Published online Dec 9, 2024. doi: 10.5492/wjccm.v13.i4.98862
Revised: September 26, 2024
Accepted: October 20, 2024
Published online: December 9, 2024
Processing time: 115 Days and 7.9 Hours
Dengue-associated acute liver failure (PALF) accounts for a high mortality rate in children admitted to the pediatric intensive care unit (PICU). To date, there is a lack of data on clinical algorithms for estimating the risk of mortality in pediatric patients with dengue-induced severe hepatitis (DISH).
To determine the prevalence of PALF and identify the predictors of mortality among patients with DISH.
This single-institution retrospective study was performed at a tertiary pediatric hospital in Vietnam between 2013 and 2022. The primary outcome was in-hospital mortality in pediatric patients with DISH, which was defined as either aspartate aminotransferase > 350 IU/L or alanine aminotransferase > 400 IU/L. Prognostic models for estimating the risk of death among patients with DISH were deve
A total of 459 children with DISH were included in the analysis. The median patient age was 7.7 years (interquartile range: 4.3-10.1 years). The prevalence of dengue-associated PALF in children with DISH was 18.3%. Thirty-nine DISH patients developing PALF (8.5%) died. Hepatic biomarkers, including the international normalized ratio (INR) ≥ 2.11 and total serum bilirubin (≥ 1.7 mg/dL), showed high predictive values for mortality (all P values < 0.001). Multivariable models showed the significant clinical predictors of death from dengue-induced PALF in patients with DISH, including reduced level of consciousness (pain and unresponsive levels on the Alert, Verbal, Pain, Unresponsive scale), high vasoactive-inotropic score (> 30), and elevated levels of blood lactate, INR, and serum bilirubin. The final prognostic model demonstrated high discrimination, Brier score, and an acceptable calibration slope.
The prevalence of PALF in children with DISH is 18.3%. We developed robust prognostic models to estimate the risk of death in hospitalized children with severe dengue-induced hepatitis.
Core Tip: The prevalence of dengue-associated acute liver failure in children with dengue-induced severe hepatitis (DISH) was 18.3%. The in-hospital mortality rate was approximately 8.5% among DISH patients developing acute liver failure. Hepatic biomarkers, including international normalized ratio (INR) (≥ 2.11) and total serum bilirubin (≥ 1.7 mg/dL), showed high predictive values for mortality. The significant predictors of mortality in children with DISH were decreased level of consciousness (pain and unresponsive levels on the Alert, Verbal, Pain, Unresponsive scale), high vasoactive-inotropic score (> 30), elevated blood lactate and INR levels during the first 24 hours of pediatric intensive care unit admission, and rising serum bilirubin during the first 72 hours of admission.
- Citation: Nguyen TT, Ngo PTM, Vo LT. Predicting the risk of mortality in children with dengue-induced hepatitis admitted to the paediatric intensive care unit. World J Crit Care Med 2024; 13(4): 98862
- URL: https://www.wjgnet.com/2220-3141/full/v13/i4/98862.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v13.i4.98862
Dengue-associated pediatric acute liver failure (PALF) is a critical complication in hospitalized children with severe dengue[1,2]. PALF primarily manifests as extensive hepatocellular necrosis and exacerbation of liver function, leading to hepatic encephalopathy and eventual death[3]. Our study cohort over the period 2013-2021 has shown that the prevalence of dengue-associated PALF in children with dengue shock syndrome was 4.2%[4]. Despite intensive interventions in the intensive care unit, the in-hospital fatality rate of dengue-associated PALF is high (68.3%)[5]. Our recent study revealed that combined therapeutic plasma exchange (TPE) and continuous renal replacement therapy (CRRT) significantly reduced the 28-day mortality rate of severe PALF to 39%[2]. Notably, early recognition, diagnosis, and appropriate intensive interventions at hospital admission may yield a better survival prognosis[5,6]. Our recent studies have also shown that better survival outcomes in pediatric intensive care unit (PICU)-admitted children with dengue-associated PALF are associated with rapid normalization of liver transaminase levels, metabolic biomarkers, coagulation profiles, and neurological status[1,2].
Several studies have reported that predictors of adult dengue PALF include low platelet counts, severe transaminitis, and elevated serum bilirubin levels[5,6]. Another Thai adult cohort study showed that End-Stage Liver Disease score ≥ 15 could predict the risk of PALF-associated death in adult patients with dengue-induced severe hepatitis (DISH); however, no specific predictor of PALF has been clearly determined[7]. To date, there are insufficient data on the prognosis of children with dengue-associated PALF[5,6]. Determining prognostic indicators for the risk of mortality in children with dengue-associated PALF is of utmost importance for categorizing and prioritizing treatments to improve survival and clinical outcomes. Therefore, we aimed to determine the prevalence of dengue-associated PALF among hospitalized patients with severe dengue-induced hepatitis and identify significant prognostic indicators for predicting the risk of death among patients with DISH.
This single-center, retrospective study was performed at the Children’s Hospital 2, in Vietnam. All children with dengue who were admitted to the PICU between 2013 and 2022 were identified. Eligibility included age < 18 years, laboratory-confirmed dengue infection, and presence of DISH[7]. The exclusion criteria were a lack of serologically confirmed dengue infection, incomplete data on hepatic enzymes, including aspartate aminotransferase (AST) and alanine aminotransferase (ALT), and in-hospital mortality.
The primary outcome was death during hospitalization. A set of candidate predictors was preselected based on our clinical knowledge, disease pathogenesis, and established risk factors from previously published studies[5-8]. A set of predetermined covariates included age, sex, dengue severity, severe bleeding, neurological status [the Alert, Verbal, Pain, Unresponsive (AVPU) scale], high vasoactive inotropic score (VIS) (> 30), hematocrit level, platelet count, severe transaminitis, international normalized ratio (INR), serum sodium, total serum bilirubin, blood lactate, and creatinine.
Dengue infection confirmation was in accordance with the World Health Organization (WHO) criteria from 2009, based on positive test results for the non-structural 1 antigen or Dengue-IgM antibody test[9]. DISH was defined as baseline hepatic transaminase levels > 10-fold upper normal limit at PICU admission, and more specifically, AST > 350 IU/L or ALT > 400 IU/L)[7]. Severe bleeding was diagnosed according to the WHO dengue 2009 guidelines[9]. Severe transaminitis was defined as elevated AST and/or ALT levels ≥ 1000 IU/L[9].
Pediatric PALF was defined in accordance with the European Association for the Study of the Liver criteria, as an acute presentation of critical liver dysfunction with biochemical confirmation of hepatic injury in children without prior chronic hepatic diseases, coagulation disorders not improved by supplementing vitamin K, an INR level greater than 1.5, if patients have encephalopathy, or INR higher than 2.0 in the absence of encephalopathy[3].
The severity of dengue PALF was classified into three categories: (1) Mild; (2) Moderate; and (3) Severe. First, patients with mild PALF met the above criteria, with stable hemodynamics without vasoactive inotrope requirement, normal respiratory status, and a completely alert level on the AVPU scale. Second, patients with moderate PALF presented with dengue shock syndrome, mild respiratory failure with low-flow oxygenation support (nasal oxygen, and nasal conti
Clinical and laboratory data from the participants’ medical records were entered into well-structured case report forms (CRFs). In compliance with Good Clinical Practice, all patients’ names and related information were anonymized. Subsequently, data from the CRFs were entered into an electronic database for statistical analyses. The clinical outcomes of the patients were examined during the PICU stay and at discharge.
Data on covariates were gathered during the first 24 hours of PICU admission, except for the highest level of total serum bilirubin within 72 hours of hospitalization. Blood and biochemical tests, including coagulation profiles, serum creatinine levels, and liver function tests, were performed using a machine (Alinity® ci-series, Abbott, United States). The degree of hemodynamic support was evaluated by the VIS[10].
The in-hospital mortality was calculated from the time of admission to death. Dengue severity is redundant here, should be removed. The AVPU scale, with four simple categories, including alert, verbal response, response to pain, and unresponsiveness, was employed to assess the level of consciousness of the patients[11]. Notably, considering the correlation with the pediatric Glasgow Coma Scale (pGCS), the pain response has been shown to be equivalent to a pGCS from 4 to 12 points and unresponsive on the AVPU scale corresponding pGCS of 3-5 points[11].
Continuous variables are presented as median and interquartile (IQR) range and categorical data as numerical counts (n) and percentages (%). The primary study bias was missing information on patients’ hepatic biomarkers. The area under the curve (AUC) values, sensitivity, specificity, and accuracy were evaluated as indicators for predicting the risk of in-hospital death within 72 hours of PICU admission. Bivariate logistic regression with complete-case data and multi
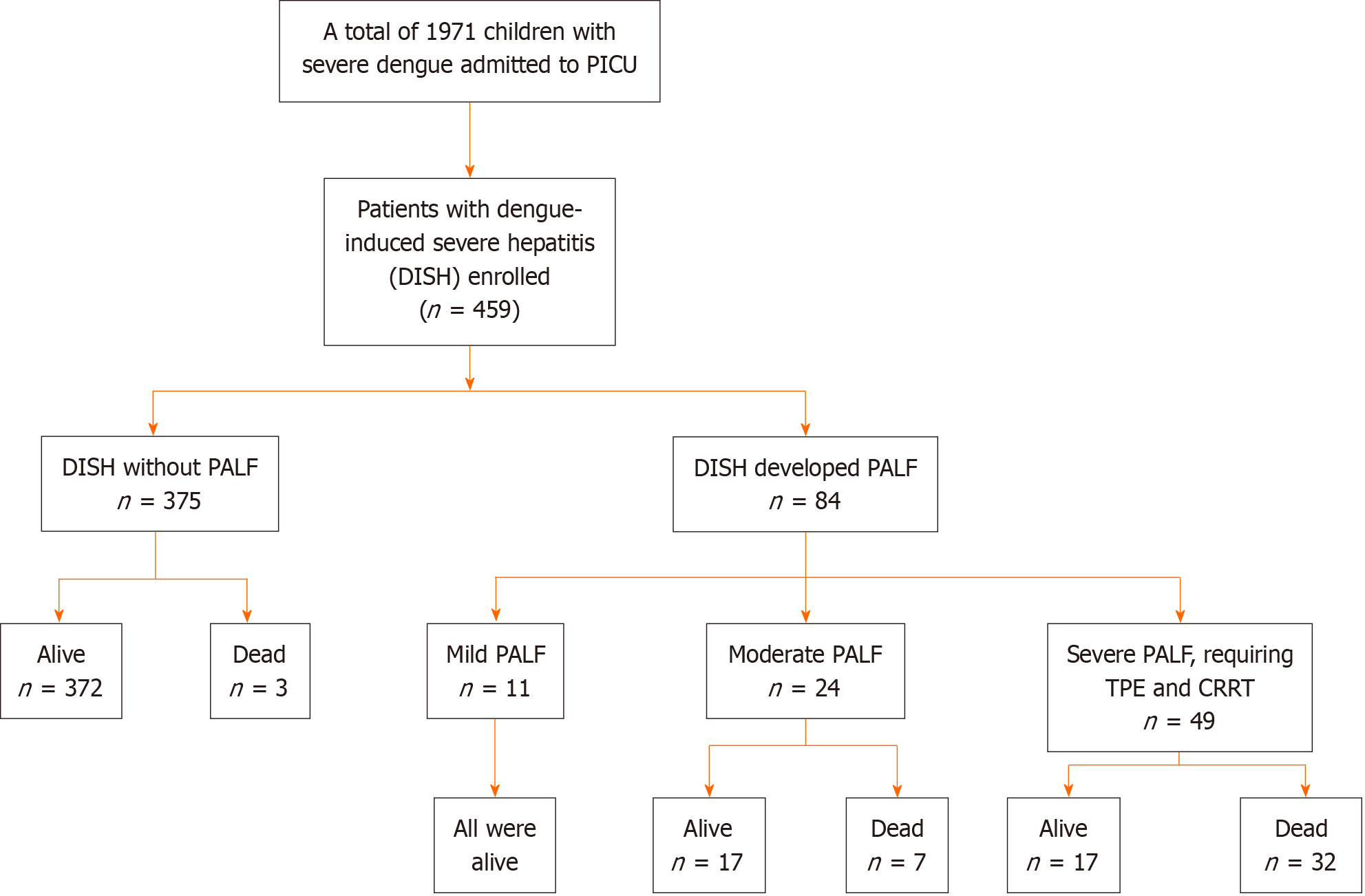
We identified 1971 children with severe dengue (dengue shock syndrome and dengue-induced liver disease) who were admitted to the PICU during the study period. A total of 459 patients experienced DISH and were included in the analysis (Figure 1). Of these, 84 patients developed DISH and fulfilled the criteria for PALF, while the remaining 375 patients did not develop PALF, progressing to clinical stability and complete recovery. Among 459 patients with DISH, 84 (18.3%) developed dengue-associated PALF. Of those diagnosed with dengue-associated PALF, 11 had mild, 24 had moderate, and 49 had severe life-threatening PALF that required TPE and CRRT (Figure 1).
The clinical and laboratory data of the study participants upon admission to the PICU are presented in Table 1. The median age of the patients was 7.7 years (IQR: 4.3-10.1 years), and females accounted for 42% of the study participants. Thirty-eight patients (8.3%) with DISH had underlying diseases, including asthma, congenital heart disease, epilepsy, and β-thalassemia. Most patients experienced dengue shock syndrome (DSS) on the median day 5 (IQR: 4-5 day) after the onset of fever. Notably, compensated DSS was observed in 87.6% of the children, and the remaining 12.4% of the patients had decompensated DSS. Severe bleeding was observed in 76 (16.7%) patients. Systolic and diastolic shock indices were markedly elevated, and 45 patients (9.8%) had a high VIS (> 30). Complete blood counts revealed increasing hematocrit and low platelet counts. Significant hepatic transaminases, coagulation disorders, and elevated serum bilirubin levels were observed in participants experiencing DISH. The median blood lactate was 2.9 mmol/L (IQR: 2.0-4.5 mmol/L) on PICU admission.
| Characteristics | No. participants | Statistics |
| Age (years) | 457 | 7.7 (4.3-10.1) |
| Female patients | 459 | 193 (42) |
| Underlying diseases | 459 | 38 (8.3) |
| Day of occurrence of dengue shock since onset of fever (days) | 451 | 5 (4-5) |
| Grading of DSS | 459 | |
| Compensated DSS | 402 (87.6) | |
| Decompensated DSS | 57 (12.4) | |
| Severe bleeding | 456 | 76 (16.7) |
| Respiratory rate (breaths/minute) | 405 | 26 (24-30) |
| Systolic shock index (bpm/mmHg) | 390 | 1.33 (1.15-1.6) |
| Diastolic shock index (bpm/mmHg) | 390 | 1.83 (1.5-2.25) |
| Vasoactive-inotropic score > 30 | 459 | 45 (9.8) |
| White blood cell count, (× 109/L) | 456 | 5.35 (3.76-7.8) |
| Peak hematocrit | 446 | 47 (44-51) |
| Nadir hematocrit | 446 | 38 (33-41) |
| Platelet cell count, (× 109/L) | 457 | 32.6 (20-48) |
| Aspartate aminotransferase (IU/L) | 459 | 751 (465-1416) |
| Alanine aminotransferase (IU/L) | 459 | 344 (221-638) |
| International normalized ratio | 406 | 1.40 (1.18-1.96) |
| Prothrombin time (second) | 404 | 18 (15-27) |
| Activated partial thromboplastin time (second) | 405 | 52 (43-69) |
| Peak serum bilirubin level (mg/dL) | 119 | 1.9 (1.04-5.3) |
| Peak serum ammonia level (µmol/L) | 64 | 117 (80-197) |
| Blood creatinine (µmol/L) | 447 | 54 (44-63) |
| Troponin I (ng/mL) | 164 | 0.036 (0.01-0.22) |
| Blood lactate (mmol/L) | 293 | 2.9 (2.0-4.5) |
| Length of pediatric intensive care unit stay (days) | 459 | 3 (2-5) |
| Length of hospital stay (days) | 459 | 5 (4-10) |
| Fatal outcome | 459 | 42 (9.2) |
As shown in Figure 1, thirty-nine PICU-admitted children with DISH who developed PALF died during hospitalization, corresponding to a mortality rate of approximately 8.5%. Patients with severe PALF who experienced dengue shock syndrome, critical respiratory failure with mechanical ventilation support, and extracorporeal therapies with plasma exchange and/or CRRT accounted for a very high fatality rate, as noted in of the 32/49 patients (65%). In addition, other three DISH patients without PALF died of dengue-associated encephalitis, pulmonary hemorrhage, and multiorgan failure. Among the fatal cases, PICU-associated sepsis was notable, as 10/42 (23.8%) patients had a positive hemoculture with identified Acinetobacter baumannii and Pseudomonas aeruginosa.
As presented in Table 2, two significant biomarkers, including INR during the first 24 hours of PICU admission and highest levels of total serum bilirubin levels within 72 hours of admission, showed high predictive values for in-hospital mortality (all presented significant P-values < 0.001). The optimal cut-off points for INR (≥ 2.11) and total bilirubinemia (≥ 1.7 mg/dL) revealed good predictive values for sensitivity, specificity, and accuracy.
| Parameters | Area under the curve of mortality (95%CI) | P value | Optimal cutoff points of the parameters | Sensitivity | Specificity | Accuracy | Youden index | |
| INR peak during first 24 hours of PICU admission | 0.907 (0.858-0.956) | < 0.001 | INR peak ≥ 2.11 during first 24 hours of admission | 0.902 | 0.855 | 0.859 | 0.757 | |
| Peak of total serum bilirubin during 72 hours of PICU admission (mg/dL) | 0.790 (0.704-0.875) | < 0.001 | Peak of total serum bilirubin during the first 72 hours of PICU admission ≥ 1.7 (mg/dL) | 0.941 | 0.565 | 0.672 | 0.506 | |
As presented in Table 3, bivariate analyses revealed important risk factors for in-hospital mortality in PICU-admitted children with DISH, including dengue severity, severe bleeding, significantly reduced mental status (pain response and unresponsive levels on the AVPU scale), high VIS (> 30), low platelet counts (< 20 × 109/L), serum creatinine, INR, and the highest levels of total bilirubin, serum lactate, and ammonia within 72 hours of PICU admission. However, multivariate logistic analysis showed that the significant prognostic indicators of mortality in PICU-admitted children with dengue-associated PALF were altered mental status, as indicated by the pain response and unresponsive levels on the AVPU scale, high levels of VIS > 30, and blood lactate. No significant interactions were found between the covariates.
| Factors | Non-survivors (n = 42) | Survivors (n = 417) | Unadjusted OR (95%CI), Pa | Adjusted OR (95%CI), Pb |
| Age (years) | 7 (4.2-10) | 7.8 (4.4-10.2) | 0.98 (0.9-1.06), P = 0.55 | - |
| Gender | ||||
| Female | 16 (38) | 177 (42) | 1.2 (0.62-2.3), P = 0.58 | - |
| Male | 26 (62) | 240 (58) | ||
| Grading of DSS | ||||
| Compensated DSS | 29 (69) | 373 (89) | 3.8 (1.84-7.85), P < 0.001 | - |
| Decompensated DSS | 13 (31) | 44 (11) | ||
| Severe bleeding | ||||
| No | 11 (26) | 372 (89) | 23.1 (10.9-49.1), P < 0.001 | - |
| Yes | 31 (74) | 45 (11) | ||
| Levels of consciousness (on the Alert, Verbal, Pain, Unresponsive scale) | ||||
| Alert and verbal response | 15 (36) | 387 (93) | 23.2 (11.1-48.2), P < 0.001 | 4.43 (1.12-17.5), P = 0.03 |
| Pain and unresponse | 27 (64) | 30 (7) | ||
| High vasoactive inotropic score (> 30) | ||||
| No | 7 (17) | 407 (98) | 204 (73-568), P < 0.001 | 39.2 (9.2-167), P < 0.001 |
| Yes | 35 (83) | 10 (2) | ||
| Peak hematocrit (%) | 46 (40-51) | 47 (44-51) | 0.95 (0.9-0.99), P = 0.024 | - |
| Low platelet counts, (< 20 x 109/L) | ||||
| No | 25 (60) | 319 (76) | 2.21 (1.15-4.27), P = 0.018 | - |
| Yes | 17 (40) | 98 (24) | ||
| Severe transaminitis | ||||
| No | 9 (21) | 260 (62) | 6.07 (2.83-13.0), P < 0.001 | - |
| Yes | 33 (79) | 157 (38) | ||
| Serum sodium, (mmol/L) | 128 (124-133) | 128 (125-131) | 1.0 (0.95-1.07), P = 0.74 | - |
| Highest level of blood lactate (mmol/L) | 10.3 (7.2-13.4) | 2.4 (1.8-4.2) | 1.67 (1.47-1.89), P < 0.001 | 1.61 (1.34-1.94), P < 0.001 |
| Serum creatinine (µmol/L) | 73 (58-123) | 53 (44-61) | 1.03 (1.02-1.04), P < 0.001 | - |
Table 4 compares the prognostic values of the three predictive models for the risk of death in PICU-admitted patients with DISH. When clinically identified predictors were combined with hepatic biomarkers, the reduced models with either INR (prognostic model 1) or total serum bilirubin (prognostic model 2) significantly predicted the risk of death. However, the total bilirubin level was not statistically significant in the full model with clinically identified predictors, INR, and serum bilirubin level (prognostic model 3). These models were further evaluated using internal validation.
| Prognostic factors | Model 1 | Model 2 | Model 3 | |||
| OR (95%CI), Pa | OR (95%CI),Pb | OR (95%CI), Pc | ||||
| Pain and unresponsive levels on the Alert, Verbal, Pain, Unresponsive scale | 13.86 (3.71-51.8) | < 0.001 | 14.36 (4.12-50.1) | < 0.001 | 13.96 (3.73-52.2) | < 0.001 |
| High vasoactive inotropic score (> 30) | 18.1 (4.99-65.4) | < 0.001 | 17.4 (5.06-60.2) | < 0.001 | 18.0 (4.98-65.3) | < 0.001 |
| Highest level of blood lactate (mmol/L) | 1.5 (1.27-1.78) | < 0.001 | 1.62 (1.37-1.91) | < 0.001 | 1.5 (1.27-1.78) | < 0.001 |
| International normalized ratio levels, ≥ 2.11 | 11.3 (2.62-48.3) | 0.001 | _ | _ | 9.1 (1.72-47.9) | < 0.01 |
| Peak serum bilirubin level, ≥ 1.7 mg/dL | _ | _ | 4.72 (1.12-19.8) | 0.03 | 1.5 (0.29-7.81) | 0.62 |
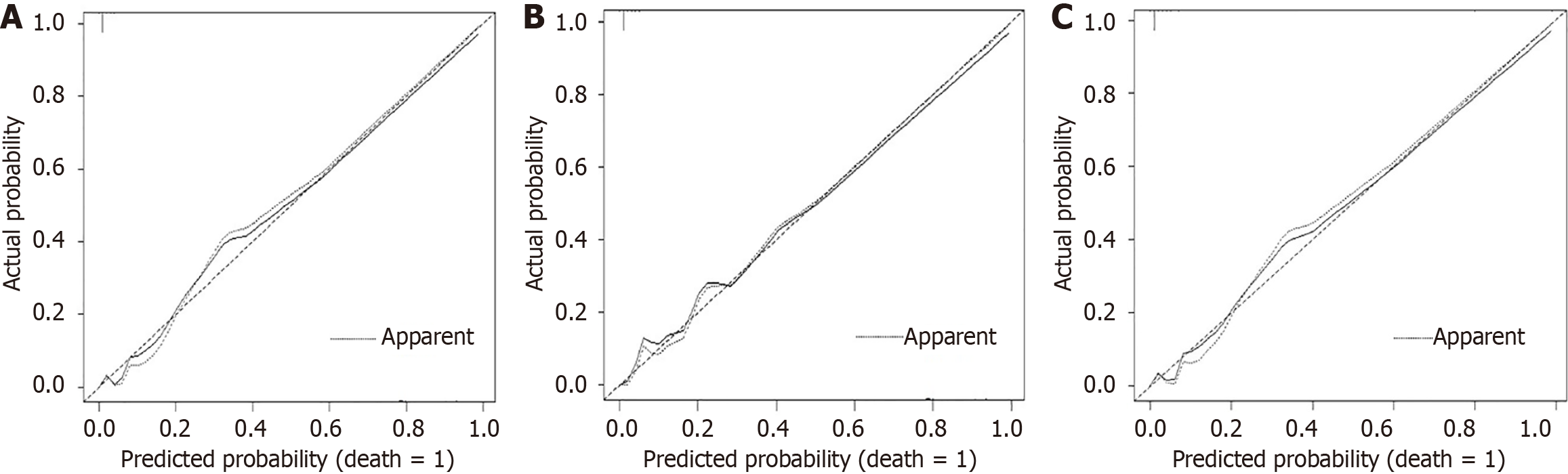
The performance parameters of the three prognostic models were compared (Table 5). All reduced and full models revealed competency in performance as indicated by good discrimination with a high C-statistic index. Prognostic model 2 showed a slightly higher calibration slope than comparative models 1 and 3. However, the full prognostic model 3 had the least variability, as indicated by the lowest Brier scores in the training and test sets. Most importantly, as presented in Figure 2, full prognostic model 3 visually showed the best calibration plot, indicating the least variability. Based on the clinical importance of serum bilirubin in disease pathogenesis and the medical literature, it was retained in the final full prognostic model.
| Prognostic models | C-statistic | Calibration slope | Brier score |
| Model 1 | 0.974 (0.967) | 1 (0.892) | 0.021 (0.023) |
| Model 2 | 0.966 (0.960) | 1 (0.906) | 0.023 (0.027) |
| Model 3 | 0.975 (0.968) | 1 (0.884) | 0.020 (0.023) |
The fatality rate of hospitalized patients with dengue shock syndrome ranges from 20% to 70%[5-8]. A dramatically elevated fatality rate has been reported in patients presenting with PALF secondary to prolonged shock, severe respiratory failure requiring mechanical ventilation, and extracorporeal therapies (CRRT and/or TPE)[1,2]. Apart from prognostic studies in adult cohorts, there are still inadequate data on the prognosis of children with dengue-associated PALF[5-8]. Therefore, in this study, we aimed to determine the prevalence of dengue-associated PALF in hospitalized children with DISH. We identified the most significant prognostic indicators for estimating the risk of mortality in patients with critical liver transaminases.
The reported incidence of dengue-associated PALF among the mild-to-severe dengue infected population in a large pediatric cohort in Thailand was 1.1%[5]. In this study, we showed that the prevalence of dengue-associated PALF in the pediatric population with DISH was as high as 18.3%. Notably, 39 of the 459 PICU-admitted children with DISH developed PALF and died during hospitalization, corresponding to a mortality rate of approximately 8.5%. These data are useful for establishing preemptive diagnostic schemes for dengue-induced PALF among hospitalized children presenting with severe transaminitis. Notably, there were 39 fatalities among the 84 patients diagnosed with PALF, indicating that the case fatality rate of dengue-induced PALF was 46.4%. This figure was markedly lower than that in previous reports from Thai pediatric (68.3%) and adult (58.8%) cohorts[5,7]. A possible explanation for the difference in mortality between our study and the Thai cohorts is that, in our study patients diagnosed with severe dengue-induced PALF were managed with extracorporeal therapies (TPE and/or CRRT). Most importantly, our research team has recently shown that combined TPE and CRRT interventions yield better survival outcomes in children with dengue-associated PALF and DSS[2]. In addition, our study had a larger sample size of dengue-associated PALF, indicating more statistical power than the Thai cohorts [5,7]. Currently, there is no classification for the severity of dengue-associated PALF, and in-hospital fatality rates vary greatly according to PALF severity. In this regard, we constructed a simplified categorization of PALF severity in which clinicians can rapidly classify severe patients and provide appropriate interventions.
To the best of our knowledge, this is to date the largest cohort of patients with dengue-associated PALF in which prognostic models to predict the risk of death among patients with DISH were developed. Akdogan et al[14] stated that patients with hepatic encephalopathy grades III and IV could have death rates of 42% and 100%, respectively. Notably, we have shown that improvements in hepatic encephalopathy, liver function tests, coagulation profiles, and biochemistry are associated with better survival outcomes in patients with dengue-associated PALF[1,2]. Therefore, we based this rationale on preselected all covariates in developing the prognostic models and identified the significant predictors of dengue-induced PALF. First, altered mental status can signify imminent hepatic encephalopathy among patients with dengue-associated PALF, which has been shown to be an independent predictor of death[15,16]. However, there are currently insufficient data on the correlation between decreased mental status and increased risk of death among patients with dengue-induced PALF. In this regard, we found that markedly reduced consciousness, as indicated by the pain and unresponsive levels on the AVPU scale, was a strong predictor of death in patients with dengue-associated PALF. Second, we have reported that a high VIS (> 30) and elevated blood lactate (≥ 4.2 mmol/L) are significant predictors of death in a pediatric cohort of 492 PICU-admitted patients with severe dengue[8]. In this study, these covariates were significant independent predictors of mortality due to dengue-induced PALF. This can be interpreted as the pathogenesis of acute liver failure involving massive hepatocyte necrosis, leading to multiorgan damage and hepatic encephalopathy, which clinically manifests as changes in consciousness and hemodynamics[17]. In addition, PALF can cause peripheral vascular dilation, microcirculatory vasoconstriction, and poor blood perfusion to organs, leading to injury and dysfunction in multiple organs[18]. Thus, a high VIS score and elevated serum lactate levels upon PICU admission, particularly an increasing trend in these parameters after admission, highly increase the risk of cerebral edema and death[1,2,8]. Nevertheless, previously published studies from Thailand have overlooked these critical factors when constructing predictive models for mortality from dengue-induced PALF[5-7]. In this respect, our study significantly fills the existing knowledge gap.
The primary mechanisms of hepatocyte injury and DISH involve direct cytopathic effects, immune responses, and reduced hemoperfusion in the liver[19]. Most importantly, rapid normalization of hepatic function, including liver metabolic biomarkers and coagulation dysfunction, is associated with better survival outcomes[1,2]. Therefore, we examined liver-related biomarkers for prognostication of death in patients with DISH. We found no statistical association between severe transaminitis and mortality risk, which is consistent with previous studies[7,8]. In terms of coagulation dysfunction, Teerasarntipan et al[7] revealed that an INR ≥ 1.5 had a good predictive value for estimating the risk of death from DISH, with an AUC of 0.83[7]. A higher INR > 3.5 was reported as a significant mortality predictor in acute liver failure according to King’s College criteria[20]. It was shown that INR levels ≥ 2.55 on hospital admission could predict the risks of death and/or liver transplant[21]. In addition, Akdogan et al[14] stated that INR values ranging from 1.5 to 5 were attributable to a death rate of 36%, and INR levels > 5 corresponded to a markedly increased fatality rate of 82%. In our study, the INR cut-off point ≥ 2.11 showed high prognostic values [sensitivity, 90.2%; specificity, 85.5%; odds ratio = 9.1 (95%CI: 1.72-47.9; P < 0.01)] among children with DISH. Our recent studies describing the dynamic variation in INR values in children with dengue-associated PALF revealed that INR rapidly increases with the occurrence of DISH[1,2]. Therefore, markedly increased INR upon admission is an important prognostic factor for in-hospital death in patients with dengue-induced PALF.
In dengue-induced PALF, the mechanisms of devastating hepatocytes can cause elevated serum bilirubin levels, including the direct cytopathic effects of the dengue virus, immune-mediated damage, decreased perfusion, hepatocyte necrosis, Kupffer cell hyperplasia and destruction, and inflammatory cell infiltration[21]. A total serum bilirubin level > 85.5 µmol/L (or 5 mg/dL) on admission can predict mortality[21]. Thus, we studied the total serum bilirubin levels in the predictive models of death in patients with DISH when combined with clinical predictors and INR parameters (Table 4). In the prognostic model 2, peak serum bilirubin level was a significant predictor of death from dengue-induced PALF; however, it became statistically insignificant in the prognostic model 3. Several possible explanations have been proposed for this finding. First, serum bilirubin levels have been reported to be highly variable in previous dengue cohorts[22-25]. This suggests that distinct mechanisms of liver injury in various cohorts could result in different changes in the serum bilirubin levels[21,26]. Second, because PALF can rapidly progress to critical multi-organ failure, prognostic indicators, including serum lactate and INR levels, can potentially show marked changes early in the occurrence of PALF[1,18,19]. In contrast, serum bilirubin elevation occurs late in the presence of massive hepatocyte necrosis and virus-induced cholangitis, causing vast blockage of the intrahepatic bile ducts[27]. Additionally, our recent study showed that serum bilirubin levels are commonly elevated since day 3 after PALF diagnosis[1]. From our real-life experience, patients with severe dengue-associated PALF may die soon after PICU admission, despite normal serum bilirubin levels on admission. It frequently takes several weeks to normalize serum bilirubin levels in dengue-associated PALF patients[1].
Notably, covariates reported as death indicators, including critical bleeding, low platelet counts, elevated blood creatinine levels, and dengue shock syndrome, were also examined[5,7,18,27]. However, these parameters were not statistically significant in the final multivariate model. Previous studies were restricted by relatively small sample sizes to determine the effect of these covariates in the predictive model of mortality from dengue-induced acute liver failure[5,7,18,27]. In addition, among the patients with DISH who died in-hospital, PICU-associated severe sepsis was found in 23.8% of these patients, and pathogens such as Acinetobacter baumannii and Pseudomonas aeruginosa were identified in the hemoculture. This finding indicates that secondary sepsis associated with PICU stay is also a significant contributing factor to the risk of death in the DISH population. These patients were likely susceptible to sepsis owing to invasive procedures such as catheterization and mechanical ventilation. According to our clinical supervision, these septic complications frequently occurred from days 4 to 5 after PICU admission. In this study, we aimed to develop a predictive model to estimate the risk of death among patients with DISH based on the covariates collected upon PICU admission. Nevertheless, secondary sepsis associated with PICU stay was also a notable risk factor for mortality in patients with DISH.
Our study had several limitations inherent to its single-center retrospective cohort design and unstandardized collection of clinical and laboratory data during hospital admission. The most significant bias was the missing information on serum ammonia, which was previously reported as an important prognostic indicator. However, this study retrospectively gathered data from medical records, and serum ammonia tests were only performed in patients with severe dengue-associated PALF. Similarly, the covariate total serum bilirubin was also markedly missing (approximately 60%), influencing the developed prognostic models at certain levels, despite being manipulated with multiple imputations. Considering these limitations, further investigations in prospective cohorts with appropriate study designs are essential to confirm our findings.
We developed robust prognostic models to estimate the risk of death in children with dengue experiencing severe hepatitis and identified significant predictors, including markedly reduced consciousness (pain and unresponsive levels on the AVPU scale), high VIS (> 30), rising blood lactate level, highly elevated INR, and serum bilirubin. The prognostic model will be externally validated in other cohorts.
We are grateful to the patients and administrative staffs, for their support with this study.
| 1. | Thanh NT, Dat NT, Thinh TN, Phuong NTM, Thanh MTH, Bao NT, Son PT, Viet DC, Tung TH, Thien V, Luan VT. Therapeutic plasma exchange and continuous renal replacement therapy in pediatric dengue-associated acute liver failure: A case series from Vietnam. Transfus Apher Sci. 2023;62:103617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 5.7] [Reference Citation Analysis (1)] |
| 2. | Vo LT, Do VC, Trinh TH, Vu T, Nguyen TT. Combined Therapeutic Plasma Exchange and Continuous Renal Replacement Therapy in Children With Dengue-Associated Acute Liver Failure and Shock Syndrome: Single-Center Cohort From Vietnam. Pediatr Crit Care Med. 2023;24:818-828. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 3. | European Association for the Study of the Liver. Clinical practice guidelines panel, Wendon, J; Panel members, Cordoba J, Dhawan A, Larsen FS, Manns M, Samuel D, Simpson KJ, Yaron I; EASL Governing Board representative, Bernardi M. EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure. J Hepatol. 2017;66:1047-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 669] [Article Influence: 74.3] [Reference Citation Analysis (1)] |
| 4. | Nguyen TT, Nguyen DT, Vo TT, Dang OT, Nguyen BT, Pham DT, Nguyen TT, Duong YN, Doan DH, Nguyen TH, Ho LT, Nguyen PH, Phan DN, Tran TV, Nguyen TK, Luong DC, Pham AT, Dinh TT, Do VC, Vo LT. Associations of obesity and dengue-associated mortality, acute liver failure and mechanical ventilation in children with dengue shock syndrome. Medicine (Baltimore). 2023;102:e36054. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Laoprasopwattana K, Jundee P, Pruekprasert P, Geater A. Outcome of Severe Dengue Viral Infection-caused Acute Liver Failure in Thai Children. J Trop Pediatr. 2016;62:200-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (2)] |
| 6. | Kye Mon K, Nontprasert A, Kittitrakul C, Tangkijvanich P, Leowattana W, Poovorawan K. Incidence and Clinical Outcome of Acute Liver Failure Caused by Dengue in a Hospital for Tropical Diseases, Thailand. Am J Trop Med Hyg. 2016;95:1338-1344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Teerasarntipan T, Chaiteerakij R, Komolmit P, Tangkijvanich P, Treeprasertsuk S. Acute liver failure and death predictors in patients with dengue-induced severe hepatitis. World J Gastroenterol. 2020;26:4983-4995. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 37] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (38)] |
| 8. | Nguyen Tat T, Vo Hoang-Thien N, Nguyen Tat D, Nguyen PH, Ho LT, Doan DH, Phan DT, Duong YN, Nguyen TH, Nguyen TK, Dinh HT, Dinh TT, Pham AT, Do Chau V, Trinh TH, Vo Thanh L. Prognostic values of serum lactate-to-bicarbonate ratio and lactate for predicting 28-day in-hospital mortality in children with dengue shock syndrome. Medicine (Baltimore). 2024;103:e38000. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 9. | World Health Organization. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control: New Edition. Geneva: World Health Organization, 2009. Available from: https://iris.who.int/handle/10665/44188. |
| 10. | McIntosh AM, Tong S, Deakyne SJ, Davidson JA, Scott HF. Validation of the Vasoactive-Inotropic Score in Pediatric Sepsis. Pediatr Crit Care Med. 2017;18:750-757. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 201] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 11. | Hoffmann F, Schmalhofer M, Lehner M, Zimatschek S, Grote V, Reiter K. Comparison of the AVPU Scale and the Pediatric GCS in Prehospital Setting. Prehosp Emerg Care. 2016;20:493-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 12. | Harrell FE. Package ‘Hmisc’. The R Foundation; Vienna, Australia. 2019; 235–236. |
| 13. | Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N, Pencina MJ, Kattan MW. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology. 2010;21:128-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3195] [Cited by in RCA: 3559] [Article Influence: 222.4] [Reference Citation Analysis (1)] |
| 14. | Akdogan M, Camci C, Gurakar A, Gilcher R, Alamian S, Wright H, Nour B, Sebastian A. The effect of total plasma exchange on fulminant hepatic failure. J Clin Apher. 2006;21:96-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Rose CF, Amodio P, Bajaj JS, Dhiman RK, Montagnese S, Taylor-Robinson SD, Vilstrup H, Jalan R. Hepatic encephalopathy: Novel insights into classification, pathophysiology and therapy. J Hepatol. 2020;73:1526-1547. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 323] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 16. | Riggio O, Celsa C, Calvaruso V, Merli M, Caraceni P, Montagnese S, Mora V, Milana M, Saracco GM, Raimondo G, Benedetti A, Burra P, Sacco R, Persico M, Schepis F, Villa E, Colecchia A, Fagiuoli S, Pirisi M, Barone M, Azzaroli F, Soardo G, Russello M, Morisco F, Labanca S, Fracanzani AL, Pietrangelo A, Di Maria G, Nardelli S, Ridola L, Gasbarrini A, Cammà C. Hepatic encephalopathy increases the risk for mortality and hospital readmission in decompensated cirrhotic patients: a prospective multicenter study. Front Med (Lausanne). 2023;10:1184860. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 17. | Bernal W, Wendon J. Acute liver failure. N Engl J Med. 2013;369:2525-2534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 736] [Cited by in RCA: 875] [Article Influence: 67.3] [Reference Citation Analysis (3)] |
| 18. | Stravitz RT, Lee WM. Acute liver failure. Lancet. 2019;394:869-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 513] [Cited by in RCA: 631] [Article Influence: 90.1] [Reference Citation Analysis (0)] |
| 19. | Leowattana W, Leowattana T. Dengue hemorrhagic fever and the liver. World J Hepatol. 2021;13:1968-1976. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 9.0] [Reference Citation Analysis (43)] |
| 20. | O'Grady JG, Alexander GJ, Hayllar KM, Williams R. Early indicators of prognosis in fulminant hepatic failure. Gastroenterology. 1989;97:439-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1517] [Cited by in RCA: 1335] [Article Influence: 36.1] [Reference Citation Analysis (0)] |
| 21. | Squires RH, Shneider BL, Bucuvalas J, Alonso E, Sokol RJ, Narkewicz MR, Dhawan A, Rosenthal P, Rodriguez-Baez N, Murray KF, Horslen S, Martin MG, Lopez MJ, Soriano H, McGuire BM, Jonas MM, Yazigi N, Shepherd RW, Schwarz K, Lobritto S, Thomas DW, Lavine JE, Karpen S, Ng V, Kelly D, Simonds N, Hynan LS. Acute liver failure in children: the first 348 patients in the pediatric acute liver failure study group. J Pediatr. 2006;148:652-658. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 702] [Cited by in RCA: 579] [Article Influence: 29.0] [Reference Citation Analysis (1)] |
| 22. | Trung DT, Thao le TT, Hien TT, Hung NT, Vinh NN, Hien PT, Chinh NT, Simmons C, Wills B. Liver involvement associated with dengue infection in adults in Vietnam. Am J Trop Med Hyg. 2010;83:774-780. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 127] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 23. | Karoli R, Fatima J, Siddiqi Z, Kazmi KI, Sultania AR. Clinical profile of dengue infection at a teaching hospital in North India. J Infect Dev Ctries. 2012;6:551-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Jagadishkumar K, Jain P, Manjunath VG, Umesh L. Hepatic involvement in dengue Fever in children. Iran J Pediatr. 2012;22:231-236. [PubMed] |
| 25. | Swamy AM, Mahesh PY, Rajashekar ST. Liver function in dengue and its correlation with disease severity: a retrospective cross-sectional observational study in a tertiary care center in Coastal India. Pan Afr Med J. 2021;40:261. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 20] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | Samanta J, Sharma V. Dengue and its effects on liver. World J Clin Cases. 2015;3:125-131. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 130] [Cited by in RCA: 169] [Article Influence: 15.4] [Reference Citation Analysis (7)] |
| 27. | Gupta E, Chakravarti A. Viral infections of the biliary tract. Saudi J Gastroenterol. 2008;14:158-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/