©The Author(s) 2025.
World J Exp Med. Dec 20, 2025; 15(4): 110482
Published online Dec 20, 2025. doi: 10.5493/wjem.v15.i4.110482
Published online Dec 20, 2025. doi: 10.5493/wjem.v15.i4.110482
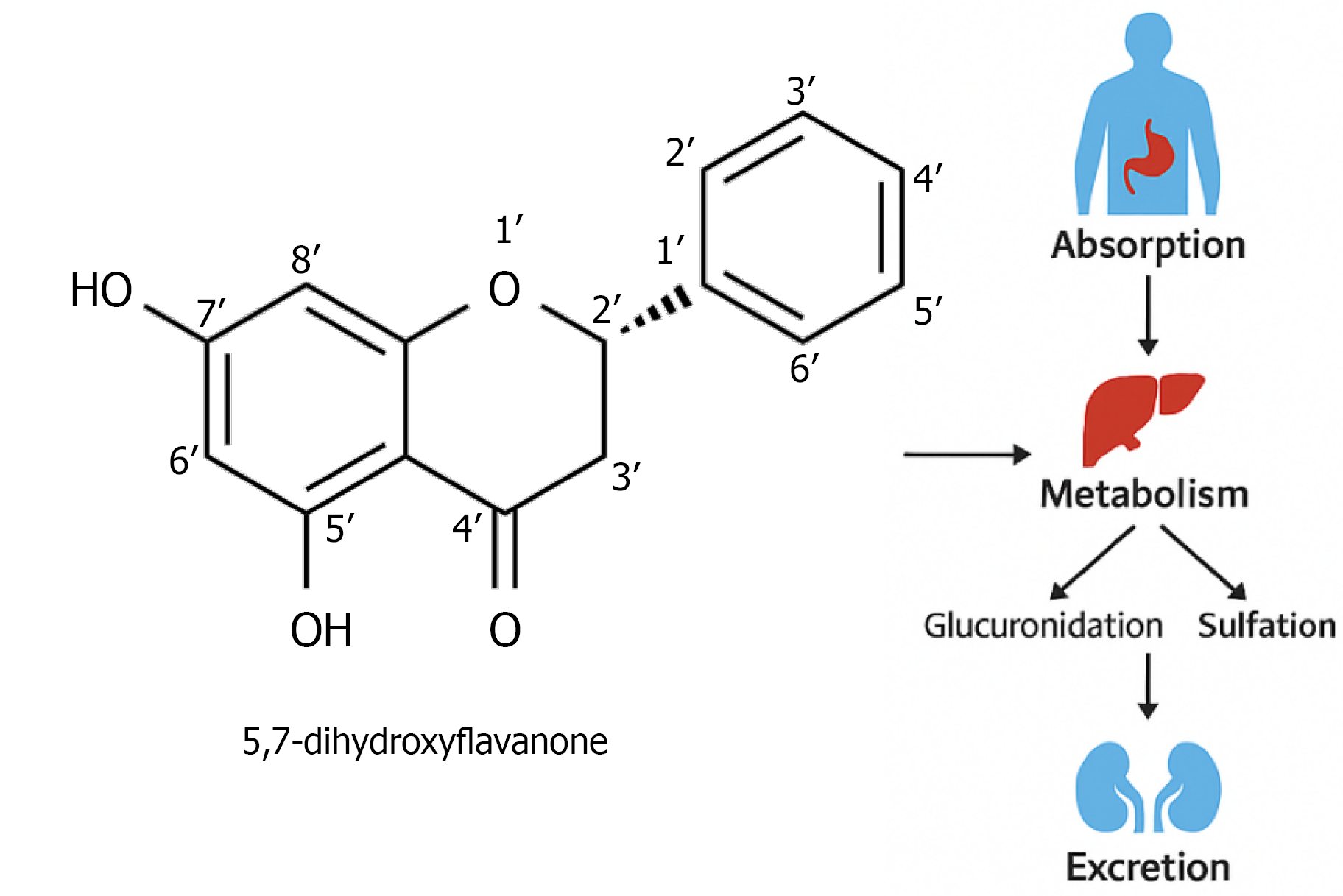
Figure 1 Atom-numbered chemical structure of pinocembrin (5,7-dihydroxyflavanone).
The hydroxyl groups at carbon positions C-5 and C-7 are shown explicitly, which are critical sites for phase II metabolic conjugation such as glucuronidation and sulfation. The absence of substituents on the B-ring is also highlighted, a feature that contributes to both its pharmacological profile and metabolic lability.
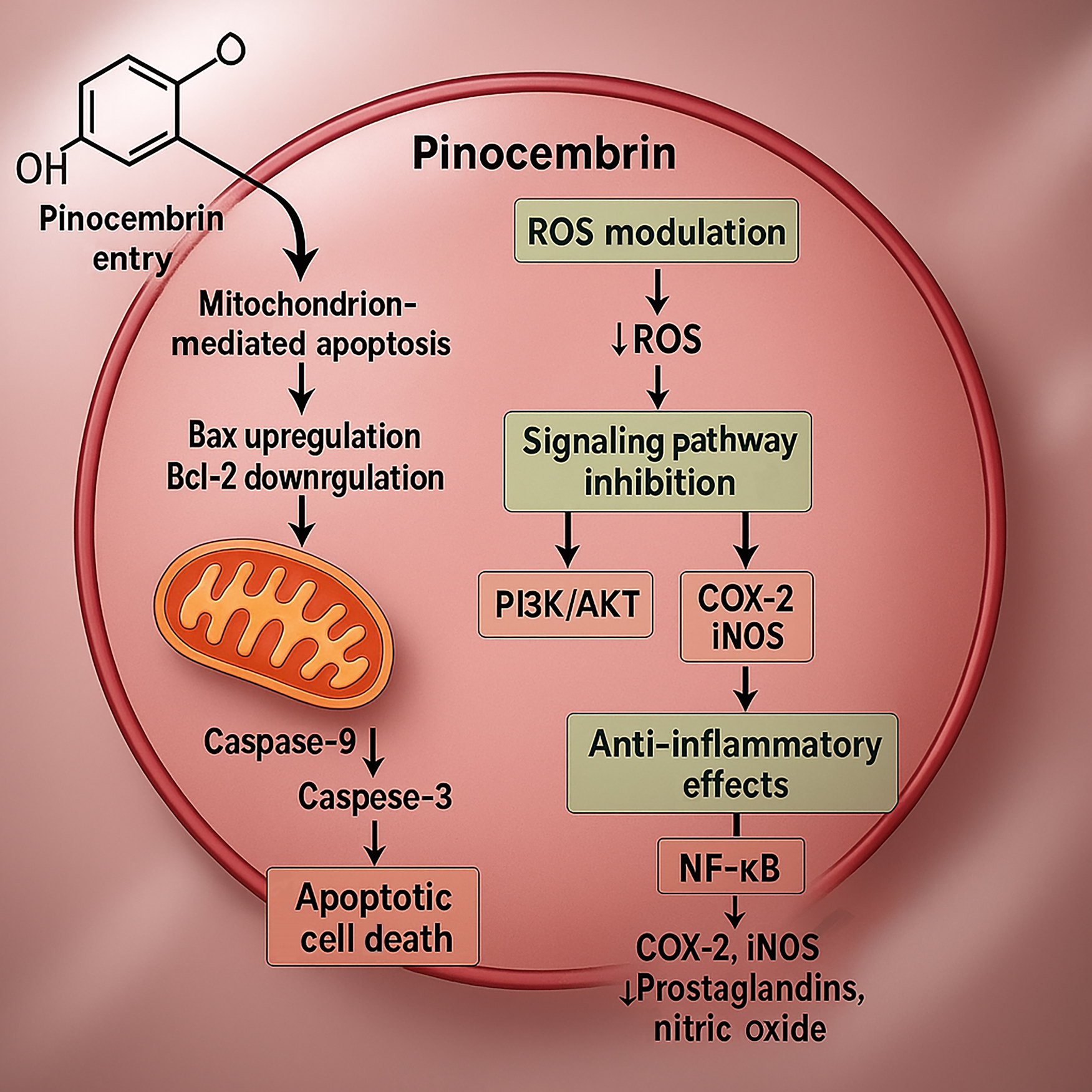
Figure 2 Cellular and molecular mechanisms of pinocembrin’s anticancer activity.
This schematic illustrates how pinocembrin (PB) enters cancer cells and triggers mitochondrion-mediated apoptosis through upregulation of Bax, downregulation of Bcl-2, and subsequent activation of caspase-9 and caspase-3. Concurrently, PB reduces intracellular reactive oxygen species levels, destabilizing the metabolic balance required for tumor growth and inhibiting phosphoinositol-3 kinase/protein kinase B signaling. Additionally, PB’s anti-inflammatory effects mediated by nuclear factor-kappa B inhibition decrease cyclooxygenase-2 and inducible nitric oxide synthase expression, further impeding cancer cell survival and proliferation. AKT: Protein kinase B; COX-2: Cyclooxygenase-2; iNOS: Inducible nitric oxide synthase; NF-κB: Nuclear factor-kappa B; PI3K: Phosphoinositol-3 kinase; ROS: Reactive oxygen species.
- Citation: Singla N, Mittal P, Babu MA, V Menon S, Ray S, Ali H, Purohit M, Goyal K, Mishra R, Hussain MS, Rekha A, Gupta G. Pinocembrin as a novel anti-cancer agent: Exploring preclinical evidence along with therapeutic potential. World J Exp Med 2025; 15(4): 110482
- URL: https://www.wjgnet.com/2220-315x/full/v15/i4/110482.htm
- DOI: https://dx.doi.org/10.5493/wjem.v15.i4.110482