Published online Jun 20, 2023. doi: 10.5493/wjem.v13.i3.7
Peer-review started: January 15, 2023
First decision: February 8, 2023
Revised: February 15, 2023
Accepted: April 10, 2023
Article in press: April 10, 2023
Published online: June 20, 2023
Processing time: 152 Days and 7.7 Hours
Overweight, obesity, and type 2 diabetes mellitus pose global health problems that are ever-increasing. A chronic low-grade inflammatory status and the presence of various pro-inflammatory markers either in circulation or within dysfunctional metabolic tissues are well established. The presence of these factors can, to some extent, predict disease development and progression. A central role is played by the presence of dysfunctional adipose tissue, liver dysfunction, and skeletal muscle dysfunction, which collectively contribute to the increased cir
Core Tip: A significant amount of literature indicates the relationship between increased inflammatory markers and overweight, obesity, and type 2 diabetes mellitus. Even though the role of inflammation in the development and progression of these conditions is uncertain, the potential use of inflammatory markers as diagnostic and prognostic tools is under vigorous investigation. Weight loss and lifestyle interventions result on reduction of inflammatory markers in individuals with overweight and obesity and/or type 2 diabetes mellitus.
- Citation: Lempesis IG, Georgakopoulou VE. Physiopathological mechanisms related to inflammation in obesity and type 2 diabetes mellitus. World J Exp Med 2023; 13(3): 7-16
- URL: https://www.wjgnet.com/2220-315x/full/v13/i3/7.htm
- DOI: https://dx.doi.org/10.5493/wjem.v13.i3.7
Overweight and obesity represent a significant, ever-increasing global public health challenge[1-4]. Obesity is a complex multifactorial disorder linked to a high risk of developing type 2 diabetes mellitus (T2DM), cardiometabolic diseases and most recently coronavirus disease 2019 (COVID-19)[2,5-10]. Excess and ectopic adiposity and adipose tissue (AT) dysfunction, characterized by a state of low-grade inflammation, underline the pathophysiology of obesity and its consequences to a great extent[8,11-18]. The presence of inflammation in obesity and related metabolic diseases is well established and is proposed to be linked to insulin resistance and/or its further exacerbation, as inflammatory mediators appear to interfere with insulin signal transduction in important metabolic organs (AT, liver, and muscle)[15,19]. Inflammatory markers may be indicators of disease development, allowing us to potentially predict the transition and/or development of complications such as T2DM and cardiovascular diseases[20,21]. Consequently, by achieving an improved understanding of the changes in metabolic and inflammatory processes in these various tissues and organs, and unveiling their properties, we could achieve a better understanding of the pathophysiology of obesity-related complications, including T2DM, and develop better prevention and treatment strategies[22-24]. In this mini-review, we will present evidence of obesity and T2DM-related inflammation, explore the underlying mechanisms in selected tissues and organs, and present potential therapeutic options based on the current literature.
Excess adiposity is related to modestly raised levels of many circulating cytokines and inflammatory factors in both mice and humans; hence, obesity is usually defined as a condition of persistent low-grade systemic inflammation[15,16,25]. Evidence suggests that many of these factors are produced in AT, collectively referred to as adipokines, including hormones (leptin, adiponectin), hormone-cleavage enzymes like dipeptidyl peptidase 4, components or factors regulating the clotting cascade like plasminogen activator inhibitor-1 (PAI-1), and chemoattraction/pro-inflammatory factors including interleukins 1, 6, and 8 (IL-1, -6, -8), tumour necrosis factor alpha (TNF-α), and monocyte chemoattractant protein-1 (MCP-1)[11,22,26-30]. Adipokine expression and/or secretion is altered in states of AT dysfunction and may contribute to obesity-associated diseases[11,26]. Leptin circulating concentrations are elevated in individuals with obesity, and its concentrations are generally strongly and positively correlated with white AT mass[31,32]. At the same time, hypoadiponectinemia is another hallmark of obesity, suggesting a loss of its positive insulin-sensitizing and anti-inflammatory properties[33-35]. In a recent meta-analysis of 60 studies with 45,210 participants, positive correlations with C-reactive protein (CRP), IL-6, and TNF-α were observed for leptin but not for adiponectin, implying an important association between AT hormonal function and inflammatory biomarkers with potentially pathophysiological implications[36]. Moreover, acute-phase proteins, including CRP and serum amyloid A, and white blood cell (WBC) count, are also elevated in obesity and related metabolic diseases[21,29,37-40].
Several genome-wide association studies (GWAS) have been conducted to explore the link between obesity and various conditions, as well as the potential cause-and-effect relationship[41]. These studies have identified over 300 single-nucleotide polymorphisms that are associated with different measures of obesity, such as body mass index (BMI), waist-hip ratio, and other indicators of adiposity[41]. In a large-scale genome-wide association study involving a total of over 40000 individuals of European descent, genetic variants associated with higher BMI were strongly associated with higher high-sensitive CRP levels, indicating a causal relationship between adiposity and inflammation, however the opposite was not recorded[42].
Notably, pro-inflammatory markers were also strongly associated with insulin resistance in most individuals, regardless of the degree of adiposity, implicating either a role or at least a relationship between these molecules and the transition to more insulin-resistant states like T2DM[21,43,44].
Subclinical chronic inflammation appears to be a distinct contributor to the development of T2DM[21,45]. Independent of the initial degree of insulin resistance and obesity, elevated levels of several inflammatory biomarkers at baseline in different human populations are predictive of T2DM occurrence[21,45]. Elevated levels of IL-6 and CRP are significantly associated with an increased risk of T2DM[46-48]. An elevated WBC count is also associated with a worsening of insulin sensitivity and predicts the development of T2DM[38]. Increased circulating concentrations of pro-inflammatory cytokines IL-1β, IL-18, TNF-α, (apart from IL-6 and CRP) and low levels of adiponectin are strongly associated with T2DM[49]. Among the markers of blunted fibrinolysis, increased PAI-1 appeared to predict T2DM development independent of insulin resistance and other known risk factors for diabetes[50]. Furthermore, biomarkers indicative of inflammation and endothelial dysfunction, including intercellular adhesion molecule 1 and E-selectin, were positively associated with the incidence of T2DM[51]. Based on these observations, it could also be claimed that these changes may be associated with the various cardiovascular complications often related to T2DM and obesity[52,53].
Finally, a plethora of GWAS has been conducted more recently regarding the association and causality between T2DM and inflammatory biomarkers[54]. Of these IL-1 and IL-6 pathways appeared to be positively associated, however evidence remains elusive[55,56].
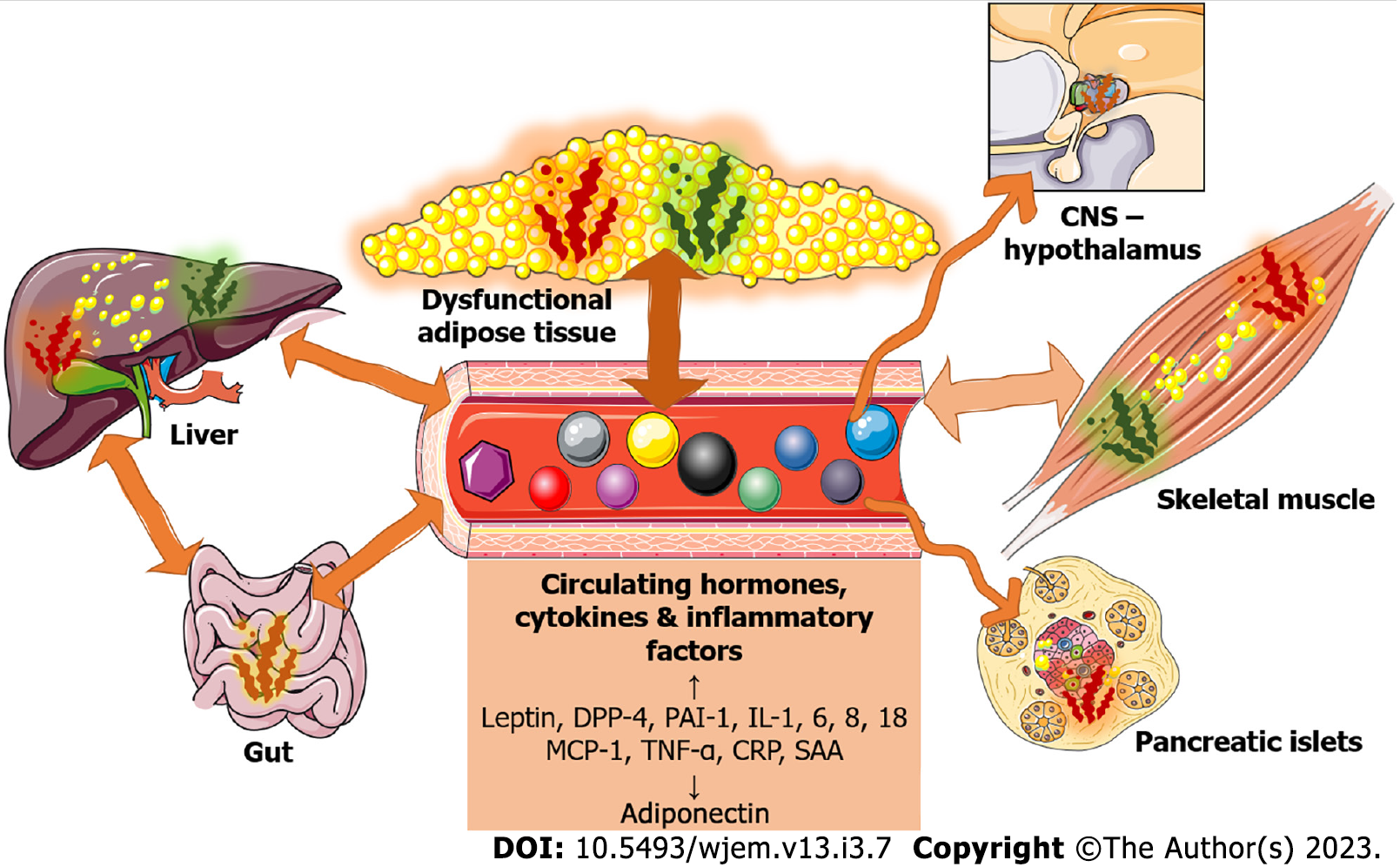
As mentioned already, inflammation appears to be linked to insulin resistance and/or its worsening in obesity since it was shown to interfere with insulin signal transduction in critical metabolic organs (AT, liver, and muscle) and potentially contribute to the development of T2DM[15,19,48]. In this section, we will present potential mechanisms driving obesity-related inflammation, primarily in the AT, and the implications of circulatory inflammatory factors on various metabolic and regulatory organs. A summary can be found in Figure 1.
Excess adiposity, AT dysfunction (characterized by a state of low-grade inflammation), body fat distribution, and ectopic fat deposition, particularly visceral fat deposition, are all central figures in the pathophysiology of obesity and its complications[8,11,14].
Dysfunctional AT is distinguished by adipocyte hypertrophy, which is associated with chronic low-grade inflammation, pro-inflammatory immune cell infiltration, adipokine dysregulation, hormonal resistance, blunted metabolism, reactive oxygen species production, endoplasmic reticulum stress, mitochondrial dysfunction, and altered oxygenation, all of which contribute to ectopic fat accumulation and related complications[13,15,57,58]. The location of lipid storage is a key factor in determining an individual's overall health, as obesity-related complications such as hypertension and the risk of T2DM relate to abdominal (upper body) fat accumulation[14,59-63]. In contrast, fat accumulation in the lower body (gluteofemoral AT) is linked to a lower risk of cardiometabolic disease[59,64-67]. Lower body cell composition, including immune cells, is thought to be primarily associated with enhanced anti-inflammatory properties[17,18]. In accordance with that theory, IL-6 release (as determined by an arteriovenous difference technique model) was much lower in femoral adipose tissue than in matched abdominal tissue[60].
Obesity-related lipid accumulation in non-adipose tissues has significant metabolic effects since it is linked to insulin resistance and, potentially, through molecular mimicry, lipid moieties may trigger inflammatory pathways[11,22,68]. Furthermore, hypertrophic adipocytes are shown to possess a pro-inflammatory phenotype, which may exacerbate insulin resistance, resulting in a vicious cycle[69,70]. Adipocyte and AT inflammation, on the other hand, appears to be required for healthy AT growth and remodeling[71]. That observation implies that inflammation is more than just a harmful process, maybe contributing to AT adaptation to stressors, including excess calorie intake. It is worth noting that drugs used to treat T2DM may reduce inflammation by lowering hyperglycemia. However, these medicines' anti-inflammatory effects are inconsistent, and it is unclear if their favorable metabolic effects are mediated by regulation of chronic low-grade inflammation[72]. Finally, in addition to white AT inflammation in obesity, it appears that brown AT inflammation also exists, at least in animal models of obesity, implicating that dysregulation of this tissue aggregates the obesity-related inflammatory status[73].
Liver dysfunction linked with obesity, which encompasses the Metabolic Associated Fatty Liver Disease (MAFLD) spectrum, is characterized by complex pathophysiological processes that are currently under vigorous investigation and involve several pathways[74]. It has been postulated that an inability to sufficiently enhance subcutaneous AT triglyceride storage capacity in response to increased caloric consumption and body weight reroutes lipids towards other organs, such as the skeletal muscle and the liver, resulting in ectopic lipid accumulation and lipotoxicity at the cellular level, which leads to insulin resistance (IR) and inflammatory responses in these organs[75-79]. Interestingly, fat molecules appear to serve as ligands for substantial inflammatory signaling pathways in Kupffer cells in the liver and AT macrophages via the toll-like receptor 2 and 4 (TLR2/TLR4) signaling cascade[80]. Numerous previous studies have shown that pro-inflammatory cytokines, specifically TNF- and IL-6, play an important role in the development and progression of NASH[81,82]. TNF- and IL-6 Levels are elevated in the livers and blood of NASH patients, but blocking these mediators improved MAFLD in animal models[81,83].
Several pro-inflammatory cytokines have been reported to be overexpressed apart from AT and the liver, also in the skeletal muscle of individuals with obesity and insulin resistance as well as in animal models[84,85]. Obesity progression increases inflammation in skeletal muscle in two ways: Indirectly through AT inflammation and adipocytokines dysregulation, which affect skeletal muscle function and may also augment IR, and directly through ectopic lipid deposition within the skeletal muscle, which initiates several pro-inflammatory pathways[81,86,87]. Myocytes stimulate the production of several hormones and cytokines, collectively called myokines, including IL-6 and IL-15, as well as other molecules like fibroblast growth factor 21 (FGF21) and irisin[75,82]. All these molecules can regulate potential inflammatory processes, and the imbalance of their production in IR, obesity, and T2DM could further aggregate this overall inflammatory status[88]. Sarcopenic obesity, which is more common in older patients, may also cause an increase in unfavorable pro-inflammatory status and impair insulin sensitivity via a loss of favorable myokines[9]. Finally, as in the AT and other organs, immune cell infiltration with pro-inflammatory activation in skeletal muscle has been observed, resulting in the release of pro-inflammatory markers such as IL-1, IL-6, and TNF-α[89,90].
Evidence exists that several other tissues and organs in obesity, T2DM, and insulin resistance states are affected by or involved in systemic inflammation. For example, it is well established that macrophage infiltration is associated with islet inflammation and cell abnormalities in T2DM and obesity[91-93]. Furthermore, the western diet and cultural habits may be part of a vicious cycle that promotes oxidative stress and inflammation in the gut and even the brain[94]. Obesity-related inflammation is enhanced by diminished mucosal barriers and intestinal immune homeostasis[95]. These findings could be attributed to effects on the gut microbiome[96]; the importance of diet on organ-specific and systemic inflammation is apparent in diet-induced models of obesity in which even parts of the brain, in particular the hypothalamic arcuate nucleus, have been affected via infiltration of macrophages and increased expression of pro-inflammatory markers[16,97-99]. Finally, even non-metabolic or metabolic regulatory organs seem to be affected, as obesity appears to induce ovarian and kidney inflammation, respiratory hyperresponsiveness, and various hematological consequences[100-103].
Strategies to tackle obesity, diabetes, and related cardiometabolic diseases include a variety of combinations, including lifestyle changes with dietary and exercise options, anti-obesity, anti-diabetic, and anti-hyperlipidemic medications, bariatric or metabolic surgery, and potentially the use of drugs with anti-inflammatory properties[72,104-107].
Current medicinal therapies for T2DM act in a multitude of ways to improve glycemic control, but they can also be beneficial for the treatment of obesity and related cardiometabolic diseases[23,24,72,107]. Many of these medications, for instance, metformin, may possess anti-inflammatory properties that can be exerted indirectly via metabolic control of hyperglycemia and hyperlipidemia and weight loss or by directly impacting the immune system and inflammatory responses[72,107]. Weight loss per se and therapeutic interventions that achieve it, including anti-diabetic and anti-hyperlipidemic medication use, resulted in reduced circulating concentrations of IL-6, IL-8, CRP, and MCP-1 and increased adiponectin concentrations[39,40,108-110]. A recent meta-analysis of the effect of intermittent fasting dietary patterns on plasma concentrations of inflammatory biomarkers found a decrease in CRP in individuals with overweight or obesity, but no changes in IL-6 or TNF-α[111]. A meta-analysis of 116 studies[112], however, found that serum concentrations of CRP, IL-6, and TNF- were significantly lower after bariatric surgery.
Targeting inflammation or inflammatory pathways in general has emerged as a viable alternative to traditional metabolic therapeutic options[72,107]. Anti-TNF therapy has produced contentious results in the treatment of T2DM in humans[72,113]. In animal models of IR and T2DM, inhibition combining anti-TNF and IL-1 was shown to be more effective[107,114]. Favorable effects were recorded with IL-1 blockage alone in a human study[115]. Moreover, salsalate, a prodrug of salicylate, diacerein, an anti-arthritis medication, and hydroxychloroquine, usually used for the treatment of autoimmune diseases, appeared to be beneficial; however, long-term safety profiles for these metabolic diseases are still to be elucidated[72]. Finally, the option of directly altering the pro- or anti-inflammatory activation and the balance of the immune cells within the AT arises as a potential therapeutic option[107].
In this brief review, we have demonstrated that inflammatory biomarkers reflecting underlying processes and pathway activations are present in obesity and type 2 diabetes mellitus. The impact of obesity and T2DM on inflammatory pathways appears to be linked to disease progression. Achieving a better understanding of the connection and causality between these factors and the disease risk and progression could give us the opportunity to potentially predict, follow up on, and modify their risk. Further studies are warranted to better understand the underlying pathophysiology and the use of predictive biomarkers in everyday clinical practice. It is necessary to conduct in vivo physiological studies to investigate the sequence of events underlying pathophysiological events in various metabolic and regulatory tissues, such as adipose tissue. Such studies can help elucidate whether inflammation precedes metabolic derangements or is mainly a result of perturbations caused by increased adiposity. Furthermore, it is essential to conduct randomized clinical trials that precisely target inflammatory pathways in diverse populations to either confirm or refute these findings.
| 1. | GBD 2015 Obesity Collaborators, Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, Marczak L, Mokdad AH, Moradi-Lakeh M, Naghavi M, Salama JS, Vos T, Abate KH, Abbafati C, Ahmed MB, Al-Aly Z, Alkerwi A, Al-Raddadi R, Amare AT, Amberbir A, Amegah AK, Amini E, Amrock SM, Anjana RM, Ärnlöv J, Asayesh H, Banerjee A, Barac A, Baye E, Bennett DA, Beyene AS, Biadgilign S, Biryukov S, Bjertness E, Boneya DJ, Campos-Nonato I, Carrero JJ, Cecilio P, Cercy K, Ciobanu LG, Cornaby L, Damtew SA, Dandona L, Dandona R, Dharmaratne SD, Duncan BB, Eshrati B, Esteghamati A, Feigin VL, Fernandes JC, Fürst T, Gebrehiwot TT, Gold A, Gona PN, Goto A, Habtewold TD, Hadush KT, Hafezi-Nejad N, Hay SI, Horino M, Islami F, Kamal R, Kasaeian A, Katikireddi SV, Kengne AP, Kesavachandran CN, Khader YS, Khang YH, Khubchandani J, Kim D, Kim YJ, Kinfu Y, Kosen S, Ku T, Defo BK, Kumar GA, Larson HJ, Leinsalu M, Liang X, Lim SS, Liu P, Lopez AD, Lozano R, Majeed A, Malekzadeh R, Malta DC, Mazidi M, McAlinden C, McGarvey ST, Mengistu DT, Mensah GA, Mensink GBM, Mezgebe HB, Mirrakhimov EM, Mueller UO, Noubiap JJ, Obermeyer CM, Ogbo FA, Owolabi MO, Patton GC, Pourmalek F, Qorbani M, Rafay A, Rai RK, Ranabhat CL, Reinig N, Safiri S, Salomon JA, Sanabria JR, Santos IS, Sartorius B, Sawhney M, Schmidhuber J, Schutte AE, Schmidt MI, Sepanlou SG, Shamsizadeh M, Sheikhbahaei S, Shin MJ, Shiri R, Shiue I, Roba HS, Silva DAS, Silverberg JI, Singh JA, Stranges S, Swaminathan S, Tabarés-Seisdedos R, Tadese F, Tedla BA, Tegegne BS, Terkawi AS, Thakur JS, Tonelli M, Topor-Madry R, Tyrovolas S, Ukwaja KN, Uthman OA, Vaezghasemi M, Vasankari T, Vlassov VV, Vollset SE, Weiderpass E, Werdecker A, Wesana J, Westerman R, Yano Y, Yonemoto N, Yonga G, Zaidi Z, Zenebe ZM, Zipkin B, Murray CJL. Health Effects of Overweight and Obesity in 195 Countries over 25 Years. N Engl J Med. 2017;377:13-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5669] [Cited by in RCA: 5390] [Article Influence: 598.9] [Reference Citation Analysis (3)] |
| 2. | Heymsfield SB, Wadden TA. Mechanisms, Pathophysiology, and Management of Obesity. N Engl J Med. 2017;376:254-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 904] [Cited by in RCA: 1237] [Article Influence: 137.4] [Reference Citation Analysis (0)] |
| 3. | González-Muniesa P, Mártinez-González MA, Hu FB, Després JP, Matsuzawa Y, Loos RJF, Moreno LA, Bray GA, Martinez JA. Obesity. Nat Rev Dis Primers. 2017;3:17034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 593] [Cited by in RCA: 861] [Article Influence: 95.7] [Reference Citation Analysis (0)] |
| 4. | NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body-mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet. 2016;387:1377-1396. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4095] [Cited by in RCA: 3642] [Article Influence: 364.2] [Reference Citation Analysis (0)] |
| 5. | Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14:88-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2249] [Cited by in RCA: 3697] [Article Influence: 462.1] [Reference Citation Analysis (0)] |
| 6. | Bray GA, Heisel WE, Afshin A, Jensen MD, Dietz WH, Long M, Kushner RF, Daniels SR, Wadden TA, Tsai AG, Hu FB, Jakicic JM, Ryan DH, Wolfe BM, Inge TH. The Science of Obesity Management: An Endocrine Society Scientific Statement. Endocr Rev. 2018;39:79-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 550] [Cited by in RCA: 549] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 7. | Martin SD, McGee SL. Metabolic reprogramming in type 2 diabetes and the development of breast cancer. J Endocrinol. 2018;237:R35-R46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Frühbeck G, Busetto L, Dicker D, Yumuk V, Goossens GH, Hebebrand J, Halford JGC, Farpour-Lambert NJ, Blaak EE, Woodward E, Toplak H. The ABCD of Obesity: An EASO Position Statement on a Diagnostic Term with Clinical and Scientific Implications. Obes Facts. 2019;12:131-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 204] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 9. | Sagris M, Kokkinidis DG, Lempesis IG, Giannopoulos S, Rallidis L, Mena-Hurtado C, Bakoyiannis C. Nutrition, dietary habits, and weight management to prevent and treat patients with peripheral artery disease. Rev Cardiovasc Med. 2020;21:565-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Lempesis IG, Karlafti E, Papalexis P, Fotakopoulos G, Tarantinos K, Lekakis V, Papadakos SP, Cholongitas E, Georgakopoulou VE. COVID-19 and liver injury in individuals with obesity. World J Gastroenterol. 2023;29:908-916. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 10] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Goossens GH. The role of adipose tissue dysfunction in the pathogenesis of obesity-related insulin resistance. Physiol Behav. 2008;94:206-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 350] [Cited by in RCA: 383] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 12. | Blüher M. Adipose tissue dysfunction contributes to obesity related metabolic diseases. Best Pract Res Clin Endocrinol Metab. 2013;27:163-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 232] [Cited by in RCA: 291] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 13. | Goossens GH, Blaak EE. Adipose tissue dysfunction and impaired metabolic health in human obesity: a matter of oxygen? Front Endocrinol (Lausanne). 2015;6:55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 112] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 14. | Goossens GH. The Metabolic Phenotype in Obesity: Fat Mass, Body Fat Distribution, and Adipose Tissue Function. Obes Facts. 2017;10:207-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 512] [Article Influence: 56.9] [Reference Citation Analysis (0)] |
| 15. | Lempesis IG, van Meijel RLJ, Manolopoulos KN, Goossens GH. Oxygenation of adipose tissue: A human perspective. Acta Physiol (Oxf). 2020;228:e13298. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 16. | Lempesis IG, Tsilingiris D, Liu J, Dalamaga M. Of mice and men: Considerations on adipose tissue physiology in animal models of obesity and human studies. Metabol Open. 2022;15:100208. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 17. | Lempesis IG, Hoebers N, Essers Y, Jocken JWE, Rouschop KMA, Blaak EE, Manolopoulos KN, Goossens GH. Physiological Oxygen Levels Differentially Regulate Adipokine Production in Abdominal and Femoral Adipocytes from Individuals with Obesity Versus Normal Weight. Cells. 2022;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 18. | Lempesis IG, Hoebers N, Essers Y, Jocken JW, Blaak EE, Manolopoulos KN, Goossens GH. Distinct inflammatory signatures of upper-and lower-body adipose tissue in postmenopausal women with normal weight and obesity. Endocrine Abstracts. 2022;81:EP355. [DOI] [Full Text] |
| 19. | de Luca C, Olefsky JM. Inflammation and insulin resistance. FEBS Lett. 2008;582:97-105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 851] [Cited by in RCA: 833] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 20. | Calle MC, Fernandez ML. Inflammation and type 2 diabetes. Diabetes Metab. 2012;38:183-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 376] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 21. | Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res Clin Pract. 2014;105:141-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1141] [Cited by in RCA: 1468] [Article Influence: 122.3] [Reference Citation Analysis (0)] |
| 22. | Rosen ED, Spiegelman BM. What we talk about when we talk about fat. Cell. 2014;156:20-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1414] [Cited by in RCA: 1864] [Article Influence: 155.3] [Reference Citation Analysis (0)] |
| 23. | Lempesis IG, Liu J, Dalamaga M. The catcher in the gut: Tirzepatide, a dual incretin analog for the treatment of type 2 diabetes mellitus and obesity. Metabol Open. 2022;16:100220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 24. | Vallianou NG, Tsilingiris D, Kounatidis D, Lempesis IG, Karampela I, Dalamaga M. Sodiumglucose cotransporter2 inhibitors in obesity and associated cardiometabolic disorders: where do we stand? Pol Arch Intern Med. 2022;132. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 25. | Burhans MS, Hagman DK, Kuzma JN, Schmidt KA, Kratz M. Contribution of Adipose Tissue Inflammation to the Development of Type 2 Diabetes Mellitus. Compr Physiol. 2018;9:1-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 185] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 26. | Fasshauer M, Blüher M. Adipokines in health and disease. Trends Pharmacol Sci. 2015;36:461-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 523] [Cited by in RCA: 779] [Article Influence: 70.8] [Reference Citation Analysis (0)] |
| 27. | Lamers D, Famulla S, Wronkowitz N, Hartwig S, Lehr S, Ouwens DM, Eckardt K, Kaufman JM, Ryden M, Müller S, Hanisch FG, Ruige J, Arner P, Sell H, Eckel J. Dipeptidyl peptidase 4 is a novel adipokine potentially linking obesity to the metabolic syndrome. Diabetes. 2011;60:1917-1925. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 431] [Cited by in RCA: 468] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 28. | Kim CS, Park HS, Kawada T, Kim JH, Lim D, Hubbard NE, Kwon BS, Erickson KL, Yu R. Circulating levels of MCP-1 and IL-8 are elevated in human obese subjects and associated with obesity-related parameters. Int J Obes (Lond). 2006;30:1347-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 384] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 29. | Pickup JC, Mattock MB, Chusney GD, Burt D. NIDDM as a disease of the innate immune system: association of acute-phase reactants and interleukin-6 with metabolic syndrome X. Diabetologia. 1997;40:1286-1292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 816] [Cited by in RCA: 832] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
| 30. | Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, Klein S, Coppack SW. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997;82:4196-4200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 470] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 31. | Mantzoros CS, Magkos F, Brinkoetter M, Sienkiewicz E, Dardeno TA, Kim SY, Hamnvik OP, Koniaris A. Leptin in human physiology and pathophysiology. Am J Physiol Endocrinol Metab. 2011;301:E567-E584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 416] [Article Influence: 27.7] [Reference Citation Analysis (0)] |
| 32. | Kalaitzopoulos DR, Lempesis IG, Samartzis N, Kolovos G, Dedes I, Daniilidis A, Nirgianakis K, Leeners B, Goulis DG, Samartzis EP. Leptin concentrations in endometriosis: A systematic review and meta-analysis. J Reprod Immunol. 2021;146:103338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 27] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 33. | Straub LG, Scherer PE. Metabolic Messengers: Adiponectin. Nat Metab. 2019;1:334-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 257] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 34. | Santaniemi M, Kesäniemi YA, Ukkola O. Low plasma adiponectin concentration is an indicator of the metabolic syndrome. Eur J Endocrinol. 2006;155:745-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 84] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 35. | Achari AE, Jain SK. Adiponectin, a Therapeutic Target for Obesity, Diabetes, and Endothelial Dysfunction. Int J Mol Sci. 2017;18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 477] [Cited by in RCA: 841] [Article Influence: 93.4] [Reference Citation Analysis (1)] |
| 36. | Graßmann S, Wirsching J, Eichelmann F, Aleksandrova K. Association Between Peripheral Adipokines and Inflammation Markers: A Systematic Review and Meta-Analysis. Obesity (Silver Spring). 2017;25:1776-1785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 66] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 37. | Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19:972-978. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1662] [Cited by in RCA: 1647] [Article Influence: 61.0] [Reference Citation Analysis (0)] |
| 38. | Vozarova B, Weyer C, Lindsay RS, Pratley RE, Bogardus C, Tataranni PA. High white blood cell count is associated with a worsening of insulin sensitivity and predicts the development of type 2 diabetes. Diabetes. 2002;51:455-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 403] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 39. | Christiansen T, Richelsen B, Bruun JM. Monocyte chemoattractant protein-1 is produced in isolated adipocytes, associated with adiposity and reduced after weight loss in morbid obese subjects. Int J Obes (Lond). 2005;29:146-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 283] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 40. | Bruun JM, Helge JW, Richelsen B, Stallknecht B. Diet and exercise reduce low-grade inflammation and macrophage infiltration in adipose tissue but not in skeletal muscle in severely obese subjects. Am J Physiol Endocrinol Metab. 2006;290:E961-E967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 315] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 41. | Goodarzi MO. Genetics of obesity: what genetic association studies have taught us about the biology of obesity and its complications. Lancet Diabetes Endocrinol. 2018;6:223-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 313] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 42. | Welsh P, Polisecki E, Robertson M, Jahn S, Buckley BM, de Craen AJ, Ford I, Jukema JW, Macfarlane PW, Packard CJ, Stott DJ, Westendorp RG, Shepherd J, Hingorani AD, Smith GD, Schaefer E, Sattar N. Unraveling the directional link between adiposity and inflammation: a bidirectional Mendelian randomization approach. J Clin Endocrinol Metab. 2010;95:93-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 103] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 43. | Natali A, Toschi E, Baldeweg S, Ciociaro D, Favilla S, Saccà L, Ferrannini E. Clustering of insulin resistance with vascular dysfunction and low-grade inflammation in type 2 diabetes. Diabetes. 2006;55:1133-1140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 148] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 44. | Phillips CM, Perry IJ. Does inflammation determine metabolic health status in obese and nonobese adults? J Clin Endocrinol Metab. 2013;98:E1610-E1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 215] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 45. | Dorcely B, Katz K, Jagannathan R, Chiang SS, Oluwadare B, Goldberg IJ, Bergman M. Novel biomarkers for prediabetes, diabetes, and associated complications. Diabetes Metab Syndr Obes. 2017;10:345-361. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 107] [Cited by in RCA: 132] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 46. | Wang X, Bao W, Liu J, Ouyang YY, Wang D, Rong S, Xiao X, Shan ZL, Zhang Y, Yao P, Liu LG. Inflammatory markers and risk of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care. 2013;36:166-175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 649] [Cited by in RCA: 642] [Article Influence: 49.4] [Reference Citation Analysis (0)] |
| 47. | Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286:327-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2974] [Cited by in RCA: 3071] [Article Influence: 122.8] [Reference Citation Analysis (1)] |
| 48. | Adabimohazab R, Garfinkel A, Milam EC, Frosch O, Mangone A, Convit A. Does Inflammation Mediate the Association Between Obesity and Insulin Resistance? Inflammation. 2016;39:994-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 49. | Liu C, Feng X, Li Q, Wang Y, Hua M. Adiponectin, TNF-α and inflammatory cytokines and risk of type 2 diabetes: A systematic review and meta-analysis. Cytokine. 2016;86:100-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 375] [Cited by in RCA: 346] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 50. | Festa A, D'Agostino R Jr, Tracy RP, Haffner SM; Insulin Resistance Atherosclerosis Study. Elevated levels of acute-phase proteins and plasminogen activator inhibitor-1 predict the development of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes. 2002;51:1131-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 751] [Cited by in RCA: 761] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 51. | Odegaard AO, Jacobs DR Jr, Sanchez OA, Goff DC Jr, Reiner AP, Gross MD. Oxidative stress, inflammation, endothelial dysfunction and incidence of type 2 diabetes. Cardiovasc Diabetol. 2016;15:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 201] [Cited by in RCA: 223] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 52. | Battineni G, Sagaro GG, Chintalapudi N, Amenta F, Tomassoni D, Tayebati SK. Impact of Obesity-Induced Inflammation on Cardiovascular Diseases (CVD). Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 134] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 53. | Qu D, Liu J, Lau CW, Huang Y. IL-6 in diabetes and cardiovascular complications. Br J Pharmacol. 2014;171:3595-3603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 130] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 54. | Yuan S, Merino J, Larsson SC. Causal factors underlying diabetes risk informed by Mendelian randomisation analysis: evidence, opportunities and challenges. Diabetologia. 2023;66:800-812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 31] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 55. | Yuan S, Larsson SC. An atlas on risk factors for type 2 diabetes: a wide-angled Mendelian randomisation study. Diabetologia. 2020;63:2359-2371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 165] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 56. | Interleukin-6 Receptor Mendelian Randomisation Analysis (IL6R MR) Consortium; Swerdlow DI, Holmes MV, Kuchenbaecker KB, Engmann JE, Shah T, Sofat R, Guo Y, Chung C, Peasey A, Pfister R, Mooijaart SP, Ireland HA, Leusink M, Langenberg C, Li KW, Palmen J, Howard P, Cooper JA, Drenos F, Hardy J, Nalls MA, Li YR, Lowe G, Stewart M, Bielinski SJ, Peto J, Timpson NJ, Gallacher J, Dunlop M, Houlston R, Tomlinson I, Tzoulaki I, Luan J, Boer JM, Forouhi NG, Onland-Moret NC, van der Schouw YT, Schnabel RB, Hubacek JA, Kubinova R, Baceviciene M, Tamosiunas A, Pajak A, Topor-Madry R, Malyutina S, Baldassarre D, Sennblad B, Tremoli E, de Faire U, Ferrucci L, Bandenelli S, Tanaka T, Meschia JF, Singleton A, Navis G, Mateo Leach I, Bakker SJ, Gansevoort RT, Ford I, Epstein SE, Burnett MS, Devaney JM, Jukema JW, Westendorp RG, Jan de Borst G, van der Graaf Y, de Jong PA, Mailand-van der Zee AH, Klungel OH, de Boer A, Doevendans PA, Stephens JW, Eaton CB, Robinson JG, Manson JE, Fowkes FG, Frayling TM, Price JF, Whincup PH, Morris RW, Lawlor DA, Smith GD, Ben-Shlomo Y, Redline S, Lange LA, Kumari M, Wareham NJ, Verschuren WM, Benjamin EJ, Whittaker JC, Hamsten A, Dudbridge F, Delaney JA, Wong A, Kuh D, Hardy R, Castillo BA, Connolly JJ, van der Harst P, Brunner EJ, Marmot MG, Wassel CL, Humphries SE, Talmud PJ, Kivimaki M, Asselbergs FW, Voevoda M, Bobak M, Pikhart H, Wilson JG, Hakonarson H, Reiner AP, Keating BJ, Sattar N, Hingorani AD, Casas JP. The interleukin-6 receptor as a target for prevention of coronary heart disease: a mendelian randomisation analysis. Lancet. 2012;379:1214-1224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 834] [Cited by in RCA: 903] [Article Influence: 64.5] [Reference Citation Analysis (0)] |
| 57. | Shulman GI. Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. N Engl J Med. 2014;371:1131-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 780] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 58. | Frayn KN, Karpe F. Regulation of human subcutaneous adipose tissue blood flow. Int J Obes (Lond). 2014;38:1019-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 105] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 59. | Manolopoulos KN, Karpe F, Frayn KN. Gluteofemoral body fat as a determinant of metabolic health. Int J Obes (Lond). 2010;34:949-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 480] [Cited by in RCA: 599] [Article Influence: 37.4] [Reference Citation Analysis (0)] |
| 60. | Karpe F, Pinnick KE. Biology of upper-body and lower-body adipose tissue--link to whole-body phenotypes. Nat Rev Endocrinol. 2015;11:90-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 384] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 61. | Canoy D, Luben R, Welch A, Bingham S, Wareham N, Day N, Khaw KT. Fat distribution, body mass index and blood pressure in 22,090 men and women in the Norfolk cohort of the European Prospective Investigation into Cancer and Nutrition (EPIC-Norfolk) study. J Hypertens. 2004;22:2067-2074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 86] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 62. | Grundy SM, Adams-Huet B, Vega GL. Variable contributions of fat content and distribution to metabolic syndrome risk factors. Metab Syndr Relat Disord. 2008;6:281-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 63. | Meisinger C, Döring A, Thorand B, Heier M, Löwel H. Body fat distribution and risk of type 2 diabetes in the general population: are there differences between men and women? The MONICA/KORA Augsburg cohort study. Am J Clin Nutr. 2006;84:483-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 176] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 64. | Seidell JC, Pérusse L, Després JP, Bouchard C. Waist and hip circumferences have independent and opposite effects on cardiovascular disease risk factors: the Quebec Family Study. Am J Clin Nutr. 2001;74:315-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 344] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 65. | Terry RB, Stefanick ML, Haskell WL, Wood PD. Contributions of regional adipose tissue depots to plasma lipoprotein concentrations in overweight men and women: possible protective effects of thigh fat. Metabolism. 1991;40:733-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 105] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 66. | Williams MJ, Hunter GR, Kekes-Szabo T, Snyder S, Treuth MS. Regional fat distribution in women and risk of cardiovascular disease. Am J Clin Nutr. 1997;65:855-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 130] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 67. | Yim JE, Heshka S, Albu JB, Heymsfield S, Gallagher D. Femoral-gluteal subcutaneous and intermuscular adipose tissues have independent and opposing relationships with CVD risk. J Appl Physiol (1985). 2008;104:700-707. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 102] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 68. | Haczeyni F, Bell-Anderson KS, Farrell GC. Causes and mechanisms of adipocyte enlargement and adipose expansion. Obes Rev. 2018;19:406-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 155] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 69. | Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J Clin Endocrinol Metab. 2007;92:1023-1033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 863] [Cited by in RCA: 916] [Article Influence: 48.2] [Reference Citation Analysis (12)] |
| 70. | Sun S, Ji Y, Kersten S, Qi L. Mechanisms of inflammatory responses in obese adipose tissue. Annu Rev Nutr. 2012;32:261-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 221] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 71. | Wernstedt Asterholm I, Tao C, Morley TS, Wang QA, Delgado-Lopez F, Wang ZV, Scherer PE. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab. 2014;20:103-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 539] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 72. | Pollack RM, Donath MY, LeRoith D, Leibowitz G. Anti-inflammatory Agents in the Treatment of Diabetes and Its Vascular Complications. Diabetes Care. 2016;39 Suppl 2:S244-S252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 188] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 73. | Villarroya F, Cereijo R, Gavaldà-Navarro A, Villarroya J, Giralt M. Inflammation of brown/beige adipose tissues in obesity and metabolic disease. J Intern Med. 2018;284:492-504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 235] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 74. | Sarwar R, Pierce N, Koppe S. Obesity and nonalcoholic fatty liver disease: current perspectives. Diabetes Metab Syndr Obes. 2018;11:533-542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 171] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 75. | Beals JW, Smith GI, Shankaran M, Fuchs A, Schweitzer GG, Yoshino J, Field T, Matthews M, Nyangau E, Morozov D, Mittendorfer B, Hellerstein MK, Klein S. Increased Adipose Tissue Fibrogenesis, Not Impaired Expandability, Is Associated With Nonalcoholic Fatty Liver Disease. Hepatology. 2021;74:1287-1299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 76. | Ter Horst KW, Gilijamse PW, Versteeg RI, Ackermans MT, Nederveen AJ, la Fleur SE, Romijn JA, Nieuwdorp M, Zhang D, Samuel VT, Vatner DF, Petersen KF, Shulman GI, Serlie MJ. Hepatic Diacylglycerol-Associated Protein Kinase Cε Translocation Links Hepatic Steatosis to Hepatic Insulin Resistance in Humans. Cell Rep. 2017;19:1997-2004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 137] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 77. | Korenblat KM, Fabbrini E, Mohammed BS, Klein S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology. 2008;134:1369-1375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 484] [Cited by in RCA: 465] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 78. | Sanyal AJ, Campbell-Sargent C, Mirshahi F, Rizzo WB, Contos MJ, Sterling RK, Luketic VA, Shiffman ML, Clore JN. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183-1192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1458] [Cited by in RCA: 1531] [Article Influence: 61.2] [Reference Citation Analysis (7)] |
| 79. | Sharpton SR, Schnabl B, Knight R, Loomba R. Current Concepts, Opportunities, and Challenges of Gut Microbiome-Based Personalized Medicine in Nonalcoholic Fatty Liver Disease. Cell Metab. 2021;33:21-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 143] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 80. | Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. J Clin Invest. 2008;118:2992-3002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 843] [Cited by in RCA: 870] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 81. | Luo Y, Lin H. Inflammation initiates a vicious cycle between obesity and nonalcoholic fatty liver disease. Immun Inflamm Dis. 2021;9:59-73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 82] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 82. | Feldstein AE. Novel insights into the pathophysiology of nonalcoholic fatty liver disease. Semin Liver Dis. 2010;30:391-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 92] [Article Influence: 5.8] [Reference Citation Analysis (1)] |
| 83. | Tilg H. The role of cytokines in non-alcoholic fatty liver disease. Dig Dis. 2010;28:179-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 184] [Article Influence: 11.5] [Reference Citation Analysis (2)] |
| 84. | Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2402] [Cited by in RCA: 2730] [Article Influence: 182.0] [Reference Citation Analysis (0)] |
| 85. | Jorquera G, Russell J, Monsalves-Álvarez M, Cruz G, Valladares-Ide D, Basualto-Alarcón C, Barrientos G, Estrada M, Llanos P. NLRP3 Inflammasome: Potential Role in Obesity Related Low-Grade Inflammation and Insulin Resistance in Skeletal Muscle. Int J Mol Sci. 2021;22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 43] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 86. | Therkelsen KE, Pedley A, Speliotes EK, Massaro JM, Murabito J, Hoffmann U, Fox CS. Intramuscular fat and associations with metabolic risk factors in the Framingham Heart Study. Arterioscler Thromb Vasc Biol. 2013;33:863-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 204] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 87. | Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017;542:177-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1054] [Cited by in RCA: 1717] [Article Influence: 190.8] [Reference Citation Analysis (0)] |
| 88. | Eckardt K, Görgens SW, Raschke S, Eckel J. Myokines in insulin resistance and type 2 diabetes. Diabetologia. 2014;57:1087-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 170] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 89. | Wang T, He C. Pro-inflammatory cytokines: The link between obesity and osteoarthritis. Cytokine Growth Factor Rev. 2018;44:38-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 698] [Article Influence: 87.3] [Reference Citation Analysis (0)] |
| 90. | Fink LN, Oberbach A, Costford SR, Chan KL, Sams A, Blüher M, Klip A. Expression of anti-inflammatory macrophage genes within skeletal muscle correlates with insulin sensitivity in human obesity and type 2 diabetes. Diabetologia. 2013;56:1623-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 91. | Eguchi K, Nagai R. Islet inflammation in type 2 diabetes and physiology. J Clin Invest. 2017;127:14-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 270] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 92. | McNelis JC, Olefsky JM. Macrophages, immunity, and metabolic disease. Immunity. 2014;41:36-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 509] [Cited by in RCA: 592] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 93. | Ying W, Fu W, Lee YS, Olefsky JM. The role of macrophages in obesity-associated islet inflammation and β-cell abnormalities. Nat Rev Endocrinol. 2020;16:81-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 288] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 94. | Kopp W. How Western Diet And Lifestyle Drive The Pandemic Of Obesity And Civilization Diseases. Diabetes Metab Syndr Obes. 2019;12:2221-2236. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 266] [Cited by in RCA: 467] [Article Influence: 66.7] [Reference Citation Analysis (0)] |
| 95. | Ding S, Chi MM, Scull BP, Rigby R, Schwerbrock NM, Magness S, Jobin C, Lund PK. High-fat diet: bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS One. 2010;5:e12191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 476] [Cited by in RCA: 545] [Article Influence: 34.1] [Reference Citation Analysis (0)] |
| 96. | Saad MJ, Santos A, Prada PO. Linking Gut Microbiota and Inflammation to Obesity and Insulin Resistance. Physiology (Bethesda). 2016;31:283-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 514] [Article Influence: 57.1] [Reference Citation Analysis (0)] |
| 97. | Thaler JP, Yi CX, Schur EA, Guyenet SJ, Hwang BH, Dietrich MO, Zhao X, Sarruf DA, Izgur V, Maravilla KR, Nguyen HT, Fischer JD, Matsen ME, Wisse BE, Morton GJ, Horvath TL, Baskin DG, Tschöp MH, Schwartz MW. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122:153-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1169] [Cited by in RCA: 1475] [Article Influence: 98.3] [Reference Citation Analysis (1)] |
| 98. | Araújo EP, Torsoni MA, Velloso LA. Hypothalamic inflammation and obesity. Vitam Horm. 2010;82:129-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 99. | Thaler JP, Guyenet SJ, Dorfman MD, Wisse BE, Schwartz MW. Hypothalamic inflammation: marker or mechanism of obesity pathogenesis? Diabetes. 2013;62:2629-2634. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 166] [Cited by in RCA: 165] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 100. | Snider AP, Wood JR. Obesity induces ovarian inflammation and reduces oocyte quality. Reproduction. 2019;158:R79-R90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 223] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 101. | Navarro-Díaz M, Serra A, López D, Granada M, Bayés B, Romero R. Obesity, inflammation, and kidney disease. Kidney Int Suppl. 2008;S15-S18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 102. | Purdy JC, Shatzel JJ. The hematologic consequences of obesity. Eur J Haematol. 2021;106:306-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 117] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 103. | Shore SA. Obesity, airway hyperresponsiveness, and inflammation. J Appl Physiol (1985). 2010;108:735-743. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 128] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 104. | Zhang C, Liu S, Yang M. Hepatocellular Carcinoma and Obesity, Type 2 Diabetes Mellitus, Cardiovascular Disease: Causing Factors, Molecular Links, and Treatment Options. Front Endocrinol (Lausanne). 2021;12:808526. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 6.6] [Reference Citation Analysis (1)] |
| 105. | Stefan N. Causes, consequences, and treatment of metabolically unhealthy fat distribution. Lancet Diabetes Endocrinol. 2020;8:616-627. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 407] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 106. | Romero-Gómez M, Zelber-Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol. 2017;67:829-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 844] [Cited by in RCA: 981] [Article Influence: 109.0] [Reference Citation Analysis (0)] |
| 107. | AlZaim I, Hammoud SH, Al-Koussa H, Ghazi A, Eid AH, El-Yazbi AF. Adipose Tissue Immunomodulation: A Novel Therapeutic Approach in Cardiovascular and Metabolic Diseases. Front Cardiovasc Med. 2020;7:602088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 63] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 108. | Bastard JP, Jardel C, Bruckert E, Blondy P, Capeau J, Laville M, Vidal H, Hainque B. Elevated levels of interleukin 6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss. J Clin Endocrinol Metab. 2000;85:3338-3342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 280] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 109. | Haffner S, Temprosa M, Crandall J, Fowler S, Goldberg R, Horton E, Marcovina S, Mather K, Orchard T, Ratner R, Barrett-Connor E; Diabetes Prevention Program Research Group. Intensive lifestyle intervention or metformin on inflammation and coagulation in participants with impaired glucose tolerance. Diabetes. 2005;54:1566-1572. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 239] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 110. | Belalcazar LM, Haffner SM, Lang W, Hoogeveen RC, Rushing J, Schwenke DC, Tracy RP, Pi-Sunyer FX, Kriska AM, Ballantyne CM; Look AHEAD (Action for Health in Diabetes) Research Group. Lifestyle intervention and/or statins for the reduction of C-reactive protein in type 2 diabetes: from the look AHEAD study. Obesity (Silver Spring). 2013;21:944-950. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 111. | Wang X, Yang Q, Liao Q, Li M, Zhang P, Santos HO, Kord-Varkaneh H, Abshirini M. Effects of intermittent fasting diets on plasma concentrations of inflammatory biomarkers: A systematic review and meta-analysis of randomized controlled trials. Nutrition. 2020;79-80:110974. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 70] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 112. | Askarpour M, Khani D, Sheikhi A, Ghaedi E, Alizadeh S. Effect of Bariatric Surgery on Serum Inflammatory Factors of Obese Patients: a Systematic Review and Meta-Analysis. Obes Surg. 2019;29:2631-2647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 95] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 113. | Donath MY, Dalmas É, Sauter NS, Böni-Schnetzler M. Inflammation in obesity and diabetes: islet dysfunction and therapeutic opportunity. Cell Metab. 2013;17:860-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 264] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 114. | DiK B, Bahcivan E, Eser Faki H, Uney K. Combined treatment with interleukin-1 and tumor necrosis factor-alpha antagonists improve type 2 diabetes in rats. Can J Physiol Pharmacol. 2018;96:751-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 115. | Larsen CM, Faulenbach M, Vaag A, Ehses JA, Donath MY, Mandrup-Poulsen T. Sustained effects of interleukin-1 receptor antagonist treatment in type 2 diabetes. Diabetes Care. 2009;32:1663-1668. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 304] [Cited by in RCA: 308] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Endocrinology and metabolism
Country/Territory of origin: Greece
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cordeiro A, Portugal; Duan W, China S-Editor: Liu JH L-Editor: A P-Editor: Yu HG