Published online Sep 9, 2021. doi: 10.5492/wjccm.v10.i5.260
Peer-review started: February 21, 2021
First decision: May 13, 2021
Revised: June 22, 2021
Accepted: July 27, 2021
Article in press: July 27, 2021
Published online: September 9, 2021
Processing time: 200 Days and 3.3 Hours
Immune dysfunction following major traumatic injury is complex and strongly associated with significant morbidity and mortality through the development of multiple organ dysfunction syndrome (MODS), persistent inflammation, immunosuppression, and catabolism syndrome and sepsis. Neutrophils are thought to be a pivotal mediator in the development of immune dysfunction.
To provide a review with a systematic approach of the recent literature describing neutrophil kinetics and functional changes after major trauma in humans and discuss hypotheses as to the mechanisms of the observed neutrophil dysfunction in this setting.
Medline, Embase and PubMed were searched on January 15, 2021. Papers were screened by two reviewers and those included had their reference list hand searched for additional papers of interest. Inclusion criteria were adults > 18 years old, with an injury severity score > 12 requiring admission to an intensive care unit. Papers that analysed major trauma patients as a subgroup were included.
Of 107 papers screened, 48 were included in the review. Data were heterogeneous and most studies had a moderate to significant risk of bias owing to their observational nature and small sample sizes. Key findings included a persistently elevated neutrophil count, stereotyped alterations in cell-surface markers of activation, and the elaboration of heterogeneous and immunosuppressive populations of cells in the circulation. Some of these changes correlate with clinical outcomes such as MODS and secondary infection. Neutrophil phenotype remains a promising avenue for the development of predictive markers for immune dysfunction.
Understanding of neutrophil phenotypes after traumatic injury is expanding. A greater emphasis on incorporating functional and clinically significant markers, greater uniformity in study design and assessment of extravasated neutrophils may facilitate risk stratification in patients affected by major trauma.
Core Tip: Major trauma results in complex immune dysfunction, with dysregulated pro- and anti-inflammatory processes presenting as clinical syndromes such as acute respiratory distress syndrome (ARDS) and multiple organ dysfunction syndrome (MODS). This review examines the role of neutrophils in immune dysfunction following major trauma requiring admission to the intensive care unit, with a focus on the kinetics of the neutrophil immunophenotype and how this correlates with clinical outcomes. This review also proposes new hypotheses as to the mechanisms of complications of immune dysfunction, including ARDS and MODS.
- Citation: Finlay LD, Conway Morris A, Deane AM, Wood AJ. Neutrophil kinetics and function after major trauma: A systematic review. World J Crit Care Med 2021; 10(5): 260-277
- URL: https://www.wjgnet.com/2220-3141/full/v10/i5/260.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v10.i5.260
Major traumatic injury precipitates a complex disease process, with multiple physiological and immunological stressors spanning from the moment of injury to well after discharge. Despite improvements in addressing the acute causes of morbidity and mortality, the WHO reports that trauma still accounts for 10% of all deaths globally[1]. In Australia, trauma ranks as the third highest area of health care spending, at a cost of $8.9 billion in the 2015-2016 financial year[2]. A significant portion of this expenditure is associated with extended intensive care unit (ICU) admissions complicated by syndromes of immune dysfunction, including: multiple organ dysfunction syndrome (MODS), acute respiratory distress syndrome (ARDS), persistent inflammation, immunosuppression and catabolism syndrome, sepsis, and hospital associated infections[3-7].
Neutrophils are one of the key components of the innate immune response and are suspected to be one of the main effector cells involved in MODS and sepsis following major trauma[4]. Neutrophil phenotype provides a unique snapshot of the immune response to trauma, as it represents the functional culmination of the complex cellular milieu observed in severe, systemic inflammation[8,9]. Trauma is a ‘sterile’ inflammatory process, with neutrophils being activated by products of cellular damage and necrosis, known as danger associated molecular patterns (DAMPs), rather than bacterial products[10]. Important DAMPs released in trauma include high mobility group box 1, mitochondrial nucleic acids, and cell free DNA (cfDNA)[6,10].
In response to DAMPs, neutrophils transition from resting to either a primed or activated phenotype, accompanied by changes in both cell surface markers and functional status[6,11-14]. Following priming and activation, there is initially significantly increased release of neutrophil antimicrobial products, including reactive oxygen species (ROS), cytokines, heparin binding protein, elastase, and neutrophil-derived cfDNA known as neutrophil extracellular traps (NETs), contributing to a systemic inflammatory response syndrome (SIRS)[6,12-17].
Clinically, this may be followed or accompanied by a period of decreased immune activity resulting in an increased risk of infectious complications. This period has been labelled the compensatory anti-inflammatory response syndrome (CARS)[4]. Immunologically, this period is characterised by neutrophil dysfunction, hypo-responsiveness to subsequent stimuli and active immunosuppression[1,18]. Whilst CARS was initially thought to follow SIRS, there is evidence to suggest that the underlying mechanisms to both SIRS and CARS are activated at the same point early after trauma[1,16,19].
Despite their key role, to our knowledge there are currently no systematic reviews describing neutrophil immunophenotype in adults affected by major trauma. This review aims to describe the extant literature on neutrophil immunophenotype over time in adults admitted to the ICU with major trauma, with a focus on markers that may predict complications related to immune dysfunction during the ICU admission. It also aims to generate hypotheses as to the mechanisms behind MODS and sepsis, and areas for future research.
This review was conducted in accordance with the protocol available in the Supplementary materials. All study types (e.g., cohort, case-control, randomized controlled trials) were eligible for inclusion providing patient inclusion/exclusion criteria were met and an assessment of neutrophil function or kinetics was performed. Inclusion criteria were: English language, adult human (aged > 18 years) population with an injury severity score (ISS) > 12 implying major trauma[20], who required admission to an ICU. Exclusion criteria were a publication date prior to 1990 (to provide an assessment of the relatively recent literature) and conditions which influence the immune phenotype, namely pregnancy, haematological malignancy and immunosuppression.
The Medline Ovid, PubMed and Embase databases were searched on January 15, 2021. We used the following search terms to search the above databases: trauma or major trauma, neutrophil, innate immunity, activation, function, dysfunction, immunophenotype, intensive care, critical care or illness. The complete search strategies used for each database are shown in the Supplementary materials. Results were then filtered by date (> 1990), human adults and English language. Both reviews and primary studies were initially included, with the review papers hand searched for further relevant studies which were subsequently screened.
Study eligibility was assessed by 2 independent reviewers (LF and AW) in a blinded manner using online Covidence software[21]. Disagreements between reviewers were resolved by consensus. Data on the studied patient population and assessment of neutrophil function or kinetics was extracted by the same reviewers and recorded in Table 1. Bias was estimated for included studies but was not a barrier for inclusion given the nature of the literature (predominantly small observational studies with heterogenous outcome measures and moderate-high risk of bias). Data were analysed qualitatively, and summary statements produced for key findings in the literature. Meta-analysis was not performed owing to the heterogeneity in outcome measures used in included studies.
| Title of paper | Ref. | Year published | Number of patients recruited | Average ISS | Average age | Samples collected (time post injury) | Location of study | Major outcomes |
| Postinjury neutrophil priming and activation states: therapeutic challenges | Botha et al[12] | 1994 | 10 | N/A | N/A | 3 h, 6 h, 12 h, 24 h, 48 h, 72 h, 96 h | United States | Functional states of NADP H, primed 6-24 h, unprimable > 48 h |
| Postinjury neutrophil priming and activation: an early vulnerable window | Botha et al[14] | 1995 | 17 | 26.7 | 26.7 | 3 h, 6 h, 12 h, 24 h, 48 h, 72 h | United States | Priming occurs < 24 h after injury, but cells are resistant to priming 48 h after trauma |
| Early Neutrophil Sequestration after Injury: A Pathogenic Mechanism for Multiple Organ Failure | Botha et al[25] | 1995 | 33 | 27.7 | 29.1 | 3 h, 6 h, 12 h, 24 h | United States | Neutrophil kinetics and CD11b expression suggest end organ sequestration predisposing to MODS |
| Base deficit after major trauma directly relates to neutrophil CD11b expression: a proposed mechanism of shock-induced organ injury | Botha et al[27] | 1997 | 17 | 26.7 | 26 | 3 h, 6 h, 12 h, 24 h, 48 h, 72 h, 96 h, 120 h | United States | Kinetics of neutrophilia, CD11b, CD18 and CD11a |
| Major injury induces increased production of IL10 in human granulocyte fractions | Koller et al[49] | 1998 | 15 | 28 | 36 | Daily between days 3-10 | Germany | Neutrophils from trauma patients produce IL-10 |
| The effects of trauma and sepsis on soluble L-selectin and cell surface expression on L-selectin and CD11b on leukocytes | Maekawa et al[32] | 1998 | 20 | 20.1 | 45.6 | ADM, every 30 min up to 4 h, every 3h up to 24 h, every 6 h up to 120 h | Japan | Neutrophil L selectin and CD11b both increase immediately and more slowly out to 24 h post trauma in ISS > 16 but not in ISS < 16 |
| Polymorphonuclear Neutrophil Chemiluminescence in Whole blood from Blunt Trauma Patients with Multiple Injuries | Brown et al[56] | 1999 | 12 | 36.4 | 49.5 | < 24 h | United States | CR3a is a marker of neutrophil priming and is upregulated in trauma |
| Neutrophils are primed for cytotoxicity and resist apoptosis in injured patients at risk of for multiple organ failure | Biffl et al[13] | 1999 | 12 | 22.6 | N/A | Daily for 5 d | United States | Neutrophil apoptosis is delayed in trauma patients |
| Preferential Loss of CXCR-2 Receptor Expression and Function in Patients Who Have Undergone Trauma | Quaid et al[35] | 1999 | 20 | 19 | 35 | One sample within 24 h | United States | CXCR-2 expression and function are downregulated in severely injured patients |
| Superoxide production of neutrophils after severe injury: Impact of subsequent surgery and sepsis | Shih et al[57] | 1999 | 18 | 26.2 | 41.6 | 1 d, 3 d, 7 d | Taiwan | Neutrophil superoxide production after trauma is initially increased but is then decreased in those who go on to develop multiorgan failure at day 7 |
| Early role of neutrophil L-selectin in posttraumatic acute lung injury | Rainer et al[29] | 2000 | 147 | 1 | 1 | On admission to ED | Hong Kong | Total leukocyte and neutrophil counts, expression of L-selectin, and the ratio of neutrophil to plasma L-selectin increased with injury and were highest in those who developed acute lung injury (ALI). Soluble L-selectin decreased with injury severity and was lowest in those who developed ALI |
| Early Trauma polymorphonuclear neutrophil responses to chemokines are associated with development of sepsis, pneumonia and organ failure | Adams et al[34] | 2001 | 15 | 34 | 36 | 12 h | United States | High CXCR2 activity correlated with ARDS. Low CXCR2 activity correlated with sepsis |
| Decreased leukotriene release from neutrophils after severe trauma: role of immature cells | Koller et al[40] | 2001 | 15 | 35 | 35 | 1 sample, between 3-14 d | Germany | Neutrophils secrete less leukotrienes following trauma |
| Prospective study of neutrophil chemokine responses in trauma patients at risk for pneumonia | Tarlowe et al[36] | 2005 | 32 | 27.4 | 35.1 | ADM, 3 d, 7 d | United States | Prospectively assessed CXCR function and expression in neutrophils from trauma patients at high risk for pneumonia and their matched volunteer controls. CXCR2-specific calcium flux and chemotaxis were desensitized by injury, returning toward normal after 1 wk. CXCR1 responses were relatively maintained |
| Neutrophil priming for elastase release in adult blunt trauma patients | Bhatia et al[15] | 2006 | 10 | 29.3 | 40.3 | ADM, 24 h, 3 d, 5 d | United Kingdom | Neutrophils release more elastase after trauma |
| Aberrant regulation of polymorphonuclear phagocyte responsiveness in multi-trauma patients | Hietbrink et al[30] | 2006 | 13 | 21 | 40 | ADM, 3 d, 5 d, 7 d | Netherlands | Priming markers low in first week. Decreased responsiveness to fMLP with increased ISS |
| Neutrophil-derived circulating free DNA: a potential prognostic marker for posttraumatic development of inflammatory second hit and sepsis | Margraf et al[58] | 2008 | 37 | 31.6 | 45 | ADM, daily for 10 d | Germany | Kinetics of NET formation, 3 patterns of kinetics |
| Early expression changes of complement regulatory proteins and C5a receptor (CD88) on leukocytes after multiple injury in humans | Amara et al[39] | 2010 | 12 | 48 | 38 | 4 h, 12 h, 24 h, 120 h, 240 h after trauma | Germany | Complement regulators and CD88 on neutrophils are significantly altered following trauma. CD55 is elevated, shows decreased expression |
| Nature of Myeloid Cells Expressing Arginase 1 in Peripheral Blood After Trauma | Bryk et al[45] | 2010 | 10 | 18.63 | 43.7 | < 24 h, 3-7 d, 14-21 d | United States | MDSCs derived from major trauma patients show increased arginase activity, allowing modulation of T cell responses |
| Divergent adaptive and innate immunological responses are observed in humans following blunt trauma | Kasten et al[11] | 2010 | 22 | 22.8 | 36.3 | 1 sample, between 24-96 h | United States | CD11b kinetics, lipid rafts, phosphorylated Akt increased in trauma |
| A genomic storm in critically injured humans | Xiao et al[19] | 2011 | 167 | 31.3 | 34 | < 12 h, 1 d, 4 d, 7 d, 14 d, 21 d, 28 d | United States | Genomics of response to trauma, anti- and pro-inflammatory mechanisms activated simultaneously |
| A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1 | Pillay et al[33] | 2011 | N/A | N/A | N/A | N/A | Netherlands | ROS-induced immunosuppressive CD16bright/CD62L dim neutrophil population first isolated |
| Kinetics of the innate immune response after trauma: implications for the development of late onset sepsis | Hietbrink et al[8] | 2012 | 36 | 24.2 | 45 | 3-12 h, daily for 10 d | Netherlands | Kinetics of neutrophilia, CRP, IL-6, CD11b, FcγRII, CXCR1, respiratory burst, CD88 |
| Molecular mechanisms underlying delayed apoptosis in neutrophils from multiple trauma patients with and without sepsis | Paunel-Görgülü et al[59] | 2012 | 24 | 46.7 | 41.7 | Routinely until 10 d | Germany | Neutrophil apoptosis is reduced after trauma and patients undergoing a post-trauma course complicated by sepsis exhibit different expression of pro- and anti-apoptotic regulators |
| Increased MerTK expression in circulating innate immune cells of patients with septic shock | Guignant et al[60] | 2013 | 51 | 38 | 35 | 24-48 h | France | TAM receptors are differentially upregulated in sepsis and trauma |
| IL33-mediated ILC2 activation and neutrophil IL5 production in the lung response after severe trauma: A reverse translation study from a human cohort to a mouse trauma model | Xu et al[48] | 2017 | 472 | 20.2 | N/A | ADM, < 24 h, daily for 7 d | United States | IL33 kinetics, neutrophils produce IL-5 |
| Prehospital immune responses and development of multiple organ dysfunction syndrome following traumatic injury: a prospective cohort study | Hazeldine et al[23] | 2017 | 89 | 24 | 41 | < 1 h after trauma, 4-12 h, 24-48 h | United States | Early kinetics of neutrophil phenotype, including neutrophilia, cytokines, NETs, CD11b, and CD16/CD62L subsets |
| Early decreased neutrophil responsiveness is related to late onset sepsis in multitrauma patients: An international cohort study | Groeneveld et al[31] | 2017 | 109 | 1 | 1 | On arrival | Netherlands, South Africa | Reduced fMLP responsiveness in a cohort study at early time points and in association with septic shock |
| Heparin-binding protein as a biomarker of post-injury sepsis in trauma patients | Halldorsdottir et al[28] | 2018 | 97 | 33 | 47 | 1 d, 3 d, 5 d | Sweden | HBP is a marker of neutrophil activation and correlates with ISS |
| A rise in neutrophil size precedes organ dysfunction after trauma | Hesselink et al[26] | 2018 | 81 | 1 | 1 | ADM, 6 h, 12 h, 24 h, 48 h | Netherlands | In patients who developed organ failure a significant increase in neutrophil count, size and complexity, and a decrease in lobularity were seen after trauma |
| Neutrophil-derived long noncoding RNA IL-7R predicts development of multiple organ dysfunction syndrome in patients with trauma | Jin et al[55] | 2020 | 60 | 23.5 | 51.5 | ADM | China | Neutrophil derived lnc-IL7R negatively correlates with MODS and mortality |
| New automated analysis to monitor neutrophil function point-of-care in the intensive care unit after trauma | Hesselink et al[5] | 2020 | 15 | 33 | 1 | <12 h, 3 d, 6 d, 10 d, 15 d | Netherlands | Patterns of phagosomal acidification correlate with infection, neutrophil CD16/CD62L subsets |
| Point-of-Care analysis of neutrophil phenotypes: A first step toward immune-based precision medicine in the Trauma ICU | Spijkerman et al[24] | 2020 | 32 | N/A | N/A | ADM to trauma bay | Netherlands | CD16/CD62L neutrophil subtype correlates with infection |
| Olfactomedin 4 Positive Neutrophils are Upregulated Following Hemorrhagic Shock | Kassam et al[61] | 2020 | 56 | N/A | 41.5 | ADM, 3 d, 7 d | United States | Increased OLFM4+ neutrophil fraction after blunt trauma associated with increased ICU length of stay, ventilator days |
| Current Concepts of the inflammatory response after major trauma – an update | Giannoudis[18] | 2003 | Review Paper | 2 | 2 | 2 | United Kingdom | Malignant SIRS can develop into MODS or ARDS, however main effect of trauma on neutrophils is suppressive |
| Trauma: The role of the innate immune system | Hietbrink et al[4] | 2006 | Review Paper | 2 | 2 | 2 | Netherlands | Neutrophils are the main effector cells leading to MODS, an overactive SIRS can lead to CARS/MARS |
| The systemic inflammatory response induced by trauma is reflected by multiple phenotypes of blood neutrophils | Pillay et al[3] | 2007 | Review Paper | 2 | 2 | 2 | Netherlands | Description of cell surface markers and their role in normal neutrophil function and in trauma |
| Postinjury immune monitoring: can multiple organ failure be predicted? | Visser et al[46] | 2008 | Review Paper | 2 | 2 | 2 | Netherlands | Excessive neutrophilia in the hours post trauma increase risk of MODS and mortality. Severity of the initial SIRS causes the depth of immunosuppression |
| Trauma equals danger – damage control by the immune system | Stoecklein et al[62] | 2012 | Review Paper | 2 | 2 | 2 | United States | Trauma induces immunosuppression, characterised clinically as CARS or MARS (mixed antagonist response syndrome) |
| The impact of trauma on neutrophil function | Hazeldine et al[16] | 2014 | Review Paper | 2 | 2 | 2 | United Kingdom | Sequestration of neutrophils in organs may lead to ARDS, whilst leaving the circulation open to infection |
| The systemic immune response to trauma: an overview of pathophysiology and treatment | Lord et al[17] | 2014 | Review Paper | 2 | 2 | 2 | United Kingdom | Heightened SIRS suppresses immune responses resulting in inflammation and cellular immunoparalysis, contradictory accumulation in organs causes organ dysfunction |
| Assessing the Immune Status of critically ill trauma patients by flow cytometry | Kuethe et al[63] | 2014 | Review Paper | 2 | 2 | 2 | United States | CD66b and CD11b are selective markers for neutrophils when expressed together. Neutrophils differentially regulate cell surface markers based on activation |
| The role of neutrophils in immune dysfunction during severe inflammation | Leliefeld et al[42] | 2016 | Review Paper | 2 | 2 | 2 | Netherlands | NETosis occurs in response to IL-8, TNFα and LPS, under the control of NADPH oxidase. Massive neutrophil release from the bone marrow may result in exhaustion |
| Neutrophils in critical illness | McDonald[64] | 2018 | Review Paper | 2 | 2 | 2 | Canada | TREM-1 may assist in differentiating sterile from septic SIRS, as TREM-1 only upregulates in sepsis |
| Innate Immunity in the Persistent Inflammation, Immunosuppression and Catabolism Syndrome and its implications for therapy | Horiguchi et al[6] | 2018 | Review paper | 2 | 2 | 2 | United States | Major DAMPs in trauma include HMGB1, mtDNA, ATP and cfDNA. Result in neutrophils releasing IL-6, TNFα, IFNγ, and ROS. Neutrophils exist in resting, primed and active states |
| Danger signals in the ICU | Schenck et al[10] | 2018 | Review Paper | 2 | 2 | 2 | United States | mtDNA is a main DAMP in trauma due to similarities to bacterial DNA. Early neutrophil chemotaxis is DAMP dependent |
| Neutrophil heterogeneity and its role in infectious complications after severe trauma | Hesselink et al[9] | 2019 | Review Paper | 2 | 2 | 2 | Netherlands | Activated neutrophils leave the blood, leaving dysfunctional neutrophils behind. Analysis of low density neutrophils, CD16/CD62L subtypes |
| Does neutrophil phenotype predict the survival of trauma patients? | Mortaz et al[1] | 2019 | Review Paper | 2 | 2 | 2 | Iran | CD11b is considered a marker of poor prognosis, increased CXCR2 relates to risk of ARDS. Understanding phenotype could allow use as a predictive tool |
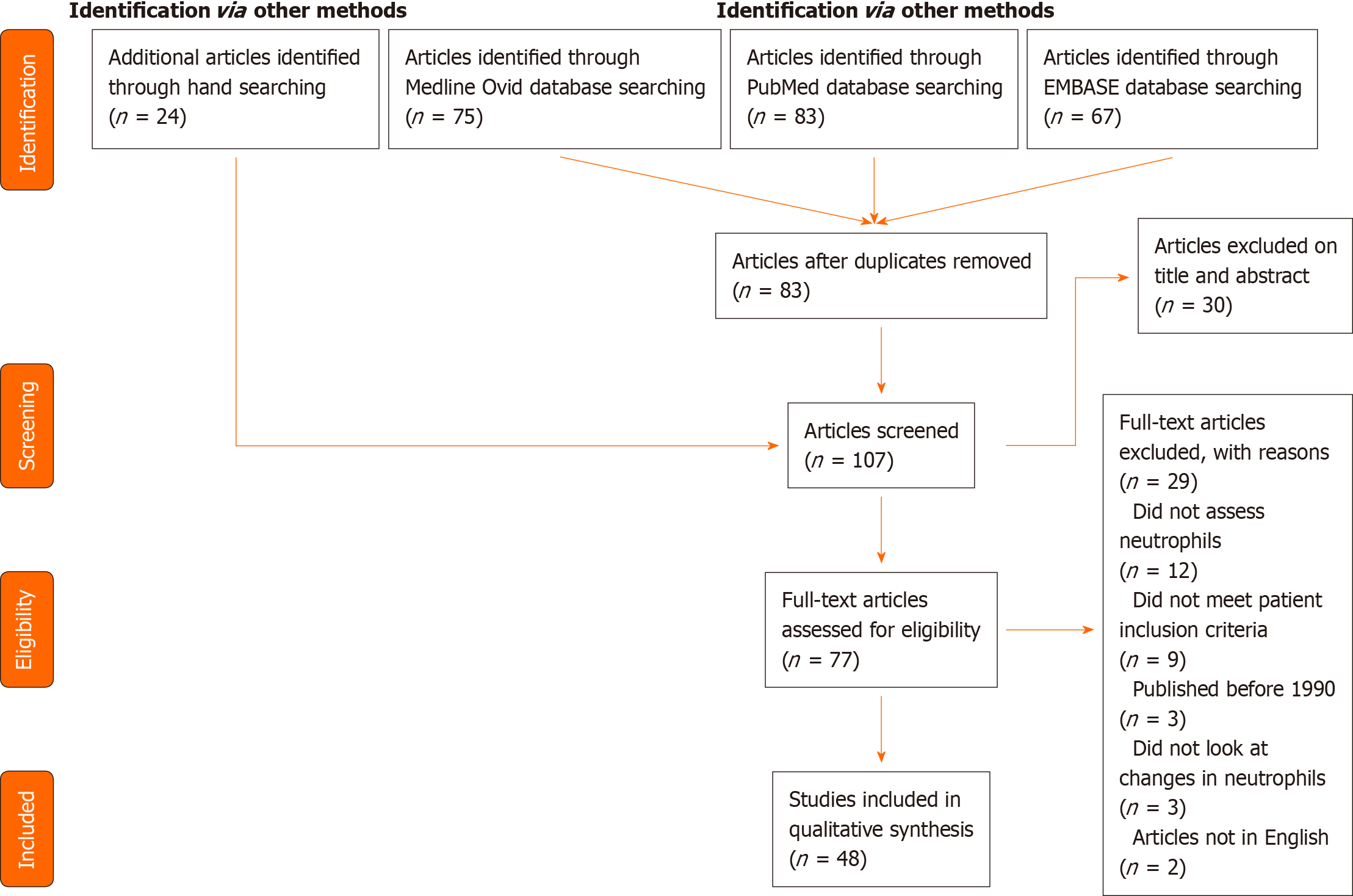
Two hundred and twenty five papers were identified using the search strategy outlined above and in the Supplementary materials. Following removal of duplicates, 83 articles remained. A further 24 papers were identified through hand-searching reference lists, resulting in 107 articles having titles and abstracts screened for relevance, of which 30 studies were excluded as irrelevant. Only primary studies were summarised in this review. Review papers were included but were used to identify further relevant primary studies only.
The full text review resulted in the exclusion of 29 papers. Main reasons for exclusion were: not specifically reporting neutrophil phenotypes (n = 12) or patients not meeting eligibility criteria, either due to age or ISS (n = 9). In total, 48 manuscripts were included in this review. This information is summarised in the PRISMA diagram (Figure 1)[22].
A summary of the results and each study’s patient demographics, including average age, ISS, and frequency of samples, is included in Table 1. Multiple changes to neutrophil phenotype were noted, and these changes can be broadly classified into physical parameters, cell surface markers, and changes in neutrophil function.
Neutrophil count: There were eight papers that assessed changes in neutrophil count over time. In most cases these studies compared neutrophil counts in trauma patients to a control population, however the control samples only originate from one time point, from non-matched control volunteers, potentially introducing bias. There were also discrepancies in the number of samples collected, and the window in which samples could be collected at each time point. Lack of standardisation in data collection make it difficult to compare studies quantitatively.
Neutrophilia is the first and most easily assessable change following trauma, driven by endogenous cortisol and catecholamine release promoting neutrophil demargination from the vasculature and accelerated migration from the bone marrow[23,24]. As well as being one of the first events following trauma, neutrophilia is prolonged, with reports of neutrophil counts being 2- to 5-times higher in trauma patients out to 5 d compared to healthy controls, and remaining significantly elevated out to 10 d post-trauma[8,11]. Neutrophils make up more than 80% of the total white cell count for at least 5 d following trauma[25], demonstrating their critical role in the immune response post injury.
Peak neutrophilia occurs soon after injury, with maximum neutrophil counts usually being detected 3 h after trauma[25-27]. Indeed, Hazeldine and colleagues collected blood samples in the prehospital setting and showed that leucocytosis began within minutes of trauma and persisted for days[23].
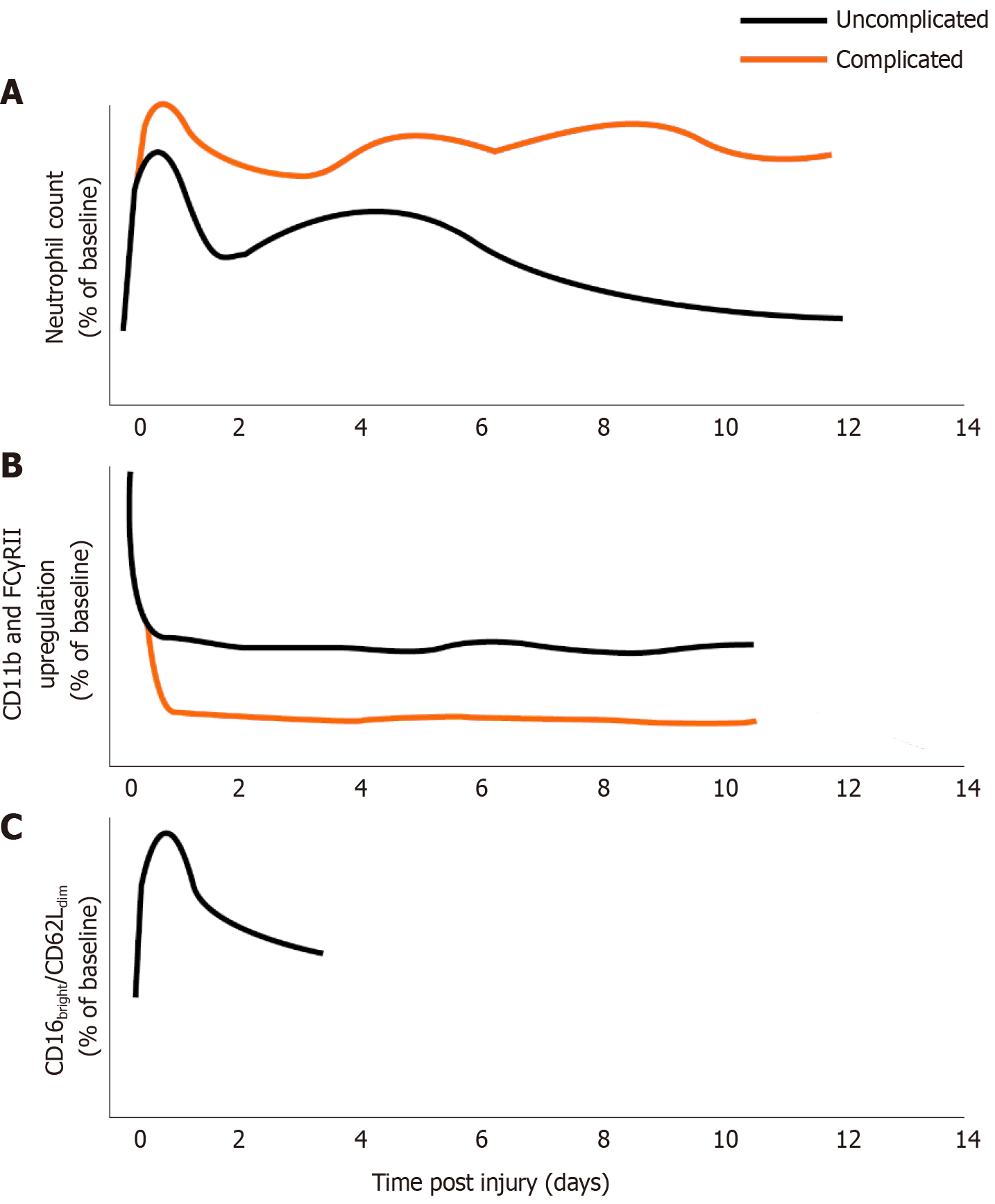
Circulating neutrophil counts in patients with major trauma followed reproducible trends. Counts tended to drop from the initial peak between 6-24 h[25-27], but remained higher than controls. Multiple studies showed a further drop in neutrophil count between days 3 and 5, followed by a rebound to levels seen in the 6-24 h phase[8,23,25,28] (see Figure 2A for schematic).
Neutrophil size: There was one paper identified which discussed changes in neutrophil size. This was a recent paper by Hesselink and colleagues published in 2019[26]. Neutrophil size is a recent marker of neutrophil activation and has been shown to be a good early predictor for MODS, with increased neutrophil size on admission to the emergency department correlating with the development of organ dysfunction later in the disease course[26].
In all patients, neutrophil size trended upward over the first 48 h following trauma[26]. In patients who developed MODS, there was a significant increase in neutrophil size relative to both healthy controls and to trauma patients who didn’t develop MODS[26]. Though not routinely reported, neutrophil size is an easily assessable parameter as it is calculated during routine full blood examinations with differentials[26].
CD11b: There were ten papers analysing changes in CD11b. These papers faced similar challenges to those looking at neutrophil count – there are major discrepancies in methodology between papers, with large variations in the window for sample collection at time points which could have significant impacts on the resulting data. These papers generally compare results to a non-standardised control sample which is collected at only one time point.
CD11b is a component of the β2 integrin receptor MAC-1, which is involved in neutrophil adhesion to the endothelial wall during extravasation[29]. It also plays a significant role in phagocytosis and the respiratory burst, and is a well-documented marker of neutrophil activation[16,29].
Expression of CD11b increases within minutes of trauma, implying neutrophils become activated early after injury[23]. Most studies show CD11b is significantly increased relative to healthy controls in the first 12 h[8,11,14,23,25,30]. In some studies, this difference was maintained up to 10 d post trauma[8].
In general, CD11b tended to peak early after injury, usually within the first 6-12 h. This was followed by a trough from 24-48 h[8,14,23,25], before rising again and remaining at elevated levels after 48 h[8].
There is conflicting data on whether CD11b correlates with clinical markers. Botha et al[25] reported that expression at 12 h correlated negatively with base deficit (a marker of tissue ischaemia and reperfusion injury severity) and neutrophil count; this was hypothesised to represent extravasation of activated neutrophils into damaged tissues. Hietbrink et al[4,30] reported that an ISS >16 was associated with increased CD11b expression, whereas Spijkerman et al[24] reported that absolute levels of CD11b did not have value in predicting which patients would develop infection.
However, CD11b upregulation following stimulation with fMLP (N-Formylmethionyl-leucyl-phenylalanine, a potent neutrophil activator) shows improved value as a predictive marker. At all timepoints, the fold-increase in CD11b after exposure to fMLP is decreased in trauma patients relative to healthy controls, and is particularly low on day 2 post trauma[8,23,24,30]. fMLP-induced CD11b expression was significantly lower in patients who developed infection and correlated with increased ISS[24,30]. A similar phenomenon of hypo-responsiveness has been observed with FC Gamma receptor II (FCγRII, CD32) upregulation, which has also been shown to correlate with development of infectious complications[8,31] (Figure 2B). Thus, assessment of surface marker changes in response to stimuli may be more predictive of immune dysfunction than expression of cell surface markers alone.
CD62L: Six papers analysed the expression of CD62L in neutrophils following major trauma. CD62L is a lectin involved in the rolling interactions with endothelium during extravasation[3,23]. It is shed on activation of the neutrophil to allow increased mobility into the tissues, and can therefore be used as a marker of extravasation as well as neutrophil activation[3,4,9,16].
Earlier studies demonstrated an apparent reduction in neutrophil CD62L expression accompanied by a rise in soluble L-selectin in plasma associated with severity of injury and development of complications[29,32]. More recent studies have supported these findings, specifically that traumatic injury correlates with a reduction in CD62L expression; this is consistent with the hypothesis that systemic inflammation leads to generalised neutrophil priming and the presence of a CD16bright/CD62Ldim subtype in the circulation (see below)[23,33]. Although the absolute number of CD62L molecules on neutrophils is decreased following trauma[16], it has been shown that trauma-derived neutrophils show significantly reduced shedding of CD62L when stimulated in vitro up to 72 h after trauma[23].
CXCR2 (CD182): Seven papers included analysis of the kinetics and function of CXCR2 following trauma. The quality of these studies varied (some included matched control groups whereas others did not) however results were broadly consistent and are discussed below.
CXCR2 is a chemokine receptor which responds to IL-8, allowing chemotaxis to sites of inflammation[3,8,34]. A change in surface expression of CXCR4 to CXCR2 is a critical step in allowing neutrophil efflux from the bone marrow (discussed further below)[35]. CXCR2 expression is easily downregulated following interactions with IL-8, and re-expression is delayed by up to 24 h[11,35]. CXCR2 is elevated within the first hour post-trauma, with decreased expression from 3 h onwards[23,36]. CXCR2 expression has been correlated with outcomes, with evidence suggesting that significantly increased CXCR2 responses to GRO-α (a CXCR2-specific ligand) correlated with ARDS, and significantly decreased responses correlated with sepsis[34]. Importantly, in this study there was no significant difference observed in CXCR2 expression between trauma patients who didn’t develop complications and healthy controls[34], suggesting that CXCR2 could be used as a predictive tool for the development of complication post injury. Conversely, CXCR1 has been assessed several times and does not appear to change over time following trauma, nor correlate with clinical outcomes[34,36].
C5aR1 (CD88): The receptor for the complement anaphylatoxin C5a (C5aR1 or CD88) under physiological circumstances serves to drive important neutrophil antimicrobial responses such as chemotaxis and ROS production[37]. In severe inflammation such as multiple trauma, massive activation of the complement system occurs[38]. Amara and colleagues demonstrated changes in multiple complement regulatory proteins immediately after trauma, including a reduction in CD88 expression on neutrophils and an inverse association with ISS[39].
Neutrophil heterogeneity is marked after trauma, though the functional implications of identified differences in circulating cells, as well as their relationship to cellular developmental stages remains under investigation. In healthy controls, the circulating neutrophil population is almost exclusively composed of mature segmented cells with lobular nuclei[40]. Following trauma there is a rapid increase in the number of immature neutrophils in the blood stream. This occurs in part due to emergency granulopoiesis, a G-CSF induced acceleration of neutrophil production and release accompanied by a diversion of other cell lineages toward neutrophil development, as reviewed elsewhere[41].
Another factor driving a circulating neutrophilia is the CXCR4/CXCL12 axis. During development in the bone marrow immature neutrophils express CXCR4 (chemokine receptor 4), which responds to the high levels of CXCL12 (chemokine ligand 12) in the bone marrow, causing the cells to remain in-situ[42]. Trauma results in disruption of the CXCR4/CXCL12 balance, allowing neutrophils to enter the circulation and resulting in a heterogenous neutrophil population in terms of maturity and function[35]. One method of assessing cellular maturity is the complexity and lobularity of nuclei; immature cells have a less lobulated nucleus and thus are often referred to as ‘band cells’ which can represent up to 98% of circulating neutrophils in conditions of severe stress such as major trauma or septic shock[35]. It has been reported that the average lobularity (and therefore maturity) of neutrophils in the circulation trends downwards over the first 48 h after trauma[26].
Combining marker expression has allowed the subtyping of neutrophils based on CD16 and CD62L expression. The differential expression and corresponding intensity of the signal detected on flow cytometry give rise to the terms ‘bright’ and ‘dim’. Seven papers included discussions on neutrophils categorised using this process.
Under homeostatic circumstances, a homogenous population of CD16bright/CD62Lbright neutrophils exists[33]. However, in the first 12 h after trauma they account for less than 40% of the neutrophil population[5]. By day 3, they have increased and stabilised at approximately 80% of neutrophils in circulation[5].
In contrast, the hypersegmented CD16bright/CD62Ldim subtype exhibits an immunosuppressive phenotype[33]. Proteomic analysis suggests they are not simply more mature neutrophils, but rather a completely separate subtype[43]. These cells produce ROS to suppress lymphocytes similarly to myeloid-derived suppressor cells (MDSCs)[36]. They show adequate phagocytosis but dysfunctional phagolysosomal acidification[31], potentially resulting in neutrophils being able to phagocytose but not kill pathogens and thus allowing the neutrophils to act as a method of transport around the body and into the tissues[9]. Whilst they make up less than 20% of the total circulating neutrophil population following trauma, they are significantly elevated compared to controls within minutes of trauma and remain elevated up to 72 h later[5,23]. To our knowledge, no associations with organ dysfunction or secondary sepsis have yet been demonstrated (Figure 2C).
A third subset (CD16dim/CD62Lbright) of neutrophils comprise half of the neutrophil population up to 12 h after trauma. From day 3 onwards their percentage in the circulation rapidly decreases until they comprise less than 10% of the circulating neutrophil population[5,24]. These cells possess a band shaped nucleus, indicating they are likely immature neutrophils released from the bone marrow[17,42]. Despite their immaturity, these cells appear to have adequate phagosomal acidification compared to the other subtypes discussed[5].
Another neutrophil subtype described in autoimmunity, neoplasia and sepsis are low density neutrophils (LDNs), so named due to their isolation from the peripheral blood mononuclear cell fraction of centrifuged blood samples rather than the polymorphonuclear cell fraction, indicating a lower physical density than expected of neutrophils[9]. This population of cells is in itself heterogeneous, demonstrating different phenotypes in different disease states and has been reviewed elsewhere[44]. Low density neutrophils are much less investigated in the context of major trauma, with our review of the literature yielding a single publication which showed LDNs found in the PBMC layer displayed evidence of an activated phenotype and significant arginase activity, known to suppress T-cell function[45].
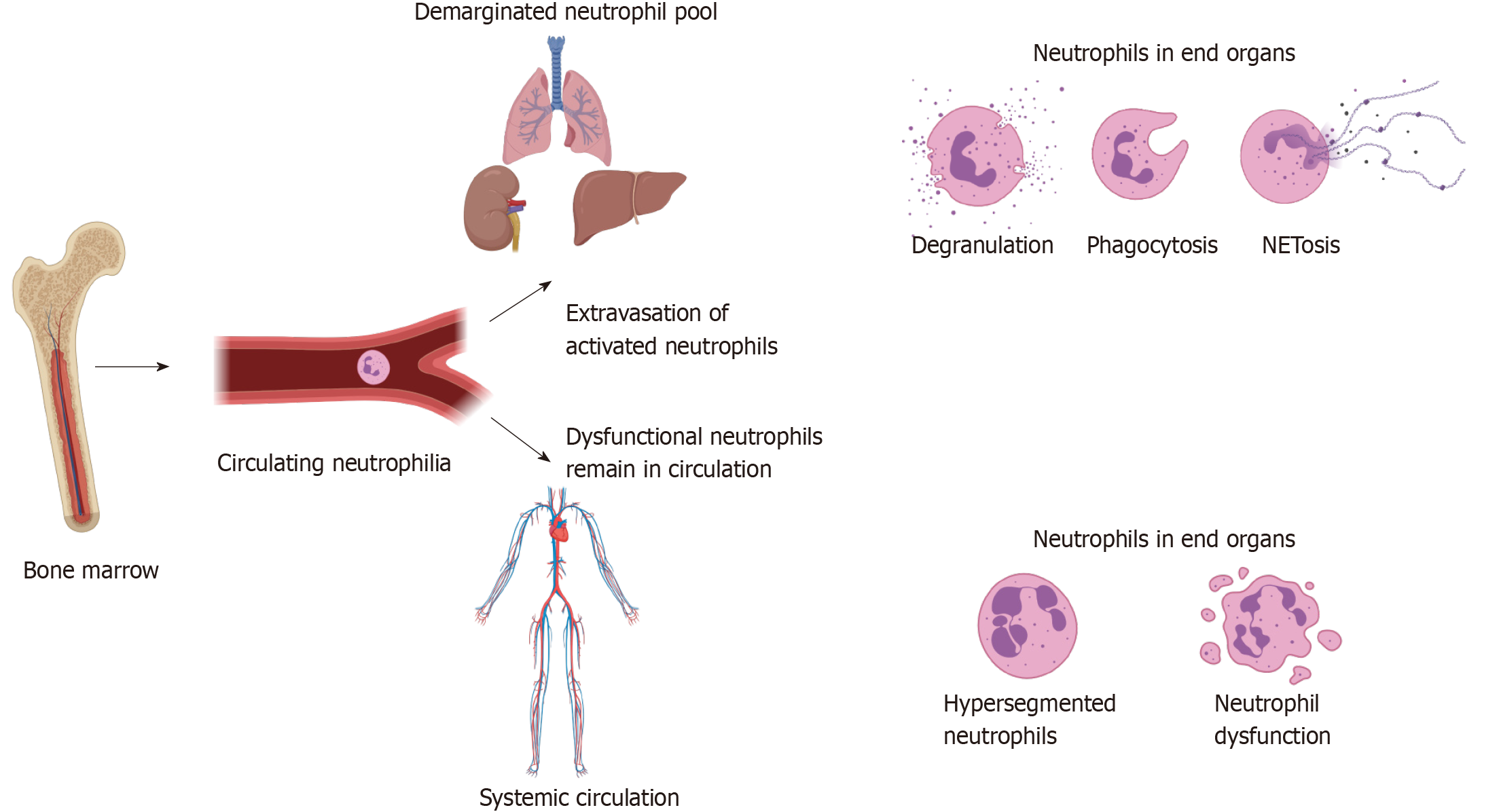
Neutrophil count is readily available from routine blood work and remains significantly elevated for a prolonged period after trauma. This increase in neutrophil count is associated with a relative decrease in CD16bright/CD62Lbright neutrophils and an increase in CD16dim/CD62Lbright neutrophils, which are thought to be immature due to early release from the bone marrow[5,9,16,23,24]. This evidence is supported by the observation that the average lobularity of neutrophils in the circulation decreases over the first 48 h, implying an increasing proportion of less mature neutrophils in the blood[26].
This increase in the proportion of immature neutrophils in the bloodstream may be due not only to influx of developing neutrophils from the bone marrow, but also due to extravasation of more mature, activated neutrophils into the end organs[25,27]. This extravasation of highly activated cells may result in collateral tissue damage and predispose to MODS[16,17,27,46]. The extravasation of neutrophils may explain the observed reduction in neutrophil count over the first 6-24 h. A correlation has been identified between the magnitude of the neutrophil count, the steepness of the decrement at 12 h and the development of organ dysfunction, which may make this an attractive avenue of investigation for prognostication in this patient group[27,46].
A reduction in lobularity accompanied the drop in neutrophil count between days 3 and 5 post injury, correlating with changes in activation markers such as CD62L and fMLP-induced CD11b expression (see “Results” section). There are several potential reasons for this phenomenon. One is that the lifespan of a circulating neutrophil in trauma is 3 to 5 d, however it takes 7 d for the bone marrow to produce new neutrophils leading to a potential gap in neutrophil supply and demand between days 4-7[1,8,13]. Whilst it is tempting to accept this explanation, differences in lifespan are unlikely to solely account for the observed reduction in circulating neutrophil count, especially given the phenomenon of emergency granulopoiesis[41].
Another theory is that activated neutrophils extravasate into tissues, leaving behind potentially immature or hypofunctional neutrophils with defects in activation, chemotaxis[9,27,30] and antimicrobial functions[23], consistent with our work in broader critically ill cohorts at risk of secondary infection[7,47]. This hypothesis would be supported by the observation that CD11b, a marker of activation and key regulator of extravasation, initially peaks within 6 h of injury before decreasing at the same time the neutrophil count and average lobularity decrease[8,25-27], indicating activated cells have extravasated. The observed decrease in CXCR2 expression after 3 h may indicate that cells expressing high levels of CXCR2 have already extravasated into the tissues[16,23]. The remaining cells in circulation may be ineffective at clearing infection, predisposing to bacteraemia and sepsis[9,12,27,30]. Further, the average lobularity of neutrophils dropped faster in patients who later developed organ dysfunction[26], consistent with the hypothesis that more mature cytotoxic neutrophils moved into the tissues and were replaced with less mature cells from the bone marrow.
Extravasation of activated neutrophils could be complicated by changes in the inflammatory state around this time. Day 5 is typically when the anti-inflammatory response starts to dominate, accompanied by a rise in the immunosuppressive cytokines IL-5 and IL-10, and the emergence of heterogeneous and immunosuppressive neutrophils[9,16,42,48,49]. The massive complement activation and reduced CD88 expression following trauma (see above) may also play a part, as reduced CD88 expression has been associated with nosocomial infection[7] and defective neutrophil antimicrobial function in general critically ill cohorts[47]. Thus, the neutrophils that remain in circulation may show suppressed activity partly due to the increased anti-inflammatory signalling in the bloodstream.
Deficient circulatory immunity due to one or more of bone marrow exhaustion, intrinsically hypofunctional neutrophils or active immunosuppression may allow for haematogenous seeding of bacteria into multiple organs filled with primed and activated neutrophils. This may act as a ‘second hit’, resulting in a secondary SIRS response causing organ dysfunction and sepsis if the infection is not controlled. These changes are summarised in Figure 3 below.
This study has several limitations, relating to both the search strategy and biases within the primary studies. Firstly, the studies reviewed are limited in that they study circulating neutrophils, which although pragmatic may not represent the phenotypes and activity of neutrophils sequestered in the tissue[13].
There are also limitations relating to observational studies. Monitoring neutrophils over extended periods of time makes it difficult to account for confounding variables such as patient comorbidities, and most of the studies done in this area use a pool of control samples rather than individually matched controls.
There does not appear to be a consensus on methodology across studies when it comes to frequency of sample collections and the time window in which samples can be collected. This makes quantitative meta-analysis of these data difficult, as some studies collected every 30-60 min after trauma whereas other studies only collected one sample every 3-5 d.
This review was limited in scope, so that not all markers of neutrophil dysfunction could be discussed. However, this allowed for a focussed review of clinically relevant markers that show the most promise and had the most literature available for analysis. Furthermore, this review focussed specifically on the neutrophil, and whilst an important cell type in this clinical context, one cell type is clearly not the only determinant of immune function and clinical outcome. The original search strategy allowed for focused results however this may have also limited the number of papers found. To address this, three databases were searched and the reference lists of selected papers were hand searched to ensure seminal papers had been identified.
This review has several implications for clinicians working with major trauma patients in the ICU. A major finding of this review is that there are several markers of neutrophil function which can be assessed with a simple blood sample, and many of these markers have predictive value for risk stratifying trauma patients at risk of immune dysfunction. Current tools used to categorise major trauma fail to adequately distinguish the various phenotypes seen in major trauma patients, and one of the major outcomes of this work may be the identification of immunological signatures which can be used to allow individualised tailored care.
This paper proposes several avenues for future research. Firstly, one issue encountered in applying neutrophil markers to clinical outcomes in trauma is that only individual markers have been assessed for predictive value. The immune system is complex and following trauma the system is dynamic and overlapping; as such, a single marker is unlikely to provide insight into the complexity of the overlapping SIRS and CARS responses[3]. Therefore, the authors suggest developing a clinical tool which combines multiple phenotypic markers, in a similar way to the APACHE-II or SOFA tools for measuring and classifying critical illness. This tool may encompass values such as the ratio of neutrophils at 3h:12h, neutrophil lobularity, fMLP-induced CD11b/FCγRIII expression, CXCR2, and the relative proportions of neutrophil CD16/CD62L subtypes. Combining these values may have better prognostic capability than the single values alone, and allow for the identification of specific subcategories of trauma patients that may benefit from specific clinical interventions, as has been reported for ARDS[50,51]. Recently published work related to COVID-19 has demonstrated the utility of this approach and may inform subsequent research in the trauma context[52].
A second area would be to develop techniques that allow the phenotyping of extravasated neutrophils. There has been some success in analysing neutrophils obtained from broncho-alveolar lavage in patients with ARDS, however it would be interesting to analyse samples from other end organs in trauma patients to determine if the phenotypes of the neutrophils in these organs match the phenotypes seen in circulation, or diverge in a way we would expect. Characterisation of neutrophils that have moved back into circulation from the tissues (reverse transmigration) may allow for less invasive analysis of these cells[53,54]. Thirdly, the expanding utility of -omic profiling may allow for more in depth analysis of the genomic and proteomic changes that precede phenotypic variability, potentially allowing for risk stratification even earlier following trauma[19,55].
The immunophenotype of neutrophils isolated from patients with major trauma differs significantly from healthy controls and varies over the course of intensive care admission. Several of these changes are correlated with adverse outcomes, including organ dysfunction and secondary sepsis.
This review aimed to provide an overview of the extant literature and characterise key aspects of neutrophil immunophenotype in trauma, with special attention to factors which may hold prognostic value for patients with severe trauma. Key findings included a persistently elevated neutrophil count, stereotyped alterations in cell-surface markers of activation and the elaboration of heterogeneous and immunosuppressive populations of cells in the circulation. Many of these changes may be driven by extravasation of highly activated neutrophils into the peripheral tissues, predisposing to organ dysfunction and leaving the circulating compartment hypofunctional and less able to respond to infectious challenges. Future research may benefit from comprehensive combinations of phenotypic and functional markers, as well as interrogation of cells that have extravasated into tissues. These promising initial findings combined with further research may allow clinicians to better risk-stratify their patients.
Neutrophils play an important role in immune dysfunction after major traumatic injury and alterations in this cell type are associated with the development of complications including organ failure and secondary infection. The kinetics of neutrophil dysfunction in the context of trauma is not completely understood and may have important implications for therapy.
Developing a granular and nuanced understanding of neutrophil kinetics and changes after trauma is necessary if key associations with disease and therapeutic targets are to be identified.
This review aimed to provide an overview of established aspects of neutrophil immunophenotypes in trauma, with special attention to factors which may hold prognostic value.
This study was a systematic review of the PubMed, Ovid Medline and Embase databases for all papers on neutrophil kinetics or function after major trauma (injury severity score > 12) in adults (≥ 18 years) since 1990.
Key findings include a notable increase in immature (CD16dim/CD62Lbright) neutrophils poorly responsive to subsequent bacterial stimuli which may confer susceptibility to bacteraemia. Highly inflammatory neutrophils which express adhesion markers and chemoattractant receptors such as CD11b and CXCR2 extravasate into end organs where they may damage host tissues and cause organ dysfunction.
Neutrophil dysfunction after major trauma is complex and changes over time. Several stereotyped changes have been observed in multiple studies, as discussed above. Immunophenotyping of multiple cell types combined with clinical and laboratory data may yield endotypes likely to respond to different therapies.
Areas of ongoing research include integration of multiple markers of immune dysfunction, enrichment strategies for clinical trials of immunomodulatory agents and the assessment of live cells in tissues rather than the circulation.
The authors would like to thank librarian Jim Berryman (University of Melbourne) for his guidance on search strategies.
| 1. | Mortaz E, Zadian SS, Shahir M, Folkerts G, Garssen J, Mumby S, Adcock IM. Does Neutrophil Phenotype Predict the Survival of Trauma Patients? Front Immunol. 2019;10:2122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 2. | Australian Institute of Health and Welfare 2020. Injury expenditure in Australia 2015–16. Cat. no. HWE 78. Canberra: AIHW. [cited 21 February 2021]. Available from: https://www.aihw.gov.au/reports/health-welfare-expenditure/injury-expenditure-in-australia-2015-16. |
| 3. | Pillay J, Hietbrink F, Koenderman L, Leenen LP. The systemic inflammatory response induced by trauma is reflected by multiple phenotypes of blood neutrophils. Injury. 2007;38:1365-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 4. | Hietbrink F, Koenderman L, Rijkers G, Leenen L. Trauma: the role of the innate immune system. World J Emerg Surg. 2006;1:15. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 142] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 5. | Hesselink L, Spijkerman R, de Fraiture E, Bongers S, Van Wessem KJP, Vrisekoop N, Koenderman L, Leenen LPH, Hietbrink F. New automated analysis to monitor neutrophil function point-of-care in the intensive care unit after trauma. Intensive Care Med Exp. 2020;8:12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Horiguchi H, Loftus TJ, Hawkins RB, Raymond SL, Stortz JA, Hollen MK, Weiss BP, Miller ES, Bihorac A, Larson SD, Mohr AM, Brakenridge SC, Tsujimoto H, Ueno H, Moore FA, Moldawer LL, Efron PA; Sepsis and Critical Illness Research Center Investigators. Innate Immunity in the Persistent Inflammation, Immunosuppression, and Catabolism Syndrome and Its Implications for Therapy. Front Immunol. 2018;9:595. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 85] [Cited by in RCA: 122] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 7. | Conway Morris A, Anderson N, Brittan M, Wilkinson TS, McAuley DF, Antonelli J, McCulloch C, Barr LC, Dhaliwal K, Jones RO, Haslett C, Hay AW, Swann DG, Laurenson IF, Davidson DJ, Rossi AG, Walsh TS, Simpson AJ. Combined dysfunctions of immune cells predict nosocomial infection in critically ill patients. Br J Anaesth. 2013;111:778-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 8. | Hietbrink F, Koenderman L, Althuizen M, Pillay J, Kamp V, Leenen LP. Kinetics of the innate immune response after trauma: implications for the development of late onset sepsis. Shock. 2013;40:21-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 9. | Hesselink L, Spijkerman R, van Wessem KJP, Koenderman L, Leenen LPH, Huber-Lang M, Hietbrink F. Neutrophil heterogeneity and its role in infectious complications after severe trauma. World J Emerg Surg. 2019;14:24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 10. | Schenck EJ, Ma KC, Murthy SB, Choi AMK. Danger Signals in the ICU. Crit Care Med. 2018;46:791-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Kasten KR, Goetzman HS, Reid MR, Rasper AM, Adediran SG, Robinson CT, Cave CM, Solomkin JS, Lentsch AB, Johannigman JA, Caldwell CC. Divergent adaptive and innate immunological responses are observed in humans following blunt trauma. BMC Immunol. 2010;11:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Botha AJ, Moore FA, Moore EE, Fontes B, Banerjee A, Peterson VM. Postinjury neutrophil priming and activation states: therapeutic challenges. Shock. 1995;3:157-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 90] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Biffl WL, Moore EE, Zallen G, Johnson JL, Gabriel J, Offner PJ, Silliman CC. Neutrophils are primed for cytotoxicity and resist apoptosis in injured patients at risk for multiple organ failure. Surgery. 1999;126:198-202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 76] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 14. | Botha AJ, Moore FA, Moore EE, Kim FJ, Banerjee A, Peterson VM. Postinjury neutrophil priming and activation: an early vulnerable window. Surgery. 1995;118:358-64; discussion 364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 183] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 15. | Bhatia R, Dent C, Topley N, Pallister I. Neutrophil priming for elastase release in adult blunt trauma patients. J Trauma. 2006;60:590-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 16. | Hazeldine J, Hampson P, Lord JM. The impact of trauma on neutrophil function. Injury. 2014;45:1824-1833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 80] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 17. | Lord JM, Midwinter MJ, Chen YF, Belli A, Brohi K, Kovacs EJ, Koenderman L, Kubes P, Lilford RJ. The systemic immune response to trauma: an overview of pathophysiology and treatment. Lancet. 2014;384:1455-1465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 436] [Cited by in RCA: 576] [Article Influence: 48.0] [Reference Citation Analysis (0)] |
| 18. | Giannoudis PV. Current concepts of the inflammatory response after major trauma: an update. Injury. 2003;34:397-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 151] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 19. | Xiao W, Mindrinos MN, Seok J, Cuschieri J, Cuenca AG, Gao H, Hayden DL, Hennessy L, Moore EE, Minei JP, Bankey PE, Johnson JL, Sperry J, Nathens AB, Billiar TR, West MA, Brownstein BH, Mason PH, Baker HV, Finnerty CC, Jeschke MG, López MC, Klein MB, Gamelli RL, Gibran NS, Arnoldo B, Xu W, Zhang Y, Calvano SE, McDonald-Smith GP, Schoenfeld DA, Storey JD, Cobb JP, Warren HS, Moldawer LL, Herndon DN, Lowry SF, Maier RV, Davis RW, Tompkins RG; Inflammation and Host Response to Injury Large-Scale Collaborative Research Program. A genomic storm in critically injured humans. J Exp Med. 2011;208:2581-2590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 777] [Cited by in RCA: 887] [Article Influence: 59.1] [Reference Citation Analysis (0)] |
| 20. | Palmer CS, Gabbe BJ, Cameron PA. Defining major trauma using the 2008 Abbreviated Injury Scale. Injury. 2016;47:109-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 183] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 21. | Veritas Health Innovation. Covidence systematic review software. [cited 21 January 2021]. Available from: https://www.covidence.org/. |
| 22. | Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44932] [Cited by in RCA: 50885] [Article Influence: 10177.0] [Reference Citation Analysis (2)] |
| 23. | Hazeldine J, Naumann DN, Toman E, Davies D, Bishop JRB, Su Z, Hampson P, Dinsdale RJ, Crombie N, Duggal NA, Harrison P, Belli A, Lord JM. Prehospital immune responses and development of multiple organ dysfunction syndrome following traumatic injury: A prospective cohort study. PLoS Med. 2017;14:e1002338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 108] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 24. | Spijkerman R, Hesselink L, Bongers S, van Wessem KJP, Vrisekoop N, Hietbrink F, Koenderman L, Leenen LPH. Point-of-Care Analysis of Neutrophil Phenotypes: A First Step Toward Immuno-Based Precision Medicine in the Trauma ICU. Crit Care Explor. 2020;2:e0158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 33] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 25. | Botha AJ, Moore FA, Moore EE, Peterson VM, Goode AW. Base deficit after major trauma directly relates to neutrophil CD11b expression: a proposed mechanism of shock-induced organ injury. Intensive Care Med. 1997;23:504-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 50] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 26. | Hesselink L, Heeres M, Paraschiakos F, Ten Berg M, Huisman A, Hoefer IE, de Groot MCH, van Solinge WW, Dijkgraaf M, Hellebrekers P, Van Wessem KJP, Koenderman L, Leenen LPH, Hietbrink F. A Rise in Neutrophil Cell Size Precedes Organ Dysfunction After Trauma. Shock. 2019;51:439-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Botha AJ, Moore FA, Moore EE, Sauaia A, Banerjee A, Peterson VM. Early neutrophil sequestration after injury: a pathogenic mechanism for multiple organ failure. J Trauma. 1995;39:411-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 188] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 28. | Halldorsdottir HD, Eriksson J, Persson BP, Herwald H, Lindbom L, Weitzberg E, Oldner A. Heparin-binding protein as a biomarker of post-injury sepsis in trauma patients. Acta Anaesthesiol Scand. 2018;62:962-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Rainer TH, Lam NY, Chan TY, Cocks RA. Early role of neutrophil L-selectin in posttraumatic acute lung injury. Crit Care Med. 2000;28:2766-2772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Hietbrink F, Oudijk EJ, Braams R, Koenderman L, Leenen L. Aberrant regulation of polymorphonuclear phagocyte responsiveness in multitrauma patients. Shock. 2006;26:558-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 31. | Groeneveld KM, Koenderman L, Warren BL, Jol S, Leenen LPH, Hietbrink F. Early decreased neutrophil responsiveness is related to late onset sepsis in multitrauma patients: An international cohort study. PLoS One. 2017;12:e0180145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 32. | Maekawa K, Futami S, Nishida M, Terada T, Inagawa H, Suzuki S, Ono K. Effects of trauma and sepsis on soluble L-selectin and cell surface expression of L-selectin and CD11b. J Trauma. 1998;44:460-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 74] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 33. | Pillay J, Kamp VM, van Hoffen E, Visser T, Tak T, Lammers JW, Ulfman LH, Leenen LP, Pickkers P, Koenderman L. A subset of neutrophils in human systemic inflammation inhibits T cell responses through Mac-1. J Clin Invest. 2012;122:327-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 672] [Article Influence: 44.8] [Reference Citation Analysis (0)] |
| 34. | Adams JM, Hauser CJ, Livingston DH, Lavery RF, Fekete Z, Deitch EA. Early trauma polymorphonuclear neutrophil responses to chemokines are associated with development of sepsis, pneumonia, and organ failure. J Trauma. 2001;51:452-6; discussion 456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 44] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 35. | Quaid GA, Cave C, Robinson C, Williams MA, Solomkin JS. Preferential loss of CXCR-2 receptor expression and function in patients who have undergone trauma. Arch Surg. 1999;134:1367-71; discussion 1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 36. | Tarlowe MH, Duffy A, Kannan KB, Itagaki K, Lavery RF, Livingston DH, Bankey P, Hauser CJ. Prospective study of neutrophil chemokine responses in trauma patients at risk for pneumonia. Am J Respir Crit Care Med. 2005;171:753-759. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 37. | Wood AJT, Vassallo A, Summers C, Chilvers ER, Conway-Morris A. C5a anaphylatoxin and its role in critical illness-induced organ dysfunction. Eur J Clin Invest. 2018;48:e13028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 34] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 38. | Huber-Lang M, Lambris JD, Ward PA. Innate immune responses to trauma. Nat Immunol. 2018;19:327-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 435] [Article Influence: 54.4] [Reference Citation Analysis (0)] |
| 39. | Amara U, Kalbitz M, Perl M, Flierl MA, Rittirsch D, Weiss M, Schneider M, Gebhard F, Huber-Lang M. Early expression changes of complement regulatory proteins and C5A receptor (CD88) on leukocytes after multiple injury in humans. Shock. 2010;33:568-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 40. | Köller M, Wick M, Muhr G. Decreased leukotriene release from neutrophils after severe trauma: role of immature cells. Inflammation. 2001;25:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 41. | Manz MG, Boettcher S. Emergency granulopoiesis. Nat Rev Immunol. 2014;14:302-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 462] [Cited by in RCA: 662] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 42. | Leliefeld PH, Wessels CM, Leenen LP, Koenderman L, Pillay J. The role of neutrophils in immune dysfunction during severe inflammation. Crit Care. 2016;20:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 159] [Cited by in RCA: 205] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 43. | Tak T, Wijten P, Heeres M, Pickkers P, Scholten A, Heck AJR, Vrisekoop N, Leenen LP, Borghans JAM, Tesselaar K, Koenderman L. Human CD62Ldim neutrophils identified as a separate subset by proteome profiling and in vivo pulse-chase labeling. Blood. 2017;129:3476-3485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 88] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 44. | Hassani M, Hellebrekers P, Chen N, van Aalst C, Bongers S, Hietbrink F, Koenderman L, Vrisekoop N. On the origin of low-density neutrophils. J Leukoc Biol. 2020;107:809-818. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 118] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 45. | Bryk JA, Popovic PJ, Zenati MS, Munera V, Pribis JP, Ochoa JB. Nature of myeloid cells expressing arginase 1 in peripheral blood after trauma. J Trauma. 2010;68:843-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 46. | Visser T, Pillay J, Koenderman L, Leenen LP. Postinjury immune monitoring: can multiple organ failure be predicted? Curr Opin Crit Care. 2008;14:666-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 47. | Wood AJ, Vassallo AM, Ruchaud-Sparagano MH, Scott J, Zinnato C, Gonzalez-Tejedo C, Kishore K, D'Santos CS, Simpson AJ, Menon DK, Summers C, Chilvers ER, Okkenhaug K, Morris AC. C5a impairs phagosomal maturation in the neutrophil through phosphoproteomic remodeling. JCI Insight. 2020;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 48. | Xu J, Guardado J, Hoffman R, Xu H, Namas R, Vodovotz Y, Xu L, Ramadan M, Brown J, Turnquist HR, Billiar TR. IL33-mediated ILC2 activation and neutrophil IL5 production in the lung response after severe trauma: A reverse translation study from a human cohort to a mouse trauma model. PLoS Med. 2017;14:e1002365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 80] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 49. | Köller M, Clasbrummel B, Kollig E, Hahn MP, Muhr G. Major injury induces increased production of interleukin-10 in human granulocyte fractions. Langenbecks Arch Surg. 1998;383:460-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 50. | Calfee CS, Delucchi K, Parsons PE, Thompson BT, Ware LB, Matthay MA; NHLBI ARDS Network. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med. 2014;2:611-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 695] [Cited by in RCA: 1141] [Article Influence: 95.1] [Reference Citation Analysis (0)] |
| 51. | Famous KR, Delucchi K, Ware LB, Kangelaris KN, Liu KD, Thompson BT, Calfee CS; ARDS Network. Acute Respiratory Distress Syndrome Subphenotypes Respond Differently to Randomized Fluid Management Strategy. Am J Respir Crit Care Med. 2017;195:331-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 605] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 52. | Laing AG, Lorenc A, Del Molino Del Barrio I, Das A, Fish M, Monin L, Muñoz-Ruiz M, McKenzie DR, Hayday TS, Francos-Quijorna I, Kamdar S, Joseph M, Davies D, Davis R, Jennings A, Zlatareva I, Vantourout P, Wu Y, Sofra V, Cano F, Greco M, Theodoridis E, Freedman JD, Gee S, Chan JNE, Ryan S, Bugallo-Blanco E, Peterson P, Kisand K, Haljasmägi L, Chadli L, Moingeon P, Martinez L, Merrick B, Bisnauthsing K, Brooks K, Ibrahim MAA, Mason J, Lopez Gomez F, Babalola K, Abdul-Jawad S, Cason J, Mant C, Seow J, Graham C, Doores KJ, Di Rosa F, Edgeworth J, Shankar-Hari M, Hayday AC. A dynamic COVID-19 immune signature includes associations with poor prognosis. Nat Med. 2020;26:1623-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 696] [Cited by in RCA: 699] [Article Influence: 116.5] [Reference Citation Analysis (0)] |
| 53. | Juss JK, House D, Amour A, Begg M, Herre J, Storisteanu DM, Hoenderdos K, Bradley G, Lennon M, Summers C, Hessel EM, Condliffe A, Chilvers ER. Acute Respiratory Distress Syndrome Neutrophils Have a Distinct Phenotype and Are Resistant to Phosphoinositide 3-Kinase Inhibition. Am J Respir Crit Care Med. 2016;194:961-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 118] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 54. | Nourshargh S, Renshaw SA, Imhof BA. Reverse Migration of Neutrophils: Where, When, How, and Why? Trends Immunol. 2016;37:273-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 144] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 55. | Jin J, Qian H, Wu G, Bao N, Song Y. Neutrophil-derived long noncoding RNA IL-7R predicts development of multiple organ dysfunction syndrome in patients with trauma. Eur J Trauma Emerg Surg. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 56. | Brown GE, Silver GM, Reiff J, Allen RC, Fink MP. Polymorphonuclear neutrophil chemiluminescence in whole blood from blunt trauma patients with multiple injuries. The Journal of Trauma. 1999;46:297-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 57. | Shih HC, Su CH, Lee CH. Superoxide production of neutrophils after severe injury: impact of subsequent surgery and sepsis. Am J Emerg Med. 1999;17:15-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 58. | Margraf S, Lögters T, Reipen J, Altrichter J, Scholz M, Windolf J. Neutrophil-derived circulating free DNA: a potential prognostic marker for posttraumatic development of inflammatory second hit and sepsis. Shock. 2008;30:352-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 207] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 59. | Paunel-Görgülü A, Kirichevska T, Lögters T, Windolf J, Flohé S. Molecular mechanisms underlying delayed apoptosis in neutrophils from multiple trauma patients with and without sepsis. Mol Med. 2012;18:325-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 60. | Guignant C, Venet F, Planel S, Demaret J, Gouel-Chéron A, Nougier C, Friggeri A, Allaouchiche B, Lepape A, Monneret G. Increased MerTK expression in circulating innate immune cells of patients with septic shock. Intensive Care Med. 2013;39:1556-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 61. | Kassam AF, Levinsky NC, Mallela JP, Angel K, Opoka A, Lahni P, Sahay RD, Fei L, Nomellini V, Wong HR, Alder MN. Olfactomedin 4 Positive Neutrophils are Upregulated after Hemorrhagic Shock. Am J Respir Cell and Mol Biol. 2021;64:216-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 62. | Stoecklein VM, Osuka A, Lederer JA. Trauma equals danger – damage control by the immune system. J Leukoc Biol. 2012;92:539-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 137] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 63. | Kuethe JW, Mintz-Cole R, Johnson BL 3rd, Midura EF, Caldwell CC, Schneider BS. Assessing the immune status of critically ill trauma patients by flow cytometry. Nur Res. 2014;63:426-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 64. | McDonald B. Neutrophils in critical illness. Cell Tissue Res. 2018;371:607-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Corresponding Author's Membership in Professional Societies: Trainee Member of the College of Intensive Care Medicine of Australia and New Zealand.
Specialty type: Critical care medicine
Country/Territory of origin: Australia
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Haque N S-Editor: Wang LL L-Editor: A P-Editor: Wang LYT