©The Author(s) 2025.
World J Exp Med. Dec 20, 2025; 15(4): 108187
Published online Dec 20, 2025. doi: 10.5493/wjem.v15.i4.108187
Published online Dec 20, 2025. doi: 10.5493/wjem.v15.i4.108187
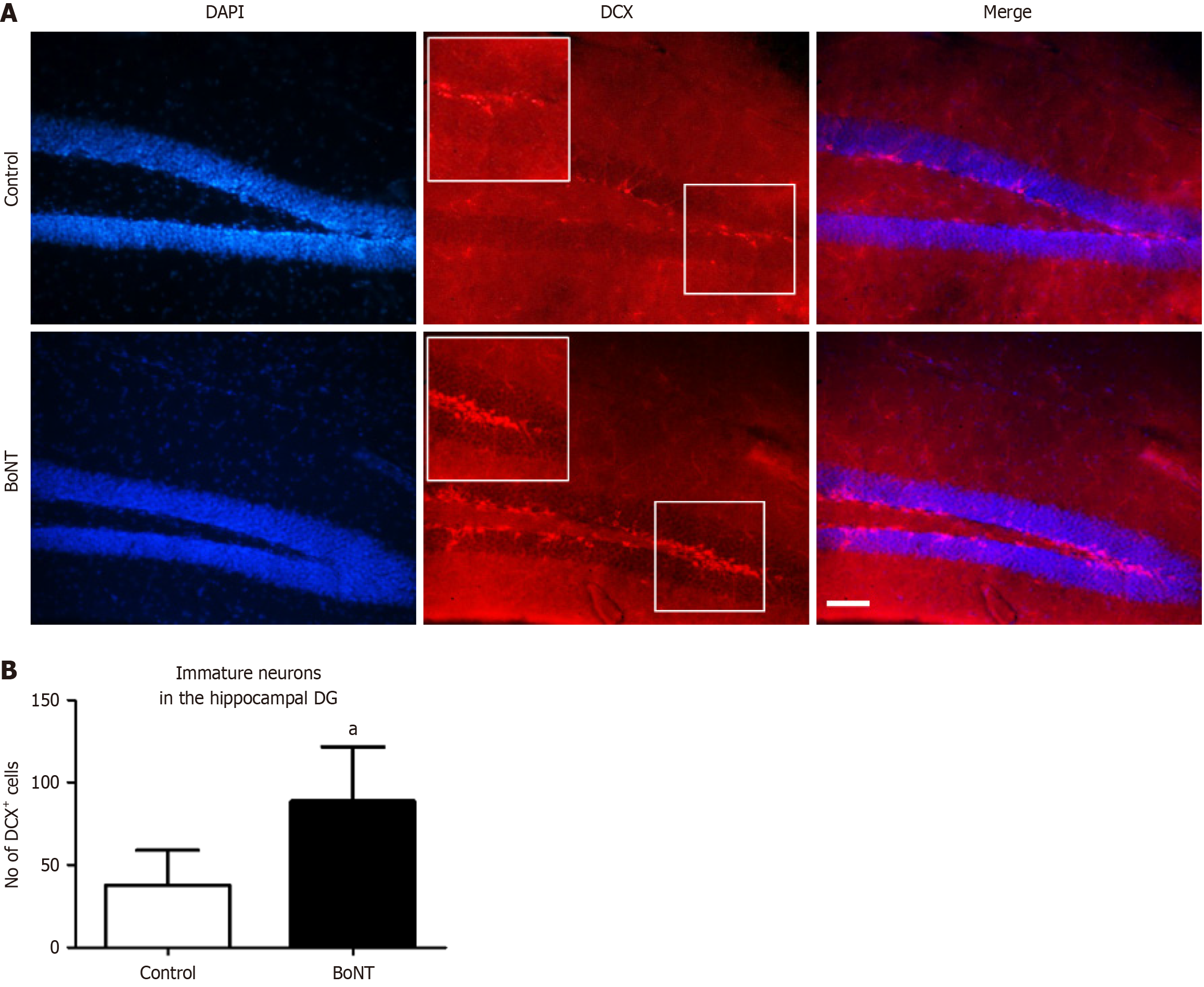
Figure 1 Immunohistochemical analysis of doublecortin-positive cells in the hippocampal dentate gyrus.
A: Representative image of doublecortin (DCX) immuno-positive cells in hippocampal dentate gyrus of control and botulinum neurotoxin (BoNT)-treated mice. The scale bar = 25 μm; B: The bar graph represents the number of DCX-positive cells. The number of DCX-positive cells in the BoNT was significantly increased when compared to the control group (aP value ≤ 0.01). DG: Dentate gyrus; BoNT: Botulinum neurotoxin; DCX: Doublecortin.
Figure 2 Confocal microscopy analysis of bromodeoxyuridine - neuronal nuclei positive neurons in the hippocampal dentate gyrus.
A: Representative confocal Z-scan image showing a bromodeoxyuridine (BrdU)-neuronal nuclei (NeuN) double-positive neuron in the hippocampal dentate gyrus (DG) region of experimental mice. Scale bar = 50 μm; B: Bar graph representing the percentage of BrdU-NeuN positive newborn neurons in the hippocampal DG. A significant increase in the percentage of BrdU-NeuN positive neurons was observed in the botulinum neurotoxin group compared to the control group (bP value ≤ 0.01). DG: Dentate gyrus; BrdU: Bromodeoxyuridine; NeuN: Neuronal nuclei.
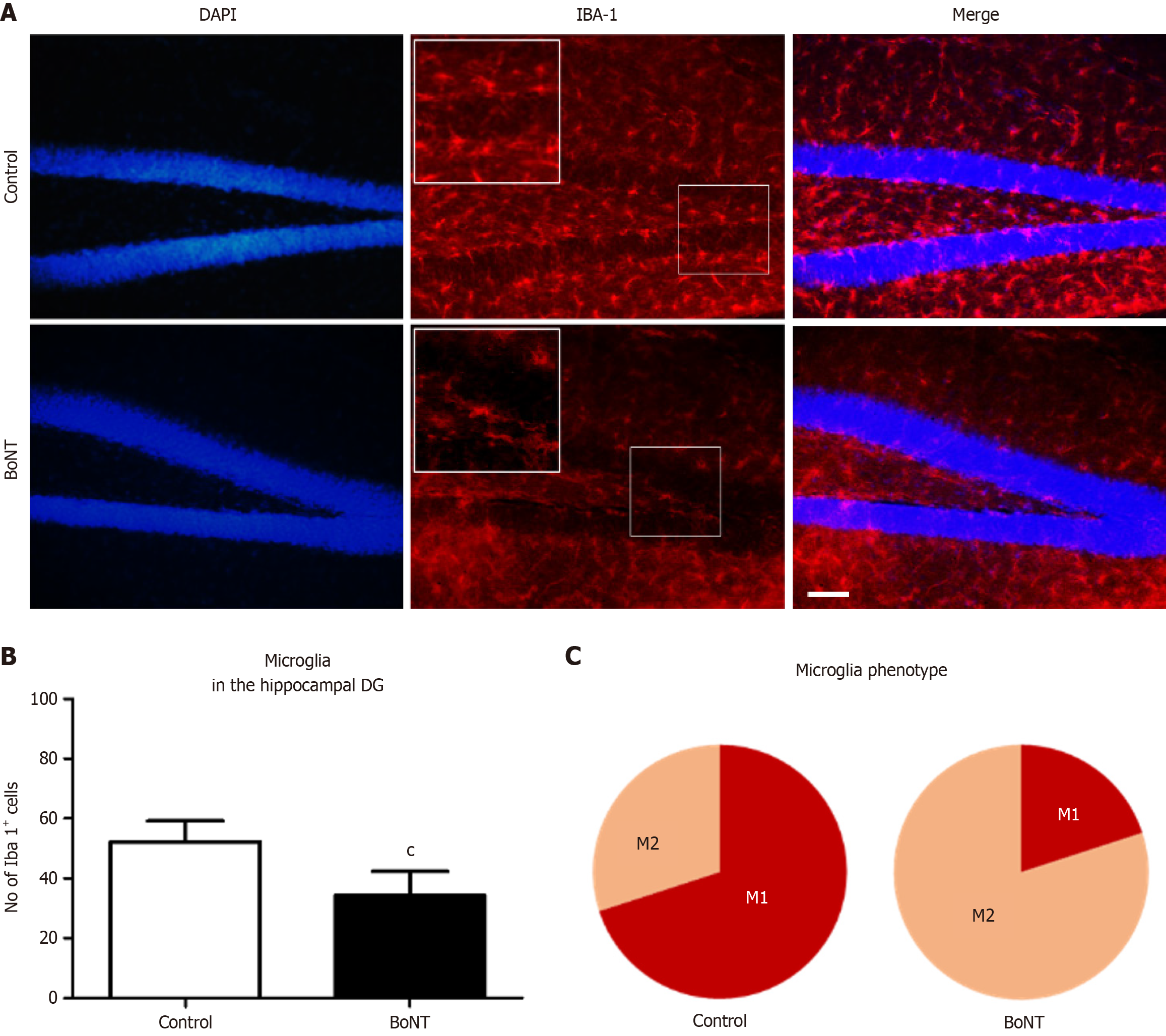
Figure 3 Immunohistochemical analysis of ionized calcium-binding adapter molecule-1 positive cells in the hippocampal dentate gyrus.
A: Representative image of ionized calcium-binding adapter molecule-1 (Iba)-1 immunopositive cells in hippocampal dentate gyrus (DG) of control and botulinum neurotoxin (BoNT)-treated mice. The scale bar = 25 μm; B: The bar graph represents the number of Iba-1 positive cells in the hippocampal DG region. The number of Iba-1 positive cells in the BoNT has been significantly reduced when compared to the control group (cP value ≤ 0.01); C: The pie chart illustrates the percentage distribution of M1 and M2 microglial phenotypes in control and BoNT-treated groups. The light maroon segment represents the protective M2 phenotype, while the dark maroon segment indicates the pro-inflammatory M1 phenotype. A prominent reduction in M1 microglia, accompanied by a shift toward the M2 phenotype, was observed following BoNT treatment. DG: Dentate gyrus; BoNT: Botulinum neurotoxin; Iba-1: Ionized calcium-binding adapter molecule-1.
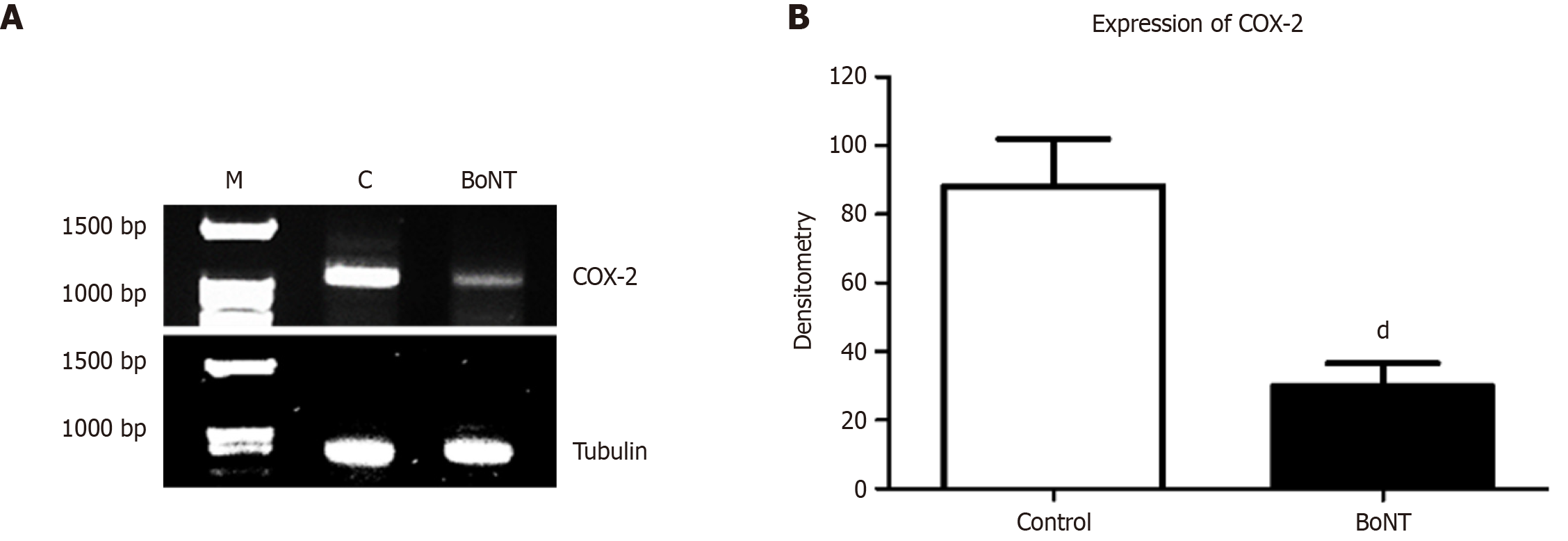
Figure 4 Reverse transcription polymerase chain reaction results of tubulin and cyclooxygenase-2.
A: Agarose gel images of the polymerase chain reaction products of cyclooxygenase (COX)-2 and Tubulin among control and botulinum neurotoxin (BoNT) groups, M indicates the 100bp DNA ladder; B: Bar graph of the densitometric analysis for the same in the hippocampal dentate gyrus of control and BoNT-treated groups. The expression of COX-2 has been significantly reduced in BoNT in comparison with the control group (dP value ≤ 0.01). DG: Dentate gyrus; BoNT: Botulinum neurotoxin.
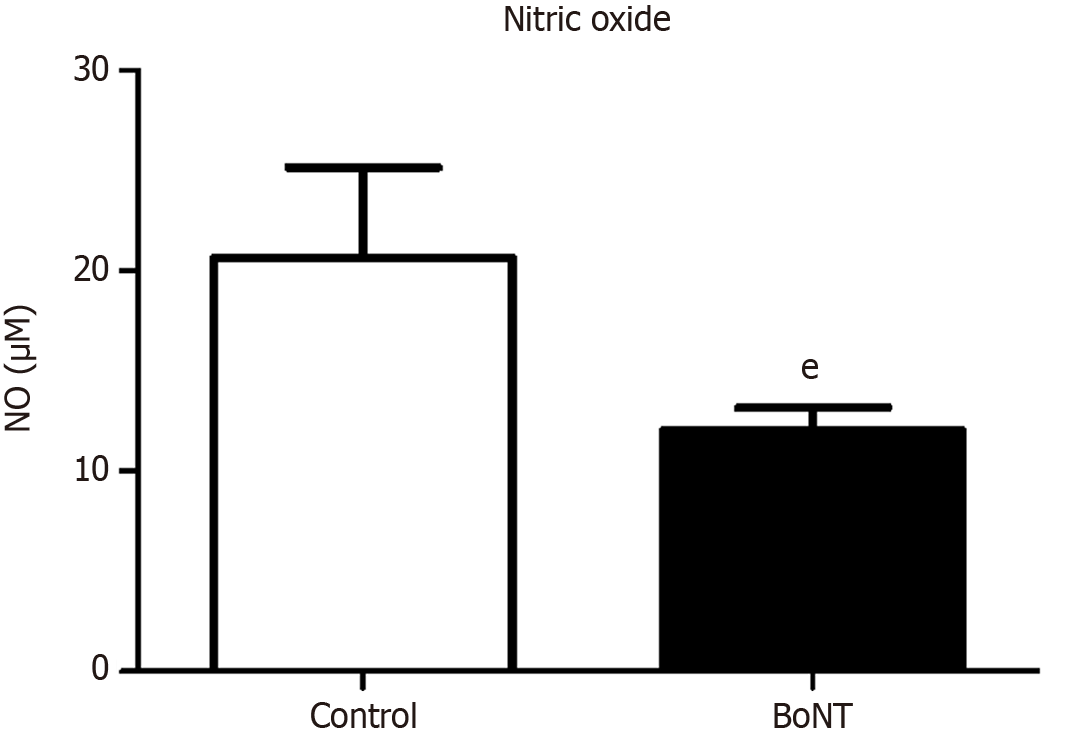
Figure 5 Level of nitric oxide.
The bar graph represents the level of nitric oxide (NO) in the hippocampus of aging experimental mice. The level of NO was found to be decreased in the botulinum neurotoxin treated group when compared to the control group (eP value ≤ 0.05). NO: Nitric oxide; BoNT: Botulinum neurotoxin.
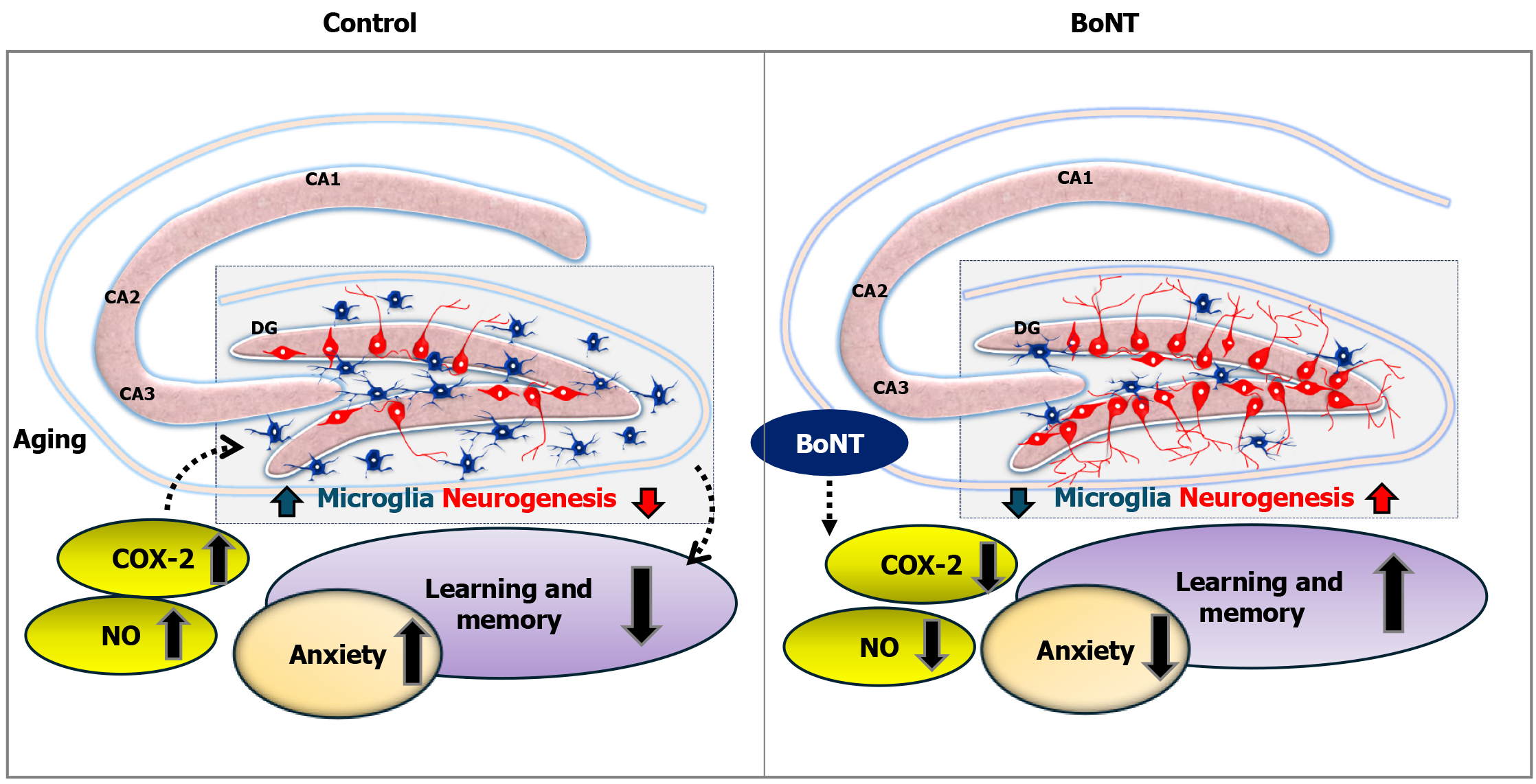
Figure 6 Pro-neurogenic effect of botulinum neurotoxin in the hippocampus.
Aging has been associated with a gradual increase in microglial activation, elevated levels of cyclooxygenase (COX)-2 and nitric oxide (NO), reduced neurogenesis, and progressive memory decline. Botulinum neurotoxin, a well-known anti-aging and analgesic agent, has also demonstrated pro-cognitive and anxiolytic properties. These effects may be attributed to its ability to suppress COX-2 and NO levels by deactivating microglia, thereby promoting hippocampal neurogenesis and potentially counteracting age-related cognitive decline. CA: Cornu Ammonis; DG: Dentate gyrus; NO: Nitric oxide; BoNT: Botulinum neurotoxin; COX-2: Cyclooxygenase-2. (This image has been adopted and modified from Kandasamy[30]).
- Citation: Joseph JHM, Babu Deva Irakkam MP, Kandasamy M. Proneurogenic and microglial modulatory properties of botulinum neurotoxin in the hippocampus of aging experimental mice. World J Exp Med 2025; 15(4): 108187
- URL: https://www.wjgnet.com/2220-315x/full/v15/i4/108187.htm
- DOI: https://dx.doi.org/10.5493/wjem.v15.i4.108187