Published online Mar 9, 2024. doi: 10.5492/wjccm.v13.i1.87459
Peer-review started: August 12, 2023
First decision: September 28, 2023
Revised: October 3, 2023
Accepted: December 4, 2023
Article in press: December 4, 2023
Published online: March 9, 2024
Processing time: 201 Days and 2.3 Hours
The prevalence of multidrug-resistant (MDR) bacteria has increased globally, with extensive drug-resistant (XDR) bacteria posing a threat to patients.
This case report describes a young man admitted for suspected tropical fever infections who experienced rapid deterioration in health. Despite negative results for tropical fever infections, he had neutrophilic leucocytosis, acute kidney injury, and chest imaging findings suggestive of bilateral consolidations. On day two, he was diagnosed with infective endocarditis with possible rheumatic heart disease and MDR methicillin-resistant Staphylococcus aureus bacteraemia, and community-acquired pneumonia. Despite treatment with broad-spectrum antibiotics, he did not respond and succumbed to death on day five.
This case highlights that clinicians/public should be aware of MDR community-acquired pneumonia, bacteraemia, and endocarditis which ultimately culminate in high rates of morbidity and mortality. Early identification of pathogenic strain and prompt antibiotic treatment are a mainstay for the management and prevention of early fatalities. Simultaneously, route cause analysis of community-acquired MDR/XDR pathogens is a global need.
Core Tip: A case of community-acquired multidrug-resistant methicillin-resistant Staphylococcus aureus infection leading to death is reported. The detection of CTX-M, VIM, NDM, mecA/C, and MREJ genes in microbial gene testing suggests that the patient was infected with MDR bacteria.
- Citation: Jatteppanavar B, Choudhury A, Panda PK, Bairwa M. Community-acquired multidrug-resistant pneumonia, bacteraemia, and infective endocarditis: A case report. World J Crit Care Med 2024; 13(1): 87459
- URL: https://www.wjgnet.com/2220-3141/full/v13/i1/87459.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v13.i1.87459
Antimicrobial drug resistance remains a global healthcare problem and poses a significant challenge to physicians worldwide, as the prevalence of multidrug-resistant (MDR), extensive drug-resistant (XDR), and pan-drug-resistant bacteria has increased in many tertiary care centres globally[1-3]. XDR bacteria are the current threats to patients. XDR bacteria are typically isolated in nosocomial settings. However, community acquisition of these infections is less prevalent but increasing day by day. Community-acquired pneumonia is a common clinical illness caused by bacteria and other pathogens. When it is associated with XDR bacteria, it is a matter of concern as there is a high risk of complications such as bacteraemia and infective endocarditis[4]. Staphylococcus aureus (S. aureus) is one of the leading causes of bacteraemia, both in the community and in the hospital setting, which can result in complicated or metastatic infections such as pneumonia, infective endocarditis, or sepsis with multi-organ dysfunction[5]. When compared with methicillin-sensitive S. aureus, MRSA is one of the leading causes of S. aureus bacteraemia and is associated with significant mortality and morbidity and poor clinical outcomes[6,7]. There is a limited amount of literature specifically addressing the combination of MDR community-acquired pneumonia (CAP), bacteraemia, and infective endocarditis.
The incidences of bacteraemia in CAP patients are 4% to 18% and one prediction model predicts bacteraemia in these patients with the help of variables like using recent antibiotic treatment, liver disease, and three vital signs (systolic blood pressure < 90 mmHg, temperature < 35 °C or ≥ 40 °C, and pulse ≥ 125/min) and three laboratory abnormalities (blood urea nitrogen ≥ 30 mg/dL, sodium < 130 mmol/L, and white blood cell count < 5000/mm3 or > 20000/mm3)[8]. This bacteraemia associated with pneumonia can lead to septicemia and other systemic complications like infective endocarditis, mostly due to delayed antibiotic administration[9]. This triad of pneumonia, bacteraemia, and infective endocarditis is uncommon, and community-acquired MDR organism causing the triad is even rarer. We herein report such a case to raise public health concerns.
Fever and abdominal pain for 3 d, and vomiting, swelling in the lower limbs, and itching and rashes all over the body for 1 d.
A young man in his 20s, previously healthy and with no substance abuse, suddenly felt ill. For the past 3 d, he had been experiencing an intermittent, documented, high-grade fever with associated chills that did not resolve despite taking medication. He also had abdominal pain for 3 d, initially as acute onset persistent nonprogressive dull aching pain in the right hypochondriac region, which later became diffuse without any aggravating or relieving factors. He experienced 3-4 episodes of non-bilious, non-blood-stained vomiting containing food particles. Additionally, he had bilateral symmetrical painless swelling in the lower limbs, without any decreased urine output, burning micturition, frothy urine, haematuria, or pyuria. He initially sought medical attention at a local hospital and took some medication, but approximately 30 min later, he developed skin itching and rashes all over his body, which was suspected to be a drug reaction. Further evaluation revealed deranged renal function, and he was subsequently referred to our centre.
Non-contributory.
Non-significant.
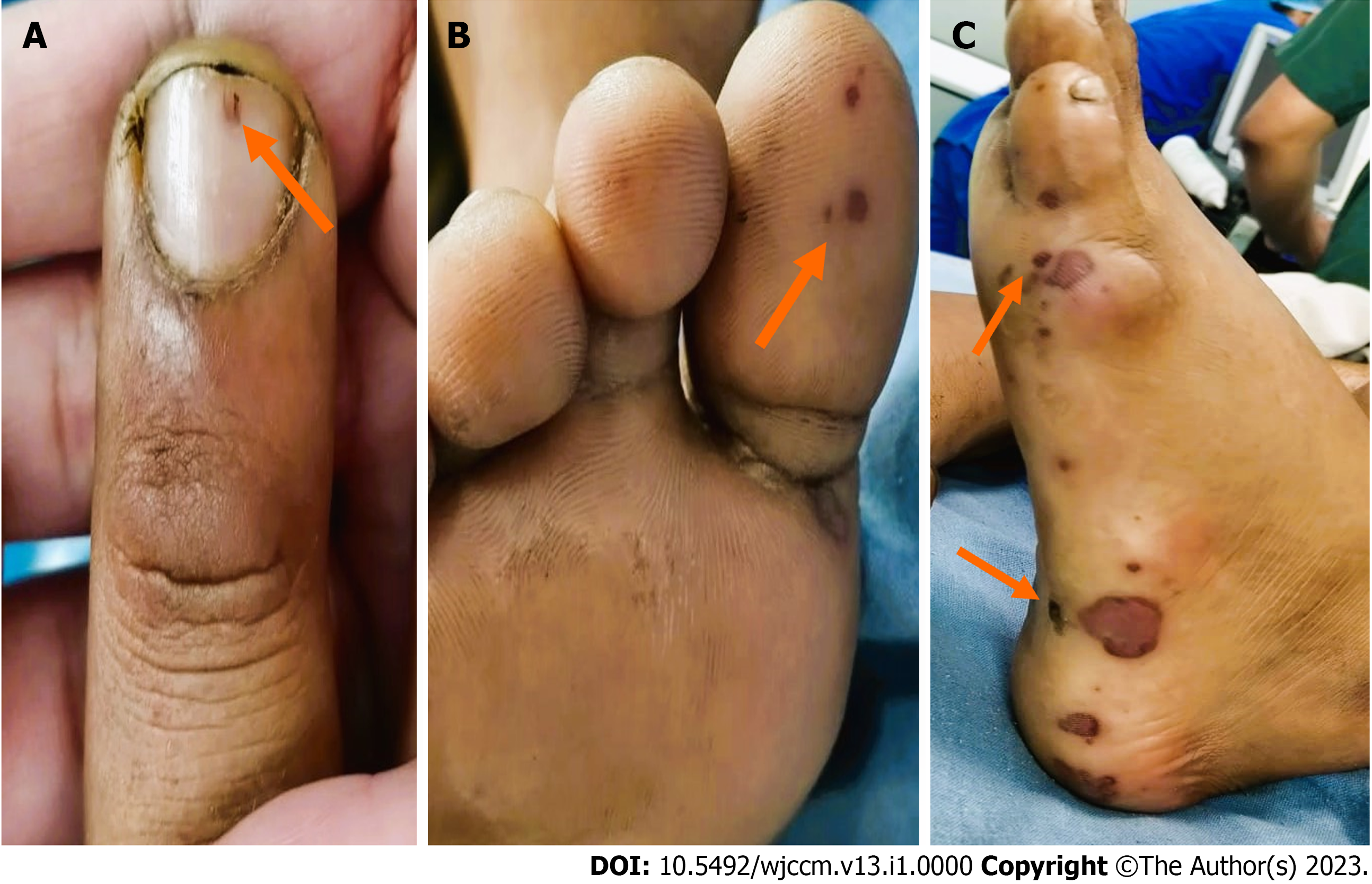
Upon presentation, the patient was fully conscious of tachycardia and tachypnoea and maintained saturation at room air. A general physical examination did not reveal any major findings, except for bilateral pitting edema. Abdominal examination showed diffuse tenderness and guarding without any rigidity, distension, or palpable organomegaly. The patient was intubated due to acute hypoxic respiratory failure and subsequently shifted to the intensive care unit. On day two of admission, he demonstrated high-grade fever, accompanied by subconjunctival dot haemorrhages, erythematous skin and non-blanching hemorrhagic petechiae, mucosal and skin erosions, splinter haemorrhages, Janeway lesions, and bilateral pitting pedal oedema with pan systolic murmur at the mitral area. It is possible that the murmur might have been missed due to subjective variations in the examiner's assessment on the first day of examination (Figure 1).
The patient's initial laboratory tests showed an increase in neutrophilic white blood cells with a decrease in platelet count, along with an elevated level of procalcitonin at 38 ng/mL (normal range, < 0.05 ng/mL; a marker for bacterial infection) and acute kidney injury (Table 1). A peripheral blood smear revealed normocytic normochromic cells with toxic granules, indicating toxic changes in white blood cells. Further investigations revealed disseminated intravascular coagulation, as evidenced by elevated levels of prothrombin time/international normalized ratio, activated partial thromboplastin clotting time, and D-dimer. As per institution policy and surviving sepsis guidelines 2021, the patient had clinical and biochemical evidence of definitive sepsis. Hence, two sets of blood cultures were sent before administration of antibiotics. Workups for tropical fever infections such as corona virus disease 2019, H3N2 and H1N1 influenza virus infection, dengue, malaria, scrub typhus, leptospira, and typhoid were negative. Arterial blood gas analysis showed normal anion gap metabolic acidosis with lactic acidosis and acute hypoxic respiratory failure.
| Investigation | Normal range and unit | March 17, 2023 | March 18, 2023 | March 20, 2023 | March 21, 2023 | March 22, 2023 |
| Hemoglobin | 13-17 g/dL | 14 | 14.2 | 11 | 10.2 | 10.2 |
| Total leucocyte count | 4-11 × 103/μL | 12700 | 18740 | 36677 | 27232 | 27800 |
| Neutrophil percentage | 40%-70% | 91 | 92.6 | 87.3 | 84.2 | 81 |
| Lymphocyte percentage | 20%-40% | 5 | 3.2 | 7.4 | 13.4 | 14.2 |
| Monocyte percentage | 2%-8% | 2 | 2.2 | 4.7 | 2.2 | 4.6 |
| Eosinophil percentage | 1%-6% | 1 | 1.2 | 0.3 | 0 | 0 |
| Basophil percentage | < 2% | 0.2 | 0.8 | 0.3 | 0.2 | 0.2 |
| Platelets | 150-400 × 103/μL | 69 × 103 | 45 × 103 | 20 × 103 | 57 × 103 | 57 × 103 |
| Total bilirubin | 0.3-1.2 mg/dL | 5.9 | 3.96 | |||
| Serum glutamic oxaloacetic transaminase | 0-50 U/L | 58 | 320 | |||
| Serum glutamate pyruvate transaminase | 0-50 U/L | 50 | 1319 | |||
| Alkaline phosphatase | 30-120 U/L | 195 | 191 | |||
| Gamma-glutamyl transferase | 0-55 U/L | 62 | 99 | |||
| Urea | 17-43 mg/dL | 64 | 223 | 216 | 289 | |
| Creatinine | 0.72-1.18 mg/dL | 1.67 | 2.04 | 1.51 | 1.61 | |
| Sodium | 136-146 mmo/L | 136 | 147 | 155 | ||
| Potassium | 3.5-5.1 mmo/L | 4.2 | 3.9 | 3.9 | ||
| Prothrombin time | 12.3 s | 14.7 | 13.7 | 20.5 | ||
| International normalized ratio | 1.14 | 1.37 | 1.27 | 1.94 | ||
| Activated partial thromboplastin clotting time | 22.1-28.1 s | 29 | 26 | |||
| Fibrinogen | 180-350 mg/dL | 398.6 | 265 | |||
| D-dimer | 0-0.5 mg/dL | > 5.5 | > 5.5 | > 5.5 | ||
| Procalcitonin | 0.5 ng/mL | 38 | ||||
| Antinuclear antibodies | Negative |
Blood cultures were sent for suspected infective endocarditis, and after 48 h of incubation, two sets of blood cultures revealed MDR methicillin-resistant Staphylococcus aureus (MRSA), which was sensitive to linezolid, vancomycin, clindamycin, and tigecycline, but resistant to penicillin, ciprofloxacin, levofloxacin, erythromycin, co-trimoxazole, and gentamicin. On the fourth day of admission, nested multiplex PCR (BioFireR) test of an endotracheal aspirate revealed the presence of Pseudomonas aeruginosa, Staphylococcus aureus, human rhinovirus, and enterovirus but sterile on culture. Microbial gene testing detected the presence of mec A/C (MRSA) cassette, which confers resistance to methicillin and other beta-lactam antibiotics, and MREJ genes (Mobile RmtE/J group genes, which encode rRNA methyltransferases that confer resistance to aminoglycoside antibiotics). The urine culture was sterile.
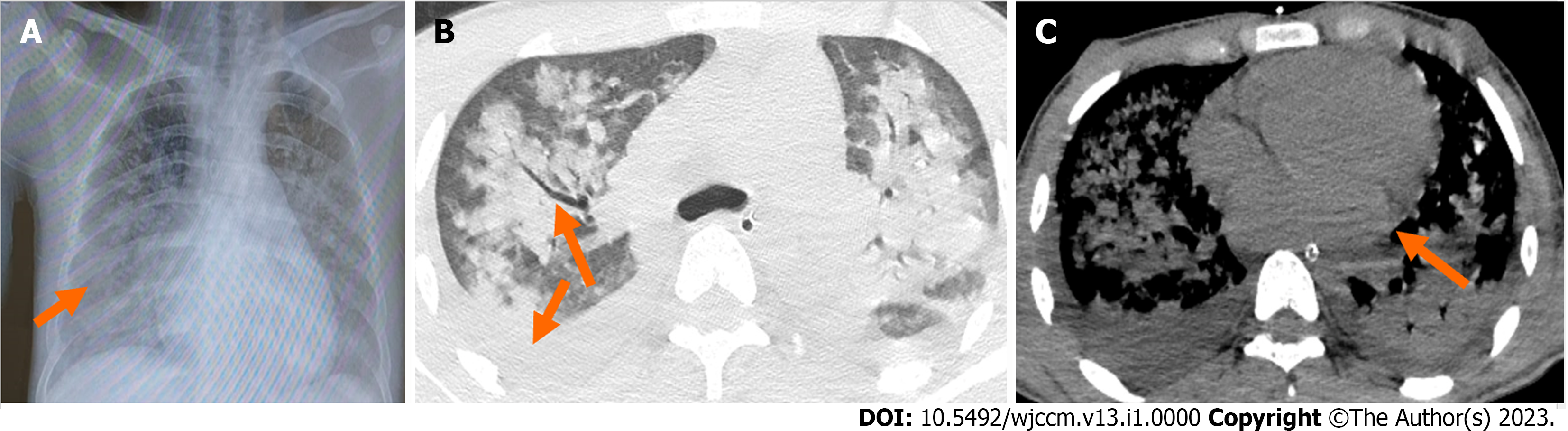
Ultrasonography of the abdomen showed hepatosplenomegaly, and an X-ray of the abdomen did not reveal any acute surgical emergencies. Chest X-ray showed bilateral areas of opacity in the middle and lower lobes of the lungs with air bronchograms. High-resolution computed tomography (HRCT) of the thorax revealed consolidation and air bronchograms in bilateral lung areas, along with interspersed ground glass opacities and bilateral pleural effusions (Figure 2). A two-dimensional echocardiography was done due to high suspicion of infective endocarditis, which revealed findings suggestive of rheumatic heart disease: Moderate mitral regurgitation, moderate mitral stenosis, mild aortic regurgitation, thickened anterior mitral leaflet with hockey stick sign with restricted leaflet motion, dilated left atrium, vegetations on the mitral valve, and a left ventricular ejection fraction of 50%.
Based on the presentation and baseline investigation, two differential diagnoses were considered. The first was pulmonary-renal syndrome, characterized by diffuse alveolar haemorrhage and glomerulonephritis, which can be caused by any underlying autoimmune disorder. This often presents with new onset bleeding from the respiratory tract, respiratory distress with hypoxia, and bilateral confluent opacities seen on HRCT of the thorax. However, severe thrombocytopenia and bilateral effusion, which are not typical findings of vasculitis, did not support this diagnosis. Furthermore, these opacities could be explained by community-acquired pneumonia as the patient's endotracheal aspirate bio-fire test was positive for Staphylococcus aureus and Pseudomonas aeruginosa. Antinuclear antibodies tested were negative by indirect immunofluorescence assay, so further serological workup for autoimmune conditions was not pursued.
The second differential diagnosis was severe fever with thrombocytopenia syndrome, an acute febrile illness characterized by fever, thrombocytopenia, leukopenia, and gastrointestinal symptoms. It is transmitted to humans by tick bites, primarily from Haemaphysalis longicornis, Ixodes nipponensis, Rhipicephalus microplus, and Amblyomma testudinarium. This syndrome is associated with a high fatality rate and can lead to multiple organ failure and death[8]. However, the patient tested negative for other endemic tick-borne diseases like scrub typhus.
The patient had community-acquired pneumonia associated with MRSA, bacteraemia, and infective endocarditis.
The diagnosis of infective endocarditis was made according to the modified Dukes' criteria, in addition to community-acquired pneumonia, sepsis with multi-organ dysfunction syndrome, shock, encephalopathy, severe acute respiratory distress syndrome, acute kidney injury, and disseminated intravascular coagulation. Intravenous (IV) vancomycin 15 mg/kg every 12 h, gentamicin 1 mg/kg every 8 h, and meropenem 1 g every 8 h were started as empirical antibiotics. Ventilator settings were optimized according to the acute respiratory distress syndrome (ARDS) protocol, and sedation and neuromuscular blockade were administered. Prone positioning was also done. Dual vasopressor support was implemented to maintain a mean arterial pressure above 65 mmHg. Approximately 14 units of fresh frozen plasma and 10 units of random donor platelets were used to treat continuous endotracheal bleeding and Ryle's tube bleeding. Antipyretics were given to control fever spikes, and therapeutic hypothermia measures were also followed. After the culture reports, the injection of meropenem was stopped, and ceftazidime-avibactam and aztreonam were started in their place. Gentamicin was stopped, vancomycin was continued, and colistin was administered through nebulization. The patient rapidly progressed to septic shock and multiorgan dysfunction. Despite being on 100% FiO2, hypoxia and saturation levels worsened, leading to severe ARDS.
Despite aggressive treatment, the patient's bacteraemia did not respond and had a fulminant course, and the patient eventually succumbed to death on the fifth day of admission due to severe ARDS.
Community-acquired MDR (CA-MDR) infections are infections that are acquired outside of healthcare settings and are caused by microorganisms that are resistant to multiple types of antibiotics. CA-MDR infections are a significant public health concern, particularly in developing countries where inadequate healthcare facilities, poor sanitation, and limited access to antibiotics contribute to the spread of these infections. CA-MDR infections can be transmitted through direct contact with contaminated surfaces or through person-to-person contact, and risk factors include overuse and misuse of antibiotics, poor sanitation and hygiene, lack of access to clean water, crowded living conditions, poor infection control practices in healthcare settings, immunosuppression, chronic illnesses, and malnutrition.
India is one of the countries where CA-MDR infections are a significant public health concern. Several studies and reports have highlighted the high prevalence of CA-MDR infections in India, as well as the challenges in addressing this issue. Of particular concern is the emergence of community-acquired MRSA infections in patients with no apparent risk factors at the community level, as seen in our case[10]. Community acquisition of MRSA infection is associated with significant morbidity and mortality, similar to nosocomial MRSA infection. Person-to-person transmission of community-associated MRSA has been reported[11]. Numerous studies, systematic analyses, and meta-analyses conducted in India have revealed a progressive rise in the incidence of MRSA and changes in resistance patterns. A systematic review and meta-analysis found that the prevalence of MRSA in India was relatively high at 27%, with a higher proportion observed among men aged > 18 years[12]. However, all MRSA isolates in India were found to be sensitive to vancomycin and teicoplanin. Resistance to cotrimoxazole, erythromycin, gentamicin, and other penicillins and cephalosporins appeared to be common features of MRSA isolates in India, consistent with other Indian studies and our patient[13]. Another study conducted in a tertiary care centre in southern India also revealed a high level of resistance among MRSA isolates, with linezolid, piperacillin/tazobactam, and tetracycline found to be effective agents against MRSA[14]. CA-MRSA (community-acquired methicillin-resistant S. aureus) isolates are now being increasingly reported from India. D’Souza et al[15] studied 412 confirmed cases of MRSA and found that 54% were true CA-MRSA possessing the SCCmec (staphylococcal chromosomal cassette mec) IV and SCCmec V genes. These were mainly isolated from skin and soft tissue infections. CA-MRSA isolates also showed variable resistance to ciprofloxacin, erythromycin, clindamycin, and tetracycline. Chatterjee et al[16] found that the overall prevalence of S. aureus nasal colonization was 52.3% and that of MRSA was 3.89% in the community.
Addressing the issue of CA-MDR infections in India requires collaboration between healthcare providers, policymakers, and the public to promote responsible antibiotic use, improve infection control practices, and ensure effective treatment of infectious diseases.
Global analysis of burden of bacterial anti-microbial resistance (AMR) in 2019 has shown that AMR caused an estimated 1.27 million deaths and was associated with an estimated 4.95 million deaths worldwide in 2019, with drug resistance in lower respiratory and bloodstream infections having the greatest impact. Among the 23 pathogens studied, drug resistance in six (E. coli, S. aureus, K. pneumoniae, S. pneumoniae, A. baumannii, and P. aeruginosa) alone led directly to 929000 deaths and was associated with 3.57 million deaths. Resistance to fluoroquinolones and beta-lactam antibiotics accounted for over 70% of deaths caused by AMR. The health impact of pathogens varied widely based on location, with high-income countries most affected by S. aureus and E. coli, while in Sub-Saharan Africa, K. pneumoniae and S. pneumoniae caused the most deaths. The study emphasized the need for improved global data collection to address the most pressing challenges posed by AMR[17].
Preventing CA-MDR infections requires a multifaceted approach that involves improving sanitation and hygiene practices, promoting responsible antibiotic use, improving infection control practices in community settings, and increasing access to healthcare for vulnerable populations. This includes educating the public about the importance of appropriate antibiotic use and supporting initiatives to reduce the overuse and misuse of antibiotics, as well as implementing effective infection control measures in community settings and providing access to affordable and quality healthcare for all individuals. Additionally, developing new antibiotics and alternative treatments, monitoring and tracking CA-MDR infections, and educating healthcare providers and the general public on CA-MDR and its risks are essential.
Overall, CA-MDR infections represent a significant public health concern, and addressing this issue requires collaboration between healthcare providers, policymakers, and the public to promote responsible antibiotic use, improve infection control practices, and ensure effective treatment of infectious diseases.
There is a growing threat of MDR bacteria in the community setting in patients with no apparent risk factors. The presence of a CA-MDR MRSA strain increases the risk of treatment failure and further spread of infection and associated complications. Better surveillance, infection control measures, and antibiotic stewardship programs are urgently needed in the community.
| 1. | Nkansa-Gyamfi NA, Kazibwe J, Traore DAK, Nji E. Prevalence of multidrug-, extensive drug-, and pandrug-resistant commensal Escherichia coli isolated from healthy humans in community settings in low- and middle-income countries: a systematic review and meta-analysis. Glob Health Action. 2019;12:1815272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 2. | Dheda K, Gumbo T, Maartens G, Dooley KE, McNerney R, Murray M, Furin J, Nardell EA, London L, Lessem E, Theron G, van Helden P, Niemann S, Merker M, Dowdy D, Van Rie A, Siu GK, Pasipanodya JG, Rodrigues C, Clark TG, Sirgel FA, Esmail A, Lin HH, Atre SR, Schaaf HS, Chang KC, Lange C, Nahid P, Udwadia ZF, Horsburgh CR Jr, Churchyard GJ, Menzies D, Hesseling AC, Nuermberger E, McIlleron H, Fennelly KP, Goemaere E, Jaramillo E, Low M, Jara CM, Padayatchi N, Warren RM. The epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant, extensively drug-resistant, and incurable tuberculosis. Lancet Respir Med. 2017;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 430] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 3. | Horcajada JP, Montero M, Oliver A, Sorlí L, Luque S, Gómez-Zorrilla S, Benito N, Grau S. Epidemiology and Treatment of Multidrug-Resistant and Extensively Drug-Resistant Pseudomonas aeruginosa Infections. Clin Microbiol Rev. 2019;32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 647] [Cited by in RCA: 655] [Article Influence: 93.6] [Reference Citation Analysis (0)] |
| 4. | Chang FY, MacDonald BB, Peacock JE Jr, Musher DM, Triplett P, Mylotte JM, O'Donnell A, Wagener MM, Yu VL. A prospective multicenter study of Staphylococcus aureus bacteremia: incidence of endocarditis, risk factors for mortality, and clinical impact of methicillin resistance. Medicine (Baltimore). 2003;82:322-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 257] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 5. | Selton-Suty C, Célard M, Le Moing V, Doco-Lecompte T, Chirouze C, Iung B, Strady C, Revest M, Vandenesch F, Bouvet A, Delahaye F, Alla F, Duval X, Hoen B; AEPEI Study Group. Preeminence of Staphylococcus aureus in infective endocarditis: a 1-year population-based survey. Clin Infect Dis. 2012;54:1230-1239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 430] [Article Influence: 30.7] [Reference Citation Analysis (0)] |
| 6. | Hassoun A, Linden PK, Friedman B. Incidence, prevalence, and management of MRSA bacteremia across patient populations-a review of recent developments in MRSA management and treatment. Crit Care. 2017;21:211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 351] [Cited by in RCA: 408] [Article Influence: 45.3] [Reference Citation Analysis (0)] |
| 7. | Abraham J, Mansour C, Veledar E, Khan B, Lerakis S. Staphylococcus aureus bacteremia and endocarditis: the Grady Memorial Hospital experience with methicillin-sensitive S aureus and methicillin-resistant S aureus bacteremia. Am Heart J. 2004;147:536-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 84] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 8. | Metersky ML, Ma A, Bratzler DW, Houck PM. Predicting bacteremia in patients with community-acquired pneumonia. Am J Respir Crit Care Med. 2004;169:342-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 146] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 9. | Hassan SA, Akhtar A, Falah NU, Khan M. An Unusual Case of Klebsiella pneumoniae Endocarditis. Cureus. 2020;12:e6999. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 10. | David MZ, Daum RS. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev. 2010;23:616-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1363] [Cited by in RCA: 1466] [Article Influence: 91.6] [Reference Citation Analysis (0)] |
| 11. | Verma S, Joshi S, Chitnis V, Hemwani N, Chitnis D. Growing problem of methicillin resistant staphylococci--Indian scenario. Indian J Med Sci. 2000;54:535-540. [PubMed] |
| 12. | Boucher HW, Corey GR. Epidemiology of methicillin-resistant Staphylococcus aureus. Clin Infect Dis. 2008;46 Suppl 5:S344-S349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 562] [Article Influence: 31.2] [Reference Citation Analysis (0)] |
| 13. | Ghia CJ, Waghela S, Rambhad G. A Systemic Literature Review and Meta-Analysis Reporting the Prevalence and Impact of Methicillin-Resistant Staphylococcus aureus Infection in India. Infect Dis (Auckl). 2020;13:1178633720970569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | C MB, V PB, Jyothi P. Drug resistance patterns of clinical isolates of staphylococcus aureus in a tertiary care center of south India. Int J Pharm Pharm Sci. 2015;70-72. [DOI] [Full Text] |
| 15. | D'Souza N, Rodrigues C, Mehta A. Molecular characterization of methicillin-resistant Staphylococcus aureus with emergence of epidemic clones of sequence type (ST) 22 and ST 772 in Mumbai, India. J Clin Microbiol. 2010;48:1806-1811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 137] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 16. | Chatterjee SS, Ray P, Aggarwal A, Das A, Sharma M. A community-based study on nasal carriage of Staphylococcus aureus. Indian J Med Res. 2009;130:742-748. [PubMed] |
| 17. | Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis. Lancet. 2022;399:629-655. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8908] [Cited by in RCA: 8943] [Article Influence: 2235.8] [Reference Citation Analysis (3)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Infectious diseases
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mohammadi M, Iran S-Editor: Liu JH L-Editor: Wang TQ P-Editor: Cai YX