Published online Jun 20, 2024. doi: 10.5493/wjem.v14.i2.94357
Revised: April 22, 2024
Accepted: May 6, 2024
Published online: June 20, 2024
Processing time: 94 Days and 9.4 Hours
In traditional descriptions, the upper surface of the liver is smooth and convex, but deep depressions are variants that are present in 5%-40% of patients. We sought to determine the relationship between surface depressions and the dia
To use exploratory laparoscopy to determine the relationship between surface de
An observational study was performed in all patients undergoing laparoscopic upper gastro-intestinal operations between January 1, 2023 and January 20, 2024. A thirty-degree laparoscope was used to inspect the liver and diaphragm. When surface depressions were present, we recorded patient demographics, presence of diaphragmatic bands, rib protrusions and/or any other source of compression during inspection.
Of 394 patients, 343 had normal surface anatomy, and 51 (12.9%) had prominent surface depressions on the liver. There was no significant relationship between the presence of surface depressions and gender nor the presence of rib projections. However, there was significant association between the presence of surface depressions and diaphragmatic muscular bands (P < 0.001).
With these data, the diaphragmatic-band theory has gained increased importance over other theories for surface depressions. Further studies are warranted using cross sectional imaging to confirm relationships with intersectional planes as well as beta-catenin assays in the affected liver parenchyma.
Core Tip: The upper surface of the liver is usually smooth and convex, but deep depressions are present in 5%-40% of patients. Laparoscopic surgery provides an opportunity to examine the relationship between surface depressions and the diaphragm. This study showed that 51 (12.9%) of 394 patients had prominent surface depressions on the liver. There was significant association between the presence of surface depressions and diaphragmatic muscular bands, giving credence to the diaphragmatic-band theory for surface depressions.
- Citation: Cawich SO, Thomas DA, Mohammed F, Gardner MT, Craigie M, Johnson S, Kedambady RS. Hepatic grooves: An observational study at laparoscopic surgery. World J Exp Med 2024; 14(2): 94357
- URL: https://www.wjgnet.com/2220-315x/full/v14/i2/94357.htm
- DOI: https://dx.doi.org/10.5493/wjem.v14.i2.94357
In conventional anatomic descriptions, the liver has a smooth, rounded convex upper surface contacting the diaphragm. However, there are deep depressions on the diaphragmatic surface of the liver in 5%[1] to 40%[2] of unselected patients. Some authorities theorized that these deep depressions may be the result of post-mortem compression from the ribs[3,4] or the diaphragm[4-6]. These theories were summarily dismissed because evidence of compression could not be proven in many cases.
However, when laparoscopic surgery became the standard of care for many operations in the late 20th Century, this presented an additional tool to examine the compression theory in living humans. In this paper, we examined the relationship of the liver and diaphragm in patients undergoing laparoscopic upper abdominal surgery.
This study was carried out at a tertiary referral hospital in Trinidad & Tobago after permission was secured from the local institutional review board. All participants provided written informed consent prior to enrollment. Two independent observers were present in the operating room during all laparoscopic upper gastro-intestinal operations performed between January 1, 2023 and January 20, 2024. After insertion of the visual trocar, a thirty-degree laparoscope was directed to the upper abdomen to visually inspect the liver and diaphragm. This was strictly an observational study and no change in operative treatment was imposed by the study methodology.
When surface depressions were present, we recorded the patient demographics, presence of diaphragmatic bands, rib protrusions and/or any other source of compression during inspection.
All patients who underwent laparoscopic upper abdominal operations during the study period were potential candi
Diaphragmatic bands were defined as visible, well-defined muscular bundles that connected the central tendon of the diaphragm to the inner aspect of the lower thoracic cage[7]. When present, we identified the band by using the name of the corresponding hepatic segment. For example, a band that was present to the immediate left of the gallbladder fossa was named a “segment 4b band.” Any localized projection extending ≥ 5 mm into the peritoneum and associated with a visible rib was considered rib protrusion.
The data were divided into two groups: Patients with surface depressions and those with conventional surface anatomy. We compared the presence of diaphragmatic bands and rib projections between the two groups. We used the Statistical Package for the Social Sciences Version 16 to perform statistical analyses, with significance assigned to a P value < 0.05. The data in each group were compared using the Chi Square test for categorical variables between the groups.
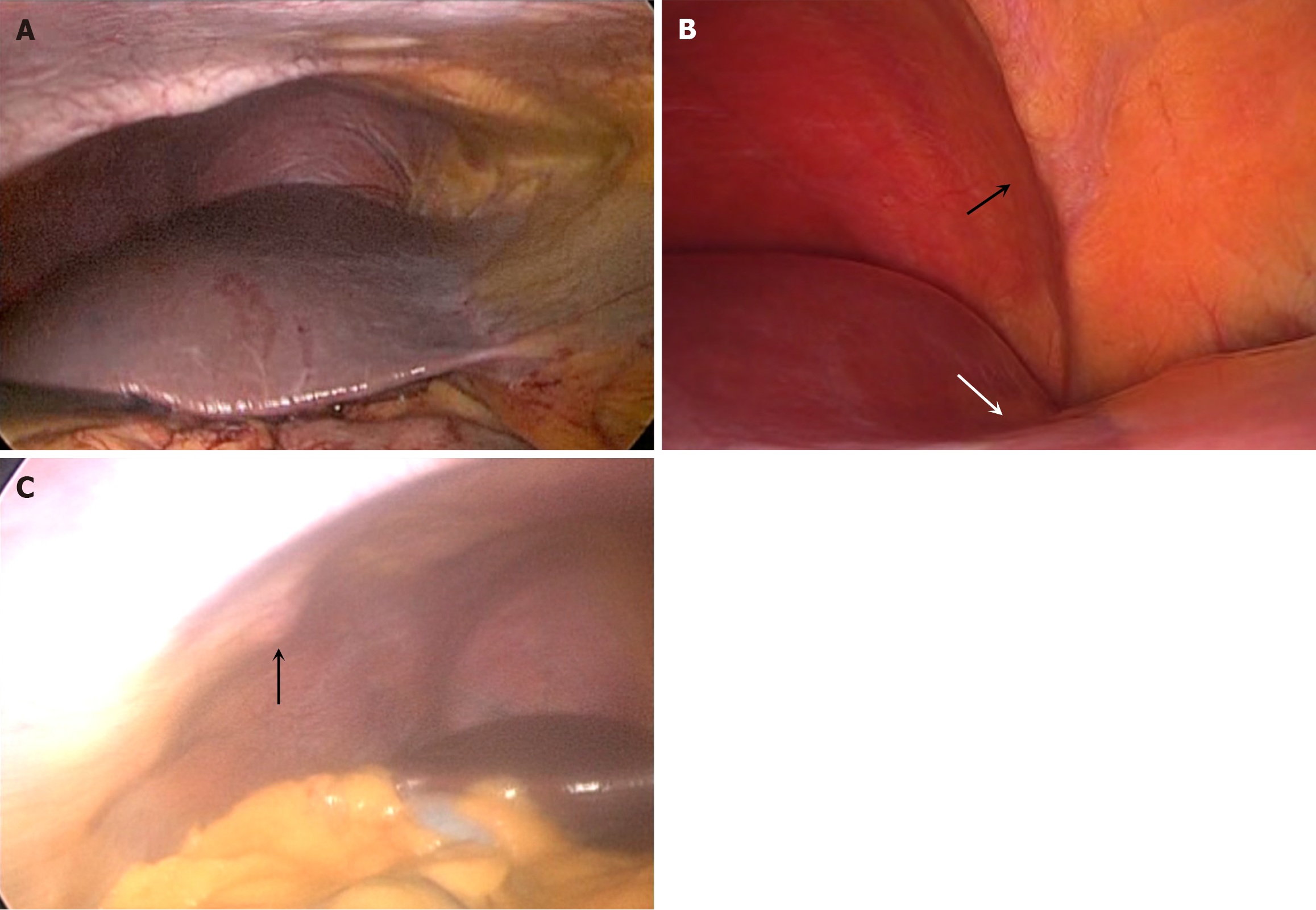
Over the study period, 190 men and 204 women underwent varied upper abdominal operations using the laparoscopic approach, as outlined in Table 1. Of the total 394 patients, 343 (87.1%) had normal surface anatomy (Figure 1A), and 51 (12.9%) had prominent surface depressions on the liver (Figure 1B).
| Operations | n |
| Cholecystectomy | 259 |
| Liver resection | 15 |
| Pancreaticoduodenectomy | 3 |
| Gastrectomy | 4 |
| Distal pancreatectomy | 14 |
| Colectomy | 59 |
| Adrenalectomy | 2 |
| Small bowel resection | 8 |
| Adhesiolysis | 12 |
| Ventral hernia repair | 18 |
Surface depressions were present in 30 (11.5%) males and 21 (16%) females, with no significant gender predilection (P = 0.1977). The mean age of patients with surface depressions was 47.73 years (range 26-70; median 48; SD ± 9.04).
Rib projections were present in 28 (7.1%) patients. This included 22 males and 6 females, with a mean age of 49.6 years (range 30-70; median 48; SD ± 9.16). When rib projections were present, they were associated with floating ribs (Figure 1C). Only 3 of the patients with rib projections also had surface depressions (P = 0.7153). And, when present their location did not correspond to the location of the surface depression. Most of the rib projections were present at the lateral costal margin corresponding to hepatic segments V and VI.
There were 51 patients with surface depressions. This included 32 males and 19 females at a mean age of years (range 26-70; median 49; SD ± 9.53). Table 2 demonstrates that there was significant association between the presence of surface depressions and diaphragmatic muscular bands (Figure 1B). Of 51 patients with surface depressions, there were 40 (78.4%) with co-existent diaphragmatic bands (P < 0.001). Additionally, when present there was a spatial relationship in all cases.
| Parameter | Conventional surface anatomy (343) | Surface depressions (51) | P value |
| Rib protrusion | 25 | 3 | 0.7153 |
| No rib protrusion | 318 | 48 | |
| Diaphragmatic bands | 153 | 40 | < 0.0001 |
| No bands | 190 | 11 | |
| Male (n = 264) | 233 | 30 (11.5%) | 0.1977 |
| Female (n = 131) | 110 | 21 (16%) |
There is documentation that the prevalence of surface depression in cadaveric studies in the Caribbean diaspora ranges from 12% to 15%[8]. In this study, we were able to demonstrate that the prevalence of surface depressions in living persons (12.9%) was comparable to that documented in cadaveric studies.
We could not demonstrate a reliable association with rib projections in this study. When rib projections were present, there was no spatial relationship with the surface depressions. Most rib projections appeared at the lateral aspect of the chest, over hepatic segments 5/6. This, we believe, is sufficient to discount the theory of rib compression. Some proposed that these are post-mortem changes that are due to the ribs continuously compressing a single area on the diaphragmatic surface after death, creating a corresponding depression[3,4]. It is true that proponents of this theory make the argument that this accounts for the increased prevalence on cadaveric studies[4,9]. However, our study demonstrates that these surface depressions are visible in living patients and that there is no association with projecting ribs.
There was a significant association with diaphragmatic muscular bands. Interestingly, this relationship was not borne out when we performed cadaveric studies in our population[8] - probably because the muscular bands would lose muscle tone in the post-mortem state.
In light of this new data, we believe that the diaphragmatic band theory should be reconsidered as one of the main causes of surface depressions. Originally proposed by Macchi et al[2], the diaphragmatic band theory suggested that there are “weak zones” on the liver surfaced susceptible to compression. Newell and Morgan-Jones[4] also noted that the orientation of the bands was related to the surface depressions – similar to the orientation in our study.
There is existing data from animal studies demonstrating that hepatocytes are sensitive to changes in concentration of beta-catenin, resulting in altered liver size and shape[10,11]. Further data in humans demonstrated that there is upregulation of beta-catenin when there is hepatic venous congestion in right heart failure[12]. This may give some insight into the relationship with diaphragmatic bands. It is well known that the liver is supplied in sections[13], and that there is relatively less vascularity at intersectional planes[14]. This forms the basis of hepatic segmentectomy[15]. It also stands to reason that, with less vascularity, there would be down-regulation of beta-catenin levels at these watershed areas. We propose that this, combined with the presence of diaphragmatic bands, could explain the presence of surface depressions. This could also explain the observations by Ono et al[16] and later by Macchi et al[2] that surface depressions were closely related to inter-sectional planes. This could be the basis of further study by attempting to correlate the occurrence of surface depressions with intersectional planes and also with measurements of beta-catenin levels in the corresponding liver parenchyma. These measurements could not be made with the existing study model.
The findings of this study show a significant relationship between diaphragmatic bands and liver surface depressions. With this data, the diaphragmatic-band theory has gained increased importance over other theories for surface depressions. Further studies are warranted using cross sectional imaging to confirm relationships with intersectional planes as well as beta-catenin assays in the affected liver parenchyma.
| 1. | Othman FB, Latiff AA, Suhaimi FH, Das S. Accessory sulci of the liver. An anatomical study with clinical implications. Saudi Med J. 2008;29:1247-1249. [PubMed] |
| 2. | Macchi V, Feltrin G, Parenti A, De Caro R. Diaphragmatic sulci and portal fissures. J Anat. 2003;202:303-308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Auh YH, Rubenstein WA, Zirinsky K, Kneeland JB, Pardes JC, Engel IA, Whalen JP, Kazam E. Accessory fissures of the liver: CT and sonographic appearance. AJR Am J Roentgenol. 1984;143:565-572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Newell RLM, Morgan-Jones R. Grooves in the superior surface of the liver. Clin Anat. 1993;6:333-336. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Joshi S, Valimbe N, Joshi S. Morphological Study of Foetal Thymus: A Cross-sectional Study. IJARS. 2022;. [DOI] [Full Text] |
| 6. | Yang DM, Kim HS, Cho SW. Pictorial review: various causes of hepatic capsular retraction: CT and MR findings. Br J Radiol. 2002;75:994-1002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Hawkins SP, Hine AL. Diaphragmatic muscular bundles (slips): ultrasound evaluation of incidence and appearance. Clin Radiol. 1991;44:154-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Cawich SO, Ali RRA, Gardner MT, Charles J, Sandy S, Pearce NW, Naraynsingh V. Hepatic surface grooves in Trinidad and Tobago. Surg Radiol Anat. 2020;42:1435-1440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Seema, Singh M, Mahajan A. An Anatomical Study of Variations of Sacral Hiatus in Sacra of North Indian Origin and Its Clinical Significance. Int J Morphol. 2013;31:110-114. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Michalopoulos G, Monga S, Pediaditakis P, Stolz D, Mule K. Changes in Wnt/β-catenin signaling during regulated growth in liver regeneration. Gastroenterology. 2001;120:A46-A46. [DOI] [Full Text] |
| 11. | Suksaweang S, Lin CM, Jiang TX, Hughes MW, Widelitz RB, Chuong CM. Morphogenesis of chicken liver: identification of localized growth zones and the role of beta-catenin/Wnt in size regulation. Dev Biol. 2004;266:109-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 80] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 12. | Mahmoodzadeh S, Eder S, Nordmeyer J, Ehler E, Huber O, Martus P, Weiske J, Pregla R, Hetzer R, Regitz-Zagrosek V. Estrogen receptor alpha up-regulation and redistribution in human heart failure. FASEB J. 2006;20:926-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 112] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 13. | Gotra A, Sivakumaran L, Chartrand G, Vu KN, Vandenbroucke-Menu F, Kauffmann C, Kadoury S, Gallix B, de Guise JA, Tang A. Liver segmentation: indications, techniques and future directions. Insights Imaging. 2017;8:377-392. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 96] [Cited by in RCA: 113] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 14. | Sureka B, Sharma N, Khera PS, Garg PK, Yadav T. Hepatic vein variations in 500 patients: surgical and radiological significance. Br J Radiol. 2019;92:20190487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 28] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Lowe MC, D'Angelica MI. Anatomy of Hepatic Resectional Surgery. Surg Clin North Am. 2016;96:183-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Ono ML, Murakami G, Sato TJ, Sawada K. Hepatic grooves and portal segmentation. Kaibogaku Zasshi. 2000;75:517-523. [PubMed] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/