Published online Jun 20, 2023. doi: 10.5493/wjem.v13.i3.17
Peer-review started: March 4, 2023
First decision: May 12, 2023
Revised: May 15, 2023
Accepted: May 22, 2023
Article in press: May 22, 2023
Published online: June 20, 2023
Processing time: 104 Days and 4.9 Hours
Cancer diagnosis is increasing around the world and in the Democratic Republic of the Congo (DRC). The proportion of thyroid cancer has increased over the past three decades. There are very few studies on cancer epidemiology, and in particular on thyroid cancer in the DRC.
To establish the most recent proportion of thyroid cancer in the DRC compared to other cancers.
This is a retrospective and descriptive study of 6106 consecutive cancer cases listed in the pathological registers of 4 Laboratories in the city of Kinshasa. This study included all cancer cases recorded in the registers between 2005 and 2019.
From a sample of 6106 patients, including all cancer types, 68.3% cases were female and 31.7% were male. Breast and cervical cancer were the most common types of cancer in women and, prostate and skin cancer were the most common types in men. Thyroid cancer was sixth in proportion in women and eleventh in men compared to all cancers. Papillary carcinoma was the most common of thyroid cancers. Rare cancers such as anaplastic and medullary thyroid carcinomas had a proportion of 7% and 2%, respectively.
Newer diagnostic tools led to a surge in cancer diagnoses in the DRC. Thyroid cancer has more than doubled its proportion over the last several decades in the country.
Core Tip: Cancer diagnosis has been increasing worldwide. This is also true in Africa, particularly in the second biggest African country. However, there are currently no data on cancer in the Democratic Republic of Country (DRC). This study offers the most updated cancer data in general and thyroid cancer in particular in the DRC. Using this current database, more research can be carried out in the country.
- Citation: Bukasa-Kakamba J, Bangolo AI, Bayauli P, Mbunga B, Iyese F, Nkodila A, Atoot A, Anand G, Lee SH, Chaudhary M, Fernandes PQ, Mannam HP, Polavarapu A, Merajunnissa M, Azhar A, Alichetty MN, Singh G, Arana Jr GV, Sekhon I, Singh M, Rodriguez-Castro JD, Atoot A, Weissman S, M’buyamba JR. Proportion of thyroid cancer and other cancers in the democratic republic of Congo. World J Exp Med 2023; 13(3): 17-27
- URL: https://www.wjgnet.com/2220-315x/full/v13/i3/17.htm
- DOI: https://dx.doi.org/10.5493/wjem.v13.i3.17
Thyroid pathology is the most common endocrinopathy worldwide[1] and is mostly represented by goiters and nodules[2,3]. Five to ten percent of thyroid nodules are malignant[3-6]. Thyroid cancer only represents 1% of all cancers worldwide[7,8] but has occupied the fifth position among all cancers in France and Canada in women in terms of incidence and twentieth in terms of mortality in 2005[9-11]. The improvement of diagnostic techniques by means of thyroid ultrasound, fine needle aspiration, Computed Tomography scan and detailed histopathological analyses partly explains the increase in incidence of thyroid cancer[12,13]. Despite this increase in incidence, the mortality curve has remained stable over time[10,14].
The Democratic Republic of the Congo (DRC) is a low-income country where there are only 7 pathology laboratories for more than 80 million citizens. Five of these laboratories are located in the capital city of Kinshasa. The typical Congolese meals have been characterized with a low iodine content for decades. Iodine deficiency is a well-known risk factor for thyroid cancer[14,15]. We thus hypothesize that thyroid cancer may be frequent in the DRC but reliable data on cancers in general and thyroid cancer in particular is scarce. The first study addressing thyroid cancer proportion in the DRC was conducted by Mashinda et al[16] and it revealed, in women, a thyroid cancer proportion of 0.5% out of all cancers found in the anatomopathological records between 1969 and 2008. Although epidemiologic trend changes are expected to be gradual, the available data now seems dated. The objective of this study is thus to provide more recent thyroid cancer proportion data using the largest series analyzed so far in the DRC.
This is a retrospective and descriptive study of thyroid cancers and of all types of cancer retrieved from the records of 4 anatomopathological laboratories including that of Kinshasa University Clinics, National Institute of Biomedical Research, Kinshasa General Hospital (HGRK) and LEBOMA laboratory. All these laboratories are located in the capital city of Kinshasa, a city of nearly 12 million inhabitants. This study included cancers diagnosed in those centers between 2005 and 2019, except for the data obtained from LEBOMA laboratory, which covered from 2015 to 2019. The choice of these centers was governed by the fact that they are the only pathology laboratories in the town of Kinshasa with available data over the period of the study. We calculated the relative proportion of thyroid cancer by dividing the number of thyroid cancers by the number of all types of cancer. It’s important to report that calcitonin was not measured preoperatively in patients with thyroid cancer.
The study took into account the following socio-demographic characteristics: Age, gender, year of diagnosis and histopathological diagnosis.
The following types of cancer were taken into account: Breast cancer, cervical cancer, prostate cancer, skin cancer, hematologic cancers, uterine cancer, colon cancer, lung cancer, stomach cancer, bone cancer, thyroid cancer, anorectal cancer, Kaposi sarcoma, soft tissue cancers, eye cancers, ovarian cancer, mouth cancer, vaginal cancer, urinary bladder cancer, laryngeal cancer, nose cancer, peritoneal cancer, liver cancer, renal cancer, vulva cancer, ureteral cancer, nasopharyngeal cancer, intestinal cancer, pancreatic cancer, greater omentum cancer, esophageal cancer, penile cancer, testicular cancer, tonsillar cancer, brain cancer, coecum cancer, vocal cords cancer, ear cancer, parotid glands cancer, duodenal cancer, cancer of the palate, forehead cancer, glottis cancer, trachea cancer, sweat glands cancer, maxillary cancer and splenic cancer.
Data was entered into Excel and transported to Statistical Package for the Social Sciences version 21. Quantitative variables were expressed as mean (+/- SD) or median (+/- interquartile range) for variables that did not have a normal distribution. Qualitative variables were expressed as proportions. Student's t-test was used for comparing averages between men and women. χ2 test or Fisher's exact test was used to compare the difference in proportions between the 2 groups regarding qualitative variables. A P value < 0.05 was of statistical significance.
6106 cancer cases were included in this study. A female predominance was observed with 68.3% of cases vs 32.7% for men with a female/male ratio of 4. We noted 106 cases of thyroid cancer, representing 1.7% of the total number of cancer cases. The proportion of all types of cancer according to their location and gender, are grouped together in Table 1.
| All, n = 6106 | Females, n = 4169 (68.3%) | Males, n = 1937 (31.7%) | |
| Breast | 1631 (26.7) | 1560 (37.4) | 71 (3.7) |
| Cervix | 1138 (18.6) | 1138 (27.3) | - |
| Prostate | 678 (11.1) | - | 678 (35) |
| Skin | 356 (5.8) | 186 (4.5) | 170 (8.8) |
| Blood and LO | 206 (3.4) | 95 (2.3) | 111 (5.7) |
| Uterus | 196 (3.2) | 196 (4.7) | - |
| Colon | 173 (2.8) | 92 (2.2) | 81 (4.2) |
| Lungs | 161 (2.6) | 60 (1.4) | 101 (5.2) |
| Stomach | 112 (1.8) | 45 (1.1) | 67 (3.5) |
| Bone | 110 (1.8) | 58 (1.4) | 52 (2.7) |
| Thyroid | 106 (1.7) | 84 (2) | 22 (1.1) |
| Anus/rectum | 97 (1.6) | 51 (1.2) | 46 (2.4) |
| KS | 87 (1.4) | 22 (0.5) | 65 (3.4) |
| Soft tissue | 87 (1.4) | 52 (1.2) | 35 (1.8) |
| Eye | 77 (1.3) | 35 (0.8) | 42 (2.2) |
| Ovary | 77 (1.3) | 77 (1.8) | - |
| Mouth | 76 (1.2) | 44 (1.1) | 32 (1.7) |
| Vagina | 75 (1.2) | 75 (1.8) | - |
| Urinary bladder | 73 (1.2) | 32 (0.8) | 41 (2.1) |
| Larynx | 65 (1.1) | 11 (0.3) | 54 (2.8) |
| Nose | 62 (1.0) | 28 (0.7) | 34 (1.8) |
| Peritoneum | 51 (0.8) | 24 (0.6) | 27 (1.4) |
| Liver | 49 (0.8) | 24 (0.6) | 25 (1.3) |
| Kidneys | 41 (0.7) | 21 (0.5) | 20 (1) |
| Vulva | 41 (0.7) | 41 (1) | - |
| Ureter | 33 (0.5) | 14 (0.3) | 19 (1) |
| Pharynx/nasopharynx | 33 (0.5) | 19 (0.4) | 14 (0.7) |
| Intestines | 29 (0.5) | 16 (0.4) | 13 (0.7) |
| Pancreas | 29 (0.5) | 13 (0.3) | 16 (0.8) |
| Greater omentum | 22 (0.4) | 15 (0.4) | 7 (0.4) |
| Esophagus | 21 (0.3) | 8 (0.2) | 13 (0.7) |
| Penis | 20 (0.3) | - | 20 (1) |
| Testicles | 20 (0.3) | - | 20 (1) |
| Tonsils | 13 (0.2) | 5 (0.1) | 8 (0.4) |
| Brain | 10 (0.2) | 6 (0.1) | 4 (0.2) |
| Coecum | 10 (0.2) | 4 (0.1) | 6 (0.3) |
| Vocal cords | 8 (0.1) | 1 (0.0) | 7 (0.4) |
| Ears | 7 (0.1) | 5 (0.1) | 2 (0.1) |
| Parotid glands | 7 (0.1) | 2 (0.0) | 5 (0.3) |
| Duodenum | 6 (0.1) | 2 (0.0) | 4 (0.2) |
| Palate | 4 (0.1) | 4 (0.1) | 0 (0.0) |
| Forehead | 2 (0.0) | 1 (0.0) | 1 (0.1) |
| Glottis | 2 (0.0) | 0 (0.0) | 2 (0.1) |
| Trachea | 2 (0.0) | 1 (0.0) | 1 (0.1) |
| Sweat glands | 1 (0.0) | 1 (0.0) | 0 (0.0) |
| Maxillary | 1 (0.0) | 1 (0.0) | 0 (0.0) |
| Spleen | 1 (0.0) | 0 (0.0) | 1 (0.1) |
The most common types of cancer, in decreasing order of proportion were breast cancer, cervical cancer, prostate cancer, skin cancer and lymphoid cancers. Thyroid cancer ranked eleventh in proportion for all cancer types.
The five most common types of cancer in women were breast cancer, cervical cancer, uterine cancer, skin cancer and lymphoid organ (LO) cancer. Thyroid cancer was ranked Sixth with a proportion of 2%.
The five most common types of cancer in men were prostate cancer, skin cancer, LO cancer, lung cancer and colon cancer. For men, thyroid cancer was ranked eleventh (1.1% of all types of cancer).
The male gender was more represented in the age groups ≤ 30 and > 60, while the female gender was more represented in the age groups between 30 and 60 years old. Most cases of cancer in women occur between the ages of 40 and 60 and in men over 50. The number of patients diagnosed with cancer increases with age in both genders.
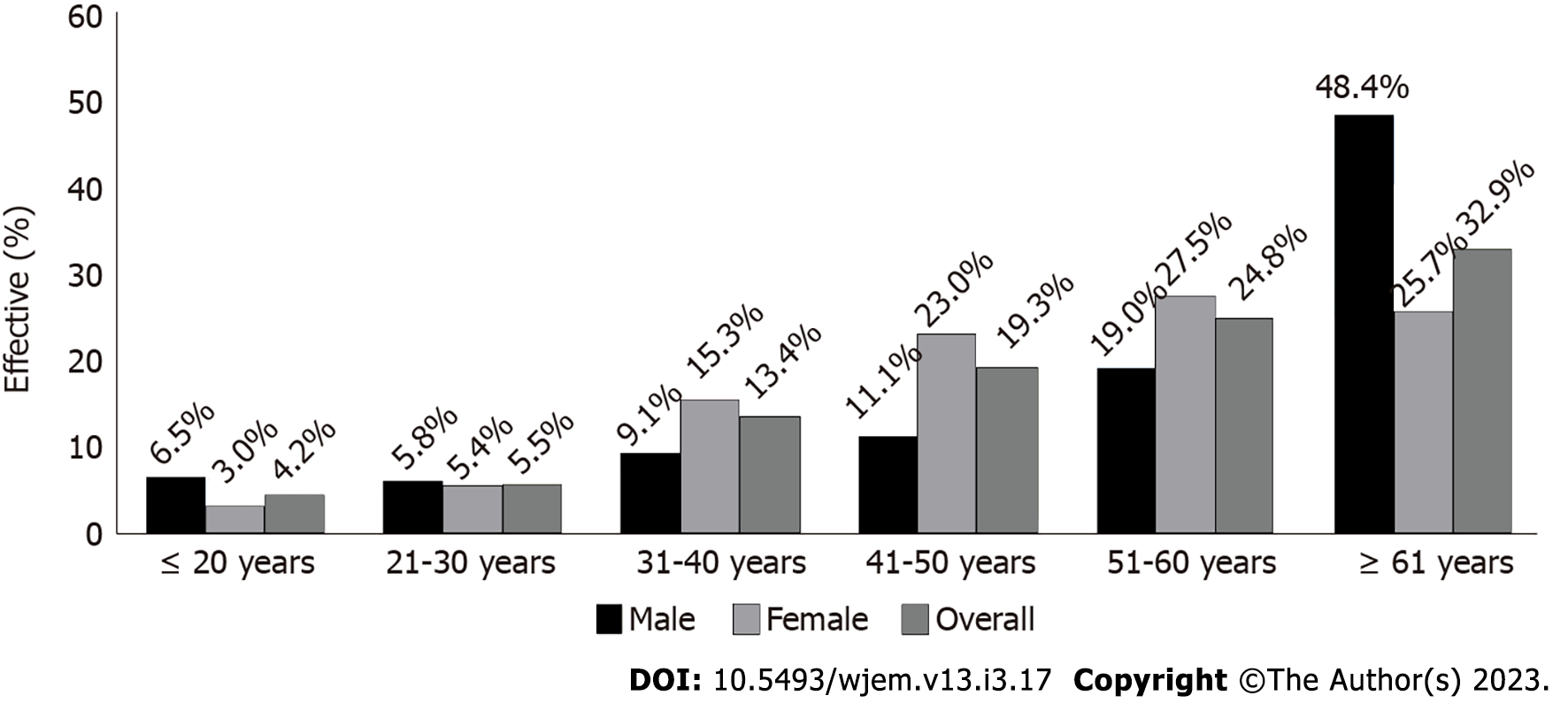
In the age group up to 40 years old and between 41 and 60 years old, breast cancer was the most common type of cancer. In the age group of over 61, prostate cancer was the most common. Thyroid cancer occupied the sixth position in the age group under 41 years, the tenth in the age group between 41 years and 60 years and the sixteenth in the age group over 60 years old. Figure 1 represents cancer cases’ distribution based on age and gender.
The frequencies of cancer cases according to age groups are found in Table 2.
| Cancers | ≤ 40 yr, n (1409) | % | 41-60 yr, n (2689) | % | ≥ 61 yr, n (2008) | % |
| Prostate | 25 | 1.8 | 122 | 4.5 | 531 | 26.4 |
| Cervical | 156 | 11.1 | 611 | 22.7 | 371 | 18.5 |
| Breast | 403 | 28.6 | 895 | 33.3 | 333 | 16.6 |
| Skin | 141 | 10.0 | 133 | 4.9 | 82 | 4 |
| Uterus | 22 | 1.6 | 107 | 4.0 | 67 | 3.3 |
| Lungs | 30 | 2.1 | 64 | 2.4 | 67 | 3.3 |
| Blood and LO | 106 | 7.5 | 48 | 1.8 | 52 | 2.6 |
| Colon | 37 | 2.6 | 92 | 3.4 | 44 | 2.2 |
| Stomach | 19 | 1.3 | 60 | 2.2 | 33 | 1.6 |
| KS | 32 | 2.3 | 24 | 0.9 | 31 | 1.5 |
| Soft tissue | 27 | 1.9 | 30 | 1.1 | 30 | 1.5 |
| Vessels | 13 | 0.9 | 32 | 1.2 | 28 | 1.4 |
| Bones | 51 | 3.6 | 33 | 1.2 | 26 | 1.3 |
| Vagina | 16 | 1.1 | 33 | 1.2 | 26 | 1.3 |
| Larynx | 18 | 1.3 | 21 | 0.8 | 26 | 1.3 |
| Anus-rectum | 27 | 1.9 | 45 | 1.7 | 25 | 1.2 |
| Thyroid | 37 | 2.6 | 45 | 1.7 | 24 | 1.2 |
| Mouth | 28 | 2.0 | 24 | 0.9 | 24 | 1.2 |
| Liver | 12 | 0.9 | 19 | 0.7 | 18 | 0.9 |
| Ureter | 3 | 0.2 | 12 | 0.4 | 18 | 0.9 |
| Vulva | 4 | 0.3 | 20 | 0.7 | 17 | 0.8 |
| Ovary | 25 | 1.8 | 36 | 1.3 | 16 | 0.8 |
| Peritoneum | 12 | 0.9 | 23 | 0.9 | 16 | 0.8 |
| Nose | 26 | 1.8 | 23 | 0.9 | 13 | 0.6 |
| Eye | 35 | 2.5 | 30 | 1.1 | 12 | 0.6 |
| Esophagus | 5 | 0.4 | 6 | 0.2 | 10 | 0.5 |
| Testicles | 8 | 0.6 | 2 | 0.1 | 10 | 0.5 |
| Pharynx | 14 | 1.0 | 8 | 0.3 | 9 | 0.4 |
| Pancreas | 5 | 0.4 | 16 | 0.6 | 8 | 0.4 |
| Intestines | 7 | 0.5 | 15 | 0.6 | 7 | 0.3 |
| Kidney | 23 | 1.6 | 11 | 0.4 | 7 | 0.3 |
| Vocal cords | 1 | 0.1 | 2 | 0.1 | 5 | 0.2 |
| Greater omentum | 7 | 0.5 | 11 | 0.4 | 4 | 0.2 |
| Penis | 5 | 0.4 | 12 | 0.4 | 3 | 0.1 |
| Duodenum | 1 | 0.1 | 2 | 0.1 | 3 | 0.1 |
| Tonsils | 4 | 0.3 | 7 | 0.3 | 2 | 0.1 |
| Coecum | 5 | 0.4 | 3 | 0.1 | 2 | 0.1 |
| Ears | 4 | 0.3 | 1 | 0.0 | 2 | 0.1 |
| Palate | 1 | 0.1 | 1 | 0.0 | 2 | 0.1 |
| Parotid glands | 3 | 0.2 | 3 | 0.1 | 1 | 0.0 |
| Trachea | 1 | 0.1 | 0 | 0.0 | 1 | 0.0 |
| Maxillary | 0 | 0.0 | 0 | 0.0 | 1 | 0.0 |
| Spleen | 0 | 0.0 | 0 | 0.0 | 1 | 0.0 |
| Brain | 6 | 0.4 | 4 | 0.1 | 0 | 0.0 |
| Glottis | 0 | 0.0 | 2 | 0.1 | 0 | 0.0 |
| Nasopharynx | 1 | 0.1 | 1 | 0.0 | 0 | 0.0 |
| Forehead | 2 | 0.1 | 0 | 0.0 | 0 | 0.0 |
| Sweat glands | 1 | 0.1 | 0 | 0.0 | 0 | 0.0 |
Papillary carcinoma was the most common type of thyroid cancer, representing 67% of all thyroid cancer cases followed by the follicular type in 21% of cases. The anaplastic type occupied the third, lymphoma the fourth and medullary cancer the fifth position.
The frequencies of the different types of thyroid cancer cases in this series are found in Table 3.
| Variables | Number of cases | Percentage (%) |
| Histology | ||
| Papillary carcinoma | 71 | 67.0 |
| Follicular carcinoma | 23 | 21.7 |
| Anaplastic carcinoma | 8 | 7.5 |
| Lymphoma | 3 | 2.8 |
| Medullary carcinoma | 1 | 0.9 |
The main objective of this research was to establish the proportion of thyroid cancer in the Congolese population, secondarily aiming to identify the most frequent types of cancer. Our series included 6106 cases, of which 68.3 % were female and 31.7% were male. This predominance of the female gender was also found in the series of Mashinda who studied the proportion of cancer in the urban setting of Kinshasa[16] and it was also the case for the Lukanu series, which included cases from a rural setting of Congo in Kimpese[17]. These are the first two epidemiological studies analyzing neoplastic diseases carried out in the DRC and which preceded our research.
Female predominance in overall cancer diagnosis was observed in the DRC, a similar trend was also observed in Brazil[18]. The cascade of sexual hormone activity, in particular estrogens and the aromatization of androgens via Mitogen-activated protein kinase, causing the decline of immune cells and promoting the proliferation of cancer cells and the inhibition of apoptotic activity can explain the female predominance[19,20]. However, some European series have found a male predominance[21-25].
The male gender was more represented in the age groups up to 30 years and over 60 years; on the other hand, the female gender was more represented in the age groups between 30 and 60 years. Most cancers in women occurred between the ages of 40 and 60 and in men over 50. Overall, 77% of cancer patients were over 40 years of age. Most series around the world, according to which the older the age the greater the probability of developing a neoplastic disease[26,27].
In our series, breast cancer occupies the first position in terms of proportion of cancer in women and cervical cancer occupies the second position. However, in the series published by Mashinda et al[16], cervical cancer was the most frequent followed by breast cancer. This difference can be explained by the methodology, the study period, the progress of the national policy on cancer screening in women and by the development of diagnostic means. Mashinda studied the records of two pathology laboratories, while we researched the records of 4 pathology laboratories. Mashinda analyzed results from 1965 to 2008, while we studied data between 2005 and 2019. We must consider that the means of raising awareness have evolved and the educational level of the population has increased over time. All these parameters can explain this difference. The findings of our study are similar to the Lukanu series; breast cancer was the most frequent followed by cervical cancer[17]. Our results also mirrored those found in several African and worldwide series[28-31].
When we consider both sexes, breast cancer was the most frequent type of cancer in our series, this result being similar to those in the literature[29].
Regarding cancer in male patients, prostate cancer was the most frequent in our series. This result was similar to the series of Lukanu[17]. On the other hand, Mashinda found lymphoid organ cancer as the most frequent followed by prostate cancer[16]. Our results are similar to those found in the literature[30]. There has been an improvement in the awareness of the Congolese population regarding prostate cancer over the past two decades.
Thyroid cancer is the most common type of cancer of the endocrine system[32]. Our series found a proportion of 1.7%. In women, the proportion is 2% and it is 1.1% in men, with a female/male ratio of 4. The series described by Mashinda et al[16] found a thyroid cancer proportion of 0.5% in women. The proportion of thyroid cancer in women in our series compared to that of Mashinda’s is multiplied by 4, a female predominance that is confirmed in the literature[33,34]. Thyroid cancer occupied the sixth position of all listed cancers among women in our series, whereas it is the 5th most frequent cancer in women worldwide[35].
The increase in thyroid cancer proportion has also been observed in several studies around the world over the past three decades[10]. The mechanisms underlying this increase have not yet been elucidated. However, nutritional, hormonal, anthropometric, environmental, and other factors are suspected. Many authors also consider that excessive iodine intake, and the development and accessibility of diagnostic tools participate to the increase in diagnosis[36,37].
Our study found that nearly 7% of thyroid cancers were anaplastic and 1% were medullary, while these cancers are rare in the literature[38]. This can be considered as a particularity of the DRC regarding thyroid cancer, especially since calcitonin is not generally measured in the assessment of thyroid nodules or preceding thyroidectomy.
It is known that 90% of thyroid cancers are differentiated and have good prognosis and that only 5% to 10% are undifferentiated and have therefore a bad prognosis[39,40]. This high proportion of undifferentiated cancers in our series constitutes a particularity of the Congo. This can be explained by the fact that, undifferentiated thyroid cancers, given their aggressive behavior, are more likely to warrant a surgical evaluation. Thyroid cancers in our study originated from surgical pathology reports. Nevertheless, this particularity requires more in-depth studies to better understand the causes and mechanisms.
Since iodine deficiency in the soil is considered a risk factor for anaplastic cancer[41], the question to be raised is whether iodine deficiency could be responsible for this higher proportion of undifferentiated cancers. knowing that iodine saturation in the Congo was only obtained in 1993[42]. Another potential mechanism is that initially differentiated cancers have lost differentiation over time[43] due to late diagnosis.
This work has the limitations of retrospective studies. In addition, it is biased due to the fact that we only took into consideration the patients who had carried out the anatomopathology while those who had not carried out one, were not included in this study, this may have influenced a high proportion of cancers and certain histological types. Finally, limitations in diagnostic facilities in data reporting in a resource-poor healthcare facility are also potentially limiting.
Despite these limitations, this work gives a scoping vision of cancer in the DRC and in particular of thyroid cancer. It has established the frequencies of different forms of cancer in a country where cancer data are rare.
Cancer diagnosis is on the rise in the DRC and the proportion of thyroid cancer as compared to total number of cancers has doubled over the period from 2005 to 2019. A marked female predominance was observed. Papillary thyroid cancer is the most frequent type of thyroid cancer followed by follicular carcinoma. There is a high proportion of undifferentiated thyroid cancers such as anaplastic carcinomas, long recognized as rare carcinomas. Breast cancer is the most common of all types of cancer, followed by cervical cancer. Prostate cancer is the most common type of cancer in men. Thyroid cancer ranked sixth most common cancer in women and eleventh most common in men. This study establishes the most recent and updated proportion of thyroid cancer in the second-largest African country.
Cancer diagnosis has been increasing worldwide and in Africa as well, particularly in the Democratic Republic of Congo (DRC). However, there are currently no studies addressing the proportion of different cancers in the DRC, and in particular thyroid cancer.
The main motivation of this study was to identify the proportions of different cancers in the DRC and in particular thyroid cancer.
The purpose of this study was to analyze different epidemiologic characteristics of thyroid cancers in the second-largest African country while establishing the proportions of all cancers in the country.
This is a retrospective and descriptive study of 6106 consecutive cancer cases listed in the pathological registers of 4 Laboratories in the city of Kinshasa. This study included all cancer cases recorded in the registers between 2005 and 2019.
In our series two third of cancer patients were females. Breast is the most common cancer in females while prostate cancer is the most common among their male counterparts. Thyroid cancer was ranked sixth in occurrence and a higher proportion of anaplastic thyroid cancer was encountered.
Female patients seem to be more affected by cancer than their male counterparts. Rare anaplastic thyroid cancers which are often associated with a dismal prognosis, although rare in the literature, are found in higher proportion in the DRC. A surge of all cancers was also observed owing to the advances in diagnostic tools used.
Oncology and cancer research in the DRC remains an unexplored area. There is a serious paucity of data of any cancer in the country. This study offers the most updated data on cancer in the second-largest African country. This study paves the way for future prospective studies in the country, helping to identify groups at higher risk and shaping the national guidelines.
| 1. | Unnikrishnan AG, Menon UV. Thyroid disorders in India: An epidemiological perspective. Indian J Endocrinol Metab. 2011;15:S78-S81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 136] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 2. | Hegedüs L. Clinical practice. The thyroid nodule. N Engl J Med. 2004;351:1764-1771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 984] [Cited by in RCA: 954] [Article Influence: 43.4] [Reference Citation Analysis (0)] |
| 3. | Bouaity B, Darouassi Y, Chihani M, Touati MM, Ammar H. [Analysis of predictors of malignancy of nodular goiters: about 500 cases]. Pan Afr Med J. 2016;23:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 4. | Wémeau JL, Sadoul JL, d'Herbomez M, Monpeyssen H, Tramalloni J, Leteurtre E, Borson-Chazot F, Caron P, Carnaille B, Léger J, Do C, Klein M, Raingeard I, Desailloud R, Leenhardt L. Guidelines of the French society of endocrinology for the management of thyroid nodules. Ann Endocrinol (Paris). 2011;72:251-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Belfiore A, La Rosa GL, Padova G, Sava L, Ippolito O, Vigneri R. The frequency of cold thyroid nodules and thyroid malignancies in patients from an iodine-deficient area. Cancer. 1987;60:3096-3102. [PubMed] [DOI] [Full Text] |
| 6. | Rago T, Chiovato L, Aghini-Lombardi F, Grasso L, Pinchera A, Vitti P. Non-palpable thyroid nodules in a borderline iodine-sufficient area: detection by ultrasonography and follow-up. J Endocrinol Invest. 2001;24:770-776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 48] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid. 2016;26:1-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10769] [Cited by in RCA: 10274] [Article Influence: 1027.4] [Reference Citation Analysis (1)] |
| 8. | Touati MM, Aljalil A, Darouassi Y, Chihani M, Lahkim M, Fihri JA, Bouaity B, Ammar H. [Thyroid carcinoma: epidemiological, clinical and therapeutic profiles, about 102 cases]. Pan Afr Med J. 2015;21:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Colonna M, Bossard N, Guizard AV, Remontet L, Grosclaude P; le réseau FRANCIM. Descriptive epidemiology of thyroid cancer in France: incidence, mortality and survival. Ann Endocrinol (Paris). 2010;71:95-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Kilfoy BA, Zheng T, Holford TR, Han X, Ward MH, Sjodin A, Zhang Y, Bai Y, Zhu C, Guo GL, Rothman N. International patterns and trends in thyroid cancer incidence, 1973-2002. Cancer Causes Control. 2009;20:525-531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 525] [Cited by in RCA: 499] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 11. | Government of Canada SC. Changing trends in thyroid cancer incidence in Canada: A histologic examination, 1992 to 2016 [Internet]. 2020. Available from: https://www150.statcan.gc.ca/n1/pub/82-003-x/2020001/article/00002-eng.htm. |
| 12. | Jung CK, Little MP, Lubin JH, Brenner AV, Wells SA Jr, Sigurdson AJ, Nikiforov YE. The increase in thyroid cancer incidence during the last four decades is accompanied by a high frequency of BRAF mutations and a sharp increase in RAS mutations. J Clin Endocrinol Metab. 2014;99:E276-E285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 291] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 13. | Seib CD, Sosa JA. Evolving Understanding of the Epidemiology of Thyroid Cancer. Endocrinol Metab Clin North Am. 2019;48:23-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 333] [Article Influence: 47.6] [Reference Citation Analysis (0)] |
| 14. | Leenhardt L, Bernier MO, Boin-Pineau MH, Conte Devolx B, Maréchaud R, Niccoli-Sire P, Nocaudie M, Orgiazzi J, Schlumberger M, Wémeau JL, Chérie-Challine L, De Vathaire F. Advances in diagnostic practices affect thyroid cancer incidence in France. Eur J Endocrinol. 2004;150:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 162] [Cited by in RCA: 157] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 15. | Cardis E, Kesminiene A, Ivanov V, Malakhova I, Shibata Y, Khrouch V, Drozdovitch V, Maceika E, Zvonova I, Vlassov O, Bouville A, Goulko G, Hoshi M, Abrosimov A, Anoshko J, Astakhova L, Chekin S, Demidchik E, Galanti R, Ito M, Korobova E, Lushnikov E, Maksioutov M, Masyakin V, Nerovnia A, Parshin V, Parshkov E, Piliptsevich N, Pinchera A, Polyakov S, Shabeka N, Suonio E, Tenet V, Tsyb A, Yamashita S, Williams D. Risk of thyroid cancer after exposure to 131I in childhood. J Natl Cancer Inst. 2005;97:724-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 419] [Cited by in RCA: 380] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 16. | Mashinda KD, Kayembe KP, Mapatano MA. Prévalence du cancer en République Démocratique du Congo: données anatomopathologiques recueillies aux Cliniques Universitaires et à l’Hôpital Général de Référence de Kinshasa Cancer prevalence in Democratic Republic of the Congo: anatomopathologic. Annales africaines de médecine. 2012;5:3. |
| 17. | Lukanu NP, Ntontolo NP, Diakengua V, Kalombo C, Nyambu J, Landu J, Nlandu J, Atungu P, Nsiangana Z, Nkodila A. Epidemiology of Cancer in Rural Congo: Case of IME Kimpese Hospital, Democratic Republic of Congo. J Cancer Ther. 2021;12:127. [DOI] [Full Text] |
| 18. | Kolankiewicz ACB, de Souza Magnago TSB, Dos Santos Dullius AI, De Domenico EBL. Liens entre les variables démographiques, économiques et cliniques et les symptômes rapportés par les patients en cours de traitement contre le cancer. Can Oncol Nurs J. 2017;27:375-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 19. | Irelli A, Sirufo MM, D'Ugo C, Ginaldi L, De Martinis M. Sex and Gender Influences on Cancer Immunotherapy Response. Biomedicines. 2020;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 20. | Özdemir BC, Dotto GP. Sex Hormones and Anticancer Immunity. Clin Cancer Res. 2019;25:4603-4610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 100] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 21. | Dorak MT, Karpuzoglu E. Gender differences in cancer susceptibility: an inadequately addressed issue. Front Genet. 2012;3:268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 228] [Cited by in RCA: 328] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 22. | Dyba T, Randi G, Bray F, Martos C, Giusti F, Nicholson N, Gavin A, Flego M, Neamtiu L, Dimitrova N, Negrão Carvalho R, Ferlay J, Bettio M. The European cancer burden in 2020: Incidence and mortality estimates for 40 countries and 25 major cancers. Eur J Cancer. 2021;157:308-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 429] [Article Influence: 85.8] [Reference Citation Analysis (0)] |
| 23. | Ketfi A, Zanoun N, Laouedj I, Gharnaout M, Fraga S. [Primary lung cancer and occupational exposure in a North African population]. Pan Afr Med J. 2020;37:120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 24. | Cao B, Hill C, Bonaldi C, León ME, Menvielle G, Arwidson P, Bray F, Soerjomataram I. Cancers attributable to tobacco smoking in France in 2015. Eur J Public Health. 2018;28:707-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 25. | Nga WTB, Eloumou SAFB, Engbang JPN, Bell EMD, Mayeh AMM, Atenguena E, Biwole ME, Ayissi GBN, Kenfack G, Noah DN, Luma HN, Sone AM, Ndom P, Ndam ECN. [Prognosis and survival of esophageal cancer in Cameroon: a prognostic study]. Pan Afr Med J. 2019;33:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Ndahindwa V, Ngendahayo L, Vyankandondera J. Aspects Epidemiologiques Et Anatomopathologiques Des Cancers Dans Les Centres Hospitaliers Universitaires (Chu) Du Rwanda. RMJ. 2012;69:10. |
| 27. | Chase D. Letter in response to the original article: "Evaluation of femoral approach to coronary sinus catheterisation in electrophysiological and ablation procedures: Single centre experience" authored by Osama Abdel Atty, Mohamed Morsy and Mark M. Gallagher (Journal of the Saudi Heart Association, Volume 23, Issue 4, October 2011, pp. 213-216). J Saudi Heart Assoc. 2012;24:145. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 28. | Airtum Working Group. [Numbers (Airtum Working Group). Survival prospects change with time]. Epidemiol Prev. 2008;32:136. [PubMed] |
| 29. | Fwelo P, Nwosu KOS, Adekunle TE, Afolayan O, Ahaiwe O, Ojaruega AA, Nagesh VK, Bangolo A. Racial/ethnic and socioeconomic differences in breast cancer surgery performed and delayed treatment: mediating impact on mortality. Breast Cancer Res Treat. 2023;199:511-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 30. | Organisation mondiale de la Sante. Breast Cancer. 2021. Available from: https://www.who.int/fr/news-room/fact-sheets/detail/breast-cancer. |
| 31. | Chbani L, Hafid I, Berraho M, Mesbahi O, Nejjari C, Amarti A. [Epidemiological and pathological features of cancer in Fez Boulemane region, Morocco]. East Mediterr Health J. 2013;19:263-270. [PubMed] |
| 32. | Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71-96. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8283] [Cited by in RCA: 8236] [Article Influence: 457.6] [Reference Citation Analysis (11)] |
| 33. | Kilfoy BA, Devesa SS, Ward MH, Zhang Y, Rosenberg PS, Holford TR, Anderson WF. Gender is an age-specific effect modifier for papillary cancers of the thyroid gland. Cancer Epidemiol Biomarkers Prev. 2009;18:1092-1100. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 146] [Cited by in RCA: 154] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 34. | Moses W, Weng J, Kebebew E. Prevalence, clinicopathologic features, and somatic genetic mutation profile in familial versus sporadic nonmedullary thyroid cancer. Thyroid. 2011;21:367-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 101] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 35. | Worldwide cancer data. World Cancer Research Fund International [Internet]. WCRF International. [cite 16 in 2021]. Available from: https://www.wcrf.org/dietandcancer/worldwide-cancer-data/. |
| 36. | Jegerlehner S, Bulliard JL, Aujesky D, Rodondi N, Germann S, Konzelmann I, Chiolero A; NICER Working Group. Overdiagnosis and overtreatment of thyroid cancer: A population-based temporal trend study. PLoS One. 2017;12:e0179387. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 127] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 37. | Dal Maso L, Bosetti C, La Vecchia C, Franceschi S. Risk factors for thyroid cancer: an epidemiological review focused on nutritional factors. Cancer Causes Control. 2009;20:75-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 189] [Cited by in RCA: 201] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 38. | Wemeau JL, Do Cao C. [Anaplastic thyroid carcinoma]. Ann Endocrinol (Paris). 2008;69:174-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 39. | Rego-Iraeta A, Pérez-Méndez LF, Mantinan B, Garcia-Mayor RV. Time trends for thyroid cancer in northwestern Spain: true rise in the incidence of micro and larger forms of papillary thyroid carcinoma. Thyroid. 2009;19:333-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 108] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 40. | Zhu J, Sun K, Wang J, He Y, Li D, Liu S, Huang Y, Zhang M, Song B, Liao X, Liang H, Zhang Q, Shi M, Guo L, Zhou Y, Lin Y, Lu Y, Tuo J, Xia Y, Sun H, Xiao H, Ji Y, Yan C, Qiao J, Zeng H, Zheng R, Zhang S, Chang S, Wei W. Clinicopathological and surgical comparisons of differentiated thyroid cancer between China and the USA: A multicentered hospital-based study. Front Public Health. 2022;10:974359. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 41. | Kim J, Gosnell JE, Roman SA. Geographic influences in the global rise of thyroid cancer. Nat Rev Endocrinol. 2020;16:17-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 319] [Article Influence: 53.2] [Reference Citation Analysis (1)] |
| 42. | Banza BI, Lumbu JB, Donnen P, Twite EK, Kwete DM, Kazadi CM, Ozoza JO, Habimana L, Kalenga PM, Robert A. [Iodine concentration in cooking salt consumed in Lubumbashi and the iodine status of vulnerable people: case study of pregnant women living in underprivileged areas]. Pan Afr Med J. 2016;23:129. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 43. | Harach HR, Galíndez M, Campero M, Ceballos GA. Undifferentiated (anaplastic) thyroid carcinoma and iodine intake in Salta, Argentina. Endocr Pathol. 2013;24:125-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Oncology
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Haddadi S, Algeria; Wang KJ, China S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ