Published online May 9, 2022. doi: 10.5492/wjccm.v11.i3.139
Peer-review started: December 23, 2021
First decision: March 7, 2022
Revised: March 9, 2022
Accepted: April 21, 2022
Article in press: April 21, 2022
Published online: May 9, 2022
Processing time: 134 Days and 22.3 Hours
Neonatal sepsis is a life-threatening disease. Early diagnosis is essential, but no single marker of infection has been identified. Sepsis activates a coagulation cascade with simultaneous production of the D-dimers due to lysis of fibrin. D-dimer test reflects the activation of the coagulation system.
To assess the D-dimer plasma level, elaborating its clinicopathological value in neonates with early-onset and late-onset neonatal sepsis.
The study was a prospective cross-sectional study that included ninety neonates; divided into three groups: Group I: Early-onset sepsis (EOS); Group II: Late-onset sepsis (LOS); and Group III: Control group. We diagnosed neonatal sepsis according to our protocol. C-reactive protein (CRP) and D-dimer assays were compared between EOS and LOS and correlated to the causative microbiological agents.
D-dimer was significantly higher in septic groups with a considerably higher number of cases with positive D-dimer. Neonates with LOS had substantially higher levels of D-dimer than EOS, with no significant differences in CRP. Neonates with LOS had a significantly longer hospitalization duration and higher gram-negative bacteriemia and mortality rates than EOS (P < 0.01). Gram-negative bacteria have the highest D-dimer levels (Acinetobacter, Klebsiella, and Pseudomonas) and CRP (Serratia, Klebsiella, and Pseudomonas); while gram-positive sepsis was associated with relatively lower levels. D-dimer had a significant negative correlation with hemoglobin level and platelet count; and a significant positive correlation with CRP, hospitalization duration, and mortality rates. The best-suggested cut-off point for D-dimer in neonatal sepsis was 0.75 mg/L, giving a sensitivity of 72.7% and specificity of 86.7%. The D-dimer assay has specificity and sensitivity comparable to CRP in the current study.
The current study revealed a significant diagnostic value for D-dimer in neonatal sepsis. D-dimer can be used as an adjunct to other sepsis markers to increase the sensitivity and specificity of diagnosing neonatal sepsis.
Core Tip: The study aimed to define the diagnostic and prognostic value of the D-dimer assay in early and late-onset sepsis. We prospectively studied C-reactive protein and D-dimer levels in 90 neonates divided into control, Early-onset sepsis, and late-onset sepsis. D-dimer was significantly higher in the septic groups, more in late-onset than early-onset sepsis, and with gram-negative sepsis than gram-positive sepsis. The best-suggested cut-off point for D-dimer in neonatal sepsis was 0.75 mg/L, giving a sensitivity of 72.7% and specificity of 86.7%.
- Citation: Al-Biltagi M, Hantash EM, El-Shanshory MR, Badr EA, Zahra M, Anwar MH. Plasma D-dimer level in early and late-onset neonatal sepsis. World J Crit Care Med 2022; 11(3): 139-148
- URL: https://www.wjgnet.com/2220-3141/full/v11/i3/139.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v11.i3.139
Neonatal sepsis is a severe systemic inflammatory response to blood-stream infection with high morbidity and mortality during the neonatal period. Early and proper diagnosis of neonatal sepsis is critical for timely-administered antibiotics, decreases the length of the hospital stay, and improves the prognosis, especially the neurodevelopmental outcome[1]. To diagnose neonatal sepsis, the physicians usually depend on the blood culture, the gold standard, and some screening tools such as acute phase reactants and cytokines, for instance, the white blood cell count, C-reactive protein (CRP), procalcitonin, interleukin-6, interleukin-8, CD11b, and CD64. However, the blood culture yield is not always accurate, with the possibility of false-negative and positive results. Acute phase reactants and cytokines may have high sensitivity to diagnose bacterial sepsis, but they may lack high specificity and good predictive value[2,3].
As sepsis is a clinical condition resulting from the interaction between the microbial agent and the host immune, inflammatory, and coagulation responses, some studies showed changes in the circulating coagulation proteins coupled with impaired fibrinolytic activity in patients with confirmed sepsis[4]. Activation of the coagulation cascade resulting from the released sepsis-induced; inflammatory cytokines enhance the degradation of cross-linked fibrin polymers with increased production of D-dimer[5]. Consequently, D-dimer is considered a specific marker for increased procoagulatory activity and fibrinolysis. Elevation of D-dimer and fibrinogen degradation products rapidly occurs after disseminated intravascular coagulation (DIC) initiation, which may arise as a complication of sepsis[6]. Activation of the coagulation reflected by the increase in D-dimer levels contributes significantly to the sepsis outcome. Different ways to assess D-dimer levels are available, including enzyme-linked immunofluorescence immunoassay, microplate enzyme-linked immunosorbent assay, latex quantitative, latex semiquantitative, latex qualitative, and whole-blood assays[7]. In this study, we aimed to assess the plasma level of D-dimer in infants with neonatal sepsis to throw more light on its clinicopathological value in patients having neonatal sepsis.
The present research was a prospective cross-sectional study conducted on ninety-four full-term neonates recruited serially from the Neonatal Intensive Care Unit (NICU), Pediatric department; the tertiary care hospital of Tanta University between January 2019 to January 2021. The recruited neonates were divided into three comparable groups: Group I included neonates with early sepsis (who developed sepsis within the first week after childbirth), Group II included neonates with late neonatal sepsis (who developed sepsis within the first week after birth), and Group III included healthy neonates with no clinical manifestation of sepsis and negative CRP and Gerdes sepsis screen less than two, recruited from the postnatal ward of the Obstetric Department and the outpatient clinic. All parents, guardians, or next of kin signed informed consent for the minors to participate in this study. The Institutional Ethical and Research Review Board of the Faculty of Medicine, Tanta University, approved the study.
We diagnosed neonatal sepsis based on the presence of suspected clinical signs of sepsis, positive CRP (≥ 10 mg/L), positive Gerdes' sepsis screen (≥ 2), and positive blood culture. Sepsis was suspected in the presence of fever or temperature instability, irritability, lethargy, feeding difficulty, apnea or respiratory distress, hepatomegaly, abdominal distention, convulsion, hypotonia, hemodynamic instability, or bleeding diathesis. As the diagnosis of neonatal sepsis is hampered by the frequent presence of non-infectious conditions that may mimic sepsis, we only included those with proven sepsis and positive blood culture in the study. According to Neonatal Intensive Care Unit protocol, all children with suspected sepsis receive the appropriate management.
We excluded premature neonates and neonates with congenital heart diseases, hypoxic-ischemic encephalopathy, liver diseases, renal diseases, hereditary coagulopathy, or other non-sepsis-related systemic disorders that could affect the level of CRP or D-dimer levels. All included neonates had thorough prenatal, natal, and postnatal history, thorough clinical examination, complete blood cell count (CBC) with differential, CRP levels, urine analysis and culture, blood culture, cerebrospinal fluid (CSF) analysis, and culture, and other infection markers as indicated. Chest X-ray, echocardiography, or abdominal X-ray were tailored according to the clinical indications. The D-dimer assay was performed using the D-dimer test device (NycoCard D-dimer, Axis-Shield, Oslo, Norway) and the Nycocard READER II (NycoCard READER II, Axis-Shield, Oslo, Norway). It is a single rapid test for detection of D-dimer in plasma and is based upon an immunometric flow-through, sandwich-format, immunofiltration principle. CRP levels were measured using high-sensitive Tinaquant CRP (Latex) immunoturbidimetric assay using Roche Modular P analyzer (CRP latex HS, Roche kit; Roche Diagnostics, GmbH, D-68298 Mannheim, Germany), following the manufacturer's instructions.
We used the Power and Precision V3 program to estimate the power level of the primary endpoint (serum level of D-dimer with a mean level of 1.0 ± 0.3 mg/L) (http://www.Power-Analysis.com, Englewood, New Jersey). The power level was 90% when using 30 patients for each group. The collected data were organized, tabulated, and statistically analyzed using SPSS version 19 (Statistical Package for Social Studies, IBM, Chicago, IL, United States). For numerical values, the range means and standard deviations were calculated. The differences between the two mean values were used using the student's t-test. Differences in mean values between more than two groups were tested by analysis of variance (F). We used the Scheffe test to compare every two groups when we found significance. The number and percentage were calculated for categorical variables, and differences between subcategories were tested by chi-square. Fisher and Monte Carlo exact tests were used when chi-square was not appropriate. We used the receiver operating characteristic (ROC) test to evaluate the diagnostic power of the different diagnostic techniques. We used Pearson's correlation coefficient to test the relations between two variables. Sensitivity, specificity, and predictive values were calculated to differentiate the ability to diagnose sepsis by CRP, Gerdes, and I/T ratio. We adopted the level of significance at P < 0.05.
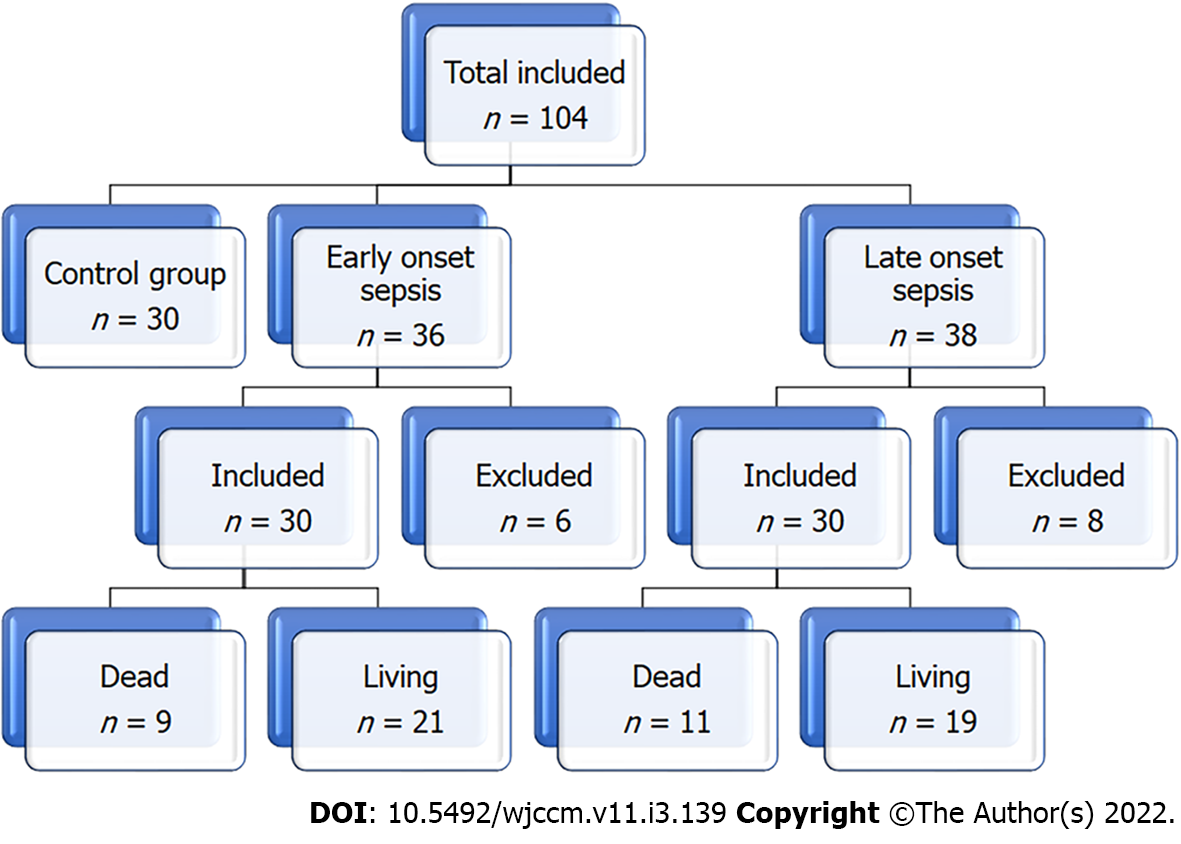
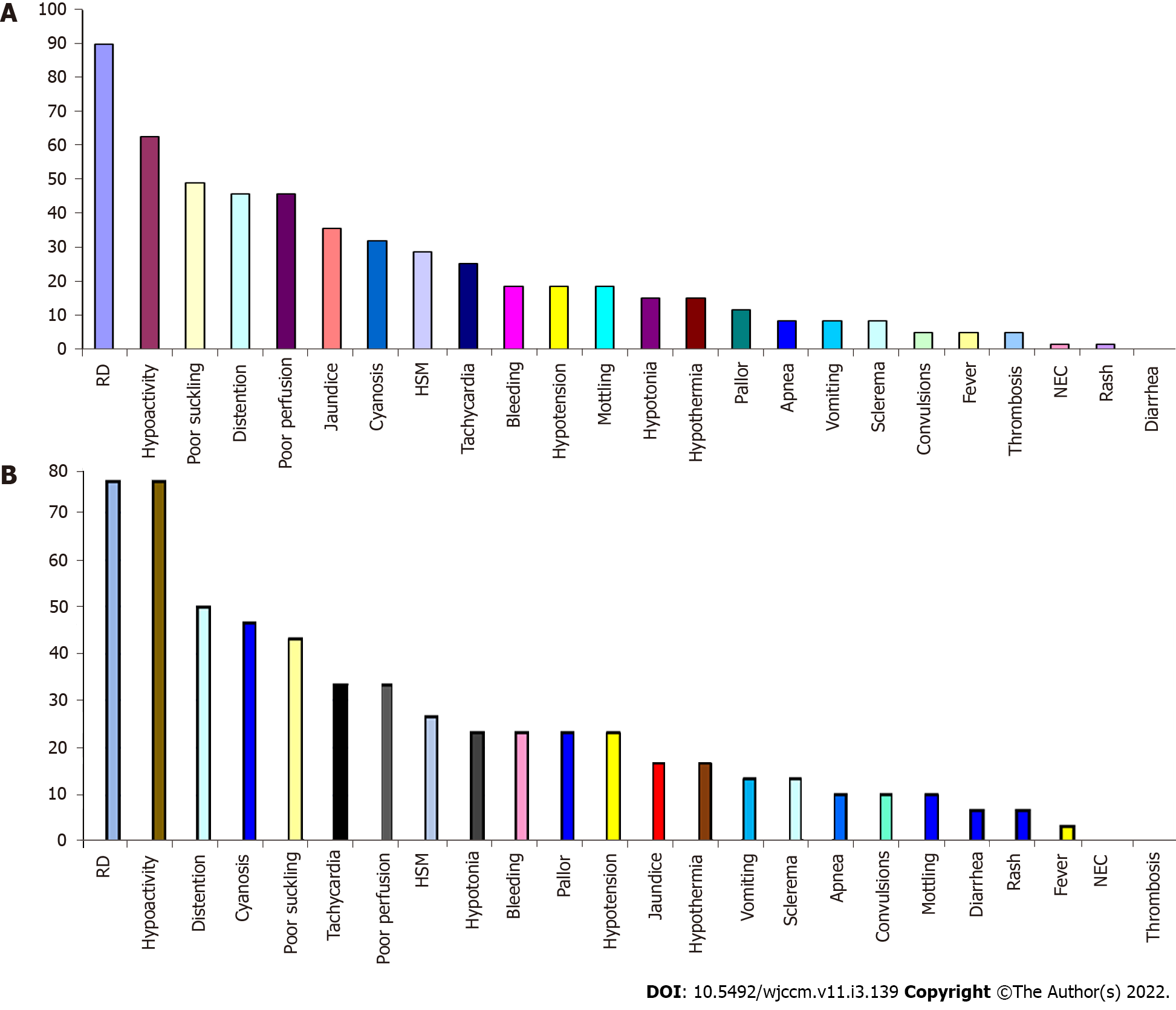
Figure 1 shows the flow chart of the study, which included three groups: the control group (30 healthy neonates), Neonates with EOS (30 neonates after exclusion of 36 neonates), and neonates with LOS (30 neonates after exclusion of 8 neonates). The neonates were recruited sequentially. Table 1 and Figure 2 show the groups' demographics, clinical presentation, and laboratory testing. There were no significant differences between the groups in the male-to-female ratio, presentation weight, and cesarean section rate. However, LOS was more common in males than females. We found no significant differences in the clinical presentation between EOS and LOS, although respiratory distress was more common in the EOS while cyanosis was more common in LOS. However, the number of neonates with a positive Gerdes score (≥ 2) was significantly higher in the EOS than in LOS. Premature rupture of membranes was present in 20% of cases with EOS. While umbilical vein catheterization or endotracheal intubation was more common in EOS, combined umbilical vein catheterization and endotracheal intubation were significantly more common in LOS. Neonates with EOS had a substantially higher rate of thrombocytopenia than LOS. However, Neonates with LOS had considerably higher levels of D-dimer than EOS. Meanwhile, we found no significant differences in CRP levels in neonates with EOS or LOS. However, neonates with LOS had a significantly longer duration of hospitalization and higher mortality rates than neonates with EOS.
| Control group (n = 30) | EOS group (n = 30) | LOS group (n = 30) | t/Z | P value | ||
| Age (mean ± SD, d) | 2.10 ± 0.8 | 2.47 ± 0.57 | 12.47 ± 4.03 | 147.024 | 0.001 | |
| Weight (mean ± SD, g) | 2.98 ± 0.4 | 2.85 ± 0.41 | 2.95 ± 0.3 | 0.895 | NS | |
| Male: Female | 0.9:1 | 0.87: 1 | 1.5:1 | 0.356 | NS | |
| % of cesarean section | 23 (76.7%) | 22 (73.3%) | 21 (70%) | 0.381 | NS | |
| PROM | 0 | 6 (20%) | 0 | |||
| Risk factors (invasive procedure) | UVC | 0 | 4 (13.3%) | 0 | 0.001 | |
| ETT | 0 | 5 (16.7%) | 4 (13.3%) | 0.001 | ||
| UVC + ETT | 0 | 7 (23.3%) | 13 (43.3%) | 0.001 | ||
| None | 100% | 14 (46.7%) | 13 (43.3%) | 0.001 | ||
| Respiratory distress | 0 | 27 (90%) | 23 (77%) | 1.920 | NS | |
| Apnea | 0 | 3 (10%) | 3 (10%) | FE | NS | |
| Cyanosis | 0 | 10 (33.3%) | 14 (46.7%) | 1.111 | NS | |
| Positive Gerdes score (≥ 2) | 0 | 22 (73.3%) | 19 (63.3 %) | 38.258 | 0.001 | |
| Thrombocytopenia | 2 (6.7%) | 22 (73%) | 12 (40%) | 6.787 | 0.009 | |
| CRP (mg/dL) | 4 ± 2 | 57.53 ± 38.82 | 65.47 ± 39.62 | 0.783 | NS | |
| D-dimer (mg/L) | 0.60 ± 0.70 | 1.48 ± 1.44 | 2.27 ± 1.86 | 10.512 | 0.001 | |
| Hospital duration | 0 | 21.6 ± 10 | 22 ± 9 | 0.051 | NS | |
| Mortality | 0 | 9 (30%) | 11 (36.7%) | 0.300 | NS | |
Table 2 shows the microbial profile of neonates with EOS and LOS. The gram-negative bacteremia rate was significantly higher in LOS than in EOS, while gram-positive bacteremia was markedly higher in EOS than in LOS (P < 0.01). Klebsiella was the most common isolated gram-negative organism, while Group B Streptococcus was the most common isolated gram-positive organism. Table 3 shows the blood levels of D-dimer and CRP according to the isolated organism, with gram-negative bacteria having the highest levels of D-dimer (Acinetobacter, Klebsiella, and Pseudomonas) and CRP (Serratia, Klebsiella, and Pseudomonas). On the other hand, gram-positive sepsis was associated with relatively lower levels of D-dimer and CRP. Table 4 showed that D-dimer had a significant negative correlation with hemoglobin level and platelet count while having a significant positive correlation with CRP, duration of the hospital stays, and mortality. The D-dimer levels were non-significantly higher in the neonates who died (1.91 ± 1.72) than those who survived (1.81 ± 1.68). We used the ROC curves to evaluate D-dimer's diagnostic power (discriminative ability) to diagnose neonatal sepsis. It revealed a significant diagnostic value for D-dimer for neonatal sepsis. The best-suggested cut-off point for D-dimer in neonatal sepsis is 0.75 mg/L, giving a sensitivity of 72.7% and specificity of 86.7% (Table 5).
| EOS group (n = 30) | LOS group (n = 30) | Total (n =60) | t | P value | ||
| Gram-negative bacteria | Total | 21 (70.0%) | 28 (93.3%) | 49 (81.67%) | 2.3 | < 0.01 |
| Klebsiella | 15 (50%) | 20 (67%) | 35 (58%) | 1.3 | NS | |
| E. coli | 4 (13.30%) | 1 (3.3%) | 5 (8.33%) | -1.4 | NS | |
| Acinetobacter | 2 (6.66%) | 4 (13.30%) | 6 (10%) | 0.85 | NS | |
| Serratia | 0% | 1 (3.3%) | 1 (1.66%) | |||
| Pseudomonas | 0% | 2 (6.66%) | 2 (3.33%) | |||
| Gram-positive bacteria | Total | 8 (26.7%) | 2 (6.7%) | 10 (16.7%) | -2.07 | < 0.01 |
| Group B Streptococcus | 5 (16.60%) | 0% | 5 (8.33%) | |||
| CoNS | 2 (6.66%) | 1 (3.3%) | 3 (5%) | -0.6 | NS | |
| Enterococcus | 1 (3.3%) | 0% | 1 (1.66%) | |||
| MRSA | 0% | 1 (3.3%) | 1 (1.66%) | |||
| Candida | 1 (3.3%) | 0% | 1 (1.66%) | |||
| Organism | D-dimer (mean ± SD) | CRP (mean ± SD) | |
| Gram-negative bacteria | E. coli | 1.3 ± 0.81 | 44.2 ± 4.3 |
| Klebsiella | 2.0971 ± 1.98916 | 71.1 ± 3.9 | |
| Acinetobacter | 2.1333 ± 1.63 | 37.7 ± 3.4 | |
| Pseudomonas | 1.95 ± 0.92 | 64.0 ± 5.65 | |
| Serratia | 1.8 ± 0.4 | 99.0 ± 0.79 | |
| Gram-positive bacteria | Group B Streptococcus | 1.6 ± 10.6 | 39.7 ± 2.5 |
| CoNS | 1.3 ± 0.51 | 53.7 ± 2.7 | |
| MRSA | 1.2 ± 0.60 | 58.0 ± 8.2 | |
| ROC curve results | The area under the curve | P value | Cut off point | Sensitivity | Specificity |
| D-dimer (mg/L) | 0.822 | 0.001 | 0.75 | 72.7% | 86.7% |
The current study examined D-dimer yield in diagnosing neonatal sepsis in 90 neonates, divided into three groups, early-onset, last onset sepsis, and a control group. Despite there being no significant differences in gender among the studied group, we observed an increased rate of LOS in males than in females, which could be related to a diminished cell-mediated immune response in males as it is an X-chromosome-linked trait with the expression of some sex-specific pro-and anti-inflammatory cytokines[8].
D-dimers are the D fragments of fibrinogen resulting from fibrinolysis during the plasmin mediated lysis of fibrin and are more specific than fibrin/fibrinogen degradation products and can serve as an indicator of microcirculatory failure[9]. In the current study, we found a significant increase in serum level of D-dimer in both patient groups with sepsis compared to the control, being significantly higher in the LOS than the EOS. These findings agree with Peker et al[10] and Mautone et al[11], who found high D-dimer levels in neonates with sepsis. These results contrast with Brahmana et al[12], who found low D-dimer in neonates with sepsis. This difference could be related to the gestational age of the neonates recruited, as they included preterm babies in their study.
Our study found that D-dimer has a high sensitivity (72.7%) and specificity (86.7%) to diagnose neonatal sepsis with a cut-off point of 0.822 mg/L. This finding agrees with the work of Pancham et al[13], who found that D-dimer had a sensitivity (90.0%) and negative predictive value (84.4%) in predicting sepsis. Considering the relatively high sensitivity of D-dimer, it can be beneficial as an additional diagnostic tool for neonatal sepsis. However, we should consider the relatively low specificity of the D-dimer. The current study observed that D-dimer was higher in the LOS than in neonates with EOS. The increase of D-dimer in LOS than EOS may be related to increased frequency of gram-negative bacterial sepsis and rate of invasive procedures such as umbilical vein catheterization and endotracheal intubation compared to EOS, as we observed a significant increase of D-dimer plasma levels in gram-negative sepsis when compared to EOS. Previous studies showed that the inflammatory cytokines, reflecting the severity of infection, increased from 1.5-5 folds in gram-negative sepsis compared to gram-positive sepsis[14]. Unfortunately, we did not find previous studies comparing D-dimer levels between gram-negative and gram-positive sepsis. Meini et al[15] found that the D-dimer level can predict the severity and the course of severe invasive infections caused by the gram-negative bacteria Neisseria meningitides while failing to expect the course of the disease in invasive infections caused by Streptococcus pneumoniae. The increased rate of invasive procedures in LOS compared to EOS in our study could be an effect rather than a cause due to the increased rate of gram-negative sepsis with increasing severity. Meanwhile, there were higher rates of maternal risk factors such as premature rupture of membranes in the neonates with EOS than with LOS in our study. This finding could explain why gram-positive sepsis was relatively more common in EOS than in LOS.
In the current study, we observed a significant positive correlation of D-dimer with CRP level, duration of hospitalization, and mortality rate. CRP is a marker of inflammation and plays a role in the inflammatory process itself, activating the complement pathway, phagocytosis, apoptosis, and the production and release of cytokines[16]. CRP evaluation has a role in neonatal sepsis diagnosis even though many studies showed low or at least variable validity in screening neonatal sepsis and being a non-specific test[17,18]. However, it is a good indicator of the success of the antibiotic treatment[19]. The addition of D-dimer to CRP can increase the sensitivity and specificity of both tests in the diagnosis of neonatal sepsis. We also observed a significant positive correlation of the level of D-dimer with the duration of hospitalization, which agrees with the results of previous studies[20,21]. The positive correlation of D-dimer level with the mortality rate observed in the current research is related to many factors, as high D-dimer is observed in gram-negative sepsis, which carries high mortality risk and is associated with elevated CRP, indicating the severity of inflammation.
The high mortality observed in the current study is related to the high percentage of gram-negative sepsis included in the study. Our NICU is the leading tertiary NICU in the region, receiving critically sick and septic neonates from peripheral units. Most of the isolated gram-negative organisms were Acinetobacter and Klebsiella; most were MDR. Meanwhile, many neonates had severe thrombocytopenia and markedly elevated CRP, which predict a worse prognosis. Our results agree with the meta-analysis done by Shah et al[22]. They found that patients with COVID-19 infection and elevated D-dimer levels had a higher risk of severe morbidity and mortality. Our results also agree with Ay et al[23], who found that a high D-dimer level was associated with a poor survival rate and high mortality rate in patients with cancer.
In the current study, we found a significant negative correlation of D-dimer with both hemoglobin (%) and the platelet count. Platelets have an active role in the host defense mechanisms as they can perform phagocytosis. Their activation helps generate cytotoxic free radicals and oxidative molecules that destroy the invading organisms[24]. The current study found thrombocytopenia in 73% and 40% of EOS and LOS, respectively. Thrombocytopenia could be one of the presenting signs of neonatal sepsis but lack sensitivity and specificity and may appear late in the disease, which questions its usefulness as an initial marker of neonatal sepsis. However, we found a significant negative correlation between platelet count and plasma D-dimer levels. This correlation could reflect early or developing DIC, linked to increased fibrin degradation products (FDP) and D-dimer levels and increased platelet consumption[25]. Other possible causes of neonatal sepsis-associated thrombocytopenia could be increased platelet activation, diffuse endothelial cell injury, and bacterial/fungal toxins-associated platelet destruction[26]. Our results agree with Ree et al[27]. They reported that thrombocytopenia is independently associated with intravascular thrombosis and gram-negative sepsis, increasing the mortality risk nearly four to six-fold, especially in gram-negative sepsis.
We have some limitations in the current study. We had a relatively small sample size. At the same time, the study was conducted in a single institution, so the results could not be generalized.
Neonatal sepsis is a life-threatening disease with high mortality and morbidity. The D-dimer is an exciting and promising biomarker for neonatal sepsis, able to predict morbidity and mortality. The current study revealed a significant diagnostic value for the D-dimer in neonatal sepsis. D-dimer can be used as an adjunct to other sepsis markers to increase the sensitivity and specificity of diagnosing neonatal sepsis.
Neonatal sepsis is one of the critical conditions that put the life of neonates in danger. It is a severe systemic inflammatory response to blood-stream infections with significant neonatal morbidity and mortality. Early and proper diagnosis of neonatal sepsis is critical for timely-administered antibiotics, decreases the length of the hospital stay, and improves the prognosis, especially the neurodevelopmental outcome.
Early and proper diagnosis of neonatal sepsis is critical for appropriate and effective management with timely-administered antibiotics to decrease the hospitalization length and improve the prognosis, especially for the neurodevelopmental prospects.
We aimed to evaluate the significance of plasma D-dimer level in the early diagnosis of neonatal sepsis and elaborate on its clinicopathological value in neonates with early-onset and late-onset neonatal sepsis.
The study was a prospective cross-sectional study that included ninety neonates; divided into early-onset sepsis (EOS) group (Group I), late-onset sepsis (LOS) group (Group II), and control group (Group III). We diagnosed neonatal sepsis according to our protocol. C-reactive protein (CRP) and D-dimer assay were compared and related to the causative microbiological agents.
D-dimer was significantly higher in septic groups. Septic groups showed a significantly higher number of cases with positive D-dimer. Neonates with LOS had considerably higher levels of D-dimer than EOS. At the same time, there were no significant differences in CRP levels in neonates with EOS or LOS. However, neonates with LOS had a significantly longer duration of hospitalization and higher mortality rates than neonates with EOS. The rate of gram-negative bacteremia was substantially higher in LOS than in EOS, while the rate of gram-positive bacteremia was significantly higher in EOS than in LOS (P < 0.01). Gram-negative bacteria have the highest D-dimer levels (Acinetobacter, Klebsiella, and Pseudomonas) and CRP (Serratia, Klebsiella, and Pseudomonas). On the other hand, gram-positive sepsis was associated with relatively lower levels of D-dimer and CRP. D-dimer had a significant negative correlation with hemoglobin level and platelet count while having a significant positive correlation with CRP, duration of the hospital stays, and mortality. The best-suggested cut-off point for D-dimer in neonatal sepsis was 0.75 mg/L, giving a sensitivity of 72.7% and specificity of 86.7%. The D-dimer assay showed lower specificity and comparable sensitivity relative to CRP in the current study.
The study revealed a significant diagnostic value for D-dimer in neonatal sepsis. D-dimer can be used as an adjunct to other sepsis markers to increase the sensitivity and specificity of diagnosing neonatal sepsis.
To generalize our results, the authors need to include larger sample size and perform a multicenter study.
We thank the editors and the anonymous referees for their valuable suggestions.
| 1. | Simonsen KA, Anderson-Berry AL, Delair SF, Davies HD. Early-onset neonatal sepsis. Clin Microbiol Rev. 2014;27:21-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 481] [Cited by in RCA: 623] [Article Influence: 51.9] [Reference Citation Analysis (1)] |
| 2. | Marks L, de Waal K, Ferguson JK. Time to positive blood culture in early-onset neonatal sepsis: A retrospective clinical study and review of the literature. J Paediatr Child Health. 2020;56:1371-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Morad EA, Rabie RA, Almalky MA, Gebriel MG. Evaluation of Procalcitonin, C-Reactive Protein, and Interleukin-6 as Early Markers for Diagnosis of Neonatal Sepsis. Int J Microbiol. 2020;2020:8889086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | Semeraro N, Ammollo CT, Semeraro F, Colucci M. Sepsis-associated disseminated intravascular coagulation and thromboembolic disease. Mediterr J Hematol Infect Dis. 2010;2:e2010024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 92] [Cited by in RCA: 114] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 5. | Fiusa MM, Carvalho-Filho MA, Annichino-Bizzacchi JM, De Paula EV. Causes and consequences of coagulation activation in sepsis: an evolutionary medicine perspective. BMC Med. 2015;13:105. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 57] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 6. | Trimaille A, Thachil J, Marchandot B, Curtiaud A, Leonard-Lorant I, Carmona A, Matsushita K, Sato C, Sattler L, Grunebaum L, Hansmann Y, Fafi-Kremer S, Jesel L, Ohana M, Morel O. D-Dimers Level as a Possible Marker of Extravascular Fibrinolysis in COVID-19 Patients. J Clin Med. 2020;10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 7. | Coskun F, Yilmaz D, Ursavas A, Uzaslan E, Ege E. Relationship between disease severity and D-dimer levels measured with two different methods in pulmonary embolism patients. Multidiscip Respir Med. 2010;5:168-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Angele MK, Pratschke S, Hubbard WJ, Chaudry IH. Gender differences in sepsis: cardiovascular and immunological aspects. Virulence. 2014;5:12-19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 172] [Cited by in RCA: 255] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 9. | Chapin JC, Hajjar KA. Fibrinolysis and the control of blood coagulation. Blood Rev. 2015;29:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 411] [Cited by in RCA: 543] [Article Influence: 49.4] [Reference Citation Analysis (11)] |
| 10. | Peker E, Akbayram S, Geylani H, Dogan M, Kirimi E. Global fibrinolytic capacity in neonatal sepsis. Clin Appl Thromb Hemost. 2011;17:E64-E69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Mautone A, Giordano P, Montagna O, Quercia M, Altomare M, De Mattia D. Coagulation and fibrinolytic systems in the ill preterm newborn. Acta Paediatr. 1997;86:1100-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 12. | Brahmana AR, Lubis BM, Ali M. The role of d-dimer levels as a marker of neonatal sepsis. Glob J Res Anal. 2019;8:47-49. [DOI] [Full Text] |
| 13. | Pancham K, Anjula C, Parveen B, Lokesh C, Mohit K. D-dimer: A useful marker in neonatal sepsis. J Clin Neonatol. 2015;4:101-103. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Surbatovic M, Popovic N, Vojvodic D, Milosevic I, Acimovic G, Stojicic M, Veljovic M, Jevdjic J, Djordjevic D, Radakovic S. Cytokine profile in severe Gram-positive and Gram-negative abdominal sepsis. Sci Rep. 2015;5:11355. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 15. | Meini S, Sozio E, Bertolino G, Sbrana F, Ripoli A, Pallotto C, Viaggi B, Andreini R, Attanasio V, Rescigno C, Atripaldi L, Leonardi S, Bernardo M, Tascini C. D-Dimer as Biomarker for Early Prediction of Clinical Outcomes in Patients With Severe Invasive Infections Due to Streptococcus Pneumoniae and Neisseria Meningitidis. Front Med (Lausanne). 2021;8:627830. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 19] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 16. | Sproston NR, Ashworth JJ. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front Immunol. 2018;9:754. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 899] [Cited by in RCA: 1896] [Article Influence: 237.0] [Reference Citation Analysis (0)] |
| 17. | Khan F. C-reactive Protein as a Screening Biomarker in Neonatal Sepsis. J Coll Physicians Surg Pak. 2019;29:951-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Hisamuddin E, Hisam A, Wahid S, Raza G. Validity of C-reactive protein (CRP) for diagnosis of neonatal sepsis. Pak J Med Sci. 2015;31:527-531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 23] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 19. | Ahmed E, Rehman A, Ali MA. Validation of serum C-reactive protein for the diagnosis and monitoring of antibiotic therapy in neonatal sepsis. Pak J Med Sci. 2017;33:1434-1437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Dhainaut JF, Shorr AF, Macias WL, Kollef MJ, Levi M, Reinhart K, Nelson DR. Dynamic evolution of coagulopathy in the first day of severe sepsis: relationship with mortality and organ failure. Crit Care Med. 2005;33:341-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 191] [Article Influence: 9.1] [Reference Citation Analysis (5)] |
| 21. | Rodelo JR, De la Rosa G, Valencia ML, Ospina S, Arango CM, Gómez CI, García A, Nuñez E, Jaimes FA. D-dimer is a significant prognostic factor in patients with suspected infection and sepsis. Am J Emerg Med. 2012;30:1991-1999. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 22. | Shah S, Shah K, Patel SB, Patel FS, Osman M, Velagapudi P, Turagam MK, Lakkireddy D, Garg J. Elevated D-Dimer Levels Are Associated With Increased Risk of Mortality in Coronavirus Disease 2019: A Systematic Review and Meta-Analysis. Cardiol Rev. 2020;28:295-302. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 23. | Ay C, Dunkler D, Pirker R, Thaler J, Quehenberger P, Wagner O, Zielinski C, Pabinger I. High D-dimer levels are associated with poor prognosis in cancer patients. Haematologica. 2012;97:1158-1164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 254] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 24. | Ali RA, Wuescher LM, Worth RG. Platelets: essential components of the immune system. Curr Trends Immunol. 2015;16:65-78. [PubMed] |
| 25. | Sridhar A, Sunil Kumar BM, Rau A, Rau ATK. A Correlation of the Platelet Count with D-Dimer Levels as an Indicator for Component Therapy in Children with Dengue Hemorrhagic Fever. Indian J Hematol Blood Transfus. 2017;33:222-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 26. | Sheu JR, Hung WC, Wu CH, Ma MC, Kan YC, Lin CH, Lin MS, Luk HN, Yen MH. Reduction in lipopolysaccharide-induced thrombocytopenia by triflavin in a rat model of septicemia. Circulation. 1999;99:3056-3062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 77] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 27. | Ree IMC, Fustolo-Gunnink SF, Bekker V, Fijnvandraat KJ, Steggerda SJ, Lopriore E. Thrombocytopenia in neonatal sepsis: Incidence, severity and risk factors. PLoS One. 2017;12:e0185581. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 60] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Critical care medicine
Country/Territory of origin: Egypt
Peer-review report's scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Yang L, China; Yellanthoor RB, India S-Editor: Ma YJ L-Editor: A P-Editor: Ma YJ