in chimpanzees. J Exp Med. 1994;179:1253-1259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 260] [Cited by in RCA: 255] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 338. | Tadokoro T, Wang Y, Barak LS, Bai Y, Randell SH, Hogan BL. IL-6/STAT3 promotes regeneration of airway ciliated cells from basal stem cells. Proc Natl Acad Sci U S A. 2014;111:E3641-E3649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 223] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 339. | Rupert JE, Narasimhan A, Jengelley DHA, Jiang Y, Liu J, Au E, Silverman LM, Sandusky G, Bonetto A, Cao S, Lu X, O'Connell TM, Liu Y, Koniaris LG, Zimmers TA. Tumor-derived IL-6 and trans-signaling among tumor, fat, and muscle mediate pancreatic cancer cachexia. J Exp Med. 2021;218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 106] [Cited by in RCA: 164] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 340. | Qing H, Desrouleaux R, Israni-Winger K, Mineur YS, Fogelman N, Zhang C, Rashed S, Palm NW, Sinha R, Picciotto MR, Perry RJ, Wang A. Origin and Function of Stress-Induced IL-6 in Murine Models. Cell. 2020;182:372-387.e14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 173] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 341. | Savić D, Knežević G, Opačić G. A mathematical model of stress reaction: Individual differences in threshold and duration. Psychobiology. 2000;28:581-592. [DOI] [Full Text] |
| 342. | Malek H, Ebadzadeh MM, Safabakhsh R, Razavi A, Zaringhalam J. Dynamics of the HPA axis and inflammatory cytokines: Insights from mathematical modeling. Comput Biol Med. 2015;67:1-12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 343. | Bangsgaard EO, Hjorth PG, Olufsen MS, Mehlsen J, Ottesen JT. Integrated Inflammatory Stress (ITIS) Model. Bull Math Biol. 2017;79:1487-1509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 344. | Gonzalez Herrero ME, Kuehn C. A qualitative mathematical model of the immune response under the effect of stress. Chaos. 2021;31:061104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 345. | Galvis D, Zavala E, Walker JJ, Upton T, Lightman SL, Angelini GD, Evans J, Rogers CA, Phillips K, Gibbison B. Modelling the dynamic interaction of systemic inflammation and the hypothalamic-pituitary-adrenal (HPA) axis during and after cardiac surgery. J R Soc Interface. 2022;19:20210925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 13] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 346. | Hora J, Rambhia N, Mani I. Drug repurposing for personalized medicine. Prog Mol Biol Transl Sci. 2024;207:107-122. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 347. | Ioannou M, Foiselle M, Mallet J, Stam EL, Godin O, Dubertret C, Terro E, Sommer IEC, Haarman BCM, Leboyer M, Schoevers RA. Towards precision medicine: What are the stratification hypotheses to identify homogeneous inflammatory subgroups. Eur Neuropsychopharmacol. 2021;45:108-121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 348. | Drevets WC, Wittenberg GM, Bullmore ET, Manji HK. Immune targets for therapeutic development in depression: towards precision medicine. Nat Rev Drug Discov. 2022;21:224-244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 230] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 349. | Subramanian M, Wojtusciszyn A, Favre L, Boughorbel S, Shan J, Letaief KB, Pitteloud N, Chouchane L. Precision medicine in the era of artificial intelligence: implications in chronic disease management. J Transl Med. 2020;18:472. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 139] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 350. | Moingeon P. Artificial intelligence-driven drug development against autoimmune diseases. Trends Pharmacol Sci. 2023;44:411-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 29] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 351. | Kowara M, Kasarełło K, Czarzasta K, Opolski G, Cudnoch-Jędrzejewska A. Early-life inflammation pathways trigger a cascade leading to development of atherosclerotic plaque through the "butterfly effect" - An hypothesis. Med Hypotheses. 2019;122:106-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 352. | Desi N, Tay Y. The Butterfly Effect of RNA Alterations on Transcriptomic Equilibrium. Cells. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 353. | Cai W, Ng B, Geng T, Wu L, Santoso A, McPhaden MJ. Butterfly effect and a self-modulating El Niño response to global warming. Nature. 2020;585:68-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 354. | van Leeuwen C, Sgubin G, Bois B, Ollat N, Swingedouw D, Zito S, Gambetta GA. Climate change impacts and adaptations of wine production. Nat Rev Earth Environ. 2024;5:258-275. [DOI] [Full Text] |
| 355. | Miller-Struttmann NE. Climate change predicted to exacerbate declines in bee populations. Nature. 2024;628:270-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 356. | Agnew DC. A global timekeeping problem postponed by global warming. Nature. 2024;628:333-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 357. | WHO. For centuries we have plundered our planet. Now we are paying the price. [cited 18 September 2024]. Available from: https://www.who.int/fr/news-room/commentaries/detail/for-centuries-we-have-plundered-our-planet-now-we-are-paying-the-price. |
| 358. | Cavaillon J. Molecular Mediators: Cytokines. Rev Cell Biol Mol Med. 2015;. [DOI] [Full Text] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/