Published online Jun 20, 2024. doi: 10.5493/wjem.v14.i2.94135
Revised: April 23, 2024
Accepted: May 10, 2024
Published online: June 20, 2024
Processing time: 99 Days and 3 Hours
Anastomotic leaks remain one of the most dreaded complications in gastroin
To review current state of the art for experimental protocols in high-risk anasto
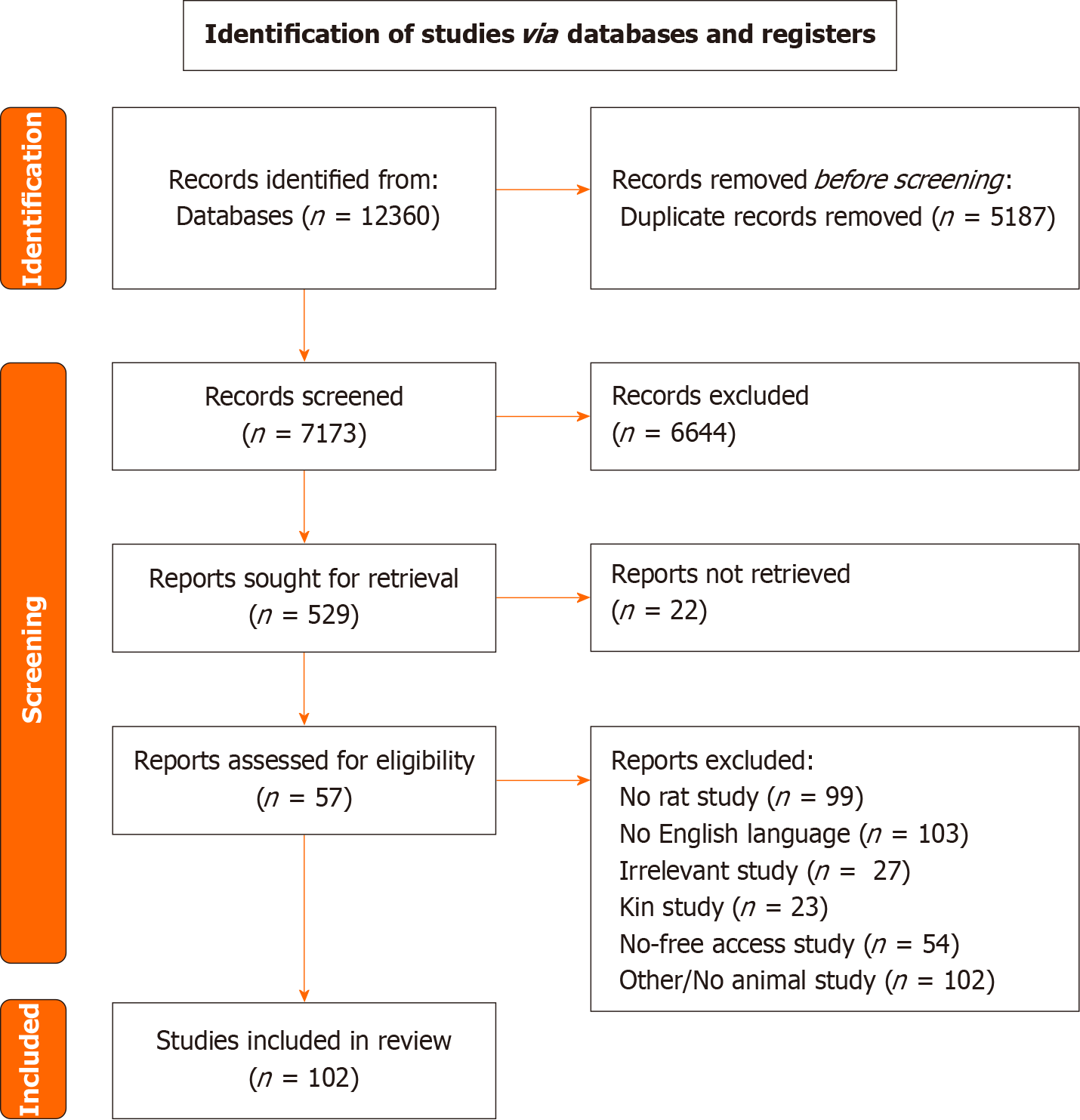
This systematic review was performed according to The Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. To identify eligible studies, a comprehensive literature search was performed in the electronic databases PubMed (MEDLINE) and Scopus, covering the period from conception until 18 October 2023.
From our search strategy 102 studies were included and were categorized based on the mechanism used to create a high-risk anastomosis. Methods of assessing anastomotic healing were extracted and were individually appraised.
Anastomotic healing studies have evolved over the last decades, but the findings are yet to be translated into human studies. There is a need for high-quality, well-designed studies that will help to the better understanding of the pathophysiology of anastomotic healing and the effects of various interventions.
Core Tip: Anastomotic leakage (AL) is a fatal complication after colorectal surgery, with high morbidity and mortality rates. AL rate is increased under emergency conditions. This review can be used as a tool to standardize and refine future research leading to studies that can be translated to human research regarding bowel anastomoses under complicated conditions.
- Citation: Ntampakis G, Pramateftakis MG, Anestiadou E, Bitsianis S, Ioannidis O, Bekiari C, Koliakos G, Karakota M, Tsakona A, Cheva A, Angelopoulos S. Experimental models of high-risk bowel anastomosis in rats: A systematic review. World J Exp Med 2024; 14(2): 94135
- URL: https://www.wjgnet.com/2220-315x/full/v14/i2/94135.htm
- DOI: https://dx.doi.org/10.5493/wjem.v14.i2.94135
Colon diseases are among the most common disorders encountered by general surgeons, since more than 600000 surgical procedures are performed in the United States annually for management of colon-related disorders[1]. The most common indication for colorectal surgery is colon cancer, while other indications for resection include diverticular disease, ischemic colitis, stoma reversal or inflammatory bowel disease[2].
Despite the increased safety of colorectal surgery due to minimally invasive surgery and perioperative management advances, anastomotic leakage (AL) remains a fatal complication of colonic anastomosis, leading to increased morbidity and mortality rates, permanent stoma impaired oncological outcomes and poorer quality of life postoperatively[3,4]. AL rate varies by the level of anastomosis and is approximately 1%–3% for ileocolic anastomosis, 6%–12% for left colon anastomosis, and 3%–19% for colorectal anastomosis[5]. In general, risk factors associated with increased AL rate can be classified to preoperative, intraoperative and postoperative factors[6]. Among them, colonic resection and anastomosis in the emergency setting has been proven to be an independent risk factor for anastomotic dehiscence, as well as death after AL[7,8]. A recent prospective multi-centre study by the American Association for the Surgery of Trauma showed that anastomotic failure rate after emergent bowel resection and colo-colonic anastomosis had a failure rate of 23%, while in patients managed with an open abdomen the same rate was approximately 22%[9].
The traditional surgical dictum suggested that in emergency colectomy due to obstruction or peritonitis of large bowel origin, construction of a colostomy was imperative independently of the severity of peritonitis or the patient's condition[10]. Stomas are also associated with numerous early and late complications, as well as impaired Quality of Life and reduced rate of closure, when performed in the emergency setting[11]. However, recently emerged evidence propose the safety of anastomosis with diverting stoma under circumstances in cases of feculent or purulent peritonitis[12]. Decision regarding anastomosis in the setting of large bowel obstruction is mostly determined by the cause and the site of the obstruction[13]. In addition to peritonitis and obstruction, a series of other local and systemic conditions impair wound healing and render the construction of anastomosis perilous[14].
Animal experimental models constitute the basis of experimental study of colorectal anastomosis healing and permit monitoring of anastomotic healing with use of functional tests, clinical scores, molecular examination and histopathological examination[15]. Pommergaard et al[16], in their systematic review, evaluated the different experimental animal models that have been used for study of colorectal AL. Animal models reported in the literature include mice, pigs, rats, dogs and rabbits, with mice and pigs being the proposed by the authors experimental models for mimicking AL[16]. In addition, numerous studies have investigated the role of different potential therapeutic agents in healing of anastomosis in experimental models both under normal and pathological conditions, such as inflammation, peritonitis, obstruction, ischemia or jaundice[17]. However, pathophysiological mechanisms behind the formation of high-risk anastomoses for research aims have been scarcely evaluated and reported in the literature.
The aim of the present systematic review was to identify and classify types of experimental anastomosis that mimic high-risk colonic anastomosis in humans, in order to provide a guide for formation of standardized and easily repro
This systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, after approval of the study protocol by all authors[18]. A completed PRISMA checklist has been submitted.
To identify eligible studies, a comprehensive literature search (last search date as of 18/10/2023) was performed in the electronic databases PubMed (MEDLINE) and Scopus, covering the period from conception until 18 October 2023 (Supplementary material). Exported results were imported into Rayyan (Qatar Computing Research Institute, Doha, Qatar), and deduplication was performed[19]. Titles and abstracts were initially screened by two independent researchers (Georgios Ntampakis and Elissavet Anestiadou), and irrelevant articles were excluded. Potentially eligible full-text articles were screened for inclusion, according to the inclusion criteria and disagreements were resolved through discussion with participation of a third researcher (Orestis Ioannidis). In addition, a manual search was performed using the snowball methodology to identify and include any relevant studies in the list of references of the included articles.
Specific inclusion criteria were established prior to literature search: (1) Rats as experimental models; (2) English language; and (3) anastomotic healing as primary outcome. Studies were excluded if: (1) Were in vitro, human studies or studies in experimental models other than rats; (2) the definition of each outcome was not clearly stated; (3) incomplete information was reported; (4) they were kin studies; and (5) they were studies with no free access or no available full text.
Two researchers (Georgios Ntampakis and Elissavet Anestiadou) reviewed all eligible studies. The following data were extracted and recorded: (1) First author; (2) year of publication and country of origin; (3) total number of animals used; (4) animal model (type, age, sex); (5) type of anastomosis; (6) type of intervention; (7) sampling day; (8) tests used for anastomosis assessment; and (9) characteristics of high-risk anastomosis.
Datasets were stratified based on the mechanism and type of high-risk anastomosis. High heterogeneity regarding anastomosis types, intervention types, method of assessment and reported outcomes of interest between the included studies rendered the conduction of a meta-analysis non-applicable. As a result, qualitative analysis of the outcomes was conducted. The provided results were assembled to identify strengths, weaknesses, and trends of each intervention.
As a result of our search strategy 102 studies were included in our systematic review and were categorized based on the mechanism used to create a high-risk anastomosis. The different categories of high-risk anastomosis were ischaemia, colitis, malnutrition, peritonitis, obstruction, radiotherapy, ischaemia/reperfusion injury, chemotherapy and immu
Ischaemia models were some of the commonest high risk anastomosis models found. In total 15 different studies were included. Ischaemia was induced with ligation or electrocauterization of a wide segment of rat’s left colon just above the pubic symphysis. The different kinds of intervention attempted are shown in Table 1[20-40].
| Ref. | Year | Sample size | Intervention | Test | Sampling day |
| Uzun et al[20] | 2008 | 56 | Sildenafil citrate | NOx, thiobarbituric acid reactive substances, glutathione | 3/7 |
| Karliczek et al[21] | 2009 | 34 | Visible light spectrometry evaluation | BPR in situ, tensile strength | 3/7 |
| Coneely et al[22] | 2010 | 50 | Ketotifen | BPR ex situ, HP, IL-6, VEGF | 4 |
| Karataş et al[23] | 2010 | 24 | Amelogenin | BPR ex situ, HP | 4 |
| Kaya et al[24] | 2010 | 48 | Tadalafil | BPR ex situ, HP, histology (as per Ehrlich et al[25]) | 4 |
| Adas et al[26] | 2011 | 40 | Bm-MSCs 1 × 106 | BPR in situ, HP | 4/7 |
| Karatepe et al[27] | 2011 | 40 | Adrenomedulin | BPR ex situ, HP, spectrophotometry, MDA, NOx, TNF-a, IL-6, VEGF, histology | 3/7 |
| Kennelly et al[28] | 2011 | 30 | Electrical field stimulation | BPR ex situ, HP, IL-6, VEGF | 4 |
| Sümer et al[29] | 2011 | 30 | Pentoxifylline and vinpocetine | BPR in situ, HP, histology (as per García et al[30]) | 5 |
| Yoo et al[31] | 2012 | 60 | AdMSC 1 × 106 | BPR ex situ, weight loss, macroscopic (adhesions as per van der Ham and Kort[32], strictures, ulcers), histology (as per Phillips et al[33]), wound infection, ileus, mortality | 7 |
| Wu et al[34] | 2013 | 24 | Triptolide | Histology, calprotectin, MPO, INF-g, IL-4, IL-17, TGF-b | 56 |
| Portilla-de Buen et al[35] | 2014 | 180 | Fibrin glue | BPR ex situ, macroscopic, microscopic, HP | 5 |
| Boersema et al[36] | 2016 | 40 | Hyperbaric oxygen | Macroscopic (as per Zühlke et al[37]), histology (as per Phillips et al[33]), serum creatinine | 3/7 |
| Ruiz-Luque et al[38] | 2019 | 93 | Alprostadil | BPR in situ, macroscopic (as per Knightly et al[39]), histology (as per García et al[30]) | 8 |
| Kayapinar et al[40] | 2021 | 60 | CORM-2 | BPR ex situ, HP, glutathione, MDA, histology (as per Ehrlich et al[25]) | 3/7 |
Various tests were used to assess the quality of the anastomosis, such as bursting pressure (BPR), Hydroxyproline levels (HP), different cytokines, oxidative stress markers, macroscopic and microscopic assessment using different protocols.
In total, 6 different studies of anastomosis in colitis environment were included. In these models, Dextran sodium sulphate or 2,4,6-Trinitrobenzene sulfonic acid were used to induce acute colitis. In one study, intra-jejunal injection of iodoacetamide had been used. The different kinds of interventions can be found in Table 2[41-50]. The tests used to assess the quality of the anastomosis were: Anastomotic BPR, HP, different cytokines, oxidative stress markers, macroscopic and microscopic assessment using different protocols.
| Ref. | Year | Colitis method | Sample size | Intervention | Test | Sampling day |
| Kirkil et al[41] | 2008 | Intra-jejunal injection of iodoacetamide | 28 | Endothelin receptor blockade by bosentan | BPR in situ, macroscopic (Mannheim index[42]), HP, histology (as per Mei et al[43]) | 4 |
| Rijcken et al[44] | 2010 | 7 d DSS 5% | Not mentioned | rhIGF-1 | BPR in situ, microscopic (as per Phillips et al[33], Ki-67), HP, MPO | 1/3/7 |
| Myrelid et al[45] | 2015 | 5 d DSS 3% | 140 | IP prednisolone, AZA, infliximab | BPR ex situ, bowel WT/length, histology, zymography | 3 |
| Alvarenga et al[46] | 2019 | 2,4,6-TNBS | 66 | AdMSC 2 × 106 | Histology, IL-10, IL-17, IFN-g, TGF-b, TNF-a, MMP-2, MMP-9 | 7 |
| Reischl et al[47] | 2021 | ANXA-1 k/o mice and 7 d DSS 2%-3%-5% | Not mentioned | Ac2-26-nanoparticles | Fluorescence imaging of MMPs, histology (as per Phillips et al[33]), whole transcriptome RNA sequencing and analysis | 3/7 |
| Weber et al[48] | 2023 | 7 d DSS 2% | 84 | Prednisolone | BPR in situ, macroscopic (adhesions scoring), histology (as per Philips et al[33]) | 3/7 |
| Ntampakis et al[49] | 2023 | 7 d DSS 5% | 24 | AdMSC 5 × 106 | BPR ex situ, macroscopic (as per Bosmans et al[50]), HP, IL-6, TNF-a, VEGF | 7 |
In total, 6 different studies of anastomosis in malnourished rat were included. Starvation for 7-15 d or 50% food re
| Ref. | Year | Colitis method | Sample size | Intervention | Test | Sampling day |
| McCauley et al[51] | 1991 | 3,5% agar diet for 7 d | 30 | BCAA | Body WT, BPR ex situ, tensile strength, protein content, HP | - |
| Karahasanoglu et al[52] | 1998 | Low-protein diet for 10 d | 40 | Growth hormone | Body WT, BPR in situ, hydroxyproline | 4 |
| Salman et al[53] | 2008 | 15 d | 72 | Cholerella sp. microalgae | Body WT, macroscopic (adhesions as per Ham et al[32]), BPR in situ, HP, histology (as per de Roy van Zuidewijn et al[54]), albumin, prealbumin, transferrin | 3/5/7/9/11/13/15 |
| Gonçalves et al[55] | 2009 | 50% food restriction for 21 d | 80 | Pre-op nutrition | Tensile strength, histology | 5 |
| Gündoğdu et al[56] | 2015 | 50% food restriction for 26 d | 18 | Pre-op nutrition | Body WT, BPR in situ, HP | 4 |
| Vizzotto Junior et al[57] | 2015 | Paired feeding | 160 | Omega-3 | Body WT, tensile strength, histology | 5 |
| Danielski et al[58] | 2016 | 50% food restriction for 26 d | 45 | Vitamin C | Body WT, macroscopic (as per Knightly et al[39]), histology, HP, MPO, TNF-a, nitrite/nitrate, oxidative damage | 7 |
In total, 6 different studies of anastomosis in rats with obstruction were included. Obstruction methods were silk ligation of the distal colon or use of a silicone ring to mimic decrease in bowel diameter. There was also a study with obstructive jaundice, where the distal common bile duct was tied. Krarup et al[59] introduced a new method of inducing bowel obstruction by laparoscopic clip application to the colon. The different kinds of interventions attempted, can be found in Table 4[60-65]. The tests used to assess the quality of the anastomosis were BPR, tensile strength, hydroxyproline, body weight changes, macroscopic and microscopic assessment using different protocols.
| Ref. | Year | Obstruction method | Sample size | Intervention | Test | Sampling day |
| Törnqvist et al[60] | 1990 | Silicone ring 6.5 mm | 54 | Diverting colostomy | Tensile strength, weight, HP | 2/7 |
| Aguilar-Nascimento et al[61] | 1997 | Silk ligature | 108 | Nutritional solutions lavage of the colon | Macroscopic, histology | 3/6 |
| Erbil et al[62] | 2000 | Silk ligature | 144 | Nutritional solutions lavage of the colon | BPR ex situ, HP, macroscopic | 3/6 |
| Cağlikülekçi et al[63] | 2002 | Common bile duct ligation | 40 | rGH | BPR ex situ, macroscopic, HP, macroscopic, histology (as per Greenhalgh et al[64]) | 7 |
| Lelyanov et al[65] | 2004 | Silk ligature | 60 | Sodium hypochlorite and ozone therapy | BPR ex situ, survival, macroscopic, histology | 1/3/6/9/12 |
| Krarup et al[59] | 2017 | Laparoscopic clip application | 32 | MMP inhibition | Body WT, HP, BPR in situ | 3 |
In total, 29 different studies of anastomosis in rats with peritonitis were included. The different techniques to induce acute peritonitis were caecal ligation and puncture which was the commonest technique used, incomplete anastomosis, as well as faecal inoculation in the abdominal cavity. In one of the studies, Vaneerdeweg et al[66] used barium sulphate and gelatine sponges with faeces to mimic bacterial and chemical peritonitis. The different kinds of interventions attempted, can be found in Table 5[66-100]. The tests used to assess the quality of the anastomosis were BPR, tensile strength, hydroxyproline, cytokines, tPA activity, oxidative stress markers, body weight changes, macroscopic and microscopic assessment using different protocols.
| Ref. | Year | Peritonitis method | Sample size | Intervention | Test | Sampling day |
| Vaneerdeweg et al[66] | 2000 | Gelatin capsule with faeces and barium sulphate | 40 | Gentamicin Sponges | BPR in situ, mortality, weight loss | 4 |
| Reijnen et al[67] | 2002 | Caecal ligation/perforation | 198 | Hyaluronan-based agents | tPA activity | 1/3/7 |
| Aydin et al[68] | 2006 | Caecal ligation/perforation | 24 | Laparostomy with Bogota bag | BPR in situ, HP, adhesions (as per Zühlke et al[37]) | 5 |
| Li et al[69] | 2006 | Enterotomy | 360 | Fibrin glue and growth hormone | BPR in situ, HP, tensile strength, histology | 1/3/5 |
| Buyne et al[70] | 2009 | Faecal inoculation | 148 | Recombinant tPA | BPR in situ, tensile strength, macroscopic, HP | 3/7 |
| Kayaoglu et al[71] | 2009 | Caecal ligation/perforation | 80 | N-butyl-2-cyanoacrylate | BPR ex situ, macroscopic (as per Knightly et al[39]), histology | 3/7 |
| Pantelis et al[72] | 2010 | Caecal ligation/perforation or incomplete anastomosis | 206 | Collagen matrix coagulation factors I and IIa (Tachosil) | BPR ex situ, histology (as per Biert et al[73], Verhofstad et al[74], Attard et al[75]), collagen type I-II, HP | 2/5/14 |
| Rocha et al[76] | 2010 | Caecal ligation/perforation or incomplete anastomosis | 45 | Hyperbaric oxygen therapy | Total energy rupture test (tensile strength) | 4 |
| Silva et al[77] | 2012 | Caecal ligation/perforation or incomplete anastomosis | 40 | Bromopride | Macroscopic (Nair et al[78]), tensile strength (Versa test), histology, quantitative collagen analysis, HP | 3/7 |
| Holmer et al[79] | 2014 | Faecal inoculation | 72 | Collagen fleece coating | BPR in situ, histology, collagen I, III, VEGF, MMP-13 | 1/3/7 |
| Camargo et al[80] | 2013 | Faecal inoculation | 40 | Peritoneal lavage with bupivacaine | Tensile strength, survival | 5 |
| Arikanoglu et al[81] | 2013 | Colon injury | 21 | Antibacterial suture | BPR in situ, HP, histology | 10 |
| Donmez et al[82] | 2013 | Colon injury | 40 | Glutamine and GH | BPR ex situ, HP | 5 |
| Senol et al[83] | 2013 | E. Coli inoculation | 40 | Fibrin glue | BPR ex situ, histology (as per Ehrlich-Hunt[25]), HP | 10 |
| Silva et al[84] | 2014 | Caecal ligation/perforation | 80 | Bromopride | MMP-1a, MMP-8, MMP-13, IL-1b, IL-6, IL-10, TNF-a, IFN-g | 3/7 |
| Erginel et al[85] | 2014 | Caecal ligation/perforation | 40 | IP O3 | BPR ex situ, histology (as per Verhofstadt et al[74] and Philips et al[33]), HP | 7 |
| Pommergaard et al[86] | 2014 | Incomplete anastomosis | 80 | Tachosil coating | Tensile strength, clinical assessment | 7 |
| Ercan et al[87] | 2015 | Caecal ligation/perforation | 40 | IP L-Carnitine | BPR ex situ, histology (as per Ehrlich- Hunt[25]), HP | 5 |
| Cakir et al[88] | 2015 | Incomplete anastomosis | 64 | Sildenafil | BPR ex situ, histology (as per Phillips et al[33]), HP, MDA, GSH | 3/7 |
| Suárez-Grau et al[89] | 2016 | Incomplete anastomosis | 56 | Fibrinogen - thrombin collagen patch | Histology (as per Biert scheme[73]), macroscopic (adhesions), survival | 15/30 |
| Sozutek et al[90] | 2016 | Caecal ligation/perforation | 50 | PRP | BPR in situ, Body WT, HP, histology (as per Verhofstadt et al[74]) | 7 |
| Ersoy et al[91] | 2016 | Caecal ligation/perforation | 60 | Melatonin | BPR ex situ, HP, histology, IL-6, IL-10, INF-γ, CRP | 7 |
| Çakır et al[92] | 2016 | Caecal ligation/perforation | 18 | IP O3 | BPR ex situ, TNF-a, IL-1β, MDA, MPO, histology | 22 |
| Sukho et al[93] | 2018 | Incomplete anastomosis | 60 | AdMSC | BPR in situ, macroscopic (as per Verco et al[94] and Zühlke et al[37]), histology | 3/7 |
| Lorenzi et al[95] | 2017 | Caecal ligation/perforation | 40 | Omiganan | BPR in situ, histology (as per García et al[30]), HP | 7 |
| Yıldırım et al[96] | 2021 | Caecal puncture | 21 | Growth factor collagen (FGF-C), abx collagen (AB-C) | BPR ex situ, HP, macroscopic (as per Bosmans et al[50]), histology (as per Ehrlich et al[25]) | 7 |
| Nakamura et al[97] | 2021 | Incomplete anastomosis | 60 | HSMM | BPR ex situ, macroscopic (as per Ham et al[32]), histology | 3/5/7/14/28 |
| Aksu et al[98] | 2021 | Colon injury | 21 | Chlorhexidine gluconate and metronidazole-soaked sponges | BPR in situ, hydroxyproline, histology | 10 |
| Yilmaz et al[99] | 2021 | Caecal ligation/perforation | 32 | Polyurethane membrane | BPR ex situ, HP, NOx, IL-6, TNF-a, tPA, macroscopic (as per Mazuji et al[100]), histology | 5 |
In total, 8 different studies of anastomosis after radiotherapy in rats were included. In each protocol different doses of radiation were used depending on the nature of the experiment. The different kinds of interventions attempted, can be found in Table 6[101-109]. The tests used to assess the quality of the anastomosis were BPR, hydroxyproline, oxidative stress markers, body weight changes, macroscopic and microscopic assessment using different protocols. Van de Putte et al[107] used positron emission tomography/computedtomography to investigate the tropism of the AdMSCs to the anastomosis along with colonoscopy for direct assessment of anastomotic site and histological examination
| Study | Year | Radiation dose | Sample size | Intervention | Test | Sampling day |
| Liu et al[101] | 2001 | 10 Gy | 74 | Lactobacillus plantarum 299v | Body weight, WBC, mucosal MPO, HP, nucleotide, DNA and RNA content, colonic bacterial microflora, bacterial translocation, histology | 4/7/11 |
| Kerem et al[102] | 2006 | 500 cGy | 84 | Soluble Fiber | Macroscopic (adhesions as per van der Ham and Kort[32]), BPR in situ, HP, histology (as per de Roy van Zuidewijn et al[54]), MMP-2 activity | 3/7 |
| Ozdemir et al[103] | 2013 | 800 rad | 30 | Amifostine | BPR ex situ, HP, histology | 5 |
| Seker et al[104] | 2014 | 485 cGy | 60 | Pycnogenol | BPR ex situ, HP, MDA, histology (as per Houdart et al[105]) | 3/7 |
| Simões Neto et al[106] | 2013 | 660 cGy | 30 | Fraction electron beam | BPR (not specified), histology | 7 |
| Van de Putte et al[107] | 2017 | 27Gy | 48 | AdMSC | 18F-FDG-PET/CT, colonoscopy, histology | 32 |
| Taşdöven et al[108] | 2019 | 6Gy | 48 | Ozon PR | BPR in situ, histology (as per Houdart et al[105]), HP, MDA, MPO | 3/7 |
| Yilmaz et al[109] | 2022 | 20Gy | 32 | Ozon PR | BPR in situ, macroscopic (as per Knightly et al[39]), HP, MPO, histology (as per de Roy van Zuidewijn et al[54]) | 5 |
In total, 10 different studies of anastomosis after ischaemia injury in rats were included. In all protocols superior me
| Ref. | Year | I/R method | Sample size | Intervention | Test | Sampling day |
| Terzi et al[110] | 2001 | SMA clampling 30 min | 65 | Allopurinol | BPR in situ, macroscopic (adhesions as per Knightly et al[39]), histology (as per de Roy van Zuidewijn et al[54]) | 3/7 |
| Tireli et al[111] | 2003 | SMA clampling 30 min | 20 | Pentoxifiline | BPR ex situ, HP | 7 |
| Miranda et al[112] | 2010 | SMA clamping 45 min | 45 | Methylene blue | BPR ex situ, macroscopic, histology | 7 |
| Celik et al[113] | 2013 | SMA clamping 45 min | 24 | Modelukast | BPR ex situ, HP, MPO, MDA, caspase-3 activity, catalase, NOx, glutathione, SOD, TNF-a, IL-6, ALT, AST | 5 |
| Akarsu et al[114] | 2017 | SMA clamping 10 min | 40 | Simvastatin | BPR ex situ, HP | 8 |
| Özkan et al[115] | 2018 | SMA clamping 30 min | 30 | Melatonin | BPR ex situ, HP, histology (as per Nursal et al[116]), SOD, glutathione | 7 |
| Özçay et al[117] | 2018 | SMA clamping 45 min | 40 | GH | Macroscopic (as per Galili et al[118]), BPR ex situ, histology (as per Greenhalgh et al[64]) | 7 |
| Sayin et al[119] | 2020 | SMA clamping 45 min | 40 | IP montelukast | BPR ex situ, macroscopic score (as per Knightly et al[39]), HP, histology (fibrosis) | 7 |
| Eryilmaz et al[120] | 2020 | SMA clamping 45 min | 30 | Hydrogen rich saline | BPR in situ, histology (as per Park et al[121] and Chiu et al[122]), TNF-a, IL-6, MDA, MPO | 5 |
| Akıncı et al[123] | 2022 | SMA clamping 30 min | 36 | Genistein | BPR ex situ, HP, SOD, glutathione, histology (as per Piroglu et al[124]) | 5 |
In total, 9 different studies of anastomosis after different schemes of chemotherapy in rats were included. Chemotherapy includes cytotoxic agents against cancer cells and was administered IV or with intra-peritoneal infusion. The different kinds of interventions attempted, can be found in Table 8[125-133]. The tests used to assess the quality of the anastomosis were BPR, tensile strength hydroxyproline, oxidative stress markers, cytokines, macroscopic and microscopic assessment using different protocols.
| Ref. | Year | Chemo agent | Sample size | Intervention | Test | Sampling day |
| Nayci et al[125] | 2003 | IP 5-FU | 40 | Electromagnetic field | Tensile strength, HP | 7 |
| Cetinkaya et al[126] | 2005 | IP mitomycin-C | 81 | GM-CSF | BPR ex situ, HP, histology (as per Ehrlich et al[25]) | 3 |
| Kanellos et al[127] | 2006 | IP 5-FU and LEV | 60 | Fibrin glue | BPR ex situ, HP, macroscopic (adhesions as per van der Ham and Kort[32]), histology (as per Phillips et al[33]) | 8 |
| Yildiz et al[128] | 2013 | 5-FU and 20 Gy | 60 | HBOT | BPR ex situ, Weight, HP, histology (Fibrosis) | 5 |
| Arapoglou et al[129] | 2017 | Irinotecan | 40 | Iloprost | BPR ex situ, macroscopic (as per van der Ham and Kort[32]), histology (as per Phillips et al[33]), HP | 8 |
| Akyuz et al[130] | 2018 | 5-FU IV | 32 | Melatonin | BPR ex situ, HP, histology, TNF-a, IL-1β | 7 |
| Ocak et al[131] | 2019 | HIPEC with CIS | 30 | PRP | BPR ex situ, HP, histology (as per Verhofstad et al[74]) | 7 |
| Gorur et al[132] | 2020 | IP 5-FU | 40 | PRP | Body weight, BPR in situ, HP, histology (as per Verhofstad et al[74]) | 7 |
| Buk et al[133] | 2020 | IP OX | 30 | PRP | BPR ex situ, histology (as per Verhofstad et al[74]), HP | 7 |
In total, 6 different studies of anastomosis after different schemes of chemotherapy in rats were included. Either steroids or other immunosuppression drugs were used, depending on the protocol. The different kinds of interventions attempted, can be found in Table 9[134-140]. The tests used to assess the quality of the anastomosis were BPR, tensile strength hydroxyproline, oxidative stress markers, cytokines, macroscopic and microscopic assessment using different protocols.
| Ref. | Year | Immunosuppression method | Sample size | Intervention | Test | Sampling day |
| Dinc et al[134] | 2002 | Methylprednizolone | 80 | GM-CSF | BPR ex situ, HP, histology (as per Ehrlich et al[25]) | 3 |
| Colak et al[135] | 2003 | Dexamethasone | 24 | Trapidil | BPR ex situ, HP, histology (as per Ehrlich et al[25]), NOx, MDA | 7 |
| Sakallioglu et al[136] | 2004 | Dexamethasone | 60 | eGF | BPR ex situ, bursting site, HP, histology | 7 |
| Inglin et al[137] | 2008 | MMF | 63 | IGF-I | BPR ex situ, histology, Ki-67 | 2/4/6 |
| Netta et al[138] | 2014 | Methylprednizolone | 50 | SCFA | BPR ex situ, CRP, IL-6, TNF-a | 4 |
| Karakaya et al[139] | 2021 | Everolimus | 60 | AdMSC | Macroscopic (as per Houston and Rotstein[140]), BPR ex si | 4/7 |
One of the most common tests used to assess the quality of anastomosis is anastomotic BPR. In the literature, there are 2 ways of performing BPR test, either in situ (in vivo) or ex situ (in vitro). In vivo, a catheter is inserted into the rat’s rectum, dyed water is infused, and the manometer is attached more proximal to the anastomosis, recording the maximum pressure in which the anastomosis bursts. In vitro, the anastomosis is dissected away from the rat, is tied distally, and is attached to a three-way system with the manometer on one side, and the syringe with the dyed water on the other. Water is infused in the bowel segment, and maximum pressure in which the anastomosis bursts is recorded. Curran et al[141] compared the two techniques in canine small bowel and they concluded that the in vitro technique had similar results compared to the in vivo one, but it was easier to perform as the researchers do not have to carry out intensive bowel dissection. In one of our team’s previous studies we described the in vitro technique in detail[49]. Some technical pearls of the in vitro technique are that it is slightly more time consuming, and it requires careful and meticulous dissection as the adhesions formed around the anastomosis might be the factor that keeps the anastomosis patent, and extensive dissection might result in anastomotic dehiscence, rendering the specimen invalid.
Along with BPR, Sakallioglu et al[136] also documented the bursting site of the anastomosed bowel, and they found out that it is usually around the anastomosis and not on the anastomosis itself.
Tensile strength is traditionally used along with BPR to assess the strength of the anastomosis. The technique includes dissecting the anastomosis out and attaching it to a device which allows application of traction force to one end of the anastomosis and recording of the force applied to the apparatus on the other end. The force at which the anastomosis is disrupted is then recorded. Either a simple commercial dynamometer[142] or more precise and expensive solutions can be used[77].
Ikeuchi et al[143] reported that there is no correlation between anastomotic bursting strength and tensile strength of anastomosis, and both tests should be used in assessing the anastomotic quality. They also suggest that minimum strength in which the anastomosis starts to rupture and maximum strength in which the anastomosis is completely ruptured should be documented.
Macroscopic assessment consists of both clinical observations of the rats, as well as macroscopic assessment of the anastomosis using different scales to grade it, depending on what parameters need to be observed.
Clinical parameters used, especially in malnutrition models, are weight changes of the rats, and survival curves. The general welfare of the animals, while easily appreciated by direct observation, can be considered but is not easily countable. Examples of clinical parameters are reduced mobility, fur erection, neglection of hygiene and reduced food intake. Specifically in colitis protocols, bloody diarrhea, and rectal mucosa erythema can be easily observed and colonoscopy can be used to verify intestinal inflammation before starting the experimental process[46,49].
Van de Putte et al[107] used colonoscopy to directly assess anastomotic healing internally.
Macroscopic assessment scores and what they assess can be found in Table 10. Of note, although the score by van der Ham and Kort[32] is one of the widest used for adhesion formation, van der Ham cite Houston and Rotstein[140] as the original creators of the same scoring system.
| Macroscopic assessment | Wound healing |
| van der Ham and Kort[32] score | Adhesions |
| Zühlke et al[37] score for adhesions | Adhesions |
| Mannheim index[42] | Peritonitis presence and severity |
| Knightly et al[39] score for adhesions | Adhesions |
| Bosmans et al[50] score | Anastomotic complication score |
| Nair et al[78] score | Adhesions |
| Verco et al[94] score | Abscess formation |
| Mazuji et al[100] score | Adhesions |
| Galili et al[118] score | Adhesions |
| Houston and Rotstein[140] score | Adhesions |
All the adhesions scoring systems are similar, and all of them assess the existence or severity of the adhesions using different criteria. The score that we considered to be more complete for the assessment of a bowel anastomosis in rats is the one created by Bosmans et al[50] in an international consensus statement. This score takes into account the presence of adhesions, abscesses, anastomotic dehiscence underneath adhesions, as well as faecal peritonitis/death of the animas.
One of the most interesting findings of the current review are the different methods used in research to assess the quality of the anastomosis. As described in Table 11[144], all models have common characteristics, such as the presence of inflammatory cells in bowel tissues, fibroblastic activity, neovascularization, and collagen deposition which play a pivotal role in anastomotic healing. The oldest and most widely used model with the above characteristics is this of Ehrlich et al[25] in 1973 and later on the same model as modified by Phillips et al[33]. in 1992, both of which set the basis for histological assessment of anastomotic healing.
| Ref. | Histologic assessment |
| Ehrlich et al[25] | Erythrocytes, polymorphonucleated cells, mononuclear cells, fibroblasts, collagen fibers, fibrin |
| Houdart et al[105] and Hutschenreiter et al[144] as modified by García et al[30] | Mucosal anastomotic re-epithelialization, neovascularization, fibroblasts, fibrosis, muscle layer destruction, neutrophil infiltration, lymphocyte infiltration, histiocyte infiltration, giant cell infiltration |
| Ehrlich et al[25] as modified by Phillips et al[33] | Inflammatory cell infiltration, blood vessel in growth, fibroblast ingrowth, collagen deposition |
| Houdart et al[105] | Granulocyte infiltration, mononuclear cell infiltration, fibroblastic proliferation, focal necrosis, exudate formation |
| Mucosal damage index by Mei et al[43] | 0: Normal mucosa, no damage on mucosal surface; 1: Mild hyperemia and edema, no erosion or ulcer on mucosal surface; 2: Moderate hyperemia and edema with erosion on mucosal surface; 3: Severe hyperemia and edema with necrosis and ulcer on mucosal surface, the major ulcerative area < 1 cm; 4: Severe hyperemia and edema with necrosis and ulcer on mucosal surface, the major ulcerative area > 1 cm |
| de Roy van Zuidewijn et al[54] | Re-epithelialization, regeneration of muscularis propria, mucosal muscularis propria damage, necrosis, inflammatory exudate, granulation tissue, granulocytes, macrophages, fibroblasts, granulation tissue |
| Greenhalgh et al[64] | Epithelization, cellular infiltration, fibroblastic proliferation, collagen deposition, neovascularization |
| Biert et al[73] | Necrosis, polymorphonuclear cells, lymphocytes, macrophages, edema, epithelium, submucosal - muscular continuity, neovascularization, fibrosis |
| Attard et al[75] | Mucosal continuity, muscular continuity, re-epithelialization, granulation tissue, polymorphonuclear cells, lymphocytes, macrophages, fibroblasts |
| Verhofstad et al[74] | Necrosis, polymorphonuclear cells, lymphocytes, macrophages, edema, mucosal continuity, submucosal – muscular continuity |
| Nursal et al[116] | Fibroblast infiltration, capillary formation, re-epithelialization, granulocyte infiltration, mononuclear cell infiltration |
| Park et al[121] and Chiu et al[122] | Grade 1: Normal mucosa; grade 2: The subepithelial space at the tip of the villus; grade 3: Increase in subepithelial space; grade 4: Overlapping and spills of the floor of the villus; grade 5: Disintegration of the lamina propria; grade 6: Crypt layer injury; grade 7: Transmucosal infarction and grade 8: Transmural infarction |
| Piroglu et al[124] | Inflammatory cell infiltration/concentration, neovascularization, fibroblastic activity, collagen fibers |
| Miltschitzky et al[15] | Blood vessel ingrowth, fibroblasts, collagen formation, inflammatory cell infiltration, first layer in which continuity has been restored, number of healed layers, epithelium closed, crypt architecture restored, overall healing quality |
The appreciation of healing layer by layer is added by de Roy van Zuidewijn et al[54] in 1992 who added the elements of re-epithelialization, muscularis propria damage and regeneration and the presence of necrosis. Their model, despite providing information on the layer-by-layer healing and inflammatory changes of the tissues, does not address the deposition of collagen or fibroblastic activity. Other models, proposed by different teams provide a more complete approach to anastomotic healing by adding the element of connective tissue regeneration as well[73-75].
As opposed to the models described above that have a semi-quantitative approach, Park et al[121] and Chiu et al[122] suggested a more qualitative approach, with grading of mucosal layer healing. This model is used to evaluate the anastomosis after ischemia reperfusion type injury[121,122].
Miltschitzky et al[15] have a very different approach in their histology grading system which includes neovascularization, fibroblastic activity and collagen formation, inflammatory cell infiltration and a more extensive layer by layer evaluation of the anastomosis in semi-quantitative way[15]. In the authors’ view this represents the most holistic approach to anastomotic healing, is easy to use and is applicable in different types of anastomosis research.
In a few cases, as shown in Tables 1-9, researchers did mention histological evaluation in their research but they did not use any of the histological grading models described above.
Hydroxyproline has proven to be one of the commonest markers used in experimental protocols of anastomotic healing. With hydroxyproline we indirectly assess collagen content of anastomosis and appreciate anastomotic healing, with higher values of hydroxyproline suggesting enhanced anastomotic healing.
Matrix Metalloproteases (MMPs) and their inhibitors (TIMPs) play an important role in wound healing as they play an important role in collagen degradation, neovascularization and are regulated by chemokines and cytokines[145].
The selection and investigation of different kinds of MMPs and TIMPs depends on the nature of the research and their objectives. In the current study we identified a few authors investigating the role of different MMPs (MMP-1, MMP-8, MMP-13) that belong to the groups of collagenases which degrade triple helical collagen and MMP-2, MMP-9 gelatinases which are involved in the processes of angiogenesis and collagenesis[46,79,84,145]. Abnormal expression of MMPs can impair anastomotic healing and can be used as biomarkers in anastomosis research to evaluate the efficacy of different interventions or the effect of a condition/factor on an anastomosis.
Different kinds of cytokines can be used in research of high-risk anastomotic healing as they provide us with valuable information on the biological processes during anastomotic healing or in response to an intervention to a high-risk anastomosis. Cytokines can be measured either with ELISA or polymerase chain reaction according to local laboratory protocols.
Some of the cytokines used in research are interleukin (IL)-1b, IL-4, IL-6, IL-17, interferon gamma, tumor necrosis factor α, as pro-inflammatory cytokines to assess the severity of inflammatory response to the anastomosis after applying the stimulus and appreciating their fluctuation after the intervention[46,49]. On the other hand, increased expression of IL-10 and tumor growth factor–beta (TGF-b) which are known as anti-inflammatory cytokines can be used as a marker of effectiveness of experimental intervention. TGF-b as reported by Alvarenga et al[46] also seems to regulate the expression of certain MMPs leading to fibrosis[46].
Vascular endothelial factor is one of the cytokines that can be used to assess the anastomosis for neo-vascularization. Increased values of vascular endothelial growth factor (VEGF) will suggest increased vascularization in the anastomosis which is important for anastomotic healing. In early stages of anastomotic healing VEGF might not be significantly increased but our group showed tendency to increase in post-operative day 7 in anastomoses treated with Adipose tissue derived stem cells[49].
A meticulous study design combining the appropriate MMPs and cytokines can extract valuable information about anastomotic healing and the various signaling pathways by which the inflammatory response is regulated.
Another state of matter that can be used in research of an anastomotic healing is oxidative stress. Authors, as shown in Tables 1-8 used markers that indicate either oxidative stress damage, such as free radicals (NOx), myeloperoxidase (MPO) and Malondialdehyde, or antioxidant markers such as superoxide dismutase and glutathione. Neutrophils contain MPO and increased levels of this marker also suggests increased neutrophilic infiltration to the tissues[34].
Our review demonstrated the evolution of different high-risk anastomosis protocols in rats as well as the different techniques used to assess anastomotic healing. We emphasize the importance of systematization of research, by standardizing experimental protocols and designing high quality studies that will give us more information on the complex pathophysiological pathways of anastomotic healing. Understanding these pathways will allow us to create interventions that will attenuate the inflammation, decrease anastomotic related complications, and negate the need for diverting stomas in surgical patients.
Mrs. Mariana Tsioutsiou for grammar checks pre-admission.
| 1. | Tanner J, Padley W, Assadian O, Leaper D, Kiernan M, Edmiston C. Do surgical care bundles reduce the risk of surgical site infections in patients undergoing colorectal surgery? A systematic review and cohort meta-analysis of 8,515 patients. Surgery. 2015;158:66-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 189] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 2. | Morris MC, Paquette IM. Laparoscopic left colectomy: Surgical technique. Ann Laparosc Endosc Surg. 2019;4:18. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 3. | Fang AH, Chao W, Ecker M. Review of Colonic Anastomotic Leakage and Prevention Methods. J Clin Med. 2020;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 4. | Law WL, Choi HK, Lee YM, Ho JW, Seto CL. Anastomotic leakage is associated with poor long-term outcome in patients after curative colorectal resection for malignancy. J Gastrointest Surg. 2007;11:8-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 253] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 5. | Slieker JC, Daams F, Mulder IM, Jeekel J, Lange JF. Systematic review of the technique of colorectal anastomosis. JAMA Surg. 2013;148:190-201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 129] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 6. | Zarnescu EC, Zarnescu NO, Costea R. Updates of Risk Factors for Anastomotic Leakage after Colorectal Surgery. Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 95] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 7. | van Praagh JB, de Goffau MC, Bakker IS, Harmsen HJ, Olinga P, Havenga K. Intestinal microbiota and anastomotic leakage of stapled colorectal anastomoses: a pilot study. Surg Endosc. 2016;30:2259-2265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 63] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 8. | Nikolian VC, Kamdar NS, Regenbogen SE, Morris AM, Byrn JC, Suwanabol PA, Campbell DA Jr, Hendren S. Anastomotic leak after colorectal resection: A population-based study of risk factors and hospital variation. Surgery. 2017;161:1619-1627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 105] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 9. | Bruns BR, Morris DS, Zielinski M, Mowery NT, Miller PR, Arnold K, Phelan HA, Murry J, Turay D, Fam J, Oh JS, Gunter OL, Enniss T, Love JD, Skarupa D, Benns M, Fathalizadeh A, Leung PS, Carrick MM, Jewett B, Sakran J, O'Meara L, Herrera AV, Chen H, Scalea TM, Diaz JJ. Stapled versus hand-sewn: A prospective emergency surgery study. An American Association for the Surgery of Trauma multi-institutional study. J Trauma Acute Care Surg. 2017;82:435-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Rasslan S, Margutti Fonoff A, Soldá SC, Angelo Casaroli A. Ostomy or intestinal anastomosis in cases of peritonitis. Sao Paulo Med J. 1995;113:1017-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 11. | Babakhanlou R, Larkin K, Hita AG, Stroh J, Yeung SC. Stoma-related complications and emergencies. Int J Emerg Med. 2022;15:17. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 12. | Ginesi M, Steinhagen E. A brave new world: Colorectal anastomosis in trauma, diverticulitis, peritonitis, and colonic obstruction. Semin Colon Rectal Surg. 2022;33:100881. [RCA] [DOI] [Full Text] [Reference Citation Analysis (1)] |
| 13. | Rajan R, Clark DA. Current management of large bowel obstruction: A narrative review. Ann Laparosc Endosc Surg. 2022;7:23. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 14. | Platell C, Barwood N, Dorfmann G, Makin G. The incidence of anastomotic leaks in patients undergoing colorectal surgery. Colorectal Dis. 2007;9:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 194] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 15. | Miltschitzky JRE, Clees Z, Weber MC, Vieregge V, Walter RL, Friess H, Reischl S, Neumann PA. Intestinal anastomotic healing models during experimental colitis. Int J Colorectal Dis. 2021;36:2247-2259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 16. | Pommergaard HC, Rosenberg J, Schumacher-Petersen C, Achiam MP. Choosing the best animal species to mimic clinical colon anastomotic leakage in humans: a qualitative systematic review. Eur Surg Res. 2011;47:173-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 17. | Nerstrøm M, Krarup PM, Jorgensen LN, Ågren MS. Therapeutic improvement of colonic anastomotic healing under complicated conditions: A systematic review. World J Gastrointest Surg. 2016;8:389-401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 51000] [Article Influence: 10200.0] [Reference Citation Analysis (2)] |
| 19. | Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5:210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5711] [Cited by in RCA: 14290] [Article Influence: 1429.0] [Reference Citation Analysis (1)] |
| 20. | Uzun H, Konukoglu D, Nuri MK, Ersoy EY, Ozçevik S, Yavuz N. The effects of sildenafil citrate on ischemic colonic anastomotic healing in rats: its relationship between nitric oxide and oxidative stress. World J Surg. 2008;32:2107-2113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Karliczek A, Benaron DA, Zeebregts CJ, Wiggers T, van Dam GM. Intraoperative ischemia of the distal end of colon anastomoses as detected with visible light spectroscopy causes reduction of anastomotic strength. J Surg Res. 2009;152:288-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Coneely J, Kennelly R, Bouchier-Hayes D, Winter DC. Mast cell degranulation is essential for anastomotic healing in well perfused and poorly perfused rat colon. J Surg Res. 2010;164:e73-e76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Karataş A, Kocael A, Aytaç E, Gökcal F, Salihoğlu Z, Uzun H, Paksoy M. Amelogenin (an extracellular matrix protein) application on ischemic colon anastomosis in rats. Ulus Travma Acil Cerrahi Derg. 2010;16:487-490. [PubMed] |
| 24. | Kaya Y, Coskun T, Ayhan S, Kara E, Sakarya A, Var A. The effect of tadalafil on anastomotic healing in ischemic small intestine in rats. Surg Today. 2010;40:555-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Ehrlich HP, Tarver H, Hunt TK. Effects of vitamin A and glucocorticoids upon inflammation and collagen synthesis. Ann Surg. 1973;177:222-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 180] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 26. | Adas G, Arikan S, Karatepe O, Kemik O, Ayhan S, Karaoz E, Kamali G, Eryasar B, Ustek D. Mesenchymal stem cells improve the healing of ischemic colonic anastomoses (experimental study). Langenbecks Arch Surg. 2011;396:115-126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Karatepe O, Kurtulus I, Yalcin O, Battal M, Kamali G, Aydin T. Adrenomedulline improves ischemic left colonic anastomotic healing in an experimental rodent model. Clinics (Sao Paulo). 2011;66:1805-1810. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Kennelly R, Conneely JB, Bouchier-Hayes DJ, Winter DC. Electrical field stimulation promotes anastomotic healing in poorly perfused rat colon. Int J Colorectal Dis. 2011;26:339-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 29. | Sümer A, Altınlı E, Senger S, Köksal N, Onur E, Eroğlu E, Güneş P. Effect of pentoxifylline and vinpocetine on the healing of ischemic colon anastomosis: an experimental study. Ulus Travma Acil Cerrahi Derg. 2011;17:482-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 30. | García JC, Arias J, Garcia C, Vara E, Balibrea J. Modificación de los mediadores inflamatorios en isquemia-reperfusión intestinal en un modelo de diabetes tipo 2. Cirugía Española. 2002;71:276-286. [RCA] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 31. | Yoo JH, Shin JH, An MS, Ha TK, Kim KH, Bae KB, Kim TH, Choi CS, Hong KH, Kim J, Jung SJ, Kim SH, Rho KH, Kim JT, Yang YI. Adipose-tissue-derived Stem Cells Enhance the Healing of Ischemic Colonic Anastomoses: An Experimental Study in Rats. J Korean Soc Coloproctol. 2012;28:132-139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 32. | van der Ham AC, Kort WJ. Fibrin sealing of irradiated bowel anastomoses. Dis Colon Rectum. 1993;36:614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 33. | Phillips JD, Kim CS, Fonkalsrud EW, Zeng H, Dindar H. Effects of chronic corticosteroids and vitamin A on the healing of intestinal anastomoses. Am J Surg. 1992;163:71-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 149] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 34. | Wu R, Li Y, Guo Z, Gong J, Zhu W, Li N, Li J. Triptolide ameliorates ileocolonic anastomosis inflammation in IL-10 deficient mice by mechanism involving suppression of miR-155/SHIP-1 signaling pathway. Mol Immunol. 2013;56:340-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 35. | Portilla-de Buen E, Orozco-Mosqueda A, Leal-Cortés C, Vázquez-Camacho G, Fuentes-Orozco C, Alvarez-Villaseñor AS, Macías-Amezcua MD, González-Ojeda A. Fibrinogen and thrombin concentrations are critical for fibrin glue adherence in rat high-risk colon anastomoses. Clinics (Sao Paulo). 2014;69:259-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 36. | Boersema GSA, Wu Z, Kroese LF, Vennix S, Bastiaansen-Jenniskens YM, van Neck JW, Lam KH, Kleinrensink GJ, Jeekel J, Lange JF. Hyperbaric oxygen therapy improves colorectal anastomotic healing. Int J Colorectal Dis. 2016;31:1031-1038. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 37. | Zühlke HV, Lorenz EM, Straub EM, Savvas V. [Pathophysiology and classification of adhesions]. Langenbecks Arch Chir Suppl II Verh Dtsch Ges Chir. 1990;1009-1016. [PubMed] |
| 38. | Ruiz-Luque V, Parra-Membrives P, Escudero-Severín C, Aguilar-Luque J. Effect of Alprostadil on Colorectal Anastomoses Under Relative Ischemia. J Surg Res. 2019;236:230-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 39. | Knightly JJ, Agostino D, Clifton EE. The effect of fibrinolysin and heparin on the formation of peritoneal adhesions. Surgery. 1962;52:250-258. [PubMed] |
| 40. | Kayapınar AK, Ercan M, Baş KK, Yamak S, Erçin U, Türkoğlu MA, Bostancı EB. The effect of carbon monoxide releasing molecule-2 (CORM-2) on healing of ischemic colon anastomosis in rats. Turk J Med Sci. 2021;51:2222-2231. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 41. | Kirkil C, Cetinkaya Z, Ustundag B, Akpolat N, Ayten R, Bulbuller N. The effects of endothelin receptor blockade by bosentan on the healing of a bowel anastomosis in an experimental Crohn's disease model. J Gastrointest Surg. 2008;12:1429-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 42. | Linder MM, Wacha H, Feldmann U, Wesch G, Streifensand RA, Gundlach E. [The Mannheim peritonitis index. An instrument for the intraoperative prognosis of peritonitis]. Chirurg. 1987;58:84-92. [PubMed] |
| 43. | Mei Q, Yu JP, Xu JM, Wei W, Xiang L, Yue L. Melatonin reduces colon immunological injury in rats by regulating activity of macrophages. Acta Pharmacol Sin. 2002;23:882-886. [PubMed] |
| 44. | Rijcken E, Fuchs T, Sachs L, Kersting CM, Bruewer M, Krieglstein CF. Insulin-like growth factor 1-coated sutures improve anastomotic healing in an experimental model of colitis. Br J Surg. 2010;97:258-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 45. | Myrelid P, Salim SY, Darby T, Almer S, Melgar S, Andersson P, Söderholm JD. Effects of anti-inflammatory therapy on bursting pressure of colonic anastomosis in murine dextran sulfate sodium induced colitis. Scand J Gastroenterol. 2015;50:991-1001. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 46. | Alvarenga V Jr, Silva PTD, Bonfá ND, Pêgo B, Nanini H, Bernardazzi C, Madi K, Baetas da Cruz W, Castelo-Branco MT, de Souza HSP, Schanaider A. Protective effect of adipose tissue-derived mesenchymal stromal cells in an experimental model of high-risk colonic anastomosis. Surgery. 2019;166:914-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 47. | Reischl S, Wilhelm D, Friess H, Neumann PA. Innovative approaches for induction of gastrointestinal anastomotic healing: an update on experimental and clinical aspects. Langenbecks Arch Surg. 2021;406:971-980. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 48. | Weber MC, Bauer J, Buck A, Clees Z, Oertel R, Kasajima A, Reischl S, Wilhelm D, Friess H, Neumann PA. Perioperative Low-Dose Prednisolone Treatment Has Beneficial Effects on Postoperative Recovery and Anastomotic Healing in a Murine Colitis Model. J Crohns Colitis. 2023;17:950-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 49. | Ntampakis G, Pramateftakis MG, Ioannidis O, Bitsianis S, Christidis P, Symeonidis S, Koliakos G, Karakota M, Bekiari C, Tsakona A, Cheva A, Aggelopoulos S. The Role of Adipose Tissue Mesenchymal Stem Cells in Colonic Anastomosis Healing in Inflammatory Bowel Disease: Experimental Study in Rats. J Clin Med. 2023;12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 50. | Bosmans JWAM, Moossdorff M, Al-Taher M, van Beek L, Derikx JPM, Bouvy ND. International consensus statement regarding the use of animal models for research on anastomoses in the lower gastrointestinal tract. Int J Colorectal Dis. 2016;31:1021-1030. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 51. | McCauley R, Platell C, McCulloch R, Hall J. The influence of branched chain amino acids on colonic atrophy and anastomotic strength in the rat. Aust N Z J Surg. 1991;61:49-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 52. | Karahasanoglu T, Altinli E, Hamzaoglu I, Paksoy M, Yeşildere T, Alemdaroglu K. Effect of growth hormone treatment on the healing of left colonic anastomoses in protein-malnourished rats. Br J Surg. 1998;85:931-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 53. | Salman B, Kerem M, Bedirli A, Katircioglu H, Ofluoglu E, Akin O, Onbasilar I, Ozsoy S, Haziroglu R. Effects of Cholerella sp. microalgae extract on colonic anastomosis in rats with protein-energy malnutrition. Colorectal Dis. 2008;10:469-478. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 54. | de Roy van Zuidewijn DB, Schillings PH, Wobbes T, de Boer HH. Histologic evaluation of wound healing in experimental intestinal anastomoses: effects of antineoplastic agents. Int J Exp Pathol. 1992;73:465-484. [PubMed] |
| 55. | Gonçalves CG, Groth AK, Ferreira M, Matias JE, Coelho JC, Campos AC. Influence of preoperative feeding on the healing of colonic anastomoses in malnourished rats. JPEN J Parenter Enteral Nutr. 2009;33:83-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 56. | Gündoğdu RH, Yaşar U, Ersoy PE, Ergül E, Işıkoğlu S, Erhan A. Effects of preoperative nutritional support on colonic anastomotic healing in malnourished rats. Ulus Cerrahi Derg. 2015;31:113-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 57. | Vizzotto Junior AO, Campos AC, Mello EV, Castilho TJ. Influence of preoperative supplementation of omega-3 fatty acid in the healing of colonic anastomoses in malnourished rats receiving paclitaxel. Rev Col Bras Cir. 2015;42:116-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 58. | Danielski LG, Walczewski E, de Jesus CR, Florentino D, Giustina AD, Goldim MP, Kanis LA, Pereira GW, Pereira VD, Felisberto F, Petronilho F. Preoperative vitamin C supplementation improves colorectal anastomotic healing and biochemical parameters in malnourished rats. Int J Colorectal Dis. 2016;31:1759-1766. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 59. | Krarup PM, Eld M, Jorgensen LN, Hansen MB, Ågren MS. Selective matrix metalloproteinase inhibition increases breaking strength and reduces anastomotic leakage in experimentally obstructed colon. Int J Colorectal Dis. 2017;32:1277-1284. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 60. | Törnqvist A, Blomquist P, Jiborn H, Zederfeldt B. The effect of diverting colostomy on anastomotic healing after resection of left colon obstruction. An experimental study in the rat. Int J Colorectal Dis. 1990;5:167-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 61. | Aguilar-Nascimento JE, Oliveira-Neto JP, Mathie RT, Williamson RC. Effect of intraoperative nutritional solutions on perianastomotic colonic mucosa in experimental large bowel obstruction. Dig Dis Sci. 1997;42:2581-2584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 62. | Erbil Y, Calis A, Berber E, Mercan S. The effect of intraoperative colonic lavage with NG-nitro-L-arginine methyl ester (L-NAME) on anastomotic healing in the presence of left-sided colonic obstruction in the rat. Surg Today. 2000;30:421-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 63. | Cağlikülekçi M, Ozçay N, Oruğ T, Aydoğ G, Renda N, Atalay F. The effect of recombinant growth hormone on intestinal anastomotic wound healing in rats with obstructive jaundice. Turk J Gastroenterol. 2002;13:17-23. [PubMed] |
| 64. | Greenhalgh DG, Sprugel KH, Murray MJ, Ross R. PDGF and FGF stimulate wound healing in the genetically diabetic mouse. Am J Pathol. 1990;136:1235-1246. [PubMed] |
| 65. | Lelyanov AD, Sergienko VI, Ivliev NV, Emel'yanov VV, Guseva ED. Effects of sodium hypochlorite and ozone on healing of intestinal anastomosis in simulated strangulation colorectal obstruction. Bull Exp Biol Med. 2004;137:103-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 66. | Vaneerdeweg W, Hendriks JM, Lauwers PR, Ieven M, Eyskens EJ. Effect of gentamicin-containing sponges on the healing of colonic anastomoses in a rat model of peritonitis. Eur J Surg. 2000;166:959-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 67. | Reijnen MM, Holmdahl L, Kooistra T, Falk P, Hendriks T, van Goor H. Time course of peritoneal tissue plasminogen activator after experimental colonic surgery: effect of hyaluronan-based antiadhesive agents and bacterial peritonitis. Br J Surg. 2002;89:103-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 68. | Aydin C, Aytekin FO, Tekin K, Kabay B, Yenisey C, Kocbil G, Ozden A. Effect of temporary abdominal closure on colonic anastomosis and postoperative adhesions in experimental secondary peritonitis. World J Surg. 2006;30:612-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 69. | Li Y, Bao Y, Jiang T, Tan L, Gao Y, Li J. Effect of the combination of fibrin glue and growth hormone on incomplete intestinal anastomoses in a rat model of intra-abdominal sepsis. J Surg Res. 2006;131:111-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 70. | Buyne OR, Bleichrodt RP, De Man BM, Lomme RM, Verweij PE, van Goor H, Hendriks T. Tissue-type plasminogen activator prevents abscess formation but does not affect healing of bowel anastomoses and laparotomy wounds in rats with secondary peritonitis. Surgery. 2009;146:939-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 71. | Kayaoglu HA, Ersoy OF, Ozkan N, Celik A, Filiz NO. Effect of n-butyl-2-cyanoacrylate on high-risk colonic anastomoses. Kaohsiung J Med Sci. 2009;25:177-183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 72. | Pantelis D, Beissel A, Kahl P, Wehner S, Vilz TO, Kalff JC. The effect of sealing with a fixed combination of collagen matrix-bound coagulation factors on the healing of colonic anastomoses in experimental high-risk mice models. Langenbecks Arch Surg. 2010;395:1039-1048. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 73. | Biert J, Seifert WF, Verhofstad AA, Wobbes T, de Man BM, Hoogenhout J, Hendriks T. A semiquantitative histological analysis of repair of anastomoses in the rat colon after combined preoperative irradiation and local hyperthermia. Radiat Res. 1998;149:372-377. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 74. | Verhofstad MH, Lange WP, van der Laak JA, Verhofstad AA, Hendriks T. Microscopic analysis of anastomotic healing in the intestine of normal and diabetic rats. Dis Colon Rectum. 2001;44:423-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 90] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 75. | Attard JA, Raval MJ, Martin GR, Kolb J, Afrouzian M, Buie WD, Sigalet DL. The effects of systemic hypoxia on colon anastomotic healing: an animal model. Dis Colon Rectum. 2005;48:1460-1470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 76. | Rocha AA, Leal RF, Ayrizono Mde L, Chung WF, Coy CS, Lee HD, Fagundes JJ. Hyperbaric oxygen therapy and mechanical resistence of the colonics anastomosis in rats with peritonitis. Acta Cir Bras. 2010;25:368-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 77. | Silva SM, Carneiro FP, Oliveira PG, Morais PH, Silva NG, Sousa JB. Effects of bromopride on the healing of left colonic anastomoses in rats with induced abdominal sepsis. Acta Cir Bras. 2012;27:370-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 78. | Nair SK, Bhat IK, Aurora AL. Role of proteolytic enzyme in the prevention of postoperative intraperitoneal adhesions. Arch Surg. 1974;108:849-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 185] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 79. | Holmer C, Praechter C, Mecklenburg L, Heimesaat M, Rieger H, Pohlen U. Anastomotic stability and wound healing of colorectal anastomoses sealed and sutured with a collagen fleece in a rat peritonitis model. Asian J Surg. 2014;37:35-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 80. | Camargo MG, Fagundes JJ, Leal RF, Ayrizono Mde L, Rossi DH, Oliveira Pde S, Chung WF, Lee HD, Coy CS. Influence of the peritoneal lavage with bupivacaine on the survival and resistance of colonic anastomoses performed under fecal peritonitis in rats. Acta Cir Bras. 2013;28:783-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 81. | Arikanoglu Z, Cetinkaya Z, Akbulut S, Ilhan YS, Aygen E, Basbug M, Ayten R, Girgin M, Ilhan N, Dagli F. The effect of different suture materials on the safety of colon anastomosis in an experimental peritonitis model. Eur Rev Med Pharmacol Sci. 2013;17:2587-2593. [PubMed] |
| 82. | Donmez R, Oren D, Ozturk G, Kisaoglu A, Ozogul B, Atamanalp SS. The combined effects of glutamine and growth hormone on intestinal anastomosis in the rat intra-abdominal sepsis model. J Surg Res. 2013;182:142-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 83. | Senol M, Altintas MM, Cevık A, Altuntas YE, Barisik NO, Bildik N, Oncel M. The effect of fibrin glue on the intensity of colonic anastomosis in the presence and absence of peritonitis: an experimental randomized controlled trial on rats. ISRN Surg. 2013;2013:521413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 84. | Silva SM, Jerônimo MS, Silva-Pereira I, Bocca AL, Sousa JB. Effects of bromopride on expression of metalloproteinases and interleukins in left colonic anastomoses: an experimental study. Braz J Med Biol Res. 2014;47:911-916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 85. | Erginel B, Erginel T, Aksoy B, Dokucu Aİ. Effect of Ozone Therapy (OT) on Healing of Colonic Anastomosis in a Rat Model of Peritonitis. Balkan Med J. 2014;31:249-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 86. | Pommergaard HC, Achiam MP, Burcharth J, Rosenberg J. Decreased leakage rate of colonic anastomoses by tachosil coating: an experimental study. Int Surg. 2014;99:359-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 87. | Ercan U, Kiraz A, Çikman Ö, Türkön H, Kilinç N, Otkun MT, Özkan ÖF, Kiraz HA, Karaayvaz M. The Effect of Systemic Carnitine Administration on Colon Anastomosis Healing in an Experimental Sepsis Model. J Invest Surg. 2015;28:334-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 88. | Cakir T, Ozer I, Bostanci EB, Keklik TT, Ercin U, Bilgihan A, Akoglu M. Increased collagen maturity with sildenafil citrate: experimental high risk colonic anastomosis model. Int J Surg. 2015;13:152-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 89. | Suárez-Grau JM, Bernardos García C, Cepeda Franco C, Mendez García C, García Ruiz S, Docobo Durantez F, Morales-Conde S, Padillo Ruiz J. Fibrinogen-thrombin collagen patch reinforcement of high-risk colonic anastomoses in rats. World J Gastrointest Surg. 2016;8:627-633. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 15] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 90. | Sozutek A, Ozyazici S, Colak T, Cetınkunar S, Irkorucu O, Bobusoglu O, Cennet A. Evaluating the effect of infliximab on the healing of left colonic anastomosis in the presence of intra-abdominal sepsis. Arab J Gastroenterol. 2016;17:84-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 91. | Ersoy ÖF, Özkan N, Özsoy Z, Kayaoğlu HA, Yenidoğan E, Çelik A, Özuğurlu AF, Arabacı Çakır E, Lortlar N. Effects of melatonin on cytokine release and healing of colonic anastomoses in an experimental sepsis model. Ulus Travma Acil Cerrahi Derg. 2016;22:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 92. | Çakır T, Aslaner A, Tekeli SÖ, Avcı S, Doğan U, Tekeli F, Soylu H, Akyüz C, Koç S, Üstünel İ, Yılmaz N. Effect of ozone on colon anastomoses in rat peritonitis model. Acta Cir Bras. 2016;31:111-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 93. | Sukho P, Boersema GSA, Kops N, Lange JF, Kirpensteijn J, Hesselink JW, Bastiaansen-Jenniskens YM, Verseijden F. Transplantation of Adipose Tissue-Derived Stem Cell Sheet to Reduce Leakage After Partial Colectomy in A Rat Model. J Vis Exp. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 94. | Verco SJ, Peers EM, Brown CB, Rodgers KE, Roda N, diZerega G. Development of a novel glucose polymer solution (icodextrin) for adhesion prevention: pre-clinical studies. Hum Reprod. 2000;15:1764-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 82] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 95. | Lorenzi T, Trombettoni MMC, Ghiselli R, Paolinelli F, Gesuita R, Cirioni O, Provinciali M, Kamysz W, Kamysz E, Piangatelli C, Castellucci M, Guerrieri M, Morroni M. Effect of omiganan on colonic anastomosis healing in a rat model of peritonitis. Am J Transl Res. 2017;9:3374-3386. [PubMed] |
| 96. | Yıldırım MA, Çakır M, Fındık S, Kişi Ö, Şentürk M. Comparison of the efficacy of growth factor collagen and antibiotic collagen on colon anastomosis in experimental animals with peritonitis. Indian J Gastroenterol. 2021;40:309-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 97. | Nakamura T, Yokoyama U, Kanaya T, Ueno T, Yoda T, Ishibe A, Hidaka Y, Umemura M, Takayama T, Kaneko M, Miyagawa S, Sawa Y, Endo I, Ishikawa Y. Multilayered Human Skeletal Muscle Myoblast Sheets Promote the Healing Process After Colonic Anastomosis in Rats. Cell Transplant. 2021;30:9636897211009559. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 98. | Aksu A, Bozan MB, Kutluer N, Kanat BH, İlhan YS, Türkoğlu A, Dağlı AF, Ilhan N, Azak Bozan A, Aksoy N. The effects of sponges soaked with chlorhexidine gluconate and metronidazole on safety of colonic anastomosis in an experimental model of peritonitis. Ulus Travma Acil Cerrahi Derg. 2021;27:167-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 99. | Yılmaz G, Özdenkaya Y, Karatepe O, Tanrıkulu Y, Kamalı G, Yalçın O. Effects of polyurethane membrane on septic colon anastomosis and intra-abdominal adhesions. Ulus Travma Acil Cerrahi Derg. 2021;27:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 100. | Mazuji MK, Kalambaheti K, Pawar B. Prevention of adhesions with polyvinylpyrrolidone. preliminary report. Arch Surg. 1964;89:1011-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 70] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 101. | Liu Q, Nobaek S, Adawi D, Mao Y, Wang M, Molin G, Ekelund M, Jeppsson B. Administration of Lactobacillus plantarum 299v reduces side-effects of external radiation on colon anastomotic healing in an experimental model. Colorectal Dis. 2001;3:245-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 102. | Kerem M, Bedirli A, Karahacioglu E, Pasaoglu H, Sahin O, Bayraktar N, Yilmaz TU, Sakrak O, Goksel F, Oguz M. Effects of soluble fiber on matrix metalloproteinase-2 activity and healing of colon anastomosis in rats given radiotherapy. Clin Nutr. 2006;25:661-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 103. | Ozdemir CS, Burgazli KM, Beken-Ozdemir E, Akdere H, Mericliler M, Ozcelik MF. The effect of Amifostine (Ethyol) on intestinal anastomosis in rats with radiation enteritis. Eur Rev Med Pharmacol Sci. 2013;17:1351-1359. [PubMed] |
| 104. | Seker A, Deger KC, Bostanci EB, Ozer I, Dalgic T, Bilgihan A, Akmansu M, Ekinci O, Ercin U, Akoglu M. Effects of β-glucan on colon anastomotic healing in rats given preoperative irradiation. J Invest Surg. 2014;27:155-162. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 105. | Houdart R, Lavergne A, Galian A, Hautefeuille P. [Anatomo-pathological evolution of single-layer end-to-end digestive anastomoses. A study of 210 colonic anastomoses in rats from the 2d to the 180th day]. Gastroenterol Clin Biol. 1983;7:465-473. [PubMed] |
| 106. | Simões Neto J, Reis Neto JA, Matos D. Effects of preoperative irradiation using fractioned electron beam on the healing process of colocolonic anastomosis in rats undergoing early and late surgical intervention. Acta Cir Bras. 2013;28:72-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 107. | Van de Putte D, Demarquay C, Van Daele E, Moussa L, Vanhove C, Benderitter M, Ceelen W, Pattyn P, Mathieu N. Adipose-Derived Mesenchymal Stromal Cells Improve the Healing of Colonic Anastomoses Following High Dose of Irradiation Through Anti-Inflammatory and Angiogenic Processes. Cell Transplant. 2017;26:1919-1930. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 108. | Taşdöven İ, Emre AU, Gültekin FA, Öner MÖ, Bakkal BH, Türkcü ÜÖ, Gün BD, Taşdöven GE. Effects of ozone preconditioning on recovery of rat colon anastomosis after preoperative radiotherapy. Adv Clin Exp Med. 2019;28:1683-1689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 109. | Yilmaz TU, Erkal EY, Çinar S, Eraldemir C, Vural Ç, Taş EU, Utkan NZ. Protective effects of rectal ozone administration on colon anastomoses following radiotherapy. Adv Clin Exp Med. 2022;31:1355-1364. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 110. | Terzi C, Kuzu A, Aşlar AK, Kale IT, Tanik A, Köksoy C. Prevention of deleterious effects of reperfusion injury using one-week high-dose allopurinol. Dig Dis Sci. 2001;46:430-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 111. | Tireli GA, Salman T, Ozbey H, Abbasoglu L, Toker G, Celik A. The effect of pentoxifylline on intestinal anastomotic healing after ischemia. Pediatr Surg Int. 2003;19:88-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 112. | Miranda EF, Greca FH, Noronha L, Kotze LR, Rubin MR. The influence of methylene blue on the healing of intestinal anastomoses subjected to ischemia and reperfusion in rats. Acta Cir Bras. 2010;25:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 113. | Celik A, Ergun E, Koksal N, Celik AS, Altinli E, Uzun MA, Eroglu E, Kemik A. Effects of montelukast on the healing of ischemic colon anastomoses. Am J Surg. 2013;206:502-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 114. | Akarsu M, Saygun O, Aydinuraz K, Aydin O, Daphan CE, Tanrıkulu FB, Kisa U, Comu FM. The Effects of Simvastatin on Ischemia Reperfusion Injury in an Experimental Colon Anastomosis Model. Indian J Surg. 2017;79:390-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 115. | Özkan N, Ersoy ÖF, Özsoy Z, Çakır E. Melatonin exhibits supportive effects on oxidants and anastomotic healing during intestinal ischemia/reperfusion injury. Ulus Travma Acil Cerrahi Derg. 2018;24:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 116. | Nursal TZ, Anarat R, Bircan S, Yildirim S, Tarim A, Haberal M. The effect of tissue adhesive, octyl-cyanoacrylate, on the healing of experimental high-risk and normal colonic anastomoses. Am J Surg. 2004;187:28-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 117. | Özçay N, Özdemir H, Besim H. Role of platelet-rich fibrin on intestinal anastomosis wound healing in a rat. Biomed Mater. 2018;13:045006. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 118. | Galili Y, Ben-Abraham R, Rabau M, Klausner J, Kluger Y. Reduction of surgery-induced peritoneal adhesions by methylene blue. Am J Surg. 1998;175:30-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 119. | Sayin T, Cimen S, Bostancı T, Akbaba S, Yildirim Z, Ersoy PE. Colonic anastomosis can be protected from ischemia reperfusion injury with intra-peritoneal Montelukast treatment. Asian J Surg. 2020;43:130-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 120. | Eryilmaz S, Turkyilmaz Z, Karabulut R, Gulburun MA, Poyraz A, Gulbahar O, Arslan B, Sonmez K. The effects of hydrogen-rich saline solution on intestinal anastomosis performed after intestinal ischemia reperfusion injury. J Pediatr Surg. 2020;55:1574-1578. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 121. | Park PO, Haglund U, Bulkley GB, Fält K. The sequence of development of intestinal tissue injury after strangulation ischemia and reperfusion. Surgery. 1990;107:574-580. [PubMed] |
| 122. | Chiu CJ, McArdle AH, Brown R, Scott HJ, Gurd FN. Intestinal mucosal lesion in low-flow states. I. A morphological, hemodynamic, and metabolic reappraisal. Arch Surg. 1970;101:478-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1258] [Cited by in RCA: 1450] [Article Influence: 25.9] [Reference Citation Analysis (6)] |
| 123. | Akıncı O, Tosun Y, Kepil N. The Effect of Genistein on Anastomotic Healing in Intestinal Ischemia/Reperfusion Injury. J Surg Res. 2022;280:389-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 124. | Piroglu I, Tulgar S, Thomas DT, Cakiroglu B, Piroglu MD, Bozkurt Y, Gergerli R, Ates NG. Mechanical Bowel Preparation Does Not Affect Anastomosis Healing in an Experimental Rat Model. Med Sci Monit. 2016;22:26-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 125. | Nayci A, Cakmak M, Renda N, Aksoyek S, Yucesan S. Effect of electromagnetic fields and early postoperative 5-fluorouracil on the healing of colonic anastomoses. Int J Colorectal Dis. 2003;18:136-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 126. | Cetinkaya K, Dinc S, Gulcelik MA, Renda N, Ustun H, Caydere M, Alagol H. Granulocyte macrophage-colony stimulating factor improves impaired anastomotic wound healing in rats treated with intraperitoneal mitomycin-C. Surg Today. 2005;35:290-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 127. | Kanellos I, Christoforidis E, Kanellos D, Pramateftakis MG, Sakkas L, Betsis D. The healing of colon anastomosis covered with fibrin glue after early postoperative intraperitoneal chemotherapy. Tech Coloproctol. 2006;10:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 128. | Yildiz R, Can MF, Yagci G, Ozgurtas T, Guden M, Gamsizkan M, Ozturk E, Cetiner S. The effects of hyperbaric oxygen therapy on experimental colon anastomosis after preoperative chemoradiotherapy. Int Surg. 2013;98:33-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 129. | Arapoglou S, Kambaroudis A, Grivas I, Delis GA, Karkos C, Ballas K, Zacharioudakis G, Petras P, Aftzoglou M, Gouziotis I, Koliakos G, Karakwta M, Hahalis G. The Effect of Iloprost in the Healing of Colonic Anastomosis in Rats under Chemotherapy with Irinotecan. Chirurgia (Bucur). 2017;112:705-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 130. | Akyuz C, Yasar NF, Uzun O, Peker KD, Sunamak O, Duman M, Sehirli AO, Yol S. Effects of melatonin on colonic anastomosis healing following chemotherapy in rats. Singapore Med J. 2018;59:545-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 131. | Ocak S, Buk OF, Genc B, Avcı B, Uzuner HO, Gundogdu SB. The effects of platelet-rich-plasma gel application to the colonic anastomosis in hyperthermic intraperitoneal chemotherapy: An experimental rat model. Int Wound J. 2019;16:1426-1432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 132. | Gorur M, Sozutek A, Irkorucu O, Karakaya B. The influence of platelet-rich plasma (PRP) on colonic anastomosis healing impaired by intraperitoneal 5-flourouracil application. An experimental study. Acta Cir Bras. 2020;35:e202000504. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 133. | Buk OF, Ocak S, Genc B, Avcı B, Uzuner HO. Is platelet-rich plasma improves the anastomotic healing in hyperthermic intraperitoneal chemotherapy with oxaliplatin: an experimental rat study. Ann Surg Treat Res. 2020;98:89-95. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 134. | Dinc S, Alagol H, Gulcelik MA, Ozbirecikli B, Kuru B, Renda N, Ustun H. Locally applied granulocyte-macrophage colony-stimulating factor improves the impaired bowel anastomoses in rats with long-term corticosteroid treatment. World J Surg. 2002;26:1208-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 135. | Colak T, Nayci A, Polat G, Polat A, Comelekoglu U, Kanik A, Turkmenoglu O, Aydin S. Effects of trapidil on the healing of colonic anastomoses in an experimental rat model. ANZ J Surg. 2003;73:916-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 136. | Sakallioglu AE, Yagmurlu A, Dindar H, Hasirci N, Renda N, Deveci MS. Sustained local application of low-dose epidermal growth factor on steroid-inhibited colonic wound healing. J Pediatr Surg. 2004;39:591-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 137. | Inglin RA, Baumann G, Wagner OJ, Candinas D, Egger B. Insulin-like growth factor I improves aspects of mycophenolate mofetil-impaired anastomotic healing in an experimental model. Br J Surg. 2008;95:793-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 138. | Netta S, Papadopoulos V, Apostolidis S, Michalopoulos A. The effect of intraoperative lavage with short chain fatty acids (SCFAs) on rectal anastomosis of rats receiving corticosteroids. Hippokratia. 2014;18:350-354. [PubMed] |
| 139. | Karakaya E, Akdur A, Ok Atilgan A, Uysal AC, Ozturan Ozer HE, Yildirim S, Haberal M. Effect of Adipose-Derived Stem Cells on Colonic Anastomosis in Rats Immunosuppressed With Everolimus: An Experimental Study. Exp Clin Transplant. 2021;19:970-976. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 140. | Houston KA, Rotstein OD. Fibrin sealant in high-risk colonic anastomoses. Arch Surg. 1988;123:230-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 141. | Curran KM, Fransson BA, Gay JM. A comparison of in situ and in vitro techniques for bursting pressure testing of canine jejunum. Am J Vet Res. 2010;71:370-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 142. | Pramateftakis MG, Kanellos D, Mantzoros I, Despoudi K, Raptis D, Angelopoulos S, Koliakos G, Zaraboukas T, Lazaridis C. Intraperitoneally administered irinotecan with 5-fluorouracil impair wound healing of colonic anastomoses in a rat model: an experimental study. Tech Coloproctol. 2011;15 Suppl 1:S121-S125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 143. | Ikeuchi D, Onodera H, Aung T, Kan S, Kawamoto K, Imamura M, Maetani S. Correlation of tensile strength with bursting pressure in the evaluation of intestinal anastomosis. Dig Surg. 1999;16:478-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 144. | Hutschenreiter G, Haina D, Paulini K, Schumacher G. [Wound healing after laser and red light irradiation]. Z Exp Chir. 1980;13:75-85. [PubMed] |
| 145. | Cabral-Pacheco GA, Garza-Veloz I, Castruita-De la Rosa C, Ramirez-Acuña JM, Perez-Romero BA, Guerrero-Rodriguez JF, Martinez-Avila N, Martinez-Fierro ML. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 586] [Cited by in RCA: 1127] [Article Influence: 187.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/