Published online Sep 9, 2021. doi: 10.5492/wjccm.v10.i5.232
Peer-review started: December 17, 2020
First decision: May 6, 2021
Revised: May 10, 2021
Accepted: July 6, 2021
Article in press: July 6, 2021
Published online: September 9, 2021
Processing time: 265 Days and 21.2 Hours
Lung resection represents the main curative treatment modality of non-small cell lung cancer. Patients with high-risk to develop postoperative pulmonary complications have been classified as “high-risk patients.” Characterizing this population could be important to improve their approach and rehabilitation.
To identify the differences between high and low-risk patients in exercise capacity and self-perceived health status after hospitalization.
A longitudinal observational prospective cohort study was carried out. Patients undergoing lung resection were recruited from the “Hospital Virgen de las Nieves” (Granada) and divided into two groups according to the risk profile criteria (age ≥ 70 years, forced expiratory volume in 1 s ≤ 70% predicted, carbon monoxide diffusion capacity ≤ 70% predicted or scheduled pneumonectomy). Outcomes included were exercise capacity (Fatigue Severity Scale, Unsupported Upper-Limb Exercise, handgrip dynamometry, Five Sit-to-stand test, and quadriceps hand-held dynamometry) and patient-reported outcome (Euroqol-5 dimensions 5 Levels Visual Analogue Scale).
In total, 115 participants were included in the study and divided into three groups: high-risk, low-risk and control group. At discharge high-risk patients presented a poorer exercise capacity and a worse self-perceived health status (P < 0.05). One month after discharge patients in the high-risk group maintained these differences compared to the other groups.
Our results show a poorer recovery in high-risk patients at discharge and 1 mo after surgery, with lower self-perceived health status and a poorer upper and lower limb exercise capacity. These results are important in the rehabilitation field.
Core Tip: Lung cancer is the leading cause of cancer death among men and the second among women worldwide. A revolutionary change in this approach is being witnessed with less invasive techniques. However, it is still associated with a high incidence of postoperative pulmonary complications, which could lead to a reduced exercise capacity. Patients with higher risk to develop postoperative pulmonary complications have been classified as “high-risk patients,” and they could present a lower exercise capacity and self-perceived health status.
- Citation: Rodríguez-Torres J, Cabrera-Martos I, López-López L, Quero-Valenzuela F, Cahalin LP, Valenza MC. Reduced exercise capacity and self-perceived health status in high-risk patients undergoing lung resection. World J Crit Care Med 2021; 10(5): 232-243
- URL: https://www.wjgnet.com/2220-3141/full/v10/i5/232.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v10.i5.232
Lung cancer is the leading cause of cancer death among men and the second among women worldwide. Non-small cell lung cancer represents 80% of all lung cancer cases, and lung resection still represents the main curative treatment modality[1].
In the last years, a revolutionary change in this approach is being witnessed[2] with less invasive techniques. However, it is still associated with a high incidence of postoperative pulmonary complications (PPCs), particularly common in patients with comorbid conditions and elderly individuals[2,3]. PPCs include (1) respiratory failure, (2) pneumonia, (3) atelectasis requiring bronchoscopy, (4) myocardial infarction, and (5) arrhythmias requiring intravenous treatment. Patients with a higher risk to develop PPCs have been classified as “high-risk patients,” and many authors have focused specifically on the approach for these patients. Besides being a clinical marker for decreased survival[3], PPCs have been associated with a longer length of hospital stay and a negative influence on the patient’s ability to resume usual daily physical activity[3].
Lung cancer patients are known to frequently exhibit poor exercise capacity, low physical activity levels and an impaired health-related quality of life that can be further aggravated after lung resection surgery[4]. Pulmonary resection causes a decrease in the lung volume, which is linked to the pain related to the chest wall, the respiratory muscle injury and the loss of muscle strength caused by bed rest, resulting in a disturbance of cardiopulmonary function and can lead to this postoperative exercise limitation. Exercise capacity has been associated with PPCs, showing a lower VO2max or a major extent of lung tissue resection in patients with PPC after curative lung resection[5]. However, other factors could affect exercise capacity like quadriceps weakness[6], illness perception[7], depressive symptoms or quality of life[8]. Moreover, we have not found specific studies based on the upper and lower limb evaluation. Functional exercise testing offers an opportunity to objectively measure patients’ exercise capacities, to identify exercise limitations that would otherwise remain undetected and to identify self-perceived capacity[9]. Moreover, the survivor’s perception of functional capacity and health status provides important information beyond objective pulmonary function testing. Despite this, we have not found studies about functional exercise limitation in these patients depending on their risk profile.
To stratify patients undergoing lung resection could be important to improve the specific rehabilitation programs and targeting these patients. Therefore, the aim of this study was to identify the differences between high and low-risk patients in exercise capacity and self-perceived health status at discharge and in the following month.
A longitudinal observational prospective cohort study has been carried out. Patients undergoing lung resection were recruited from the Thoracic Surgery Service of the “Hospital XXX” (XXX) between April 2017 and July 2018. They had to be between 18 and 80-years-old, and they were informed about the study purpose. Patients were excluded if they presented with cognitive impairment, mental instability, orthopedic pathologies that limited the test performance or neurologic pathologies. Informed consent was obtained from all individual participants included in the study. The study protocol was reviewed and approved by the XXX Ethics Committee (XXX). The STROBE guideline was followed during the course of the research[10].
Lung resection patients were divided into two groups according to the risk profile criteria[11]. High risk was defined as one or more of the following: age ≥ 70 years, forced expiratory volume in 1 s ≤ 70% predicted, carbon monoxide diffusion capacity ≤ 70% predicted or scheduled pneumonectomy. The maximum and minimal age of both lung resection groups were used to calculate the age range where control group should be included.
Data collecting was performed before lung resection, at discharge and 1 mo after surgery, always by the same investigators previously trained and blinded to the patient’s allocation. All patients followed a similar recovery pathway: after lung surgery, patients remained in the resuscitation unit 24 h and followed a similar analgesic treatment during their hospital stay, with non-steroidal anti-inflammatories. A normalized interview and an initial assessment were carried out when inclusion criteria were confirmed. Some data were collected from the medical history: anthropometric data, comorbidities (Charlson comorbidities index)[12] and operative duration. Respiratory capacity was assessed by spirometry[13] and anxiety and depression through the Hospital Anxiety and Depression Scale[14].
Main outcomes included were exercise capacity and self-perceived health status.
Exercise capacity included the self-perceived fatigue and a lower and upper limb evaluation.
To evaluate the fatigue severity, the Fatigue Severity Scale was used. The Fatigue Severity Scale[15] was developed to measure the impact of disabling fatigue on daily functioning. The instrument consists of nine items, and the total score ranges between 9 and 63. A higher score indicates more self-perceived fatigue. Minimal clinically important difference (MCID) for Fatigue Severity Scale has been reported to be 20.2.
Lower limb assessment: A hand-held dynamometer (Lafayette Manual Muscle Testing System, model 01163, Lafayette, IN, United States) was used to assess the lower limbs[16]. The test was performed with the patient seated with his/her knees and hips flexed at 90°. Resistance was applied to the anterior tibia during 5 s of maximal muscle contraction. Three trials were done in the dominant leg, and the highest value in Newton was selected for the analysis. An MCID of 46 Newton has been established.
The Five Sit-to-Stand Test (5STS) has been previously used to evaluate exercise tolerance in respiratory patients[17]. It was performed with standard height (46 cm) chair without armrests. Participants were asked to stand up all the way and sit down landing firmly, as fast as possible, five times without using the arms, and the time taken was recorded as the participant’s score. The self-perceived dyspnea and lower limb fatigue were recorded, previously and after the test, using the modified version of the Borg Scale[18]. The MCID for the 5STS has been reported to be 5 s.
Upper limb assessment: Handgrip strength is a reliable marker of peripheral muscle strength[19]. A handgrip dynamometer (TEC-60; Productos Técnicos, EE.UU.) was used to do three in the dominant hand, and the peak force in Newton was recorded. A difference of 49 Newtons has been established as the MCID.
The unsupported upper-limb exercise (UULEX) test is an incremental test developed by Takahashi et al[20] to measure peak unsupported arm exercise capacity. The subjects need to move a bar from their lap to the highest level they can reach until exhaustion. The total score was the total duration of the test in seconds. The self-perceived dyspnea and lower limb fatigue were recorded using the modified version of the Borg Scale[18]. The MCID for Borg scores was set at 1 score.
The Euroqol-5dimensions 5 Levels (EQ-5D-5L) was used to evaluate the general health status. The questionnaire comprises two parts. The first section includes five dimensions (mobility, self-care, usual activities, pain/discomfort and anxiety/ depression), each with five levels (no problem, slight problems, moderate problems, severe problems and extreme problems), and the result is an index. A value of 1 indicates full health and a value of less than zero indicates a quality of life worse than death. The second part includes a Visual Analogue Scale [EQ-5D-5L visual analogue scale (VAS)], which records the responder’s self-evaluated health between 0 (the worst imaginable health) and 100 (the best imaginable health)[21]. The MCID for the EQ-5D-5L index ranges from 0.05 and for the EQ-5D-5L VAS has been reported to be 8 points.
Statistical power calculation (GPower version 3.1.9.2 for Windows) was performed at the conception stage utilizing expected differences in the primary endpoint (EQ-5D-5L VAS) based on our previous pilot study in related subjects that employed similar methodology (unpublished). This suggested that a sample size of 30 in each group will have 80% power to detect a probability of 0.5. To allow for a generous safety margin, we decided to aim for approximately 35 patients in each study group.
Statistical Package SPSS version 20.0 (International Business Machines, Armonk, NY: http://www-01.ibm.com/support/docview.wss?uid=swg21476197) was used to analyze the data obtained. Descriptive statistics (mean ± SD) or percentages (%) were used to describe sample baseline characteristics. The Kolmogorov–Smirnov test was performed to assess continuous data normality, prior to statistical analysis. Normally distributed baseline demographic variables were compared by analysis of variance (ANOVA). The one-way ANOVA was used for baseline data. For each outcome measure, a three (high-risk, low-risk, control) × two (admission and discharge or discharge and follow-up) mixed ANOVA was performed. If the three × two ANOVA showed a significant interaction for each variable, then Bonferroni’s post hoc test was used to identify the specific mean differences. A 95% confidence interval was used for statistical analysis. A P value of less than 0.05 was considered statistically significant. Global P values were adjusted for multiplicity with the Bonferroni method.
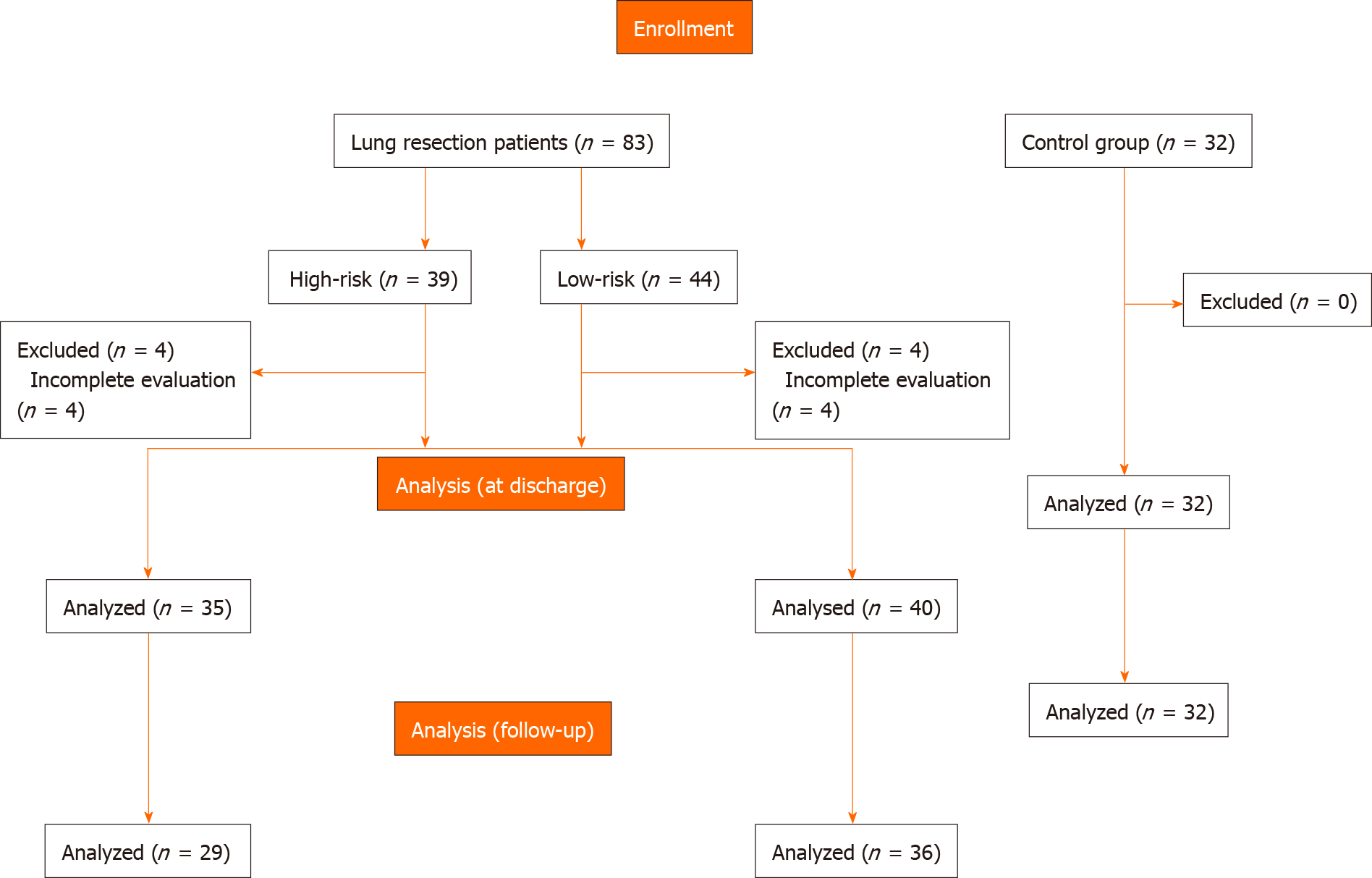
A total of 115 participants were deemed eligible and accepted to participate in this study. The distribution of participants is shown in Figure 1. Baseline characteristics of the sample are described in Table 1.
| Low-risk group, n = 39 | High-risk group, n = 44 | Control group, n = 32 | F | ||
| Age in yr | 52.18 (13.91) | 69.91 (7.97) | 48.44 (13.57) | 37.171a,c | |
| Sex, % males | 31.88 | 42.03 | 26.09 | 0.704 | |
| BMI | 27.08 (5.02) | 26.50 (4.56) | 26.08 (4.42) | 0.405 | |
| Length of hospital stay | 6.56 (1.82) | 6.95 (2.03) | - | 0.362 | |
| Charlson index | 4.10 (2.38) | 4.93 (2.43) | 1.38 (1.60) | 22.861b,c | |
| Operation duration in min | 208.79 (86.34) | 208.81 (52.29) | - | 0.999 | |
| Surgical procedure, %VATS | 74.5 | 72.4 | - | 0.722 | |
| Spirometric parameters | FEV1 | 2.71 (0.83) | 1.60 (0.50) | 2.88 (0.86) | 18.301a,c |
| FVC | 3.57 (1.02) | 2.5 (1.05) | 3.59 (0.92) | 8.605a,c | |
| HADS | Anxiety | 3.95 (2.71) | 4.64 (2.95) | 2.38 (2.94) | 5.866b,c |
| Depression | 0.92 (1.49) | 4.36 (3.84) | 0.31 (0.78) | 28.964a,b,c | |
| Total | 4.87 (3.34) | 9 (5.65) | 2.69 (3.59) | 20.253a,b,c | |
Significant differences were found in age between high-risk and the other groups. The low-risk and high-risk groups presented a similar length of hospital stay (P = 0.320) and Charlson index. Surgical procedures were similar in both groups, with most of them undergoing video-assisted thoracic surgery (74.5% vs 72.4%). As expected, forced expiratory volume in 1 s and forced vital capacity presented significant differences between low and high-risk groups (P < 0.05). Hospital Anxiety and Depression Scale presented poorer scores in the high-risk group.
Exercise capacity and self-perceived health status scores before lung resection are presented in Table 2.
| Low-risk group, n = 35 | High-risk group, n = 40 | Control group, n = 32 | F | ||
| Exercise capacity | |||||
| FSS | 19.72 (12.99) | 28 (19.49) | 9.00 | 16.469 1a,b,c,1 | |
| Lower limb assessment | |||||
| Hand-held dynamometry | 107.24 (51.59) | 112.31 (47.99) | 213.01 (60.58) | 43.669 1b,c,1 | |
| 5STS | Dyspnea baseline | 0.41 (1.33) | 0.32 (1.12) | 0 | 1.478 |
| LL fatigue baseline | 0.36 (1.20) | 1.23 (2.38) | 0 | 5.829 1a,c,1 | |
| Time | 12.43 (4.49) | 18.23 (14.27) | 9.87 (2.66) | 8.202 1a,b,c,1 | |
| Dyspnea post-test | 1.03 (1.98) | 0.86 (1.79) | 0 | 4.098b,c | |
| LL fatigue post-test | 0.72 (1.57) | 1.77 (2.94) | 0 | 7.297 1a,b,c,1 | |
| Upper limb assessment | |||||
| Handgrip dynamometry | 329.46 (93.08) | 291.57 (117.29) | 380 (79.31) | 7.178 1b,c,1 | |
| UULEX test | Dyspnea baseline | 0.44 (1.50) | 0.38 (1.03) | 0 | 1.64c |
| UL Fatigue baseline | 1.00 (1.59) | 1.13 (1.82) | 0 | 6.322 1b,c,1 | |
| Time | 442.50 (230.69) | 187.50 (201.80) | 555.00 (124.75) | 23.245 1a,b,c,1 | |
| Dyspnea post-test | 2.19 (2.54) | 0.88 (1.59) | 0.25 (0.98) | 7.439 1b,1 | |
| UL Fatigue post-test | 6.56 (2.19) | 6.38 (2.19) | 5.50 (2.66) | 1.283 | |
| Self-perceived health status | |||||
| EQ-5D-5L VAS | 86.81 (16.99) | 66.23 (22.94) | 94.69 (4.91) | 23.147 1a,c,1 | |
| EQ-5D-5L index | 1.00 | 0.76 (0.43) | 1.00 | 11.238a,b,c | |
Significant differences were found in fatigue severity and lower limb and upper limb strength between groups. The 5STS and UULEX also presented significant differences between groups, with poorer results in the high-risk group. A significant poorer self-perceived health status was shown in the high-risk group.
Exercise capacity and self-perceived health status differences at discharge among and between groups are presented in Table 3.
| Low-risk group, n = 29 | High-risk group, n = 36 | Control group, n = 32 | F | ||||||||
| Mean change | 95%CI | P value among groups | Mean change | 95%CI | P value among groups | Mean change | 95%CI | P value among groups | |||
| Exercise capacity | |||||||||||
| FSS | -4.17 (16.76) | (-10.02, 1.67) | 0.156 | -8.00 (18.56) | (-14.10, -1.90) | 0.012 | 0 | - | 1 | 21.735 1a,b,c,1 | |
| Lower limb assessment | |||||||||||
| Hand-held dynamometry | 7.20 (24.49) | (2.11, 16.52) | < 0.001 | 22.94 (31.12) | (12.41, 33.47) | < 0.001 | -0.05 (0.85) | (-0.35, 0.25) | 0.729 | 15.8 1b,c,1 | |
| 5STS test | Dyspnea baseline | -0.64 (1.45) | (-1.15, -0.12) | 0.017 | -1.05 (2.14) | (-1.75, -0.35) | 0.004 | 0 | - | 1 | 4.122 1b,c,1 |
| LL fatigue baseline | -0.30 (1.28) | (-0.76, 0.15) | 0.186 | -0.42 (1.81) | (-1.02, 0.17) | 0.160 | 0 | - | 1 | 8.735 1a,b,c | |
| Time | -3.30 (7.28) | (-5.88, -0.72) | 0.014 | -7.84 (11.39) | (-11.58, -4.09) | < 0.001 | -0.06 (0.50) | (-0.24, 0.13) | 0.531 | 14.818 1a,b,c,1 | |
| Dyspnea post-test | -0.91 (2.02) | (-1.63, -0.19) | 0.0151 | -2.74 (2.92) | (-3.69, -1.78) | < 0.001 | 0 | - | 1 | 20.128 1a,b,c,1 | |
| LL fatigue post-test | -0.42 (2.00) | (-1.13, -0.28) | 0.0081 | -1.74 (2.77) | (-2.65, -0.83) | < 0.001 | 0 | - | 1 | 23.570 1a,b,c,1 | |
| Upper limb assessment | |||||||||||
| Handgrip dynamometry | 34.14 (46.47) | (16.46, 51.81) | 0.124 | 28.05 (40.84) | (14.24, 41.87) | < 0.001 | 15.28 (64.29) | (-7.89, 38.46) | 0.188 | 7.663 1b,c,1 | |
| UULEX test | Dyspnea baseline | -1.62 (2.78) | (-3.10, -0.14) | 0.0341 | -2.00 (2.15) | (-3.24, -0.76) | 0.004 | 0 | - | 1 | 11.262 1b,c,1 |
| UL fatigue baseline | -0.75 (2.74) | (-2.21, 0.71) | 0.292 | -4.29 (3.27) | (-6.17, -2.39) | < 0.001 | 0 | - | 1 | 37.713 1a,b,c,1 | |
| Time | 202.50 (204.20) | (93.68, 311.31) | 0.002 | 145.70 (232.63) | (11.39, 280.03) | 0.036 | -3.75 (40.14) | (-18.22, 10.72) | 0.601 | 86.717 1a,b,c,1 | |
| Dyspnea post-test | -1.87 (3.44) | (-3.71, -0.04) | 0.046 | -2.29 (2.05) | (-3.47, -1.10) | 0.001 | 0 | - | 1 | 17.854 1b,c,1 | |
| UL fatigue post-test | 0 (3.40) | (-1.81, 1.81) | 1 | -1.71 (2.05) | (-2.90, -0.53) | 0.008 | 0 | - | 1 | 9.688 1a,c,1 | |
| Self-perceived health status | |||||||||||
| EQ-5D-5L VAS | 14.35 (20.48) | (3.82, 24.88) | 0.011 | 11.10 (22.07) | (0.77, 21.43) | 0.037 | -0.50 (2.64) | (-1.45, 0.45) | 0.292 | 38.091 1a,b,c,1 | |
| EQ-5D-5L index | 0.14 (0.35) | (0.07,0.21) | < 0.001 | 0.28 (0.46) | (0.11, 0.46) | 0.003 | 0 | - | 1 | 9.686 1a,c,1 | |
The high-risk group presented a significant increase in the fatigue severity at discharge (P = 0.012) and a poorer strength (P < 0.001). In the 5STS test, the high-risk group obtained significantly poorer results than the other groups, with a significant clinical difference in dyspnea and time. In the UULEX, both resection groups presented a significant statistical and clinical increase in the dyspnea levels (P < 0.05). However, only the high-risk group presented a significant increase in upper limb fatigue pretest (P < 0.001). The time reached in the UULEX was lower in both groups at discharge (P < 0.05), and a significant increase in upper limb fatigue and dyspnea post-test were found in the high-risk group (P < 0.05). The EQ-5D-5L VAS and index decreased in both groups after the intervention (P < 0.05), and it was clinically relevant in the high-risk group, which also presented significant differences in the between groups analysis.
Exercise capacity and self-perceived health status differences 1 mo after discharge, among and between groups are presented in Table 4.
| Low-risk group, n = 29 | High-risk group, n = 36 | Control group, n = 32 | F | ||||||||
| Mean change | 95%CI | P value, among groups | Mean change | 95%CI | P value among groups | Mean change | 95%CI | P value among groups | |||
| Exercise capacity | |||||||||||
| FSS | 2.84 (16.33) | (-3.90, 9.58) | 0.393 | 2.50 (14.78) | (-3.74, 8.74) | 0.416 | 0 | - | 1 | 18.606 1b,c,1 | |
| Lower limb assessment | |||||||||||
| Hand-held dynamometry | -15.56 (31.54) | (-32.37, 1.24) | 0.067 | -18.57 (27.73) | (-34.58, -2.56) | 0.026 | 0.01 (9.61) | (-2.29, 4.64) | 0.494 | 23.129 1b,c,1 | |
| 5STS test | Dyspnea baseline | 0.69 (1.98) | (-0.00, 1.39) | 0.051 | 0.37 (2.26) | (-0.37, 1.11) | 0.321 | 0 | - | 1 | 4.152c |
| LL fatigue baseline | 0 (2.15) | (-0.76, 0.76) | 1 | 0.21 (1.49) | (-0.28, 0.70) | 0.390 | 0 | - | 1 | 7.650 1b,c,1 | |
| Time | 1.75 (8.08) | (-1.10, 4.62) | 0.221 | 3.95 (14.58) | (-0.84, 8.74) | 0.104 | -0.01 (0.57) | (-0.21, 0.20) | 0.931 | 18.333 1a,b,c,1 | |
| Dyspnea post-test | 1.12 (1.87) | (0.46, 1.78) | 0.002 | 0.68 (1.47) | (0.20, 1.17) | 0.0071 | 0 | - | 1 | 26.453 1a,b,c,1 | |
| LL fatigue post-test | 0.39 (1.54) | (-0.15, 0.94) | 0.151 | 0.11 (2.27) | (-0.64, 0.85) | 0.777 | 0 | - | 1 | 47.483 1a,b,c,1 | |
| Upper limb assessment | |||||||||||
| Handgrip dynamometry | 3.80 (58.47) | (-28.58, 36.18) | 0.805 | 3.21 (69.72) | (-37.04, 43.47) | 0.866 | -16.24 (64.27) | (-39.42, 6.93) | 0.163 | 15.494 1b,c,1 | |
| UULEX test | Dyspnea baseline | 1.06 (3.29) | (-0.69, 2.82) | 0.217 | -0.60 (2.87) | (-2.66, 1.46) | 0.526 | 0 | - | 1 | 54.082 1a,b,c,1 |
| UL fatigue baseline | 0.94 (3.02) | (-0.67, 2.55) | 0.234 | 0 (3.27) | (-2.34, 2.34) | 1 | 0 | - | 1 | 194.932 1a,b,c,1 | |
| Time | -225.00 (191.62) | (-327.11, -122.89) | < 0.001 | -240.00 (187.62) | (-374.00, -105.00) | 0.003 | 7.50 (80.28) | (-21.44, 36.44) | 0.601 | 19.744 1a,b,c,1 | |
| Dyspnea post-test | 2.18 (2.61) | (0.79, 3.58) | 0.004 | -2.40 (2.79) | (-4.40, -0.39) | 0.024 | 0.13 (0.49) | (-0.05, 0.30) | 0.161 | 133.723 1a,b,c,1 | |
| UL fatigue post-test | 0.94 (2.32) | -0.30 (2.17) | 0.127 | 0 (1.76) | (-1.26, 1.26) | 1 | -0.19 (0.90) | (-0.51, 0.14) | 0.245 | 8.777 1a,c,1 | |
| Self-perceived health status | |||||||||||
| EQ-5D-5L VAS | -19.58 (23.59) | (-34.57, -4.59) | 0.015 | -3.33 (14.03) | (-12.25, 5.58) | 0.428 | -2.81 (13.68) | (-7.74, 2.12) | 0.254 | 42.089 1a,c,1 | |
| EQ-5D-5L index | -0.07 (0.35) | (-0.15, 0.01) | 0.057 | -0.22 (0.65) | (-0.54, 0.99) | 0.163 | 0 | - | 1 | 0.789 | |
Fatigue improved in the high and low-risk groups. However, the increase was not statistically or clinically significant (P > 0.05). The high-risk group presented poorer results in the 5STS and in the UULEX. The high-risk group increased, statistically and clinically significant, (P = 0.004), and the low-risk groups reduced (P = 0.024) the dyspnea. The EQ-5D-5L VAS and index improved significantly in the low-risk group (P = 0.015), with an improvement that clinically relevant. Significant differences were found between groups in the EQ-5D-5L VAS.
The aim of this study was to identify the differences between high and low-risk patients in exercise capacity and self-perceived health status at discharge and in the following month. Moreover, to compare the results with a control group is important to know if this population will reach the normative values of a similar population. Our findings show a poorer recovery in high-risk patients, with more self-perceived fatigue, a lower self-perceived health status and a poorer upper and lower limb exercise capacity. These results represent an advance in the field of rehabilitation because it allows the design of specific rehabilitation programs for each risk group.
The sample of subjects included in this study was representative of the general population undergoing lung resection, with similar sociodemographic characteristics[22].
Our results have shown significant differences in self-perceived fatigue between both surgery groups, with a higher score in the high-risk group. The occurrence of fatigue has been described following elective surgery as a negative predictor for the functional recovery[23]. Patients with persistent deficits in muscle performance will be more rapidly fatigued following motor tasks and will probably report higher levels of self-perceived fatigue. There are several possible mechanisms involved in fatigue, one of which is the release of proinflammatory cytokines by the tumor and its microenvironment. Our lung resection patients improved their fatigue level 1 mo after surgery. However, the results do not reach the control group scores. A vicious cycle may thereby be created in which these individuals avoid engaging in physical activity, further reducing their cardiorespiratory fitness and increasing their fatigability.
Our study shows poorer results in lower limb exercise capacity in the high-risk group. Similar studies[24] have shown that after lung resection surgery patients experience a decrease in maximal exercise tolerance during the first month after the intervention. This observed impairment in exercise tolerance has been reported to be induced by the cancer treatment or associated immobility; however, previous studies have suggested that deficits in exercise tolerance are likely to be apparent before surgery[24]. This aspect goes in line with our study, which suggests that exercise capacity could be determined prior to the intervention by the risk profile patient. This is important because some patients may regard immediate postoperative complications as an acceptable risk but are not prepared to accept significant postoperative functional disability[25].
Similar to our study, Cavalheri et al[26] assessed exercise capacity using the 6 min walking test in a cross-sectional study of lung cancer survivors and found that compared to age and gender-matched healthy controls there were statistically significant differences in exercise capacity. These results are similar to ours. However, they did not include a self-perceived report of dyspnea and fatigue levels, which gives us valuable information about how the patient feels their capacity or a risk profile differentiation. Benzo et al[27], in a meta-analysis, found a lower exercise capacity in patients who develop clinically relevant complications after curative lung resection. However, they only used the levels of VO2max without taking into account self-perceived exercise limitations. In the same line, Snowden et al[28] analyzed a sample of 116 major elective surgery patients and showed that patients with a higher frequency of PPCs had a much reduced level of preoperative cardiorespiratory reserve when compared with those with fewer complications.
Concerning upper limbs, our study has shown that high-risk patients present a poorer exercise capacity after lung resection. Upper limb exercise capacity plays an important role in many basic and instrumental activities of daily living and may provide unique information about upper extremity endurance not reflected in the field-based walking tests. Previous studies in similar populations have found an upper limb impairment in patients after breast cancer or cardiac surgery[29], showing decreased functionality and exercise capacity after surgery, similar to our results. However, and despite its importance, we have not found studies about UL exercise capacity after lung resection.
Finally, our results have displayed poorer self-perceived health status in the high-risk group, even 1 mo after discharge. Self-perceived health status is an important variable that rarely has been measured, but it is of tremendous significance, particularly when treating high-risk operable patients[30]. What patients fear most is to be left physically and mentally handicapped and not be able to resume an acceptable daily lifestyle[25]. In line with our study, previous research has shown that more complex resections, such as pneumonectomy, are associated with worse postoperative quality of life[25]. Brunelli et al[31] also stated that lung resection patients presented reduced quality of life values compared with the general population. However, they considered that high-risk patients had a postoperative quality of life scores similar to those observed in younger and fitter patients, which contrasts with our results. Nevertheless, the authors explained that the patients who dropped-out could have changed the results, and it should be taken into account when interpreting the results.
Our study has some limitations that have to be reported. First, the 1 mo follow-up is not enough to verify if symptom burden and exercise limitation are maintained over time. However, we have based our study design on previous studies that use the same follow-up, and in the consideration that early recovery of patients is essential to improve their quality of life. Secondly, a specific assessment of respiratory function could be included to get an objective measure of lung tissue. However, we have considered that self-perceived exercise capacity could be more important to carry out daily activities. Third, the inclusion of some comorbidities such as chronic obstructive pulmonary disease, which could affect the assessment, were not included. However, we have based our study design on previous studies, which also did not include them[22].
Our results show a poorer recovery in high-risk patients at discharge and 1 mo after surgery, with more self-perceived fatigue, lower self-perceived health status and a poorer upper and lower limb exercise capacity. Moreover, none of the groups undergoing surgery reached the results of the control group. These results represent an advance in the field of rehabilitation because it allows the design of specific rehabilitation programs for each group of patients.
Lung cancer resection still produces a high incidence of postoperative pulmonary complications. High-risk lung cancer patients are more likely to have postoperative pulmonary complications. Exercise capacity and functionality is affected in lung cancer patients after hospitalization.
High-risk patients present more complications after hospitalization. Upper and lower limb exercise capacity could be affected in these patients.
To determine if there are differences between high and low-risk patients in exercise capacity. To identify differences in self-perceived health status depending on the risk of developing postoperative pulmonary complications at discharge and 1 mo after hospitalization.
This was an observational prospective cohort study conducted between April 2017 and July 2018. Inclusion criteria included: to be between 18-years-old and 80-years-old and to be informed about the study purpose. Patients were divided into two groups according to the risk profile criteria. Outcome measures included: Fatigue Severity Scale, dynamometry, 5 Sit-to-Stand Test, unsupported upper-limb exercise, Euroqol-5 dimensions 5 levels.
Fatigue severity was higher in the high-risk group at discharge. Upper and lower limb exercise capacity presented poorer results in the high-risk group at discharge. Self-perceived health status also presented significant differences between groups. One month after hospitalization, all differences remained.
High-risk patients present a poor recovery at discharge and 1 mo after hospitalization. More fatigue and a poorer exercise capacity were found in this group. Both groups undergoing lung resection did not reach control group levels even 1 mo after hospitalization.
The approach of lung cancer patients should be different depending on the risk profile. Future studies are needed to research the differences between high and low-risk patients in a longer term. Future studies should include objective measures to identify these differences.
| 1. | Torre LA, Siegel RL, Jemal A. Lung cancer statistics. In: Lung cancer and personalized medicine. Springer: Cham, 2006: 1-19. [RCA] [DOI] [Full Text] [Cited by in Crossref: 948] [Cited by in RCA: 1283] [Article Influence: 116.6] [Reference Citation Analysis (0)] |
| 2. | Ng CS, Gonzalez-Rivas D, D'Amico TA, Rocco G. Uniportal VATS-a new era in lung cancer surgery. J Thorac Dis. 2015;7:1489-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 25] [Reference Citation Analysis (0)] |
| 3. | Simonsen DF, Søgaard M, Bozi I, Horsburgh CR, Thomsen RW. Risk factors for postoperative pneumonia after lung cancer surgery and impact of pneumonia on survival. Respir Med. 2015;109:1340-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 74] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 4. | Granger CL, McDonald CF, Irving L, Clark RA, Gough K, Murnane A, Mileshkin L, Krishnasamy M, Denehy L. Low physical activity levels and functional decline in individuals with lung cancer. Lung Cancer. 2014;83:292-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 134] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 5. | Jones LW, Hornsby WE, Goetzinger A, Forbes LM, Sherrard EL, Quist M, Lane AT, West M, Eves ND, Gradison M, Coan A, Herndon JE, Abernethy AP. Prognostic significance of functional capacity and exercise behavior in patients with metastatic non-small cell lung cancer. Lung Cancer. 2012;76:248-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 172] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 6. | Nishiyama O, Taniguchi H, Kondoh Y, Kimura T, Ogawa T, Watanabe F, Arizono S. Quadriceps weakness is related to exercise capacity in idiopathic pulmonary fibrosis. Chest. 2005;127:2028-2033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 106] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 7. | Zoeckler N, Kenn K, Kuehl K, Stenzel N, Rief W. Illness perceptions predict exercise capacity and psychological well-being after pulmonary rehabilitation in COPD patients. J Psychosom Res. 2014;76:146-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 8. | Basso RP, Jamami M, Labadessa IG, Regueiro EM, Pessoa BV, Oliveira AD Jr, Di Lorenzo VA, Costa D. Relationship between exercise capacity and quality of life in adolescents with asthma. J Bras Pneumol. 2013;39:121-127. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Lim E, Baldwin D, Beckles M, Duffy J, Entwisle J, Faivre-Finn C, Kerr K, Macfie A, McGuigan J, Padley S, Popat S, Screaton N, Snee M, Waller D, Warburton C, Win T; British Thoracic Society; Society for Cardiothoracic Surgery in Great Britain and Ireland. Guidelines on the radical management of patients with lung cancer. Thorax. 2010;65 Suppl 3:iii1-ii27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 293] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 10. | von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: guidelines for reporting observational studies. Int J Surg. 2014;12:1495-1499. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3667] [Cited by in RCA: 7419] [Article Influence: 618.3] [Reference Citation Analysis (1)] |
| 11. | Agostini P, Cieslik H, Rathinam S, Bishay E, Kalkat MS, Rajesh PB, Steyn RS, Singh S, Naidu B. Postoperative pulmonary complications following thoracic surgery: are there any modifiable risk factors? Thorax. 2010;65:815-818. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 312] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 12. | Birim O, Maat AP, Kappetein AP, van Meerbeeck JP, Damhuis RA, Bogers AJ. Validation of the Charlson comorbidity index in patients with operated primary non-small cell lung cancer. Eur J Cardiothorac Surg. 2003;23:30-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 181] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 13. | Koegelenberg CF, Swart F, Irusen EM. Guideline for office spirometry in adults, 2012. S Afr Med J. 2012;103:52-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. J Psychosom Res. 2002;52:69-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6329] [Cited by in RCA: 7460] [Article Influence: 310.8] [Reference Citation Analysis (0)] |
| 15. | Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3680] [Cited by in RCA: 4441] [Article Influence: 120.0] [Reference Citation Analysis (0)] |
| 16. | Martin HJ, Yule V, Syddall HE, Dennison EM, Cooper C, Aihie Sayer A. Is hand-held dynamometry useful for the measurement of quadriceps strength in older people? Gerontology. 2006;52:154-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 216] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 17. | Dall PM, Kerr A. Frequency of the sit to stand task: An observational study of free-living adults. Appl Ergon. 2010;41:58-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 195] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 18. | Borg G. Ratings of perceived exertion and heart rates during short-term cycle exercise and their use in a new cycling strength test. Int J Sports Med. 1982;3:153-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 345] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 19. | O'Shea SD, Taylor NF, Paratz JD. Measuring muscle strength for people with chronic obstructive pulmonary disease: retest reliability of hand-held dynamometry. Arch Phys Med Rehabil. 2007;88:32-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 20. | Takahashi T, Jenkins SC, Strauss GR, Watson CP, Lake FR. A new unsupported upper limb exercise test for patients with chronic obstructive pulmonary disease. J Cardiopulm Rehabil. 2003;23:430-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 62] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Khan I, Morris S, Pashayan N, Matata B, Bashir Z, Maguirre J. Comparing the mapping between EQ-5D-5L, EQ-5D-3L and the EORTC-QLQ-C30 in non-small cell lung cancer patients. Health Qual Life Outcomes. 2016;14:60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 51] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 22. | Brocki BC, Andreasen JJ, Langer D, Souza DS, Westerdahl E. Postoperative inspiratory muscle training in addition to breathing exercises and early mobilization improves oxygenation in high-risk patients after lung cancer surgery: a randomized controlled trial. Eur J Cardiothorac Surg. 2016;49:1483-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 65] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 23. | Rubin GJ, Hardy R, Hotopf M. A systematic review and meta-analysis of the incidence and severity of postoperative fatigue. J Psychosom Res. 2004;57:317-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 55] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Nagamatsu Y, Maeshiro K, Kimura NY, Nishi T, Shima I, Yamana H, Shirouzu K. Long-term recovery of exercise capacity and pulmonary function after lobectomy. J Thorac Cardiovasc Surg. 2007;134:1273-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 25. | Balduyck B, Hendriks J, Lauwers P, Van Schil P. Quality of life evolution after lung cancer surgery: a prospective study in 100 patients. Lung Cancer. 2007;56:423-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 26. | Cavalheri V, Hernandes NA, Camillo CA, Probst VS, Ramos D, Pitta F. Estimation of maximal work rate based on the 6-minute walk test and fat-free mass in chronic obstructive pulmonary disease. Arch Phys Med Rehabil. 2010;91:1626-1628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Benzo R, Kelley GA, Recchi L, Hofman A, Sciurba F. Complications of lung resection and exercise capacity: a meta-analysis. Respir Med. 2007;101:1790-1797. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 125] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 28. | Snowden CP, Prentis JM, Anderson HL, Roberts DR, Randles D, Renton M, Manas DM. Submaximal cardiopulmonary exercise testing predicts complications and hospital length of stay in patients undergoing major elective surgery. Ann Surg. 2010;251:535-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 210] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 29. | Izawa KP, Kasahara Y, Hiraki K, Hirano Y, Watanabe S. Relation between the Disability of the Arm, Shoulder and Hand Score and Muscle Strength in Post-Cardiac Surgery Patients. Diseases. 2017;5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Fernando HC, Landreneau RJ, Mandrekar SJ, Nichols FC, DiPetrillo TA, Meyers BF, Heron DE, Hillman SL, Jones DR, Starnes SL, Tan AD, Daly BD, Putnam JB; Alliance for Clinical Trials in Oncology. Analysis of longitudinal quality-of-life data in high-risk operable patients with lung cancer: results from the ACOSOG Z4032 (Alliance) multicenter randomized trial. J Thorac Cardiovasc Surg. 2015;149:718-25; discussion 725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 50] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 31. | Brunelli A, Socci L, Refai M, Salati M, Xiumé F, Sabbatini A. Quality of life before and after major lung resection for lung cancer: a prospective follow-up analysis. Ann Thorac Surg. 2007;84:410-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 108] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Critical care medicine
Country/Territory of origin: Spain
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Kwiecień I S-Editor: Zhang L L-Editor: Filipodia P-Editor: Li X