Published online Nov 9, 2021. doi: 10.5492/wjccm.v10.i6.390
Peer-review started: April 3, 2021
First decision: June 5, 2021
Revised: June 7, 2021
Accepted: October 11, 2021
Article in press: October 11, 2021
Published online: November 9, 2021
Processing time: 215 Days and 8.4 Hours
Acute kidney injury (AKI) is a common and severe complication after left ventricular assist device (LVAD) implantation with an incidence of 37%; 13% of which require kidney replacement therapy (KRT). Severe AKI requiring KRT (AKI-KRT) in LVAD patients is associated with high short and long-term mortality compared with AKI without KRT. While kidney function recovery is associated with better outcomes, its incidence is unclear among LVAD patients with severe AKI requiring KRT.
To identify studies evaluating the recovery rates from severe AKI-KRT after LVAD placement, which is defined by regained kidney function resulting in the discontinuation of KRT. Random-effects and generic inverse variance method of DerSimonian-Laird were used to combine the effect estimates obtained from individual studies.
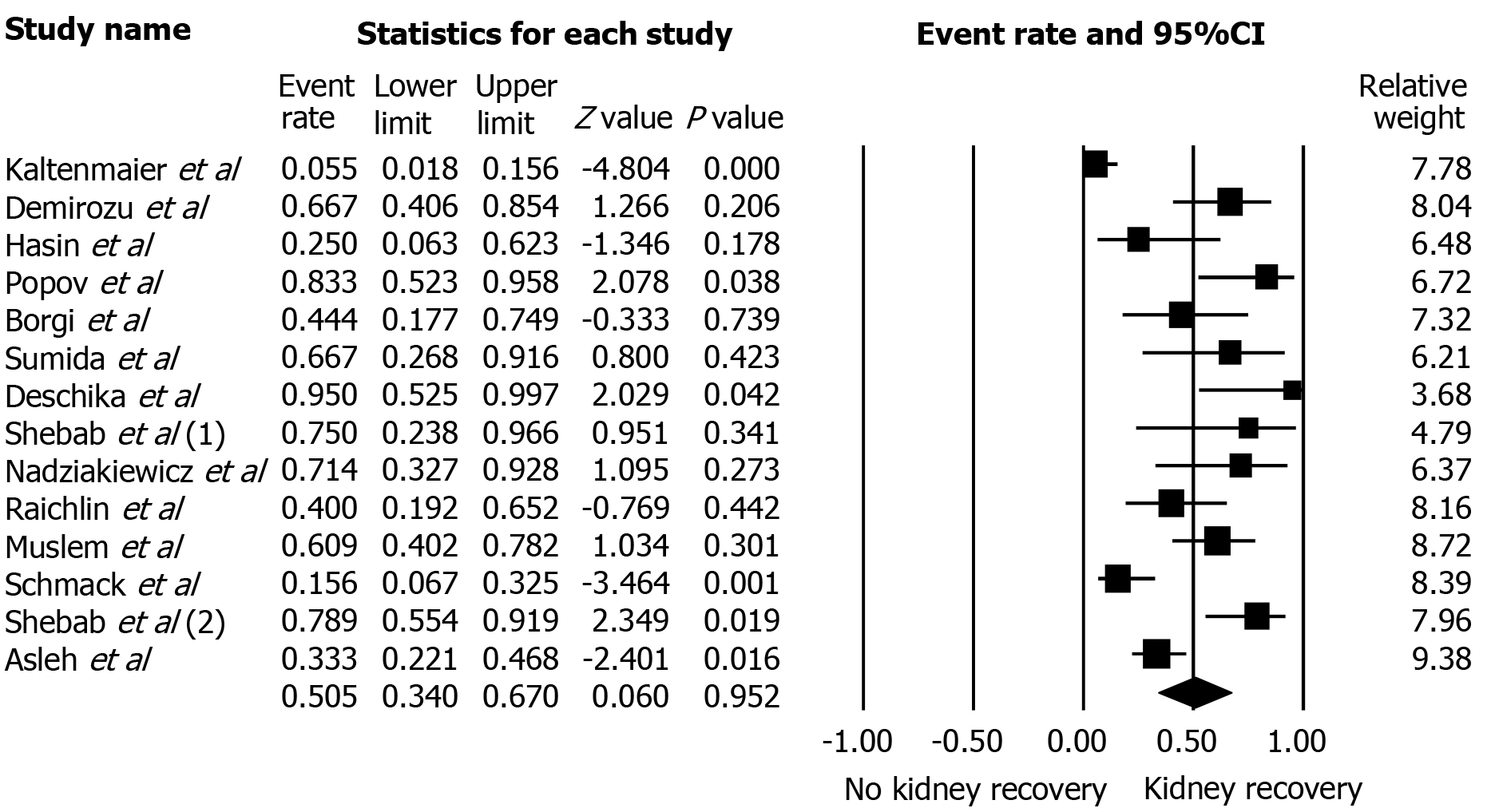
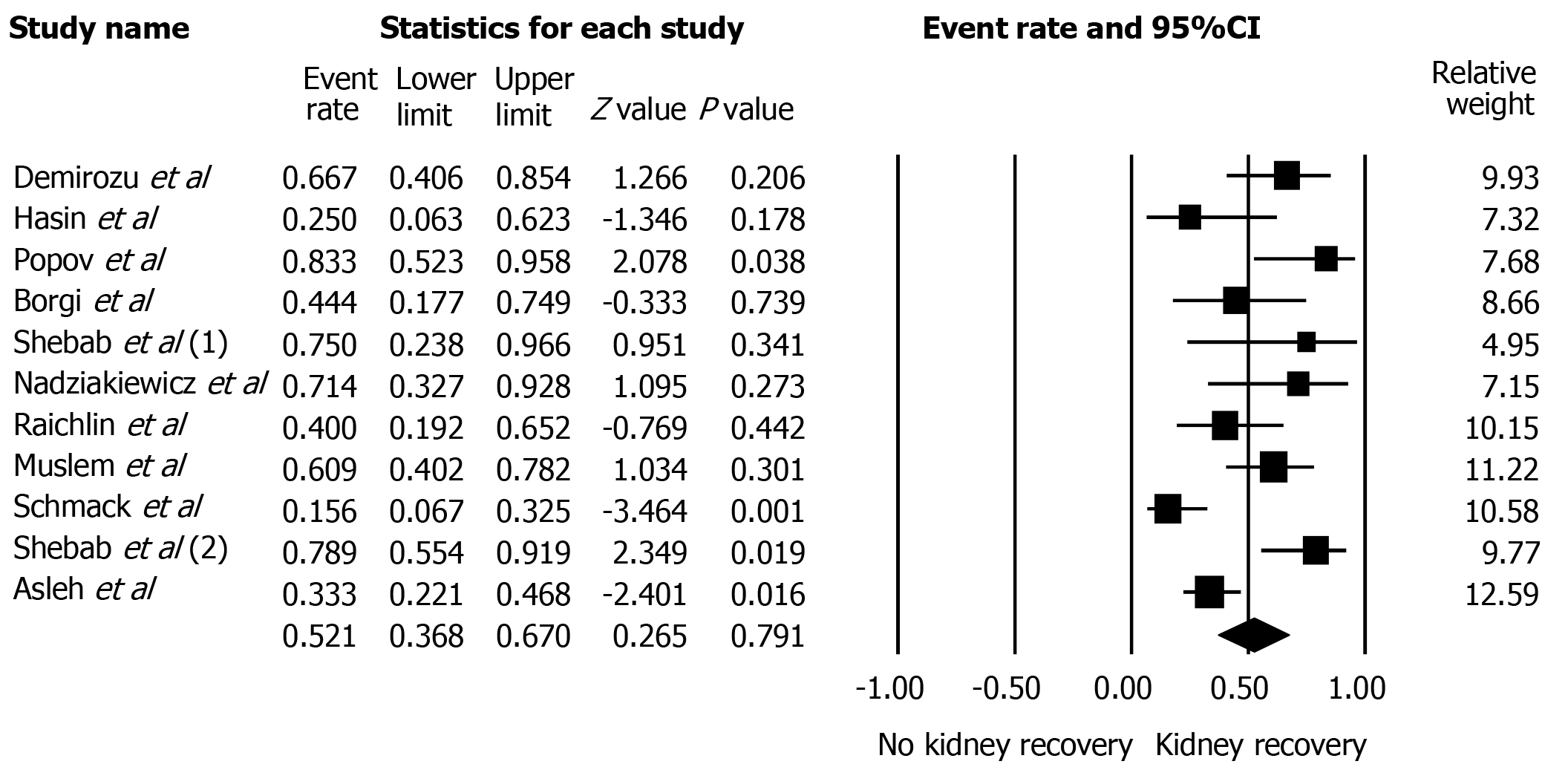
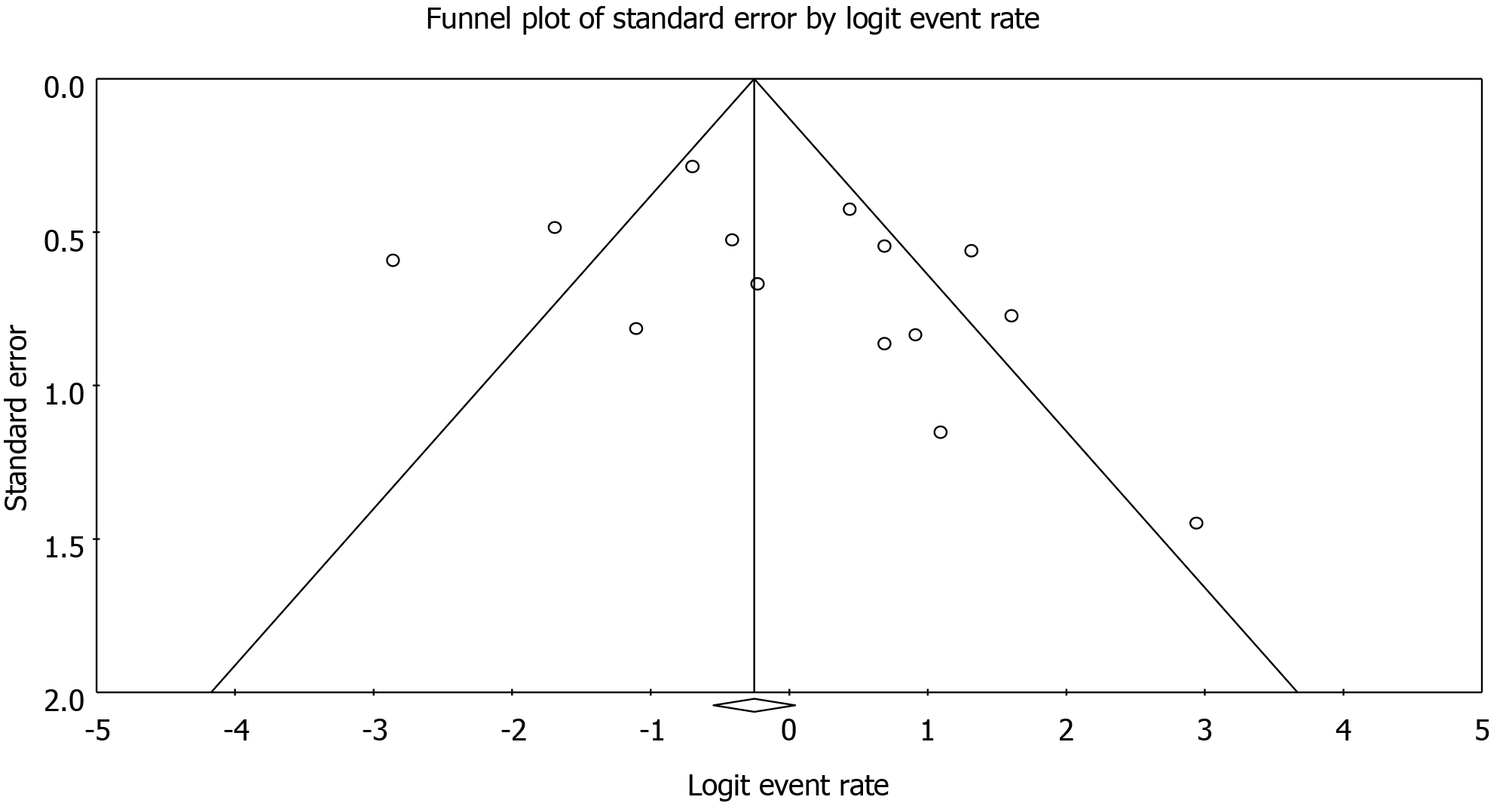
A total of 268 patients from 14 cohort studies that reported severe AKI-KRT after LVAD were included. Follow-up time ranged anywhere from two weeks of LVAD implantation to 12 mo. Kidney recovery occurred in 78% of enrollees at the time of hospital discharge or within 30 d. Overall, the pooled estimated AKI recovery rate among patients with severe AKI-KRT was 50.5% (95%CI: 34.0%-67.0%) at 12 mo follow up. Majority (85%) of patients used continuous-flow LVAD. While the data on pulsatile-flow LVAD was limited, subgroup analysis of continuous-flow LVAD demonstrated that pooled estimated AKI recovery rate among patients with severe AKI-KRT was 52.1% (95%CI: 36.8%-67.0%). Meta-regression analysis did not show a significant association between study year and AKI recovery rate (P = 0.08). There was no publication bias as assessed by the funnel plot and Egger's regression asymmetry test in all analyses.
A total of 268 patients from 14 cohort studies that reported severe AKI-KRT after LVAD were included. Follow-up time ranged anywhere from two weeks of LVAD implantation to 12 mo. Kidney recovery occurred in 78% of enrollees at the time of hospital discharge or within 30 d. Overall, the pooled estimated AKI recovery rate among patients with severe AKI-KRT was 50.5% (95%CI: 34.0%-67.0%) at 12 mo follow up. Majority (85%) of patients used continuous-flow LVAD. While the data on pulsatile-flow LVAD was limited, subgroup analysis of continuous-flow LVAD demonstrated that pooled estimated AKI recovery rate among patients with severe AKI-KRT was 52.1% (95%CI: 36.8%-67.0%). Meta-regression analysis did not show a significant association between study year and AKI recovery rate (P = 0.08). There was no publication bias as assessed by the funnel plot and Egger's regression asymmetry test in all analyses.
Recovery from severe AKI-KRT after LVAD occurs approximately 50.5%, and it has not significantly changed over the years despite advances in medicine.
Core Tip: Left ventricular assist devices (LVAD) are mechanical support tools that augment cardiac output and improve kidney perfusion. Acute Kidney Injury (AKI) is a common complication after LVAD implantation. High short- and long-term mortality is associated with severe AKI requiring Kidney replacement therapy (KRT) in LVAD patients compared with those without KRT. While kidney function recovery is associated with better outcomes, the recovery rate is unclear among LVAD patients with severe AKI requiring KRT. To investigate this further, we conducted the current systematic review and meta-analysis evaluating kidney recovery rate after AKI-KRT among LVAD patients. We report that the pooled estimated AKI recovery rate among patients with severe AKI-KRT was 50.5% (95%CI: 34.0%-67.0%) at 12 mo follow up.
- Citation: Kovvuru K, Kanduri SR, Thongprayoon C, Bathini T, Vallabhajosyula S, Kaewput W, Mao MA, Cheungpasitporn W, Kashani KB. Recovery after acute kidney injury requiring kidney replacement therapy in patients with left ventricular assist device: A meta-analysis. World J Crit Care Med 2021; 10(6): 390-400
- URL: https://www.wjgnet.com/2220-3141/full/v10/i6/390.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v10.i6.390
Heart transplantation remains the treatment of choice for patients with severe end-stage heart failure. Deteriorating kidney function is commonly noted among advanced heart failure patients secondary to cardiorenal physiology and is associated with unfavorable outcomes[1-4]. Left ventricular assist devices (LVAD) are mechanical support tools that augment cardiac output by unloading the left ventricle and improving kidney perfusion. LVAD is used as a bridge to transplantation for patients on the transplant list or destination therapy for individuals who are not ideal transplant candidates[5].
Even though kidney perfusion improves in most patients, acute kidney injury (AKI) is a common and severe complication following LVAD implantation with an incidence of 37%[6]. About one-third of them (13%) sustain severe AKI post LVAD placement needing kidney replacement therapy (KRT)[6]. As reported in previous studies, severe AKI-KRT in LVAD patients is associated with high short and long-term mortality compared to those without KRT[7]. Risk factors associated with increased risk of AKI post LVAD insertion include older age, use of intra-aortic balloon pump (IABP), lower mean total protein and albumin levels, post-implantation shock, elevated central venous pressure > 16 mmHg, longer cardiopulmonary bypass times, postoperative right ventricular failure and preexisting chronic kidney disease before implantation[8-10].
Kidney recovery is defined as independence from KRT in AKI-KRT patients within fourteen d of the initial injury [11]. In a study by Grinstein et al[12], early kidney improvement is defined as an increase in eGFR ≥ 15% within one week of LVAD implantation. In a recent prospective, multicenter assessment, serial evaluation, and subsequent sequelae (ASSESS-AKI) cohort study by Bhatraju et al[13] evaluating the incidence and progression of chronic kidney disease (CKD) and dialysis in patients who sustained AKI episodes as compared to patients without AKI, a 2- and 3-fold higher risk of major kidney adverse effects were reported among those with resolving and non-resolving AKI, respectively, as compared to patients without AKI. Addi
Early improvement in kidney function in patients with AKI after LVAD placement is associated with decreased length of stay and fewer complications [14]. Kidney recovery is associated with a favorable prognosis in estimating postoperative kidney function in adults and children undergoing LVAD placement [15]. While recovery of kidney function is associated with better outcomes, kidney recovery rates among LVAD patients with severe AKI-KRT are unknown. We, therefore, conducted the current metanalysis to report the incidence of kidney recovery among patients needing KRT post LVAD implantation at 30 d or at the time of discharge and up to 12 mo.
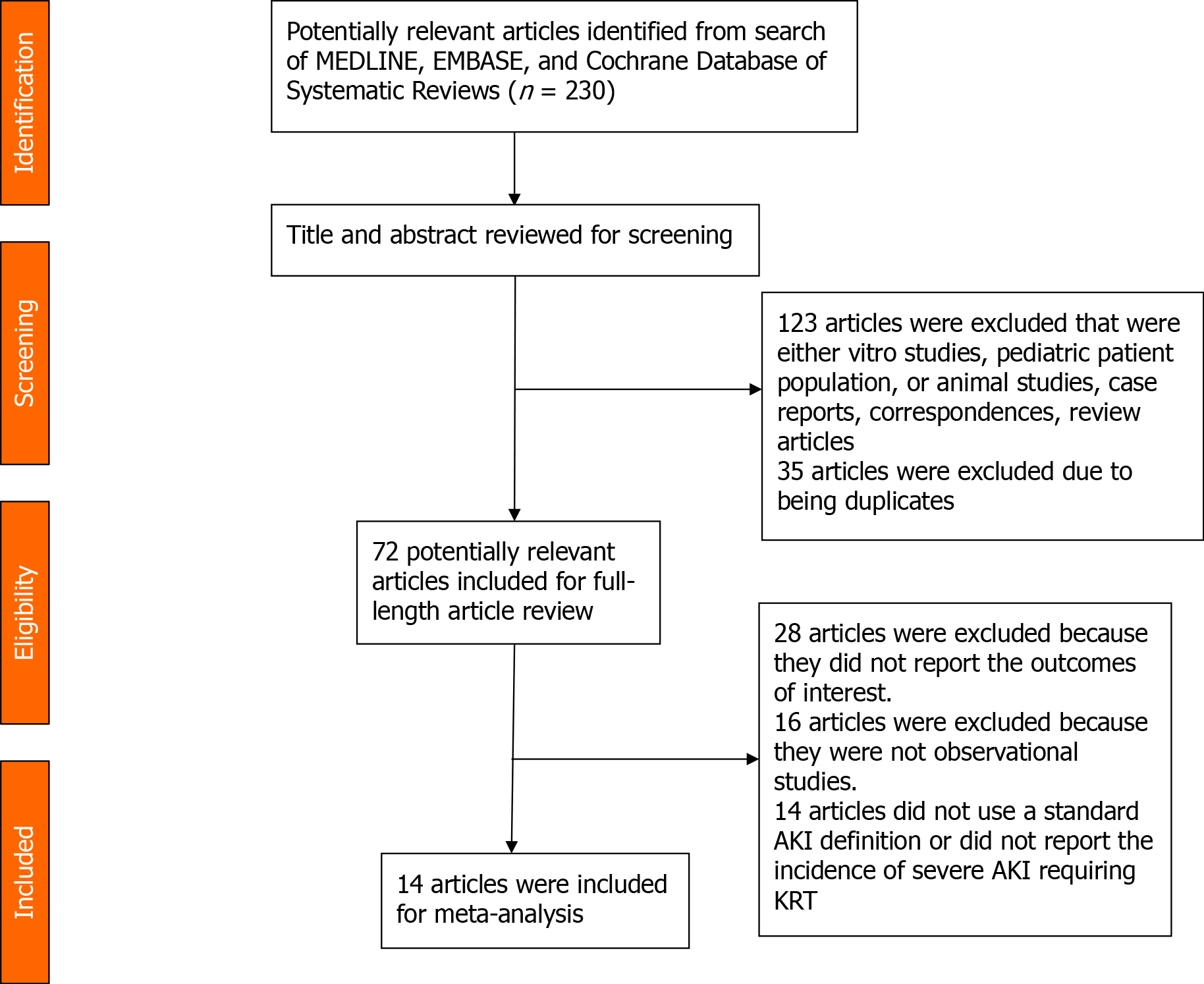
This manuscript follows the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-analysis)[16] statement and MOOSE (Meta-analysis of Observational Studies in Epidemiology)[17] guidelines. A systematic search was conducted through the Ovid MEDLINE, EMBASE, and Cochrane Library from database inception to January 2020 using the following search terms: ('left ventricular assist device' OR 'lvad' OR 'ventricular assist device') AND ('acute kidney failure' OR 'acute kidney injury' OR 'renal replacement therapy' OR dialysis). The detailed search strategy for each database is summarized in Supplementary material. No language restrictions were applied.
The following inclusion criteria determined the eligibility of each article, including: (1) The nature of the study is observational or conference abstract; (2) Study population consisted of patients with LVAD; and (3) The rates of kidney recovery after AKI episode among patients after LVAD placement is considered one of the outcomes of interest. Exclusion criteria consisted of pediatric patients, case series, and studies that did not mention outcomes of interest. Study eligibility was independently evaluated by two investigators (Kovvuru K and Kanduri SR). Any disagreements were resolved by mutual consensus. The quality of each study was appraised using the Newcastle–Ottawa quality scale[18], which assesses six components, including: (1) Representativeness of the subjects; (2) Ascertainment of the exposure; (3) Demon
The titles and abstracts of all identified studies were screened (Kovvuru K and Kanduri SR) before a full-text review. The full-text of the screened articles was reviewed to determine their eligibility. We created a standardized data collection form to extract the relevant information from the included studies, including the first author's name, year of publication, country of origin, study design, sample size, AKI definition, number of patients with AKI, rate of kidney recovery, duration of follow up. Kidney recovery was defined as independence from dialysis after an episode of severe AKI.
The rates of kidney recovery among patients with severe AKI-KRT and kidney recovery rates among the subgroup of patients with continuous-flow devices entered the meta-analysis. The results were reported in percentage along with 95% confidence interval (CI). A Forest plot of each analysis was created. Results were presented in percentage for categorical data and in mean ± SD or median (interquartile range) for continuous data.
Publication bias was evaluated by funnel plot (if the total number of studies was > 10[18] and Egger's regression intercept. An intercept P value of less than 0.05 was considered significant for potential publication bias.
All statistical analyses were performed by the Comprehensive Meta-analysis version 3 software (Eaglewood, NJ, United States). Statistical heterogeneity of the included studies was assessed using Cochran's Q-test and I2statistics. An I2value of ≤ 25% represents insignificant heterogeneity, 25%-50% represents low heterogeneity, 50%-75% represents moderate heterogeneity, and > 75% represents high heterogeneity. For analyses with I2> 50%, the results were analyzed by the random-effects model to minimize the heterogeneity and external variance[20]. A P value of less than 0.05 represents statistical significance.
A total of 14 studies[7,8,21-31], consisting of 268 subjects, were included in the current meta-analysis. Figure 1 provides a flowchart of the literature search and study selection for this analysis. Included studies were published from 2000 to 2019. The study designs included retrospective and prospective cohort studies. The total duration of follow-up was anywhere from 2 wk to 12 mo. Table 1 illustrates study characteristics and kidney recovery rates among patients included in this systematic review.
| Study | Year | Country | Patients | AKI definition | No of patients with AKI | Rate of kidney recovery |
| Kaltenmaier et al[31] | 2000 | Germany | LVAD-implantation during 1988-1995; Pulsatile Berlin Heart System HeartMate 2000, Novacor. | KRT | 55 | 3/55 = 6%; Kidney recovery at hospital discharge |
| Demirozu et al[21] | 2011 | United States | LVAD implantation during 2003-2009; Continuous HeartMate II | KRT | 15 | 10/15 = 67%; Kidney recovery at 7 mo |
| Hasin et al[8] | 2012 | United States | LVAD from 2007 to 2010; Continuous HeartMate II | KRT | 8 | 2/8 = 25%; Kidney recovery at 6 mo |
| Popov et al[22] | 2012 | United Kingdom | Patient with end-stage heart failure underwent LVAD implantation-2007-2011; Continuous Heart Ware | KRT | 12 | 10/12 = 83%; Kidney recovery post-op/ |
| Borgi et al[7] | 2013 | United States | End-stage heart failure LVAD during 2006-2011; Continuous HeartMate II; Heart Ware | AKI (KDIGO); KRT | 28; 9 | 17/28 = 60%; Kidney recovery one month; 4/9 = 44.5%; Kidney recovery after KRT--one month |
| Sumida et al[23] | 2014 | Japan | LVAD implantation during 2011-2013; LVAD type not specified | AKI; KDIGO; KRT | 11; 6 | 11/11 = 100%; Kidney recovery at Hospital discharge; 4/6 = 66.6%; Kidney recovery after KRT at hospital discharge |
| Deschika et al[24] | 2016 | Germany | LVAD recipients with pre-operative biventricular impairment who received an additionally RVAD | KRT | 9 | 9/9 = 100%; Kidney recovery at hospital discharge |
| Shebab et al[25] | 2016 | Australia | Dilated cardiomyopathy and severe biventricular failure -underwent dual HVAD implantation as a bridge to transplant during 2011-2014; Continuous Heart Ware | KRT | 4 | 3/4 = 75%; Kidney recovery at post-op |
| Nadziakiewicz et al[26] | 2016 | Portland | Patients with end-stage heart failure underwent LVAD implantation during 2007-2014; Continuous Heart ware, HeartMate II | KRT | 7 | 5/7 = 72%; Kidney recovery after KRT- 2 weeks |
| Raichlin et al[27] | 2016 | United States | End-stage heart failure with preexisting kidney dysfunction underwent LVAD implantation -2009-2014; Continuous HeartMate II | KRT | 15 | 6/15 = 40%; Kidney recovery after KRT -one month |
| Muslem et al[32] | 2018 | Netherlands, United States | LVAD implantation during 2004-2015; Continuous Heart ware, HeartMate II | KRT | 23 | 14/23 = 61%; Kidney recovery after KRT at one year |
| Schmack et al[28] | 2018 | Germany | End-stage heart failure patients underwent LVAD from 2010 to 2017; Continuous Heart Ware | KRT | 32 | 5/32 = 16%; Kidney recovery one-month post-KRT |
| Shebab et al[29] | 2018 | Australia | LVAD implantation as a bridge to transplant from 2007 to 2016; Continuous Heart Ware | KRT | 19 | 15/19 = 79%; Kidney recovery after KRT Post-op |
| Asleh et al[30] | 2019 | United States | LVAD implantation during 2007-2017; ContinuousHeartMate II; HeartMate III; Heart Ware | KRT | 54 | 18/54 = 33%; Kidney recovery at hospital discharge |
Asleh et al[30] reported among patients requiring KRT after LVAD placement, one-third had kidney recovery, one-third required outpatient hemodialysis, and one-third of the patients died before hospital discharge. In study by Borgi et al[7] patients with post LVAD AKI were more likely to suffer longer hospital stay (32.4 vs 18.7; P = 0.05), right ventricular (RV) failure (25% vs 5.6%; P = 0.01) and a higher mortality rate as compared to non-AKI groups at 30-day (17.9% vs 0%; P < 0.001), 180-day (28.6% vs 2.8%; P < 0.001), and 360- day (28.6% vs 6.9%; P = 0.012), respectively. In a study by Demirozu et al[21], patients with sustained clinical recovery after LVAD eventually had kidney recovery. Muslem et al[32] evaluated long-term mortality after LVAD placement and reported that severe AKI (i.e., stages 2 and 3) was associated with higher mortality (hazard ratio 2.2, [95%CI: 1.1 to 4.5], P = 0.027) at one year. Schmack et al[28] reported higher pre-operative blood urea nitrogen (BUN) and low albumin levels as strong predictors of the need for kidney replacement therapy post LVAD implantation.
Additionally, they reported a negative association between postoperative hemo
78.5% of patients had kidney recovery occurred at the time of hospital discharge or within 30 d. Overall, the pooled estimated rates of AKI recovery among patients with severe AKI-KRT was 50.5% (95%CI: 34.0%-67.0%) (Figure 2) and did not significantly change over the years despite advances in medicine. Meta-regression analysis did not demonstrate a significant association between study year and AKI recovery rate (P = 0.08).
The data on pulsatile-flow LVAD were limited, as the majority (85%) of patients used continuous-flow LVAD. Subgroup analysis of continuous-flow LVAD demonstrated the pooled estimated rates of AKI recovery among patients with severe AKI-KRT was 52.1% (95%CI: 36.8%-67.0%) (Figure 3).
Funnel plots (Figure 4) and Egger's regression asymmetry tests were performed to assess publication bias in analysis evaluating the rate of AKI recovery. No significant publication bias in the meta-analysis evaluating rates of AKI recovery among patients with AKI (P = 0.17) was evident.
Our analysis included 14 cohort studies that defined severe AKI as needing KRT(AKI-KRT). Kidney recovery occurred in 78.5% of patients at the time of hospital discharge or within 30 d of LVAD implantation. The initial improvement in kidney function could be secondary to hemodynamic stabilization, cardiac output optimization, and reduction in kidney venous pressures. The subsequent rise in cardiac output facilitates kidney perfusions and glomerular filtration rates (GFR)[33].
In our analysis, kidney function recovery occurred in about half of individuals with AKI-KRT. Even though 70% had initial kidney recovery within 30 d of LVAD initiation, kidney recovery rates when followed for up to 12 mo were only 50%. This observation is consistent with previous studies demonstrating a sustained and gradual decline in GFR at long-term follow-up. As evidenced by Brisco et al[34], even though half of the patients had initial improvement in GFR after one month of LVAD implantation, a significant decline in GFR was noted at one year. Similar findings were also reported by Hasin et al[8] with initial improvement in GFR at one month followed by an eventual decline at 3 and 6 mo, respectively.
The potential mechanisms for the eventual decline in kidney functions are multifactorial. Chronic hemolysis is caused by shear stress leading to red blood cell breakdown and pigment nephropathy[35]. Subsequent development of right ventricular failure after LVAD placement could contribute to a decline in kidney functions. Additionally, GFR could be overestimated post LVAD implantation secondary to reduced creatinine generation from sarcopenia and volume overload. Cystatin-based calculations of kidney clearances can be used to provide better insight into kidney functions [36].
Lack of pulsatility among continuous flow devices could lead to structural changes in the arterial system, perpetuating aortic wall stiffness. Animal studies demonstrated periarteritis and subsequent inflammation with continuous-flow devices, potentiating increased AKI risk[37]. However, the previous metanalysis reported almost similar AKI rates among patients with continuous and pulsatile flow devices[6]. Subgroup analysis on continuous-flow LVAD revealed pooled incidence of kidney recovery after AKI episode leading to KRT independence was 52%. Given limited data, we could not analyze kidney recovery among AKI-KRT with pulsatile flow LVAD. However, we hypothesize that recovery rates after AKI-KRT among patients with pulsatile flow LVAD could be similar to continuous flow devices given similar AKI rates.
Another interesting observation in our analysis is pooled incidence of kidney recovery from KRT is 50%, which is reassuring compared to kidney recovery rates of other cohorts like hematopoietic stem cell transplant (HSCT). The pooled estimated kidney recovery rates after severe AKI-KRT at 100 d among the HSCT cohort are as low as 10%[38]. This difference could be secondary to multiple factors as patients after HSCT are much sicker from underlying terminal cancer and exposed to high-dose chemotherapy or radiation. However, the HSCT cohort was followed for only 100 d, and unclear if long-term follow-up would generate encouraging results.
Few measures to enhance kidney recovery during the post-AKI/acute kidney disease (AKD) phase include medication reconciliation, avoidance of nephrotoxic drugs, avoiding supra therapeutic vancomycin levels, and contrast agents. Meticulous care should be taken to minimize hypotension during dialysis sessions[39]. Adequate catheter care education should be provided to patients and families at discharge and as an outpatient. Patients should be well informed of blood pressure goals, diuretics, bodyweight targets, and sick day protocol during the recovery period. The severity of kidney disease should be considered while managing AKI patients after LVAD, especially those requiring KRT[40]. An algorithmic approach should be protocolized in implementing diagnostic and therapeutic interventions to facilitate rapid and complete kidney function recovery.
Our study has few strengths. This is the first study analyzing kidney recovery rates after severe AKI-KRT among patients with LVAD implantation. We report that about half of the patients, when followed closely, have dialysis independence after LVAD. To mention few limitations, the cohort studies included in our analysis might not identify a causal relationship between patients with AKI-KRT and kidney recovery rates. However, they report associations between the two variables. The overall analysis showed significant statistical heterogeneity questioning the validity of included studies. However, we found similar rates of kidney recovery in the sub-group analysis. Additionally, we do not have the mean GFR of patients before LVAD insertion and after kidney recovery from KRT. Lastly, data on AKI recovery impact on outcomes among patients after LVAD insertion were not reported.
In conclusion, recovery from severe AKI-KRT after LVAD occurs in approximately 50.5%. Recovery of kidney functions is associated with improved kidney function, fewer complications, and better outcomes than patients with non-resolving AKI. Hence, adequate measures should be taken to facilitate diagnostic and therapeutic approaches aiming for early and complete kidney recovery.
Acute kidney injury (AKI) is a common (37%) and severe complication after left ventricular assist device (LVAD) implantation, and 13% require kidney replacement therapy (KRT). Severe AKI requiring KRT in LVAD patients is associated with high short-term and long-term mortality compared with those without KRT.
While recovery of kidney function is associated with better outcomes, the recovery rates of kidney function among LVAD patients with severe AKI-KRT are unclear.
To demonstrate the rates of kidney recovery among patients with AKI-KRT after LVAD implantation.
Eligible articles were searched through Ovid MEDLINE, EMBASE, and the Cochrane Library. The inclusion criteria included adult patients with recovery from severe AKI-KRT after LVAD placement, which is defined by regained kidney function resulting in discontinuation of KRT.
A total of 268 patients from 14 cohort studies with severe AKI-KRT after LVAD were enrolled. Follow-up time ranges from 2 wk of LVAD implantation up to 12 mo. 78.5% of kidney recovery occurred at the time of hospital discharge or within 30 d. The majority (85%) of patients used continuous-flow LVAD. Overall, the pooled estimated AKI recovery rates among patients with severe AKI-KRT were 50.5% (95%CI: 34.0%-67.0%). While the data on pulsatile-flow LVAD was limited, subgroup analysis of continuous-flow LVAD demonstrated the pooled estimated AKI recovery rates among patients with severe AKI-KRT was 52.1% (95%CI: 36.8%-67.0%). Meta-regression analysis did not show a significant association between study year and AKI recovery rate (P = 0.08). There was no publication bias as assessed by the funnel plot and Egger's regression asymmetry test in all analyses.
Recovery from severe AKI-KRT after LVAD occurs approximately 50.5%, and it has not significantly changed over the years despite advances in medicine.
Our study results offer a perspective of rates of kidney recovery after AKI-KRT among patients with LVAD implantation. As recovery of kidney functions is associated with improved outcomes compared to those with no AKI recovery, we suggest a meticulous approach to monitoring patients post AKI and acute kidney disease in achieving early and complete kidney recovery.
| 1. | Forman DE, Butler J, Wang Y, Abraham WT, O'Connor CM, Gottlieb SS, Loh E, Massie BM, Rich MW, Stevenson LW, Young JB, Krumholz HM. Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J Am Coll Cardiol. 2004;43:61-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 663] [Cited by in RCA: 668] [Article Influence: 30.4] [Reference Citation Analysis (0)] |
| 2. | Hillege HL, Girbes AR, de Kam PJ, Boomsma F, de Zeeuw D, Charlesworth A, Hampton JR, van Veldhuisen DJ. Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation. 2000;102:203-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 708] [Article Influence: 27.2] [Reference Citation Analysis (5)] |
| 3. | Dries DL, Exner DV, Domanski MJ, Greenberg B, Stevenson LW. The prognostic implications of renal insufficiency in asymptomatic and symptomatic patients with left ventricular systolic dysfunction. J Am Coll Cardiol. 2000;35:681-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 635] [Cited by in RCA: 639] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 4. | McCullough PA, Kellum JA, Haase M, Müller C, Damman K, Murray PT, Cruz D, House AA, Schmidt-Ott KM, Vescovo G, Bagshaw SM, Hoste EA, Briguori C, Braam B, Chawla LS, Costanzo MR, Tumlin JA, Herzog CA, Mehta RL, Rabb H, Shaw AD, Singbartl K, Ronco C. Pathophysiology of the cardiorenal syndromes: executive summary from the eleventh consensus conference of the Acute Dialysis Quality Initiative (ADQI). Contrib Nephrol. 2013;182:82-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 112] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 5. | Patel AM, Adeseun GA, Ahmed I, Mitter N, Rame JE, Rudnick MR. Renal failure in patients with left ventricular assist devices. Clin J Am Soc Nephrol. 2013;8:484-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 6. | Thongprayoon C, Lertjitbanjong P, Cheungpasitporn W, Hansrivijit P, Fülöp T, Kovvuru K, Kanduri SR, Davis PW, Vallabhajosyula S, Bathini T, Watthanasuntorn K, Prasitlumkum N, Chokesuwattanaskul R, Ratanapo S, Mao MA, Kashani K. Incidence and impact of acute kidney injury on patients with implantable left ventricular assist devices: a Meta-analysis. Ren Fail. 2020;42:495-512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 7. | Borgi J, Tsiouris A, Hodari A, Cogan CM, Paone G, Morgan JA. Significance of postoperative acute renal failure after continuous-flow left ventricular assist device implantation. Ann Thorac Surg. 2013;95:163-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Hasin T, Topilsky Y, Schirger JA, Li Z, Zhao Y, Boilson BA, Clavell AL, Rodeheffer RJ, Frantz RP, Edwards BS, Pereira NL, Joyce L, Daly R, Park SJ, Kushwaha SS. Changes in renal function after implantation of continuous-flow left ventricular assist devices. J Am Coll Cardiol. 2012;59:26-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 149] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 9. | Rose EA, Gelijns AC, Moskowitz AJ, Heitjan DF, Stevenson LW, Dembitsky W, Long JW, Ascheim DD, Tierney AR, Levitan RG, Watson JT, Meier P, Ronan NS, Shapiro PA, Lazar RM, Miller LW, Gupta L, Frazier OH, Desvigne-Nickens P, Oz MC, Poirier VL; Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) Study Group. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345:1435-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3136] [Cited by in RCA: 2986] [Article Influence: 119.4] [Reference Citation Analysis (0)] |
| 10. | Patel UD, Hernandez AF, Liang L, Peterson ED, LaBresh KA, Yancy CW, Albert NM, Ellrodt G, Fonarow GC. Quality of care and outcomes among patients with heart failure and chronic kidney disease: A Get With the Guidelines -- Heart Failure Program study. Am Heart J. 2008;156:674-681. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Forni LG, Darmon M, Ostermann M, Oudemans-van Straaten HM, Pettilä V, Prowle JR, Schetz M, Joannidis M. Renal recovery after acute kidney injury. Intensive Care Med. 2017;43:855-866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 206] [Cited by in RCA: 339] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 12. | Grinstein J. Early Renal Recovery after Left Ventricular Assist Device Implantation is Associated with Improved Clinical Outcomes in Patients with Kidney Disease at Baseline. J Heart lung transplant. 2019;S359. |
| 13. | Bhatraju PK, Zelnick LR, Chinchilli VM, Moledina DG, Coca SG, Parikh CR, Garg AX, Hsu C-y, Go AS, Liu KD, Ikizler TA, Siew ED, Kaufman JS, Kimmel PL, Himmelfarb J, Wurfel MM. Association Between Early Recovery of Kidney Function After Acute Kidney Injury and Long-term Clinical Outcomes. JAMA Network Open. 2020;e202682. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 113] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 14. | Cruz M. Renal Recovery After Replacement Therapy During CF LVAD Support. J Heart Lung Transplant. 2017;S99. |
| 15. | Hollander SA, Cantor RS, Sutherland SM, Koehl DA, Pruitt E, McDonald N, Kirklin JK, Ravekes WJ, Ameduri R, Chrisant M, Hoffman TM, Lytrivi ID, Conway J. Renal injury and recovery in pediatric patients after ventricular assist device implantation and cardiac transplant. Pediatr Transplant. 2019;23:e13477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 16. | Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52948] [Cited by in RCA: 48569] [Article Influence: 2857.0] [Reference Citation Analysis (3)] |
| 17. | Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008-2012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14425] [Cited by in RCA: 17220] [Article Influence: 662.3] [Reference Citation Analysis (0)] |
| 18. | Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet. 1991;337:867-872. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2020] [Cited by in RCA: 2039] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 19. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 48488] [Article Influence: 2108.2] [Reference Citation Analysis (4)] |
| 20. | Schroll JB, Moustgaard R, Gøtzsche PC. Dealing with substantial heterogeneity in Cochrane reviews. Cross-sectional study. BMC Med Res Methodol. 2011;11:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 163] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 21. | Demirozu ZT, Etheridge WB, Radovancevic R, Frazier OH. Results of HeartMate II left ventricular assist device implantation on renal function in patients requiring post-implant renal replacement therapy. J Heart Lung Transplant. 2011;30:182-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 22. | Popov AF, Hosseini MT, Zych B, Mohite P, Hards R, Krueger H, Bahrami T, Amrani M, Simon AR. Clinical experience with HeartWare left ventricular assist device in patients with end-stage heart failure. Ann Thorac Surg. 2012;93:810-815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 23. | Sumida M, Doi K, Kinoshita O, Kimura M, Ono M, Hamasaki Y, Matsubara T, Ishii T, Yahagi N, Nangaku M, Noiri E. Perioperative plasma neutrophil gelatinase-associated lipocalin measurement in patients who undergo left ventricular assist device implantation surgery. Circ J. 2014;78:1891-1899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Deschka H, Holthaus AJ, Sindermann JR, Welp H, Schlarb D, Monsefi N, Martens S, Scherer M. Can Perioperative Right Ventricular Support Prevent Postoperative Right Heart Failure in Patients With Biventricular Dysfunction Undergoing Left Ventricular Assist Device Implantation? J Cardiothorac Vasc Anesth. 2016;30:619-626. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 25. | Shehab S, Macdonald PS, Keogh AM, Kotlyar E, Jabbour A, Robson D, Newton PJ, Rao S, Wang L, Allida S, Connellan M, Granger E, Dhital K, Spratt P, Jansz PC, Hayward CS. Long-term biventricular HeartWare ventricular assist device support--Case series of right atrial and right ventricular implantation outcomes. J Heart Lung Transplant. 2016;35:466-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 26. | Nadziakiewicz P, Szygula-Jurkiewicz B, Niklewski T, Pacholewicz J, Zakliczynski M, Borkowski J, Hrapkowicz T, Zembala M. Effects of Left Ventricular Assist Device Support on End-Organ Function in Patients With Heart Failure: Comparison of Pulsatile- and Continuous-Flow Support in a Single-Center Experience. Transplant Proc. 2016;48:1775-1780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Raichlin E, Baibhav B, Lowes BD, Zolty R, Lyden ER, Vongooru HR, Siddique A, Moulton MJ, Um JY. Outcomes in Patients with Severe Preexisting Renal Dysfunction After Continuous-Flow Left Ventricular Assist Device Implantation. ASAIO J. 2016;62:261-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 28. | Schmack B, Grossekettler L, Weymann A, Schamroth J, Sabashnikov A, Raake PW, Popov AF, Mansur A, Karck M, Schwenger V, Ruhparwar A. Prognostic relevance of hemodialysis for short-term survival in patients after LVAD implantation. Scientific Reports, 2018: 8546. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 29. | Shehab S, Rao S, Macdonald P, Newton PJ, Spratt P, Jansz P, Hayward CS. Outcomes of venopulmonary arterial extracorporeal life support as temporary right ventricular support after left ventricular assist implantation. J Thorac Cardiovasc Surg. 2018;156:2143-2152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Asleh R, Schettle S, Briasoulis A, Killian JM, Stulak JM, Pereira NL, Kushwaha SS, Maltais S, Dunlay SM. Predictors and Outcomes of Renal Replacement Therapy After Left Ventricular Assist Device Implantation. Mayo Clin Proc. 2019;94:1003-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 31. | Kaltenmaier B, Pommer W, Kaufmann F, Hennig E, Molzahn M, Hetzer R. Outcome of patients with ventricular assist devices and acute renal failure requiring renal replacement therapy. ASAIO J. 2000;46:330-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 37] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 32. | Muslem R, Caliskan K, Akin S, Sharma K, Gilotra NA, Constantinescu AA, Houston B, Whitman G, Tedford RJ, Hesselink DA, Bogers AJJC, Russell SD, Manintveld OC. Acute kidney injury and 1-year mortality after left ventricular assist device implantation. J Heart Lung Transplant. 2018;37:116-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 33. | Iwashima Y, Yanase M, Horio T, Seguchi O, Murata Y, Fujita T, Toda K, Kawano Y, Nakatani T. Effect of pulsatile left ventricular assist system implantation on Doppler measurements of renal hemodynamics in patients with advanced heart failure. Artif Organs. 2012;36:353-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 34. | Brisco MA, Kimmel SE, Coca SG, Putt ME, Jessup M, Tang WW, Parikh CR, Testani JM. Prevalence and prognostic importance of changes in renal function after mechanical circulatory support. Circ Heart Fail. 2014;7:68-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 124] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 35. | Ross DW, Stevens GR, Wanchoo R, Majure DT, Jauhar S, Fernandez HA, Merzkani M, Jhaveri KD. Left Ventricular Assist Devices and the Kidney. Clin J Am Soc Nephrol. 2018;13:348-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 36. | Alba AC, Rao V, Ivanov J, Ross HJ, Delgado DH. Predictors of acute renal dysfunction after ventricular assist device placement. J Card Fail. 2009;15:874-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 37. | Kihara S, Litwak KN, Nichols L, Litwak P, Kameneva MV, Wu Z, Kormos RL, Griffith BP. Smooth muscle cell hypertrophy of renal cortex arteries with chronic continuous flow left ventricular assist. Ann Thorac Surg. 2003;75:178-83; discussion 183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 48] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 38. | Kanduri SR, Kovvuru K, Cheungpasitporn W, Thongprayoon C, Bathini T, Garla V, Vailta P, Vallabhajosyula S, Medaura J, Kashani K. Kidney Recovery From Acute Kidney Injury After Hematopoietic Stem Cell Transplant: A Systematic Review and Meta-Analysis. Cureus. 2021;13:e12418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 39. | Kashani K, Rosner MH, Haase M, Lewington AJP, O'Donoghue DJ, Wilson FP, Nadim MK, Silver SA, Zarbock A, Ostermann M, Mehta RL, Kane-Gill SL, Ding X, Pickkers P, Bihorac A, Siew ED, Barreto EF, Macedo E, Kellum JA, Palevsky PM, Tolwani AJ, Ronco C, Juncos LA, Rewa OG, Bagshaw SM, Mottes TA, Koyner JL, Liu KD, Forni LG, Heung M, Wu VC. Quality Improvement Goals for Acute Kidney Injury. Clin J Am Soc Nephrol. 2019;14:941-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 182] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 40. | Mehta RL. Renal Recovery After Acute Kidney Injury and Long-term Outcomes: Is Time of the Essence? JAMA Netw Open. 2020;3:e202676. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Critical care medicine
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Eccher A S-Editor: Wang LL L-Editor: A P-Editor: Wang LL