Published online Sep 9, 2024. doi: 10.5492/wjccm.v13.i3.92658
Revised: May 4, 2024
Accepted: May 29, 2024
Published online: September 9, 2024
Processing time: 207 Days and 0.1 Hours
Delayed sternal closure (DSC) can be a lifesaving approach for certain patients who have undergone cardiac surgery. The value of the type of prophylactic anti
To investigate clinical outcomes of different prophylactic antibiotic regimens in patients who had DSC after cardiac surgery.
This was a retrospective observational single-center study. Fifty-three consecutive patients who underwent cardiac surgery and had an indication for DSC were included. Patients were subjected to two regimens of antibiotics: Narrow-spectrum and broad-spectrum regimens.
The main outcome measures were length of hospital and intensive care unit (ICU) stay, duration of mechanical ventilation, and mortality. Of the 53 patients, 12 (22.6%) received narrow-spectrum antibiotics, and 41 (77.4%) received broad-spectrum antibiotics. The mean age was 59.0 ± 12.1 years, without significant differences between the groups. The mean duration of antibiotic use was significantly longer in the broad-spectrum than the narrow-spectrum group (11.9 ± 8.7 vs 3.4 ± 2.0 d , P < 0.001). The median duration of open chest was 3.0 (2.0-5.0) d for all patients, with no difference between groups (P = 0.146). The median duration of mechanical ventilation was significantly longer in the broad-spectrum group [60.0 (Δ interquartile range (IQR) 170.0) h vs 50.0 (ΔIQR 113.0) h,
Prophylactic broad-spectrum antibiotics did not improve clinical outcomes in patients with DSC post-cardiac surgery but was associated with longer ventilation duration, length of ICU and hospital stays vs narrow-spectrum antibiotics.
Core Tip: In cardiac surgical patients with delayed sternal closure the use of prophylactic broad-spectrum antibiotics did not affect the clinical outcomes in terms of length of mechanical ventilation and length of intensive care stay.
- Citation: Eissa MIA, Kaddoura R, Hassan D, Carr CS, Hanoura S, Shouman Y, Almulla A, Omar AS. Early clinical outcomes of two regimens of prophylactic antibiotics in cardiac surgical patients with delayed sternal closure. World J Crit Care Med 2024; 13(3): 92658
- URL: https://www.wjgnet.com/2220-3141/full/v13/i3/92658.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v13.i3.92658
Delayed sternal closure (DSC) after cardiac surgery is a therapeutic approach that can be lifesaving in certain situations such as severe hemorrhage, myocardial edema, arrhythmias, and low cardiac output[1-4]. The procedure can relieve excessive heart compression and allows easy access to control bleeding until the patient’s hemodynamic condition has been stabilized[2]. DSC is more popular in pediatric cardiac surgery[5]. The guidelines for antibiotic prophylaxis in cardiac surgery are well-defined[6,7]. However, there is a lack of evidence regarding the use of prophylactic antibiotics in DSC cases, and the existing literature does not address this specific matter in adult patients. Concerns have been raised about the potential susceptibility to bacterial infections when the chest is left open[8]. As a result, some surgeons have been reluctant to adopt this approach.
The existing evidence supports increased sternal infection rates in patients with DSC[9]. In a retrospective analysis performed by Das et al[10] in a pediatric population, it was found that DSC could pose a risk for blood stream infection. The antibiotic prophylaxis approaches, i.e. use of narrow-spectrum or broad-spectrum antibiotics, in DSC is highly dependent on the attending physician’s discretion. Thus, it is important to examine the outcomes of such approaches to justify the use of broad-spectrum antibiotics for prophylaxis in DSC patients.
We hypothesized that the use of broad-spectrum antibiotics may affect the outcomes differently when compared with the use of narrow-spectrum antibiotics in patients with DSC. The objective of this study was to investigate the outcomes of different prophylactic antibiotic regimens in patients who had DSC after cardiac surgery.
A single-center retrospective observational study was conducted in the cardiothoracic intensive care unit (CTICU) at a tertiary cardiac center between January 1, 2013 and December 31, 2020. The study was approved by the Corporation’s Medical Research Center with a waiver of informed consent given the retrospective design of the study, which was conducted in full agreement with the Declaration of Helsinki’s principles.
The study included all patients who were transferred to the CTICU with an open chest after cardiac surgeries during the study period. Patients were excluded if they were febrile, had evidence of sepsis, underwent thoracic surgery, received corticosteroid therapy, or died within 48 h after the decannulation of extracorporeal membrane oxygenation (where applicable). The cardiac surgical procedures in our study included coronary artery bypass grafting (CABG), valve replacement, combined CABG/valve surgery, and aortic dissection. All patients had received standard general anesthesia with standard cardiac monitoring as per our center’s protocol. Cardiopulmonary bypass (CPB) was conducted with roller pump and membrane oxygenator, and cardiac protection was maintained during CPB as per our center protocols.
The data of all patients were collected from the electronic medical records [Dendrite Clinical Systems (London, United Kingdom) and Cerner (United States)] and included demographics, past medical history, laboratory blood tests, peri-procedure-specific data, and outcomes. Peri-procedure data included, but was not limited to, the durations of CPB and aortic cross clamp, inotropes and vasopressor use, blood loss details, and transfusion requirements. The main outcome measures included the length of hospital and intensive care unit (ICU) stay, duration of mechanical ventilation, and mortality. Other outcome measures included postoperative atrial fibrillation (POAF), perioperative myocardial infarction, the need for mechanical circulatory support, and infections (wound and nosocomial). Sample size calculation was not performed as all the patients who were transferred to the CTICU with an open chest during the study period were included.
The indications of DSC in our center include severe hemodynamic instability, persistent bleeding without an obvious source, and coagulopathy. The decision for DSC is made by the operating surgeon according to the requirements. The subsequent closure of the chest is also decided by the surgeon once the reason to initially leave the chest open is resolved. The choice of prophylactic antibiotics is selected by the treating CTICU clinical team.
The DSC protocol at our center is inserting bilateral chest drains after opening both pleurae, packing the heart and the great vessels, and applying a metal stent to keep the sternum open. The drains are connected to a low-pressure high-flow suction device.
POAF was defined as the occurrence of new onset atrial fibrillation in the early postoperative period, i.e. within 7 d[11]. Acute kidney injury was defined according to the Kidney Disease Improving Global Outcomes criteria, which includes an increase in serum creatinine by ≥ 26.5 µmol/L (≥ 0.3 mg/dL) within 48 h or serum creatinine > 1.5 times the baseline value within the last 7 d after the insult, or reduced urine output < 0.5 mL/kg/h for six hours[12]. Postoperative myo
Sample size calculation was not performed as all the patients who were transferred to the CTICU with an open chest during the study period were included. Patients were divided into two groups based on the antibiotic regimen: Narrow-spectrum and broad-spectrum. Normally distributed continuous variables were presented as mean ± standard deviation and skewed variables as median and interquartile range (IQR). Continuous variables were compared using student’s t-test or Mann-Whitney U test, as appropriate. Fisher’s exact test or χ2 were used to compare the categorical variables. The priori alpha level was set at < 0.05 (two-tailed). Data was managed using Excel and IBM SPSS Statistics (version 29.0.1.0 (171), IBM Corp. 2023) programs.
Fifty-three patients were included in the analysis. Of them, 12 (22.6%) received narrow-spectrum antibiotics and 41 (77.4%) received broad-spectrum antibiotics. The mean age and body mass index for all patients were 59.0 ± 12.1 years and 26.7 ± 4.9 kg/m2, respectively, without significant differences between the groups. Most patients were males (85.0%), of an Arab ethnicity (45.3%), and non-smokers (45.1%). The most common comorbidities were hypertension (54.9%) and diabetes (43.4%) with no significant difference between the groups. Table 1 presents the collected baseline variables. In-hospital laboratory blood tests were unremarkable and did not differ between the groups as demonstrated in Table 2.
| Variable | All patients, n = 53 | Narrow-spectrum antibiotics, n = 12 | Broad-spectrum antibiotics, n = 41 | P value |
| Demographics | ||||
| Age in yr | 59.0 ± 12.1 | 60.0 ± 13.4 | 58.7 ± 11.8 | 0.366 |
| BMI in kg/m2 | 26.7 ± 4.9 (N = 52) | 25.7 ± 3.7 (n = 12) | 26.7 ± 5.2 (n = 40) | 0.227 |
| Sex | 0.175 | |||
| Male | 45/53 (85.0) | 12 | 33 | |
| Female | 0 | 8 | ||
| Ethnicity | 0.011 | |||
| African | 3/53 (5.7) | 3 | 0 | |
| Arab | 24/53 (45.3) | 4 | 11 | |
| Asian from Indian subcontinent | 20/53 (37.7) | 3 | 17 | |
| Asia | 4/53 (7.5) | 0 | 4 | |
| Western | 2/53 (3.8) | 1 | 1 | |
| Smoking status | 0.402 | |||
| Non-smoker | 23/51 (45.1) | 3 | 20 | |
| Smoker | 7/51 (13.7) | 1 | 6 | |
| Ex-smoker | 21/51 (41.2) | 6 | 15 | |
| Comorbidities | ||||
| Hypertension | 28/51 (54.9) | 4 | 24 | 0.086 |
| Diabetes | 0.305 | |||
| Type II | 16/53 (30.2) | 4 | 12 | |
| Type I | 7/53 (13.2) | 0 | 7 | |
| COPD | 2/52 (3.8) | 0 | 2 | 1.00 |
| CKD | 8/53 (15.1) | 1 | 7 | 0.665 |
| On dialysis | 2/53 (3.8) | 0 | 2 | 1.00 |
| PVD | 2/53 (3.8) | 0 | 2 | 1.00 |
| Statin use | 24/52 (46.2) | 5 | 19 | 0.722 |
| ECHO | ||||
| LVEF as % | 43.4 ± 16.9 | 45.8 ± 12.9 | 42.7 ± 18.0 | 0.578 |
| LVEF groups | 0.309 | |||
| Good, > 50% | 23/53 (43.4) | 4 | 19 | |
| Moderate, 31%-50% | 16/53 (30.2) | 6 | 10 | |
| Poor, 21%-30% | 10/53 (18.9) | 2 | 8 | |
| Very poor, ≤ 20% | 4/53 (7.5) | 0 | 4 | |
| Variable | All patients, n = 53 | Narrow-spectrum antibiotics, n = 12 | Broad-spectrum antibiotics, n = 41 | P value |
| Creatinine in micromole/L | 95.0 (72.0-110) (N = 51) | 79.0 (ΔIQR 24.0) (n = 11) | 95.0 (ΔIQR 46.0) (n = 40) | 0.688 |
| Highest creatinine in micromole/L | 110 (85.0-175) (N = 51) | 95.0 (ΔIQR 29.0) (n = 11) | 107.5 (ΔIQR 106) (n = 40) | 0.457 |
| Bilirubin in micromole/L | 9.0 (6.9-17.1) (N = 47) | 8.6 (ΔIQR 26.5) (n = 10) | 9.5 (ΔIQR 6.7) (n = 37) | 0.755 |
| SGPT in U/L | 26.0 (18.0-40.0) (N = 47) | 18.0 (ΔIQR 13.5) (n = 9) | 25.5 (ΔIQR 22.3) (n = 38) | 0.185 |
| HbA1c as % | 6.3 ± 1.6 | 5.9 ± 1.7 | 6.3 ± 1.5 | 0.243 |
| Hb in mg/dL | 12.9 ± 2.0 (N = 52) | 13.2 ± 1.9 (n = 12) | 12.7 ± 2.0 (n = 40) | 0.239 |
| WBC as 103/U | 10.8 ± 5.2 (N = 49) | 6.8 (ΔIQR 4.0) (n = 10) | 8.8 (ΔIQR 3.0) (n = 39) | 0.076 |
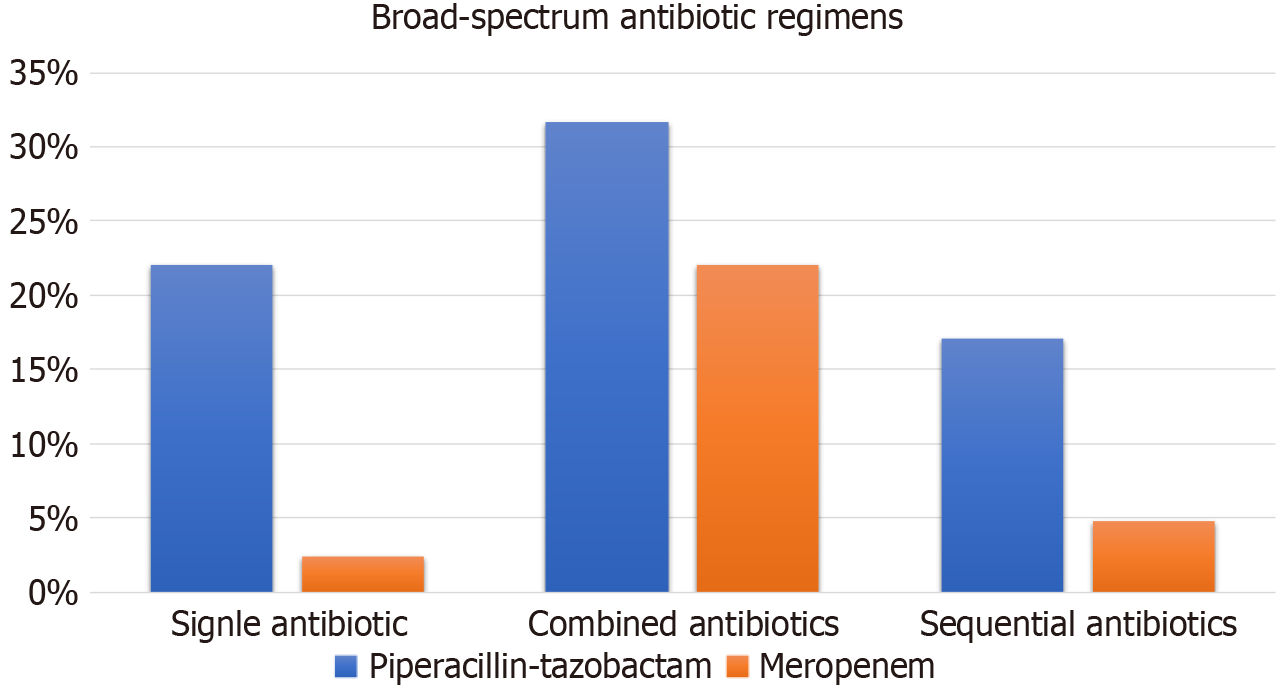
The narrow-spectrum antibiotics used were cefazolin and vancomycin. Ten (83.3%) patients were administered cefazolin alone, whereas the remaining two patients were given a combined cefazolin and vancomycin regimen. However, 23 (56.1%) patients in the broad-spectrum antibiotic group were initially administered a narrow-spectrum antibiotic regimen, and then therapy was escalated. The most used antibiotics in the broad-spectrum group were piperacillin-tazobactam followed by meropenem. Piperacillin-tazobactam was used as a single agent in 22% of patients or combined with another agent with Gram-positive coverage spectrum (e.g., vancomycin, teicoplanin) in 31.7% of patients. The respective percentages for meropenem use were 2.4% (single) and 22.0% (combined). Sequential antibiotic use comprised various regimens; piperacillin-tazobactam was the initial agent in 17.1% of patients and meropenem in 4.8% of patients (Figure 1). The estimated duration of antibiotic regimen in all patients was a median of 7.5 (IQR 3.0-14.0) d. The mean duration of antibiotic use was significantly longer in the broad-spectrum group than the narrow-spectrum group (11.9 ± 8.7 d vs 3.4 ± 2.0 d, P < 0.001).
CABG procedure was performed in 58.5% of patients, followed by valve replacement (20.8%) and combined surgeries (11.3%). The mean CPB duration for all patients was 165 ± 75.3 min. Patients in the narrow-spectrum antibiotics group had a significantly shorter mean CPB duration than those in the other group (127.3 ± 67.9 min vs 174.4 ± 74.9 min, P = 0.037). The aortic cross clamp time was 85.0 ± 45.7 min for all patients without a difference between the groups. The median durations of open chest were 3.0 (2.0-5.0) d for all patients, 2.0 (ΔIQR 2.0) d for the narrow-spectrum group and 3.0 (ΔIQR 2.0) d for the broad-spectrum group (P = 0.146). Most patients were transfused intraoperatively (78.8%) and postoperatively (96.1%). Total or at-any-time interval blood loss did not differ significantly between the groups. Pharmacological (97.9%) and mechanical (50.9%) circulatory support did not differ between the groups (Table 3).
| Variable | All patients | Narrow-spectrum antibiotics, n = 12 | Broad-spectrum antibiotics, n = 41 | P value |
| Procedure data | 0.398 | |||
| CABG | 31/53 (58.5) | 7 | 24 | |
| Valve replacement | 11/53 (20.8) | 4 | 7 | |
| Combined CABG/valve surgery | 6/53 (11.3) | 0 | 6 | |
| Aortic dissection | 5/53 (9.4) | 1 | 4 | |
| Epicardial wires | 16/52 (30.8) | 4 | 12 | 1.00 |
| CPB in min | 165 ± 75.3 (N = 50) | 127.3 ± 67.9 (n = 10) | 174.4 ± 74.9 (n = 40) | 0.037 |
| ACC in min | 85.0 ± 45.7 (N = 41) | 83.2 ± 50.5 (n = 9) | 85.4 ± 45.1 (n = 32) | 0.451 |
| Open chest duration in d | 3.0 (2.0-5.0) | 2.0 (ΔIQR 2.0) | 3.0 (ΔIQR 2.0) | 0.146 |
| Intraoperative transfusion | 41/52 (78.8) | 10 | 31 | 1.00 |
| Postoperative transfusion | 49/51 (96.1) | 12 | 37 | 1.00 |
| Blood loss in mL | ||||
| At 6 h | 700 (375-1350) (N = 45) | 900 (ΔIQR 1050) (n = 11) | 1050 (ΔIQR 1250) (n = 34) | 0.864 |
| At 12 h | 1225 (500-2050) (N = 44) | 1750 (ΔIQR 1000) (n = 11) | 1575 (ΔIQR 2088) (n = 33) | 0.881 |
| At 24 h | 1700 (775-2800) (N = 45) | 1900 (ΔIQR 2500) (n = 11) | 2175 (ΔIQR 3213) (n = 34) | 0.8443 |
| Total loss | 2400 (1362-4700) (N = 44) | 2050 (ΔIQR 4375) (n = 11) | 3325 (ΔIQR 4300) (n = 33) | 0.492 |
| Support | ||||
| Inotropes/vasopressors use | ||||
| Agent(s) use | 46/47 (97.9) | 11 | 35 | 1.00 |
| Number of agent(s) used | 0.385 | |||
| 0 | 2/49 (4.1) | 0 | 2 | |
| 1 | 3/49 (6.1) | 2 | 1 | |
| 2 | 16/49 (32.7) | 4 | 12 | |
| 3 | 20/49 (40.8) | 5 | 15 | |
| 4 | 8/49 (16.3) | 1 | 7 | |
| Number of agents used | 2.6 ± 0.9 (N = 49) | 2.4 ± 0.9 (n = 12) | 2.6 ± 1.0 (n = 37) | 0.24 |
| IABP use | 27/53 (50.9) | 6 | 21 | 0.941 |
| Defibrillation | 13/53 (24.5) | 3 | 10 | 1.00 |
Table 4 demonstrates clinical outcomes. The proportion of patients who experienced new nosocomial infection was 40.0%. Sepsis was reported in 50 patients, while data was missing for 3 patients. The mean time to sepsis was 4.8 ± 2.6 d for all patients, with similar times in both groups. Almost half of the patients (46.2%) experienced POAF and 13.5% of them experienced heart block. Only 2 patients had myocardial infarction post-surgery, and 8 patients experienced a stroke.
| Variable | All patients | Narrow-spectrum antibiotics, n = 12 | Broad-spectrum antibiotics, n = 41 | P value |
| New nosocomial infection | 20/50 (40.0) | 5 | 15 | 0.736 |
| Time to sepsis/septic shock postoperatively in d | 4.8 ± 2.6 (N = 50) | 3.0 (ΔIQR 4.0) (n = 11) | 3.5 (ΔIQR 2.0) (n = 39) | 0.586 |
| POAF | 24/52 (46.2) | 5 | 19 | 0.722 |
| New ECG changes | 39/51 (76.5) | 9 | 30 | 1.00 |
| Heart block | 7/52 (13.5) | 2 | 5 | 0.656 |
| LV dysfunction | 7/52 (13.5) | 0 | 7 | 0.181 |
| Postoperative MI | 2/52 (3.8) | 0 | 2 | 1.00 |
| New postoperative stroke | 0.576 | |||
| Transient | 3/50 (6.0) | 0 | 3 | |
| Permanent | 5/50 (10.0) | 1 | 4 | |
| Duration of ventilation in h | 70.0 (39.0-190) | 50.0 (ΔIQR 113) | 60.0 (ΔIQR 170) | 0.047 |
| LOS-ICU in d | 8.0 (3.25-15.5) (N = 52) | 5.0 (ΔIQR 5.0) (n = 12) | 7.5 (ΔIQR 10.0) (n = 40) | 0.008 |
| LOS-hospital in d | 27.0 (10.5-47.0) (N = 44) | 19.0 (ΔIQR 21.0) (n = 11) | 27.0 (ΔIQR 30.0) (n = 33) | 0.031 |
| Mortality | 18/52 (34.6) | 4 | 14 | 1.00 |
| Readmission to ICU | 5/51 (9.8) | 1 | 4 | 1.00 |
Mechanical ventilation: The median duration of mechanical ventilation was 70.0 (IQR 39.0-190) h, with a significantly longer duration in the broad-spectrum antibiotic group [60.0 (ΔIQR 170.0) h vs 50.0 (ΔIQR 113.0) h, P = 0.047].
Length of stay and mortality: The median length of stay for both ICU and hospital were 8.0 (3.25-15.5) d and 27.0 (10.5-47.0) d, with significantly longer durations in the broad-spectrum antibiotic group [7.5 (ΔIQR 10.0) d vs 5.0 (ΔIQR 5.0) d,
The aim of this study was to determine the outcomes related to the use of different regimens of prophylactic antibiotics in adult patients with DSC after cardiac surgery. The salient findings of this research were: (1) A high rate of infection-related complications (40.0%); (2) patients in the broad-spectrum antibiotic group required more prolonged antibiotic course; (3) the incidence of POAF was exceptionally high (46.2%); (4) the length of stay for both ICU and hospital were significantly longer in the broad-spectrum antibiotic group; (5) the rates of ICU readmission and mortality were only numerically higher with broad-spectrum antibiotic use; and (6) the use of broad-spectrum antibiotics did not provide an outcome-related benefit.
The DSC management strategy after cardiac surgery has been widely adopted in the pediatric population for possible avoidance of compression of the swollen myocardium and lungs with increased fluid collection. Improvement in hemodynamics is mainly notable in the diastolic functions[15,16] or variable in adult cardiac surgery[8,17,18].
In our study we reported a high rate of new nosocomial infections (40.0%). Sepsis was reported in 50 patients. The mean time to sepsis was 4.8 d for all patients, with similar times between the groups. Similarly, Das et al[10] reported a high rate of blood stream infection in patients with DSC (30% vs 9% in the control group).
While prophylactic antibiotic guidance follows a clear guideline in routine cardiac surgery, i.e. cefazolin or vancomycin[6], the antibiotic regimen in patients with DSC remains unsettled. In our study, the choice of antibiotics for patients with DSC was dependent on the discretion of the managing team. Prophylactic narrow-spectrum and broad-spectrum antibiotics were used in 22.6% and 77.4% of patients, respectively. The published literature on the use of prophylactic antibiotics was diverse; in pediatric populations a simple antibiotic regimen[19-21] or broad-spectrum antibiotics were used[8]. In adult cardiac surgery, the use was inconsistent as well[8-10,22,23].
The estimated duration of the antibiotic regimen was 7.5 d in all patients, with a significantly longer use in broad-spectrum group (11.9 d vs 3.4 d). The optimal duration of prophylactic antibiotics in DSC was not previously addressed in the published literature[7,14], which showed variable durations of therapy ranging from a median of 7.73 d total and 3.1 d until sternal closure[23].
The median length of stay for both ICU and hospital were 8.0 d and 27.0 d, respectively, with significantly longer durations in the broad-spectrum group. The ICU length of stay reflects patient morbidity and resource utilization and remains a popular outcome measure despite its limitations[24]. The median duration of mechanical ventilation was 70.0 h, and it was significantly longer in the broad-spectrum group. Previously published studies in the adult population were heterogenous, of limited number of patients, and did not report ICU length of stay or duration of ventilation use in DSC patients. The incidence of POAF was numerically higher in patients treated with broad-spectrum antibiotics (19 patients vs 5 patients, P = 0.722), which may be attributed to the prolonged ICU and hospital course as reported in previous studies[25].
The usage of broad-spectrum antibiotics did not improve clinical outcomes in our study. The concerns of acquiring wound infection and possible mediastinitis may have influenced the choice of broad-spectrum antibiotics in many cases. Eckard et al[23] did not find a significant advantage of extended-spectrum or broad-spectrum coverage on wound infection or mediastinitis with trends towards increased complications in adults with DSC. On the other hand, Boeken et al[17] routinely started broad-spectrum antibiotic prophylaxis with piperacillin/tazobactam and continued it until closing the sternum with a low incidence of deep sternal and superficial wound infections (5.3% and 4.8%, respectively). Shalabi et al[8] used a combination of cephalosporin and aminoglycoside for prophylaxis and reported high rates of superficial wound infection (22%). The authors did not report delayed sternal infection or mediastinitis. The broad-spectrum antibiotic regimen that was advocated by some investigators in the pediatric cardiac surgery population[26] did not provide benefit as well.
The study was conducted in a single center with a limited number of patients. Despite the 7-year recruitment period in our center with over 500 cardiac surgeries per year, we reported outcomes of only 53 patients with DSC. This may indicate that DSC is rarely required in our patient population. Given the small sample size and the retrospective nature of the design, it may be difficult to draw a solid conclusion regarding the study questions. Larger, randomized multicenter studies with a longer follow-up period are needed to further confirm our results.
Prophylactic broad-spectrum antibiotics use did not improve clinical outcomes in patients with DSC after cardiac surgery. It was associated with longer ventilation duration and length of ICU and hospital stay compared with narrow-spectrum antibiotics use. Further randomized controlled trials are required to confirm the results.
We thank all members of the Cardiothoracic Surgery Department.
| 1. | Murphy DA. Delayed closure of the median sternotomy incision. Ann Thorac Surg. 1985;40:76-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Furnary AP, Magovern JA, Simpson KA, Magovern GJ. Prolonged open sternotomy and delayed sternal closure after cardiac operations. Ann Thorac Surg. 1992;54:233-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 67] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Martinez MJ, Albus RA, Barry MJ, Bowen TE. Treatment of cardiac compression after cardiopulmonary bypass. Am J Surg. 1984;147:400-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Björk VO, Papaconstantinou C. Delayed sternal closure following cardiac operation. Scand J Thorac Cardiovasc Surg. 1982;16:275-277. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Tabbutt S, Duncan BW, McLaughlin D, Wessel DL, Jonas RA, Laussen PC. Delayed sternal closure after cardiac operations in a pediatric population. J Thorac Cardiovasc Surg. 1997;113:886-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 89] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Edwards FH, Engelman RM, Houck P, Shahian DM, Bridges CR; Society of Thoracic Surgeons. The Society of Thoracic Surgeons Practice Guideline Series: Antibiotic Prophylaxis in Cardiac Surgery, Part I: Duration. Ann Thorac Surg. 2006;81:397-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 204] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 7. | Engelman R, Shahian D, Shemin R, Guy TS, Bratzler D, Edwards F, Jacobs M, Fernando H, Bridges C; Workforce on Evidence-Based Medicine, Society of Thoracic Surgeons. The Society of Thoracic Surgeons practice guideline series: Antibiotic prophylaxis in cardiac surgery, part II: Antibiotic choice. Ann Thorac Surg. 2007;83:1569-1576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 265] [Cited by in RCA: 262] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 8. | Shalabi RI, Amin M, Ayed AK, Shuhiber H. Delayed sternal closure is a life saving decision. Ann Thorac Cardiovasc Surg. 2002;8:220-223. [PubMed] |
| 9. | Wong JK, Joshi DJ, Melvin AL, Aquina CT, Archibald WJ, Lidder AK, Probst CP, Massey HT, Hicks GL, Knight PA. Early and late outcomes with prolonged open chest management after cardiac surgery. J Thorac Cardiovasc Surg. 2017;154:915-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Das S, Rubio A, Simsic JM, Kirshbom PM, Kogon B, Kanter KR, Maher K. Bloodstream infections increased after delayed sternal closure: cause or coincidence. Ann Thorac Surg. 2011;91:793-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Yagdi T, Nalbantgil S, Ayik F, Apaydin A, Islamoglu F, Posacioglu H, Calkavur T, Atay Y, Buket S. Amiodarone reduces the incidence of atrial fibrillation after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2003;125:1420-1425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 51] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 12. | Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179-c184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1436] [Cited by in RCA: 3715] [Article Influence: 265.4] [Reference Citation Analysis (0)] |
| 13. | Omar AS, Sudarsanan S, Hanoura S, Osman H, Sivadasan PC, Shouman Y, Tuli AK, Singh R, Al Khulaifi A. Kinetics of Highly Sensitive Troponin T after Cardiac Surgery. Biomed Res Int. 2015;2015:574546. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Bratzler DW, Dellinger EP, Olsen KM, Perl TM, Auwaerter PG, Bolon MK, Fish DN, Napolitano LM, Sawyer RG, Slain D, Steinberg JP, Weinstein RA; American Society of Health-System Pharmacists; Infectious Disease Society of America; Surgical Infection Society; Society for Healthcare Epidemiology of America. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm. 2013;70:195-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1238] [Cited by in RCA: 1364] [Article Influence: 104.9] [Reference Citation Analysis (0)] |
| 15. | McElhinney DB, Reddy VM, Parry AJ, Johnson L, Fineman JR, Hanley FL. Management and outcomes of delayed sternal closure after cardiac surgery in neonates and infants. Crit Care Med. 2000;28:1180-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 56] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Jögi P, Werner O. Hemodynamic effects of sternum closure after open-heart surgery in infants and children. Scand J Thorac Cardiovasc Surg. 1985;19:217-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Boeken U, Assmann A, Mehdiani A, Akhyari P, Lichtenberg A. Open chest management after cardiac operations: outcome and timing of delayed sternal closure. Eur J Cardiothorac Surg. 2011;40:1146-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Anderson CA, Filsoufi F, Aklog L, Farivar RS, Byrne JG, Adams DH. Liberal use of delayed sternal closure for postcardiotomy hemodynamic instability. Ann Thorac Surg. 2002;73:1484-1488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 19. | Kundan S, Tailor K, Radhakrishnan HB, Mohanty SR, Bhavsar K, Kadam S, Joshi P, Joshi V, Karande T, Bobhate P, Kulkarni S, Rao SG. Elective delayed sternal closure portends better outcomes in congenital heart surgery: a retrospective observational study. Indian J Thorac Cardiovasc Surg. 2019;35:530-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Kennedy JT 3rd, DiLeonardo O, Hurtado CG, Nelson JS. A Systematic Review of Antibiotic Prophylaxis for Delayed Sternal Closure in Children. World J Pediatr Congenit Heart Surg. 2021;12:93-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Yang Y, Wang J, Cai L, Peng W, Mo X. Surgical site infection after delayed sternal closure in neonates with congenital heart disease: retrospective case-control study. Ital J Pediatr. 2021;47:182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Molly Schultheis SS. Delayed Sternal Closure in Cardiac Surgery. J Clin Exp Cardiolog. 2015;6. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 23. | Eckardt JL, Wanek MR, Udeh CI, Neuner EA, Fraser TG, Attia T, Roselli EE. Evaluation of Prophylactic Antibiotic Use for Delayed Sternal Closure After Cardiothoracic Operation. Ann Thorac Surg. 2018;105:1365-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Harhay MO, Ratcliffe SJ, Small DS, Suttner LH, Crowther MJ, Halpern SD. Measuring and Analyzing Length of Stay in Critical Care Trials. Med Care. 2019;57:e53-e59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 25. | Almassi GH, Schowalter T, Nicolosi AC, Aggarwal A, Moritz TE, Henderson WG, Tarazi R, Shroyer AL, Sethi GK, Grover FL, Hammermeister KE. Atrial fibrillation after cardiac surgery: a major morbid event? Ann Surg. 1997;226:501-11; discussion 511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 488] [Cited by in RCA: 535] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 26. | Murray MT, Corda R, Turcotte R, Bacha E, Saiman L, Krishnamurthy G. Implementing a standardized perioperative antibiotic prophylaxis protocol for neonates undergoing cardiac surgery. Ann Thorac Surg. 2014;98:927-933. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/