Published online May 9, 2022. doi: 10.5492/wjccm.v11.i3.149
Peer-review started: November 22, 2021
First decision: January 12, 2022
Revised: January 20, 2022
Accepted: March 16, 2022
Article in press: March 16, 2022
Published online: May 9, 2022
Processing time: 165 Days and 15.4 Hours
Critically ill patients are at risk of developing stress cardiomyopathy (SC) but can be under-recognized.
To describe a case series of patients with SC admitted to critical care units.
We conducted a retrospective observational study at a tertiary care teaching hospital. All adult (≥ 18 years old) patients admitted to the critical care units with stress cardiomyopathy over 5 years were included.
Of 24279 admissions to the critical care units [19139 to medical-surgical intensive care units (MSICUs) and 5140 in coronary care units (CCUs)], 109 patients with SC were identified. Sixty (55%) were admitted to the coronary care units (CCUs) and forty-nine (45%) to the medical-surgical units (MSICUs). The overall incidence of SC was 0.44%, incidence in CCU and MSICU was 1.16% and 0.25% respectively. Sixty-two (57%) had confirmed SC and underwent cardiac catheterization whereas 47 (43%) had clinical SC, and did not undergo cardiac catheterization. Forty-three (72%) patients in the CCUs were diagnosed with primary SC, whereas all (100%) patients in MSICUs developed secondary SC. Acute respiratory failure that required invasive mechanical ventilation and shock developed in twenty-nine (59%) MSICU patients. There were no statistically significant differences in intensive care unit (ICU) mortality, in-hospital mortality, use of inotropic or mechanical circulatory support based on type of unit or anatomical variant.
Stress cardiomyopathy can be under-recognized in the critical care setting. Intensivists should have a high index of suspicion for SC in patients who develop sudden or worsening unexplained hemodynamic instability, arrhythmias or respiratory failure in ICU.
Core Tip: In our retrospective study, we found that stress cardiomyopathy (SC) is often under-recognized in the critical care setting. Primary SC is commonly seen in the coronary care units and the secondary form predominates in the medical-surgical intensive care unit setting. Presentation of secondary SC is often atypical and the majority of patients have simultaneous acute respiratory failure and sepsis. High index of clinical suspicion for SC is needed in patients who develop sudden or worsening unexplained hemodynamic instability, arrhythmias or respiratory failure. Cardiac catheterization may not be always feasible to confirm the diagnosis. Routine utilization of point of care ultrasound on all intensive care unit patients will help identify more cases. The outcomes of these patients are excellent as majority of them show reversibility of cardiac function on follow up imaging.
- Citation: Pancholi P, Emami N, Fazzari MJ, Kapoor S. Stress cardiomyopathy in critical care: A case series of 109 patients. World J Crit Care Med 2022; 11(3): 149-159
- URL: https://www.wjgnet.com/2220-3141/full/v11/i3/149.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v11.i3.149
Stress cardiomyopathy (SC) or Takotsubo cardiomyopathy or broken heart syndrome, was first described three decades ago in Japan[1]. It is characterized by acute and transient (< 21 d) left ventricular systolic and diastolic dysfunction, often precipitated by emotional or physical stress[1-6]. The diagnosis is usually made by modified Mayo Clinic criteria comprising of echocardiographic pattern of left ventricular apical hypokinesia, akinesia, or dyskinesia (apical ballooning) and basal hyperkinesis, electrocardiogram (EKG) changes (ST segment elevation and/or T wave inversion), troponin elevation and clean coronaries during cardiac catheterization[7].
Primary or classic SC has a reported incidence of around 1%-2% in patients with a suspicion of acute coronary syndrome (ACS) and is usually precipitated by physical or psychological stress[1]. Secondary SC, on the other hand, usually develops in hospitalized medical, surgical and neurological patients who may be under the major stress of critical illness in the medical-surgical intensive care unit (MSICU) setting[2,3,6,8-24].
The diagnosis of secondary stress cardiomyopathy in critically ill intensive care unit (ICU) patients can be challenging, requires a high degree of clinical suspicion, and is often under-recognized and under-reported for a myriad of reasons[8]. First, ICU patients do not always present with or report typical cardiac symptoms such as chest pain, shortness of breath, and syncope as patients presenting from the community do[8]. Second, there are no established diagnostic criteria for secondary stress cardiomyopathy in ICU patients and extrapolation of 2008 modified Mayo criteria may not be ideal[8]. Third, cardiac catheterization cannot be routinely performed in critically ill patients to confirm the diagnosis[8]. Fourth, patients can present with atypical morphologic variants of stress cardiomyopathy and there can be overlap with other diagnoses like sepsis induced cardiomyopathy[25]. Lastly, various multicenter international registries’ data did not include critically ill patients, thereby limiting understanding of the clinical presentation and outcomes of this disease in the ICU population[8].
Very few studies have reported the incidence, clinical features and outcomes of stress cardiomyopathy in the intensive care setting[3,9,10,12-19,25-27]. None of them compared characteristics and outcomes based on critical care unit [MSICU vs coronary care unit (CCU)]. The reported incidence of secondary stress cardiomyopathy in the ICU varies from 0.37% to as high as 28%[3,13,14,16,18,19]. Jo et al[15] described underlying malignancy, male sex, old age and high APACHE2 score as the predictors of in-hospital mortality in patients with stress cardiomyopathy.
The aim of our research was to describe the case series of patients with stress cardiomyopathy admitted to the critical care units (CCUs and MSICUs) and study their clinical presentation, complications, and outcomes.
We performed a retrospective case series study where all adult (≥ 18 years old) patients with the diagnosis of Stress cardiomyopathy or Takotsubo cardiomyopathy admitted to the critical care units of three hospitals in the Montefiore Healthcare System were included. Electronic health records for the 5-year period from January 1, 2015, to December 31, 2019, were retrospectively analyzed incorporating Looking Glass Clinical Analytics (Streamline Health, Atlanta, GA) to identify the target population. Critical care units included two coronary care units (CCUs) and five medical surgical units (medical, surgical or neurosurgical ICUs). The study was approved by the Institutional Review Board of the Albert Einstein College of Medicine (IRB# 2019-10754) and waiver of informed consent was granted due to minimal risk. Data about patient demographics, baseline characteristics, laboratory values, hospital course, complications and outcomes were collected for patients admitted to the critical care units.
The diagnosis of stress cardiomyopathy was made by the ICU teams collectively using a combination of 2-dimensional echocardiography, cardiac enzymes, EKG changes, and in some cases, coronary angiography.
Confirmed SC: Patients with SC who underwent cardiac catheterization to prove the absence of underlying coronary artery disease.
Clinical SC: Patients with SC who did not undergo cardiac catheterization and diagnosis was made clinically using 2D-echocardiography, cardiac enzymes and EKG changes only.
Primary SC: Patients with SC presenting from the community with cardiac symptoms like angina, dyspnea or palpitations. Clinical presentation mimics ACS, often precipitated by physical or mental stress.
Secondary SC: Patients developing SC during the course of hospitalization with critical medical, surgical or neurosurgical illness.
Typical variant of SC: Echocardiography regional wall motion abnormality pattern showing apical akinesis with basal hyperkinesis (apical ballooning).
Atypical variant of SC: Echocardiography regional wall motion abnormality pattern showing midventricular, basal, focal, or global hypokinesia.
Continuous variables were reported as median and interquartile range (IQR), whereas categorical variables were reported as counts and percentages. Associations between categorical variables and unit were tested via chi-square or Fisher’s exact test, as appropriate. Distributional differences between critical care units (CCU vs MS/ICU) with respect to continuous variables were assessed via Wilcoxon Mann-Whitney tests. Cumulative incidence functions for hospital discharge from the time of SC diagnosis stratified by critical care unit to allow for the competing risk of in-hospital death were estimated and differences tested using Grey’s test[28]. Cumulative incidence functions for in-hospital death from the time of SC diagnosis with a competing risk of hospital discharge alive were computed similarly. All analyses were performed using SAS software, version 9.4 (SAS Institute Inc., Cary, NC, United States) by the biomedical statistician. A two-sided P value of 0.05 or less was considered statistically significant.
Of 24279 admissions to the critical care units (19139 MSICU and 5140 in CCU) over the five-year study period, 109 patients with SC were identified. Sixty (55%) of them were admitted to the coronary care units and forty-nine (45%) to the medical-surgical units. The overall incidence of SC was 0.44%, incidence in CCU and MSICU was 1.16% and 0.25% respectively. Sixty-two (57%) had confirmed SC and underwent cardiac catheterization whereas 47 (43.1%) had clinical SC and did not undergo cardiac catheterization. Forty-three (72%) patients in the CCUs were diagnosed with primary SC, whereas all (100%) patients in MSICUs developed secondary SC.
Overall, the mean (SD) age was 67.2 (14.2) years and 72% were females. Hypertension and Diabetes Mellitus were the most common comorbidities seen in 65 (60%) and 40 (37%) patients respectively. Patients in the CCUs had more hypertension compared to those in MSICUs (70% vs 47%, P = 0.01). Table 1 lists the baseline characteristics of the study patients, both overall and stratified by critical care unit (CCU vs MSICU).
| Overall, n = 109 | MSICU, (n = 49) | CCU, (n = 60) | P value1 | |
| Age (yr), mean (SD) | 67.2 (14.2) | 64.9 (14.4) | 69 (13.7) | 0.13 |
| Female gender – n (%) | 78 (71.6) | 30 (61.2) | 48 (80.0) | 0.04 |
| Race/Ethnicity – n (%) | 0.37 | |||
| White | 30 (27.5) | 17 (34.7) | 13 (21.7) | |
| Black | 20 (18.4) | 9 (18.4) | 11 (18.3) | |
| Hispanic | 42 (38.5) | 15 (30.6) | 27 (45.0) | |
| Other | 17 (15.6) | 8 (16.3) | 9 (15.0) | |
| Comorbidities – n (%) | ||||
| Diabetes mellitus | 40 (36.7) | 19 (38.8) | 21 (35) | 0.68 |
| Hypertension | 65 (59.6) | 23 (46.9) | 42 (70) | 0.01 |
| Coronary disease | 13 (11.9) | 5 (10.2) | 8 (13.3) | 0.77 |
| Heart failure | 7 (6.4) | 4 (8.2) | 3 (5) | 0.70 |
| Arrhythmia | 14 (12.8) | 4 (8.2) | 10 (16.7) | 0.25 |
| Asthma | 16 (14.7) | 6 (12.2) | 10 (16.7) | 0.52 |
| COPD | 13 (11.9) | 6 (12.2) | 7 (11.7) | 0.93 |
| Obesity | 10 (9.2) | 3 (6.1) | 7 (11.7) | 0.51 |
| CKD | 14 (12.8) | 8 (16.3) | 6 (10) | 0.33 |
| ESRD | 3 (2.8) | 1 (2) | 2 (3.3) | 1.00 |
| Cancer | 23 (21.1) | 10 (20.4) | 13 (21.7) | 1.00 |
| Cirrhosis | 6 (5.5) | 5 (10.2) | 1 (1.7) | 0.09 |
| HIV | 2 (1.8) | 1 (2) | 1 (1.7) | 1.00 |
| Social risk factors – n (%) | ||||
| Alcohol use | 25 (22.9) | 13 (26.5) | 12 (20) | 0.42 |
| Current smoker | 15 (13.8) | 4 (8.2) | 11 (18.3) | 0.17 |
| Former smoker | 36 (33) | 21 (42.9) | 15 (25) | 0.05 |
| Presenting symptoms- n (%) | ||||
| Chest pain | 35 (32.1) | 8 (16.3) | 27 (45) | 0.001 |
| SOB | 55 (50.5) | 23 (46.9) | 32 (53.3) | 0.51 |
| Shock | 41 (37.6) | 29 (59.2) | 12 (20) | < 0.001 |
| Reason for unit admission- n (%) | ||||
| Cardiac | 44 (40.3) | 0 (0.0) | 44 (73.3) | < 0.001 |
| Respiratory | 14 (12.8) | 9 (18.4) | 5 (8.3) | 0.54 |
| Sepsis | 24 (22.0) | 19 (38.8) | 5 (8.3) | < 0.001 |
| GI | 11 (10.0) | 9 (18.4) | 2 (3.3) | 0.14 |
| Neurological | 7 (6.4) | 6 (12.2) | 1 (1.7) | 0.04 |
| Metabolic | 4 (3.7) | 3 (6.1) | 1 (1.7) | 0.32 |
| Other | 5 (4.6) | 3 (6.1) | 2 (3.3) | 0.66 |
Shortness of breath was the most common presenting symptom seen in 55 (50%) of the patients overall. Twenty-seven (45%) patients in the CCU complained of chest pain compared to only eight (16%) in MSICUs. Acute respiratory failure that required invasive mechanical ventilation was seen in twenty-nine (59%) MSICU patients, as opposed to only fifteen (25%) in CCU. Twenty-nine (59%) of patients in medical-surgical units also developed shock compared to twelve (20%) of the cardiac patients. Septic shock was the most common type of shock in MSICUs vs cardiogenic shock in CCUs (47% vs 8%, P < 0.001).
All SC patients had transthoracic echocardiography performed, with only 12 (24.5%) in MSICU getting cardiac catheterization, compared to 50 (83.3%) CCU patients. The majority (n = 87, 80%) of the cases were of typical anatomical type with apical akinesia/hypokinesia and basal hyperkinesia (apical ballooning). Inotropic support was required in ten patients and mechanical circulatory support in three patients. Follow up echocardiogram was performed in sixty-nine (63.3%) patients, all of them had complete reversibility of cardiac function. Of 47 patients with clinical SC, 27 had follow up echocardiography; all of them showed return to baseline cardiac function. Table 2 presents the complications and outcomes of SC by type of unit (MSICU vs CCU).
| Overall, n = 109 | MSICU, (n = 49) | CCU, (n = 60) | P value1 | |
| Confirmed SC | 62 (56.9) | 12 (24.5) | 50 (83.3) | < 0.0001 |
| Clinical SC | 47 (43.1) | 37 (75.5) | 10 (16.7) | |
| Hospital day of diagnosis1; median [IQR] | 2 [1-3] | 3 [2-4] | 1 [1-2] | 0.0002 |
| Diagnostic Studies – n (%) | ||||
| Cardiac catherization | 62 (56.9) | 12 (24.5) | 50 (83.3) | < 0.001 |
| Transthoracic echo | 109 (100) | 49 (100) | 60 (100) | |
| Lowest ejection fraction – (%); median [IQR] | 35 [28-40] | 30 [30-40] | 35 [30-45] | 0.38 |
| TTE anatomical variant- n (%) | ||||
| Atypical | 22 (20.2) | 12 (24.5) | 10 (16.7) | 0.31 |
| Typical | 87 (79.8) | 37 (75.5) | 50 (83.3) | |
| Type of SC- n (%) | < 0.001 | |||
| Primary | 43 (39.4) | 0 (0.0) | 43 (71.6) | |
| Secondary | 66 (60.5) | 49 (100.0) | 17 (28.3) | |
| EKG Findings- n (%) | ||||
| Normal EKG | 21 (19.2) | 14 (28.6) | 7 (11.7) | 0.03 |
| ST-Segment elevation | 54 (49.5) | 15 (30.6) | 39 (65.0) | < 0.001 |
| ST-Segment depression | 4 (3.7) | 2 (4.08) | 2 (3.3) | 1.00 |
| T-Wave inversion | 23 (21.1) | 13 (26.5) | 10 (16.7) | 0.24 |
| Other | 25 (22.9) | 14 (28.6) | 11 (18.3) | 0.25 |
| Complications – n (%) | ||||
| ECMO/IABP use | 3 (2.8) | 1 (2.0) | 2 (3.3) | 1.00 |
| Inotrope use | 10 (9.2) | 7 (14.3) | 3 (5) | 0.11 |
| New arrythmia | 14 (12.8) | 5 (10.2) | 9 (15.0) | 0.57 |
| AKI | 37 (33.9) | 21 (42.9) | 16 (26.7) | 0.08 |
| RRT | 14 (12.8) | 4 (8.2) | 2 (3.3) | 0.41 |
| Acute respiratory failure – n (%) | ||||
| Mechanical ventilation | 44 (40.4) | 29 (59.2) | 15 (25.0) | < 0.001 |
| NIPPV only | 15 (13.8) | 8 (16.3) | 7 (11.7) | 0.48 |
| Shock – n (%) | 41 (37.6) | 29 (59.2) | 12 (20.0) | < 0.001 |
| Cardiogenic shock | 14 (12.8) | 5 (10.2) | 9 (15.0) | 0.46 |
| Septic shock | 28 (25.7) | 23 (46.9) | 5 (8.3) | < 0.001 |
| Other shock | 2 (1.8) | 2 (4.1) | 0 (0.0) | 0.11 |
| Follow-up echocardiogram- n (%) | ||||
| Repeat echo (% Total) | 69 (63.3) | 30 (61.2) | 39 (65.0) | 0.69 |
| Reversibility (% Echo) | 69 (100.0) | 30/30 (100.0) | 39/39 (100.0) | 1.00 |
| Clinical SC patients | ||||
| Repeat Echo (% Total) | 27/47 (57.4) | 21/47 (44.7) | 6/47 (12.8) | 0.01 |
| Reversibility (% Echo) | 27/27 (100.0) | 21/21 (100.0) | 6/6 (100.0) | 1.00 |
| Hospital outcomes – n (%) | ||||
| In-hospital mortality | 15 (13.8) | 9 (18.4) | 6 (10) | 0.27 |
| ICU mortality | 8 (7.3) | 3 (6.1) | 5 (8.3) | 0.73 |
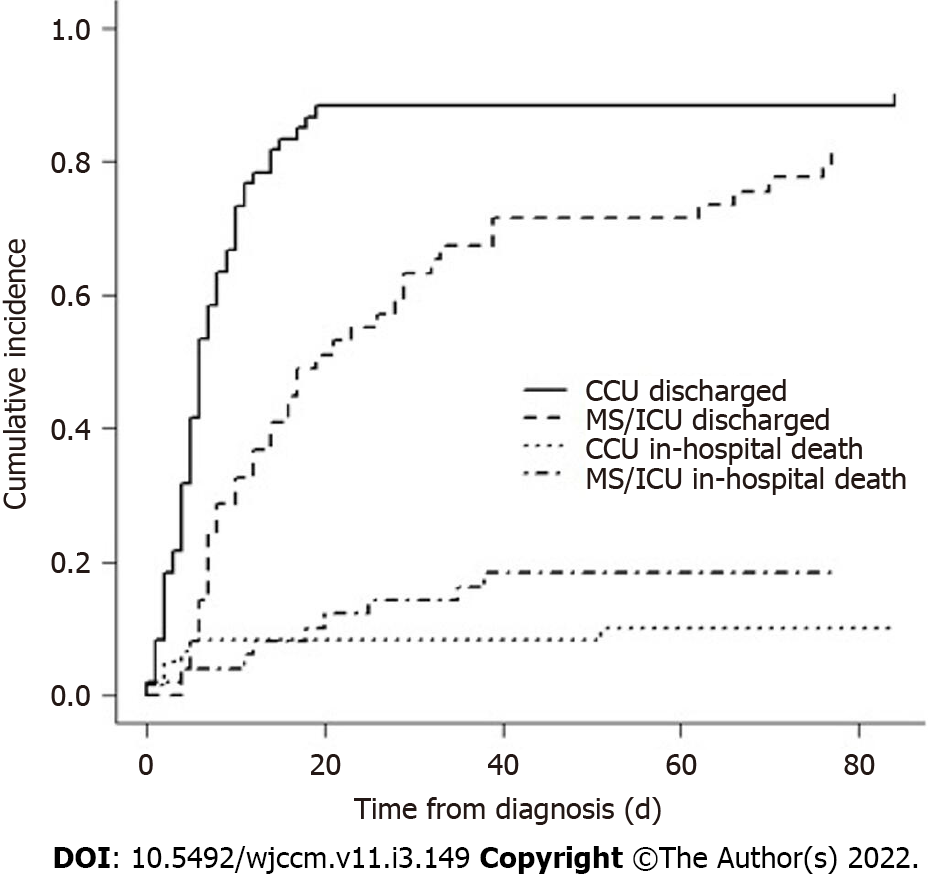
There was a statistically significant difference in the cumulative incidence function of hospital discharge stratified by critical care unit (0.56 vs 0.24 at 7 d, P = 0.01) but non-significant for in-hospital deaths stratified by critical care unit (P = 0.33) (Figure 1). Median length of stay from time of SC diagnosis to unit discharge was 1 d (range, 0-14) in CCU vs 5 d (range, 1-24) in MSICU.
A total of fifteen patients died out of which eight deaths were in the critical care units. There were no statistically significant differences in the peak laboratory values of creatine phosphokinase (CPK), troponin and pro-BNP (pro-B-type natriuretic peptide) or outcomes like ICU mortality, in-hospital mortality, use of inotropic or mechanical circulatory support based on type of unit (MSICU vs CCU) or anatomical variant (typical vs atypical) (Tables 3 and 4).
| MSICU (n = 49) | CCU (n = 60) | P value1 | |
| Troponin-T (ng/mL) | 0.42 [0.23-1.2] | 0.87 [0.29-1.54] | 0.11 |
| CPK (U/L) | 427 [148.5-1348.5] | 276.5 [161-695] | 0.48 |
| Pro-BNP (pg/mL) | 5395 [1458-15000] | 3363.5 [944.5-15369] | 0.72 |
| Typical (n = 87) | Atypical (n = 22) | P value1 | |
| Lab findings- median (IQR) | |||
| Troponin-T (ng/mL) | 0.65 [0.23–1.57] | 0.58 [0.25-0.94] | 0.61 |
| CPK (U/L) | 297.5 [151-919] | 278 [168-631] | 0.94 |
| Pro-BNP (pg/mL) | 3722 [874-11932] | 5599 [1608.5-17373.0] | 0.29 |
| Hospital complications- n (%) | |||
| Inotrope use | 8 (9.2) | 2 (9.1) | 1 |
| ECMO/IABP use | 2 (2.3) | 1 (4.5) | 0.5 |
| RRT | 3 (3.4) | 3 (13.6) | 0.1 |
| Hospital outcomes- n (%) | |||
| In-hospital mortality | 12 (13.8) | 3 (13.6) | 1 |
| ICU mortality | 7 (8) | 1 (4.5) | 1 |
To our knowledge, this is the largest case series describing the clinical presentation, complications and outcomes of patients with stress cardiomyopathy admitted to the critical care units (MSICUs and CCUs). The overall incidence of SC in our patients was 0.44%, incidence in medical-surgical ICU was 0.25%, all of them having developed secondary SC. The incidence of SC in medical-surgical units varies per previous published reports. One of the earlier studies done by Park et al[13] in 2005 screened 92 consecutive critically ill patients admitted to medical ICU by serial echocardiography on day 1, 3, and 7. They observed a high incidence (28%) of left ventricular apical ballooning (LVAB) in medical ICU patients with no cardiac diseases. Patients with LVAB had higher prevalence of sepsis, hypotension upon ICU admission, use of inotropes, pulmonary edema, cardiomegaly and lower mean 2-month survival compared to patients without LVAB. The higher incidence of SC reported in the Park et al[13] study is likely because the diagnosis was solely made based on echocardiographic findings without integrating EKG, cardiac enzymes and coronary angiogram findings. An Australian study showed a much lower incidence of silent LVAB of around 3.5% in their medical ICU without any association of negative outcomes with silent LVAB[27]. Another prospective single center study by Doyen et al[14] in medical ICU patients found a high incidence of secondary SC of 4.6%. Our reported incidence of 0.25% in MSICUs is lower than the prior studies, because of the prospective nature of those studies, where all patients got echocardiographic screening for SC upon ICU admission. Muratsu et al[18] conducted a retrospective study on 5084 patients in Japan over a 5-year period and found a low incidence of clinical Takotsubo cardiomyopathy of 0.37%; a majority of their SC patients had the diagnosis of sepsis and subarachnoid hemorrhage. This demonstrates that there are likely many cases of SC which go under-recognized since formal echocardiography is not performed on every patient in the ICU. However, use of routine point of care ultrasound (POCUS) on critically ill ICU patients will likely identify many more cases of SC.
Sepsis and acute respiratory failure were the most common ICU diagnoses of patients developing secondary SC in these studies, which is similar to our patients in the MSICUs[13,14,18]. Kleber et al[29] reported a 15% prevalence of stress cardiomyopathy in the setting of acute respiratory failure requiring mechanical ventilation.
We found that CCU patients mostly presented with primary SC from the community, many of them developing typical chest pain, shortness of breath and classic ST segment elevation on electrocardiogram.
It is reported that patients with secondary SC usually have an atypical presentation in the ICU, with the majority of them developing sudden or worsening unexplained shock/hemodynamic instability and shortness of breath[9,13,14,18,19]. Fifty-nine (59%) percent of our MSICU patients developed shock compared to 20% of CCU patients. In the prospective study by Doyen et al[14], 53.8% medical ICU patients developed cardiogenic shock. This is different from our findings as the most common type of shock in our study was septic shock. The likely explanation for this discrepancy is that 47% of our population in medical surgical ICUs had the diagnosis of severe sepsis and septic shock compared to 38% in the Doyen study.
The 2008 modified Mayo Clinic criteria and European Society of Cardiology (ESC) Heart Failure Association diagnostic criteria for stress cardiomyopathy require that patients have the absence of obstructive culprit coronary artery disease[30,31].
However, there are many reasons for forgoing cardiac catheterization in the critically ill ICU patients, such as hemodynamic instability, multi-organ failure, risk of acute kidney injury (AKI) due to contrast induced nephropathy or established AKI amongst others.
Only 25% of our patients in medical-surgical units underwent cardiac catheterization, compared to 83% in the cardiac units. The mainstay of diagnosis of clinical SC in these critically ill patients was the combination of transthoracic echocardiography, cardiac enzymes and electrocardiogram findings.
Previous reports of SC in medical-surgical ICUs also relied mainly on transthoracic echocardiography along with cardiac enzymes and EKG changes for diagnostic purposes for similar reasons[13,14,18]. With the integration of POCUS as a routine diagnostic tool in the management of ICU patients, there will be an earlier recognition and an increase in the number of patients diagnosed with Stress cardiomyopathy at bedside by Intensivists, thereby improving care of these patients[32].
Patients with secondary SC in MSICUs also had longer ICU and hospital lengths of stay compared to CCU patients, primarily because MSICU patients were sicker with stressors such as acute respiratory failure, septic shock, neurologic disorders and multi system organ failure. Interestingly, we found that 11% (n = 12) of our cases developed SC in the perioperative setting. Agarwal et al[33] performed a systematic review of perioperative SC and found 102 cases in 93 articles. Management of our perioperative SC cases was similar to non-perioperative cases.
We report a low overall mortality for patients with SC. This is similar to prior studies that also report favorable outcomes of this patient population[3,9,10,13,14,18,19]. A relatively fast and complete recovery of cardiac function may explain this finding. Fifty-seven percent of our clinical SC patients had follow up echocardiogram, all showing reversibility of cardiac function, further supporting the diagnosis of SC. We also did not find any differences in mortality based on unit type (MSICU vs CCU) or anatomical type (typical vs atypical).
The major strength of our study is that we describe a large case series of patients with stress cardiomyopathy over the five-year period. We report and compare for the first time, characteristics, complications and outcomes of stress cardiomyopathy stratified by the type of unit and anatomical type. Our study has few limitations that need to be acknowledged. First, it is a single center study. Second, it is retrospective in nature and hence some data elements may not be captured accurately. Third, we believe that our incidence is likely underestimated, as many cases of SC may have gone unrecognized. Fourth, our definition of clinical SC could include cases of myocardial ischemia, showing improvement with development of collateral circulation. Fifth, follow up echocardiograms were only available in only 69 (63.3%) patients.
Stress cardiomyopathy can be under-recognized in the critical care setting. Primary stress cardiomyopathy is commonly seen in the CCUs and the secondary form predominates in the MSICU setting. Presentation of secondary SC is often atypical and the majority of patients have simultaneous acute respiratory failure and sepsis. Intensivists should have a high index of clinical suspicion for SC in patients who develop sudden or worsening unexplained hemodynamic instability, arrhythmias, or respiratory failure. Many of the SC cases in MSICU may be diagnosed clinically as cardiac catheterization is not always feasible. Routine utilization of POCUS on all ICU patients will help identify more cases. The outcomes of these patients are excellent as majority of them show reversibility of cardiac function on follow up imaging.
Critically ill patients are at risk of developing stress cardiomyopathy (SC) but can be under-recognized.
Our goal was to learn more about patients with SC in the intensive care unit (ICU) setting.
To study the patient characteristics, clinical course, and outcomes of critically ill patients with SC.
We conducted a retrospective observational study at a tertiary care teaching hospital. All adult patients admitted to the critical care units with Stress cardiomyopathy over 5 years were included.
One hundred and nine patients were identified with SC, with 55% of them in the coronary care units (CCU) and 45% in the medical-surgical intensive care units (MSICUs). 57% of patients had SC confirmed by cardiac catherization while 43% were diagnosed clinically with echocardiography. 72% of CCU patients had primary SC whereas all MSICU patients had secondary SC. 59% of MSICU patients developed shock and acute respiratory failure that required mechanical ventilation. There were no statistically significant differences in ICU mortality, in-hospital mortality, use of inotropic or mechanical circulatory support based on type of unit or anatomical variant.
Primary SC was commonly seen in the CCUs while secondary SC was seen more commonly in the MSICUs. Secondary SC often presents atypically and many patients have acute respiratory failure and sepsis. Many of the SC cases in the MSICU may be diagnosed clinically as cardiac catherization is not always feasible. Patients with SC in the ICUs have excellent outcomes with the majority of them showing reversibility of cardiac function.
Stress Cardiomyopathy is often under-recognized in the critical care setting. In the MSICUs, secondary SC is the main form of SC encountered, where is it is often diagnosed clinically. Routine use of Point-of-care ultrasound may help with early identification of these cases.
We would like to thank Dr. Peter Dicpinigaitis for reviewing our manuscript and providing valuable feedback.
| 1. | Medina de Chazal H, Del Buono MG, Keyser-Marcus L, Ma L, Moeller FG, Berrocal D, Abbate A. Stress Cardiomyopathy Diagnosis and Treatment: JACC State-of-the-Art Review. J Am Coll Cardiol. 2018;72:1955-1971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 404] [Article Influence: 50.5] [Reference Citation Analysis (0)] |
| 2. | Boland TA, Lee VH, Bleck TP. Stress-induced cardiomyopathy. Crit Care Med. 2015;43:686-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (1)] |
| 3. | Champion S, Belcour D, Vandroux D, Drouet D, Gaüzère BA, Bouchet B, Bossard G, Djouhri S, Jabot J, Champion M, Lefort Y. Stress (Tako-tsubo) cardiomyopathy in critically-ill patients. Eur Heart J Acute Cardiovasc Care. 2015;4:189-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 4. | Antonopoulos A, Kyriacou C. Apical ballooning syndrome or Takotsubo cardiomyopathy: a new challenge in acute cardiac care. Cardiol J. 2008;15:572-577. [PubMed] |
| 5. | Pullara A, Chinaglia A, Giammaria M, Bequaraj F, Orlando F, Coda L, Lucciola MT, Forno D, Ravera L, Cecchi E, Gaita F, Belli R. Takotsubo cardiomyopathy: real life management in the intensive coronary care unit. Minerva Med. 2013;104:537-544. [PubMed] |
| 6. | Chockalingam A, Mehra A, Dorairajan S, Dellsperger KC. Acute left ventricular dysfunction in the critically ill. Chest. 2010;138:198-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 7. | Ghadri JR, Wittstein IS, Prasad A, Sharkey S, Dote K, Akashi YJ, Cammann VL, Crea F, Galiuto L, Desmet W, Yoshida T, Manfredini R, Eitel I, Kosuge M, Nef HM, Deshmukh A, Lerman A, Bossone E, Citro R, Ueyama T, Corrado D, Kurisu S, Ruschitzka F, Winchester D, Lyon AR, Omerovic E, Bax JJ, Meimoun P, Tarantini G, Rihal C, Y-Hassan S, Migliore F, Horowitz JD, Shimokawa H, Lüscher TF, Templin C. International Expert Consensus Document on Takotsubo Syndrome (Part I): Clinical Characteristics, Diagnostic Criteria, and Pathophysiology. Eur Heart J. 2018;39:2032-2046. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 641] [Cited by in RCA: 1103] [Article Influence: 157.6] [Reference Citation Analysis (0)] |
| 8. | Chockalingam A. Stress cardiomyopathy of the critically ill: Spectrum of secondary, global, probable and subclinical forms. Indian Heart J. 2018;70:177-184. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 9. | Haghi D, Fluechter S, Suselbeck T, Saur J, Bheleel O, Borggrefe M, Papavassiliu T. Takotsubo cardiomyopathy (acute left ventricular apical ballooning syndrome) occurring in the intensive care unit. Intensive Care Med. 2006;32:1069-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Ruiz Bailén M, Aguayo de Hoyos E, López Martnez A, Daz Castellanos MA, Ruiz Navarro S, Fierro Rosón LJ, Gómez Jiménez FJ, Issa-Masad Khozouz Z. Reversible myocardial dysfunction, a possible complication in critically ill patients without heart disease. J Crit Care. 2003;18:245-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Ruiz Bailén M. Reversible myocardial dysfunction in critically ill, noncardiac patients: a review. Crit Care Med. 2002;30:1280-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Marcelino PA, Marum SM, Fernandes AP, Germano N, Lopes MG. Routine transthoracic echocardiography in a general Intensive Care Unit: an 18 mo survey in 704 patients. Eur J Intern Med. 2009;20:e37-e42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Park JH, Kang SJ, Song JK, Kim HK, Lim CM, Kang DH, Koh Y. Left ventricular apical ballooning due to severe physical stress in patients admitted to the medical ICU. Chest. 2005;128:296-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 229] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 14. | Doyen D, Moschietto S, Squara F, Moceri P, Hyvernat H, Ferrari E, Dellamonica J, Bernardin G. Incidence, clinical features and outcome of Takotsubo syndrome in the intensive care unit. Arch Cardiovasc Dis. 2020;113:176-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Jo YY, Chang HJ, Na S, Sim J, Choi YS. Predictors of mortality in patients with stress-induced cardiomyopathy developed during critical care. J Crit Care. 2013;28:618-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Belcour D, Jabot J, Grard B, Roussiaux A, Ferdynus C, Vandroux D, Vignon P. Prevalence and Risk Factors of Stress Cardiomyopathy After Convulsive Status Epilepticus in ICU Patients. Crit Care Med. 2015;43:2164-2170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 2.4] [Reference Citation Analysis (1)] |
| 17. | Nasr DM, Tomasini S, Prasad A, Rabinstein AA. Acute Brain Diseases as Triggers for Stress Cardiomyopathy: Clinical Characteristics and Outcomes. Neurocrit Care. 2017;27:356-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 18. | Muratsu A, Muroya T, Kuwagata Y. Takotsubo cardiomyopathy in the intensive care unit. Acute Med Surg. 2019;6:152-157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Oras J, Lundgren J, Redfors B, Brandin D, Omerovic E, Seeman-Lodding H, Ricksten SE. Takotsubo syndrome in hemodynamically unstable patients admitted to the intensive care unit - a retrospective study. Acta Anaesthesiol Scand. 2017;61:914-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Shah R, Shelton MR, Ramanathan KB. Lesson of the month 1: broken heart in the intensive care unit. Clin Med (Lond). 2014;14:447-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 21. | Bourenne J, Jeremy B, Fresco R, Raphaëlle F, Kerbaul F, François K, Michelet P, Pierre M, Gainnier M, Marc G. Stress Cardiomyopathy Managed with Extracorporeal Support after Self-Injection of Epinephrine. Case Rep Crit Care. 2017;2017:3731069. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Choi JH, Oh ID, Shin E, Lee S, Jeon JM, Kim HT, Youn HC. Extracorporeal membrane oxygenation for takotsubo cardiomyopathy that developed after mitral valve replacement. Acute Crit Care. 2020;35:51-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Rennyson SL, Parker JM, Symanski JD, Littmann L. Recurrent, severe, and rapidly reversible apical ballooning syndrome in status asthmaticus. Heart Lung. 2010;39:537-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 24. | Taniguchi K, Takashima S, Iida R, Ota K, Nitta M, Sakane K, Fujisaka T, Ishizaka N, Umegaki O, Uchiyama K, Takasu A. Takotsubo cardiomyopathy caused by acute respiratory stress from extubation: A case report. Medicine (Baltimore). 2017;96:e8946. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (1)] |
| 25. | Y-Hassan S, Settergren M, Henareh L. Sepsis-induced myocardial depression and takotsubo syndrome. Acute Card Care. 2014;16:102-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 26. | Madias JE. What is the real incidence of Takotsubo syndrome in intensive care units? Acta Anaesthesiol Scand. 2017;61:1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Rowell AC, Stedman WG, Janin PF, Diel N, Ward MR, Kay SM, Delaney A, Figtree GA. Silent left ventricular apical ballooning and Takotsubo cardiomyopathy in an Australian intensive care unit. ESC Heart Fail. 2019;6:1262-1265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Gray RJ. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. The Annals of Statistics 16, No. 3. 1988; 1141-154. |
| 29. | Kleber HD. Drug abuse liability testing: human subject issues. NIDA Res Monogr. 1989;92:341-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (1)] |
| 30. | Prasad A, Lerman A, Rihal CS. Apical ballooning syndrome (Tako-Tsubo or stress cardiomyopathy): a mimic of acute myocardial infarction. Am Heart J. 2008;155:408-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1193] [Cited by in RCA: 1323] [Article Influence: 73.5] [Reference Citation Analysis (0)] |
| 31. | Lyon AR, Bossone E, Schneider B, Sechtem U, Citro R, Underwood SR, Sheppard MN, Figtree GA, Parodi G, Akashi YJ, Ruschitzka F, Filippatos G, Mebazaa A, Omerovic E. Current state of knowledge on Takotsubo syndrome: a Position Statement from the Taskforce on Takotsubo Syndrome of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail. 2016;18:8-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 945] [Cited by in RCA: 854] [Article Influence: 85.4] [Reference Citation Analysis (1)] |
| 32. | Lee JW, Kim JY. Stress-induced cardiomyopathy: the role of echocardiography. J Cardiovasc Ultrasound. 2011;19:7-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Agarwal S, Bean MG, Hata JS, Castresana MR. Perioperative Takotsubo Cardiomyopathy: A Systematic Review of Published Cases. Semin Cardiothorac Vasc Anesth. 2017;21:277-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Critical care medicine
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Lv Y, China; Moldovan C, Romania S-Editor: Liu JH L-Editor: A P-Editor: Liu JH