Published online Sep 9, 2021. doi: 10.5492/wjccm.v10.i5.220
Peer-review started: February 23, 2021
First decision: June 17, 2021
Revised: June 17, 2021
Accepted: July 20, 2021
Article in press: July 20, 2021
Published online: September 9, 2021
Processing time: 197 Days and 21.4 Hours
The central venous line is an essential component in monitoring and managing critically ill patients. However, it poses patients with increased risks of severe infections with a higher probability of morbidity and mortality.
To define the trends of the rates of central line-associated bloodstream infections (CLABSI) over four years, its predicted risk factors, aetiology, and the antimicrobial susceptibility of the isolated pathogens.
The study was a prospective case-control study, performed according to the guidelines of the Center for Disease Control surveillance methodology for CLABSI in patients admitted to the adult intensive care unit (ICU) and auditing the implementation of its prevention bundle.
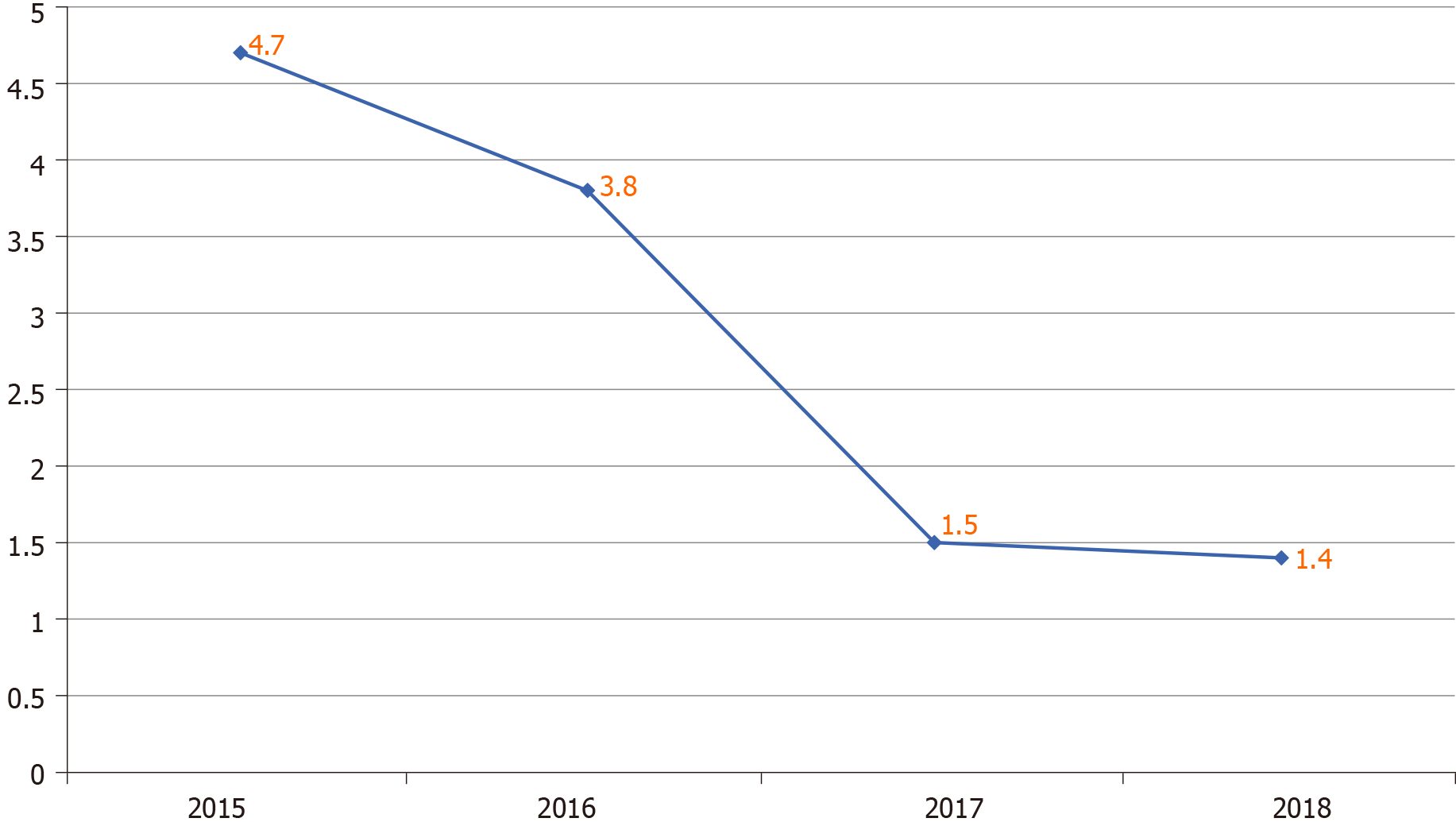
Thirty-four CLABSI identified over the study period, giving an average CLABSI rate of 3.2/1000 central line days. The infection's time trend displayed significant reductions over time concomitantly with the CLABSI prevention bundle's reinforcement from 4.7/1000 central line days at the beginning of 2016 to 1.4/1000 central line days by 2018. The most frequently identified pathogens causing CLABSI in our ICU were gram-negative organisms (59%). The most common offending organisms were Acinetobacter, Enterococcus, and Staphylococcus epidermidis, each of them accounted for 5 cases (15%). Multidrug-resistant organisms contributed to 56% of CLABSI. Its rate was higher when using femoral access and longer hospitalisation duration, especially in the ICU. Insertion of the central line in the non-ICU setting was another identified risk factor.
Implementing the prevention bundles reduced CLABSI significantly in our ICU. Implementing the CLABSI prevention bundle is crucial to maintain a substantial reduction in the CLABSI rate in the ICU setting.
Core Tip: The study aimed to define the trends of the rates of central line-associated bloodstream infections (CLABSI) over four years, its predicted risk factors, aetiology, and the antimicrobial susceptibility of the isolated pathogens. We found that implementing the prevention bundles reduced CLABSI significantly in our intensive care unit (ICU). Therefore, implementing and reinforcing the CLABSI prevention bundle are crucial to substantially reducing the CLABSI rate in the ICU setting.
- Citation: Al-Khawaja S, Saeed NK, Al-khawaja S, Azzam N, Al-Biltagi M. Trends of central line-associated bloodstream infections in the intensive care unit in the Kingdom of Bahrain: Four years’ experience. World J Crit Care Med 2021; 10(5): 220-231
- URL: https://www.wjgnet.com/2220-3141/full/v10/i5/220.htm
- DOI: https://dx.doi.org/10.5492/wjccm.v10.i5.220
The central venous line is an essential component in monitoring and managing critically ill patients. However, it poses patients with increased risks of severe infections with a higher probability of morbidity and mortality. The presence of bacteraemia initiated by the intravenous catheter is the hallmark of catheter-related bloodstream infection (BSI). Central line-associated BSIs (CLABSIs) are BSI developed in patients with central venous catheters in which there is no other apparent secondary source for bacteraemia. It is one of the most common, fatal, and costly side effects of central venous catheterisation and is one of the most frequent causes of nosocomial infections[1].
CLABSI has wide variable rates in different parts of the world, even in other institutes and units in the same country[2-5]. This rate depends on many factors such as the unit crowdedness, the scope of service provided, the ratio of the nursing staff to the patients, the staff adherence to the recommended infection control measures, and the availability of resources needed for implementing these measures[6].
The International benchmark of the National Health Safety Network (NHSN) reported a pooled CLABSI rate of 1.25/1000 central line days[7]; while the International Nosocomial Infection Control Consortium (INICC) reported pooled data from the developing countries with a CLABSI rate of 4.1 per 1000 central line-days[8]. Recently, regional studies from Arabian Gulf countries reported an average of 3.1/1000 central line days in 2017[9]. On the other hand, resource-limited countries like India reported a much higher rate of CLABSI in the range of 10-40/1000 central line days[10].
Due to the relatively high incidence of CLABSI in Arabian Gulf countries, it is crucial to prevent these infections. Many previous studies proved that most cases of CLABSI are preventable through implementing an evidence-based prevention bundle. This prevention consists of a group of elements during the insertion of the central line insertion and its maintenance. Decreasing the catheter-related infection rates can be achieved in most intensive care units (ICUs) through periodic education programs complemented by auditing and regular surveillance of the CLABSI rate[6].
According to the best of our knowledge, there is no previous published data about the rate of CLABSI in Bahrain. Therefore, the current study aimed to determine the rate of CLABSI in ICUs in Salmaniya Medical Complex (SMC), the main tertiary care hospital in Bahrain. We also sought to define the risk factors for CLABSI acquisition and define the microbiological profile of central line-related bacteraemia to map its antimicrobial susceptibility. This microbiologic and antimicrobial susceptibility mapping help select the appropriate empirical antibiotics therapy for clinically suspected CLABSI before laboratory identification of the causative organism, especially among critically ill patients in the ICU where early administration of appropriate antimicrobial treatment is crucial and lifesaving.
The study was a prospective observational case-control study, done over four years (from January 2015 till December 2018); in the adult ICU at Salmaniya Medical Complex (SMC). SMC is the main governmental tertiary-care hospital in the Kingdom of Bahrain with a 1200 bed capacity. The unit has 22 fully equipped single intensive care rooms staffed by trained nurses with a 1:1 nurse-patient ratio.
We implemented a comprehensive CLABSI prevention program in our ICU in 2016; to reduce the CLABSI rate to a level comparable to the United States NHSN rate benchmark[11]. The program included intensive education of ICU staff about CLABSI prevention bundle elements for both insertion and maintenance; regular auditing of the practice in the unit by the infection control liaison nurse (dedicated ICU nurse staff who received intensive training in the infection control with reserved hours for infection control work); and close monitoring of CLABSI rate, with periodic feedback from/to the ICU staff.
CLABSI prevention bundle for insertion included optimizing the hand hygiene before insertion, maximizing the sterile barrier precaution at insertion (full sterile body precaution for insertor including cap, mask, sterile gown, and gloves), optimal selection of the catheter insertion site, full patient body draping, proper chlorhexidine skin preparation at the insertion site. In addition, the CLABSI Prevention bundle for line care and maintenance included daily reviewing of central line necessity, optimizing the hand hygiene requirements, proper scrubbing of the hub before each use with an appropriate antiseptic, limiting accessing the catheters only with sterile devices, stressing dressing changes under complete aseptic technique using sterile gloves, and proper periodic replacement of dressings (dry gauze dressings every two days/transparent dressings every seven days)[12].
The study included all the patients (≥ 14 years) admitted to the adult ICU in SMC and needed placement of a central line for one or more days, during four years between January 2015 to December 2018. There were no exclusion criteria. We defined the cases as patients who developed CALBSI after 48 h from their admission to ICU. The control group was ICU patients who had central line insertion without the development of CLABSI. CLABSI was diagnosed -according to NHSN definition- as a laboratory-confirmed BSI. We also identified an eligible primary BSI causing organism, and a suitable central line was present on the laboratory-confirmed BSI date of the event (LCBI DOE) or the day before[11]. We defined the underlying medical or surgical conditions by proper history taking, thorough clinical examination, and the needed investigations as appropriate.
We collected the data prospectively through the unit's daily round by the ICU infection control liaison nurse. The nurse observed all central line catheterized inpatients using a particular surveillance form. The form recorded specific demographic data like age, gender, underlying diseases, hospital admission date, date of ICU admission, clinical diagnosis, and the patient outcome (death or discharge). It also included the date of insertion of the central line, the type of central line, its location, and the number of its lumens. Cases with suspected CLABSI were further referred for evaluation by the infection control team. The team included a clinical microbiologist and infectious diseases physician to finalize the cases if they fulfilled all the required clinical and microbiological criteria to diagnose CALBSI as per the NHSN definition[11].
The infection control liaison nurse collected the data about ICU staff's adherence (doctors and nurses) to the recommended CLABSI prevention bundle, using the standardized audit checklist for line insertion and maintenance. The checklist included the prevention bundle elements mentioned above. These data were collected during the observational rounds in the unit twice per week. We got a minimum of 120 observations for maintenance elements per month; and included the insertion checklist for all the lines inserted in the unit. We compared the prospective data collected for the three years (from January 2016 to December 2018) to the retrospective data collected from the same unit during the one year before implementing the comprehensive CLABSI prevention program (during the 2015 year).
We used the traditional culture and biochemical characteristics of the isolates for proper bacterial identification. In addition, we standardized the antimicrobial susceptibility testing according to the Clinical and Laboratory Standards Institute[13]. According to the multi-drug resistance (MDR) classification system, MDR strains have resistance to 3 or more classes of antimicrobial agents[14].
We collected and tabulated the data using the electronic health system, then analysing it using the statistical software SPSS version 24 (IBM Corp, Chicago, IL, United States). We calculated the descriptive statistics of demographic variables, including frequencies, percentages, means, and ranges. To calculate the incidence rate of CLABSI as events per 1000 catheter-day, we divided the total number of patients with CLABSI/total number of catheter days during the year of the study, then multiply by 1000. The compliance to prevention bundle (average overall compliance to central line insertion and maintenance prevention elements) was calculated by dividing the number of compliant elements over the number of observed elements then multiply by 100. The Research and Ethics Committee at the Ministry of Health, Kingdom of Bahrain, approved the study. We did not collect consent, as the study was observational.
The study included all patients admitted to ICU during the study period between 2015 and 2018. During the study period, there were 3323 patients with 1634 central line insertions. We documented 34 CLABSI cases, with an average rate of 3.2/1000 central line days (the rate was 4.7/1000 at the beginning of 2015 dropped to 1.4/1000 central line days by the end of 2018 (Figure 1). Table 1 showed the demographics data, the clinical characteristics, and the potential risk factors for the CLABSI cases, in addition to the control group (catheterized patients without CLABSIs). About 71% of the patients who developed CLABSI had medical and while 29% had surgical conditions (71%), with a mean age of 63.6 years; twenty were male (59%). In addition, patients with CLABSI were relatively older than the control group (median age of 63.6 years vs 52.6 years, respectively). However, there was no significant difference between the CLABSI cases and the control group in age, gender, or the type of admission (medical vs surgical). All the patients admitted to the ICU and needed central lines had temporary, non-tunnelled, with more than one-lumen type of central lines.
| Potential risk factor | CLABSI cases, n = 34, n (%) | Control, n = 1600, n (%) | P value1 | |
| Age | < 50 yr | 11 (32) | 672 (42) | 0.13 |
| > 50 yr | 23 (68) | 928 (58) | ||
| Gender | Male | 20 (59) | 880 (55) | 0.32 |
| Female | 14 (41) | 720 (45) | ||
| Primary clinical diagnosis | Medical | 24 (71) | 1056 (66) | 2.88 |
| Surgical | 10 (29) | 544 (34) | ||
| Catheter insertion site | Subclavian | 4 (11) | 496 (31) | < 0.05 |
| Jugular | 10 (30) | 464 (29) | ||
| Femoral | 20 (59) | 640 (40) | ||
| ICU time interval from ICU admission till line insertion | < 5 d | 19 (56) | 1408 (88) | < 0.05 |
| > 5 d | 15 (44) | 192 (12) | ||
| Location of central line insertion | ICU | 20 (59) | 1216 (76) | < 0.05 |
| Non-ICU | 14 (41) | 384 (24) | ||
| Length of duration central line | < 1 wk | 11 (32) | 1438 (90) | < 0.001 |
| > 1 wk | 23 (68) | 162 (10) | ||
| Death | 15 (44) | 432 (27) | < 0.01 | |
| A live discharge | 19 (54) | 1168 (73) | < 0.01 |
The table also showed that CLABSI developed on average on day 15th after the central line's insertion. All infected central line had triple lumens and non-tunnelled. The most common insertion site was femoral (59% of all CLABSI and 3% of all femoral-inserted central lines), followed by the jugular vein (30% of all CLABSI and 2.1% of jugular vein-inserted central lines). Most of the infected central lines were inserted inside the ICU (59%), while the remaining were inserted in the emergency room (P < 0.05). Patients who developed CLABSI had a significantly longer median duration of stay in the ICU before placement of the central lines (7.6 d vs 2.8 d with P < 0.05). In addition, they had a higher proportion of catheter placement in the femoral vein (59% vs 40% with P < 0.05), especially with inserting the central line outside the ICU setting (41% vs 24% with P < 0.05) than with the control group. The patients who developed CLABSI had a significantly longer central line insertion duration than those who did not develop CLABSI (P < 0.001). Patients who developed CLABSI had a substantially higher mortality rate than the control group (44% vs 27%, P < 0.01).
Table 2 showed the microbiological causes of CLABSI. Gram-negative bacteria were the most common organisms isolated from CLABSI (56%), followed by gram-positive bacteria (41%). Candida was isolated from 3% of the isolates. The gram-positive coagulase-negative Staphylococcus (18%) were the most common organisms isolated, followed by the gram-negative Acinetobacter (15%), gram-positive Enterococci (15%), Pseudomonas (12%), Escherichia (12%), Klebsiella (9%), and Staphylococcus aureus (6%). We observed MDR organisms in 59% (20/34) of all CLABSI cases (gram-positive and gram-negative organisms).
| Organism | Number (percentage out of total 34), n (%) | MDR organism, n (%) |
| Gram negative bacteria | ||
| Acinetobacter | 5 (15) | 3 MDR1 (60) |
| Escherichia coli | 4 (12) | 1 ESBL (25) 2 CRE (50) |
| Pseudomonas | 4 (12) | 2 CRP (50) |
| Klebsiella | 3 (8) | 2 CRE (66) |
| Morganella | 1 (3) | |
| Serratia | 1 (3) | |
| Stenotrophomonas maltophilia | 1 (3) | |
| Total gram negative | 19 (56) | 10 (53) |
| Gram positive bacteria | ||
| Enterococcus | 5 (15) | 3 VRE (60) |
| Coagulase negative Staphylococcus | 6 (18) | 6 MRCONS (100) |
| Staphylococcus aureus | 2 (5) | 1MRSA (50) |
| Streptococcus viridans | 1 (3) | |
| Total gram positive | 14 (41) | 10 (71) |
| Candida species | 1 (3) | |
| Total | 34 (100) | 20/34 (59) |
Table 3 showed the antibiotic sensitivity pattern for the common gram-positive organisms causing CLABSI in the current study. Their sensitivity rate to both Linezolid and Daptomycin was 100%. Staphylococcus aureus and Coagulase-negative Staphylococci were 100% sensitive to vancomycin. Table 3 also showed other antibiotics sensitivities for common gram-positive organisms. We showed the antibiotic sensitivity pattern for the common gram-negative organisms causing CLABSI in the current study in Table 4. Effective of Colistin was present in all the four main strains of the isolates. Pseudomonas aeruginosa had 100% sensitivity to piperacillin-tazobactam, ceftazidime, cefepime, meropenem, ciprofloxacin, gentamicin, and amikacin. Acinetobacter baumannii isolates had a high level of resistance. Three out of the five isolates (60%) were MDR and resistant to most tested antibiotics. However, all Acinetobacter baumannii isolates were sensitive to colistin (100% sensitivity). Three out of the four Escherichia coli (E. coli) isolates (75%) were MDR; one was ESBL producers (25%), and two were CRE (50%). Klebsiella pneumoniae isolates also had a high resistance level; two out of the three isolates (66%) were CRE. However, all Klebsiella pneumoniae isolates (100%) retain their sensitivity to colistin.
| Antibiotic | Staphylococcus aureus, n (%) = 2 (5) | Coagulase negative Staphylococcus,n (%) = 6 (18) | Enterococcus, n (%) = 5 (15) |
| Penicillin | 0/2 (0) | 0/6 (0) | 1/5 (20) |
| Ampicillin | 1/5 (20) | ||
| Erythromycin | 1/2 (50) | 0/6 (0) | 0/5 (0) |
| Clinamycin | 1/2 (50) | 3/6 (50) | |
| Trimethoprim sulphamethoxazole | 1/2 (50) | 1/6 (16) | |
| Vancomycin | 2/2 (100) | 6/6 (100) | 2/5 (40) |
| Cloxacillin | 1/2 (50) | 0/6 (0) | |
| Tetracycline | 1/2 (50) | 3/6 (50) | |
| Linazolid | 2/2 (100) | 6/6 (100) | 5/5 (100) |
| Daptomycin | 2/2 (100) | 6/6 (100) | 5/5 (100) |
| Antimicrobial agent | Acinetobacter baumanii | Pseudomonas aeruginosa | Escherichia coli | Klebsiella |
| n (%) = 5 (15) | n (%) = 4 (12) | n (%) = 4 (12) | n (%) = 3 (9) | |
| Piperacillin tazobactam | 2/5 (40) | 4/4 (100) | ||
| Ceftriaxone | 1/4 (25) | 1/3 (33) | ||
| Ceftazidime | 2/5 (40) | 4/4 (100) | 1/4 (25) | 1/3 (33) |
| Cefipime | 2/5 (40) | 4/4 (100) | 1/4 (25) | 1/3 (33) |
| Merpenem | 2/5 (40) | 4/4 (100) | 2/4 (50) | 1/3 (33) |
| Imipenem | 2/5 (40) | 2/4 (50) | 2/4 (50) | 1/3 (33) |
| Ciprofloxacin | 2/5 (40) | 4/4 (100) | 1/4 (25) | 1/3 (33) |
| Gentamicin | 2/5 (40) | 4/4 (100) | 1/4 (25) | 1/3 (33) |
| Amikacin | 2/5 (40) | 4/4 (100) | 1/4 (25) | 1/3 (33) |
| Colistin | 5/5 (100) | 4/4 (100) | 4/4 (100) | 3/3 (100) |
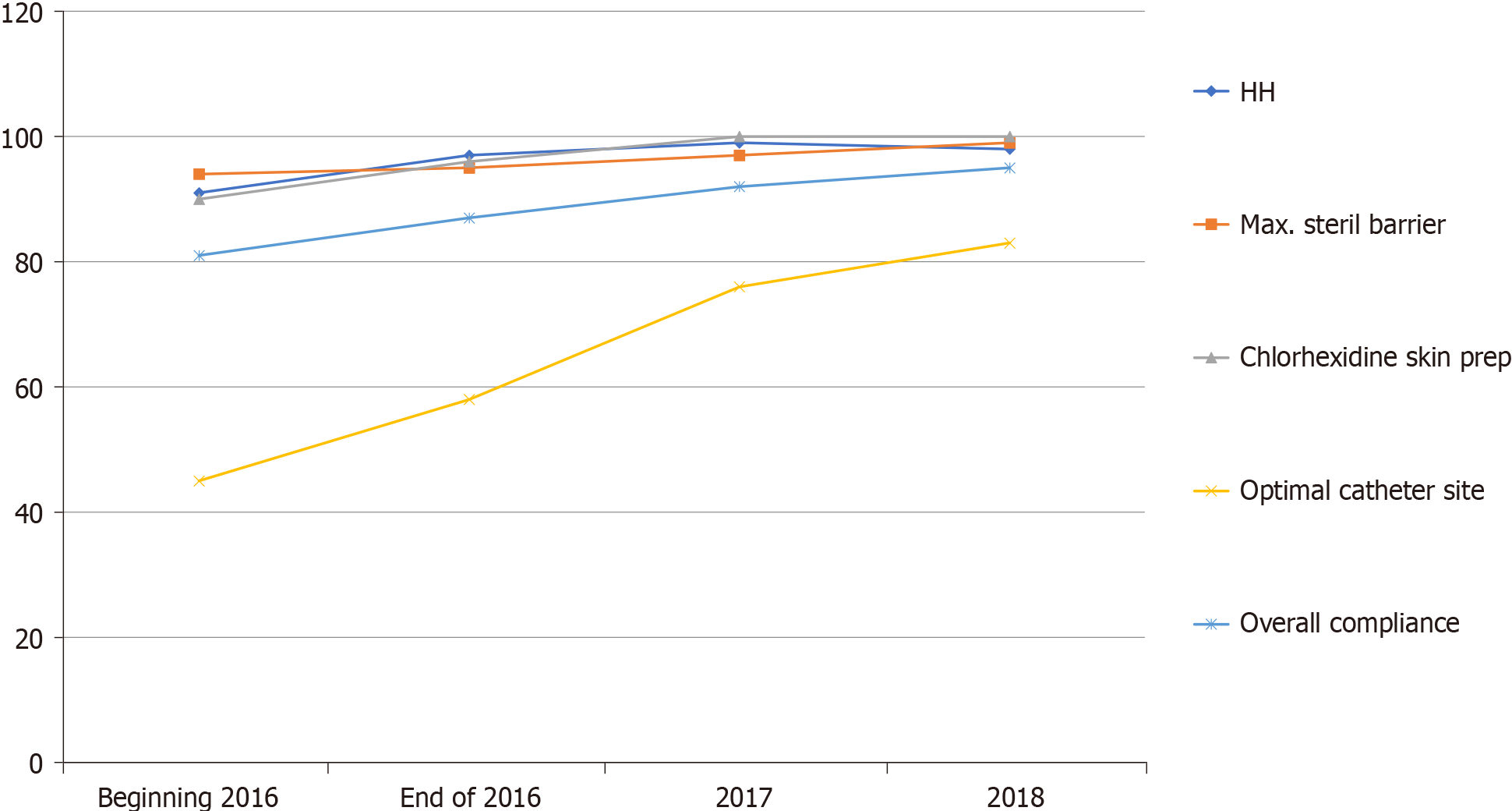
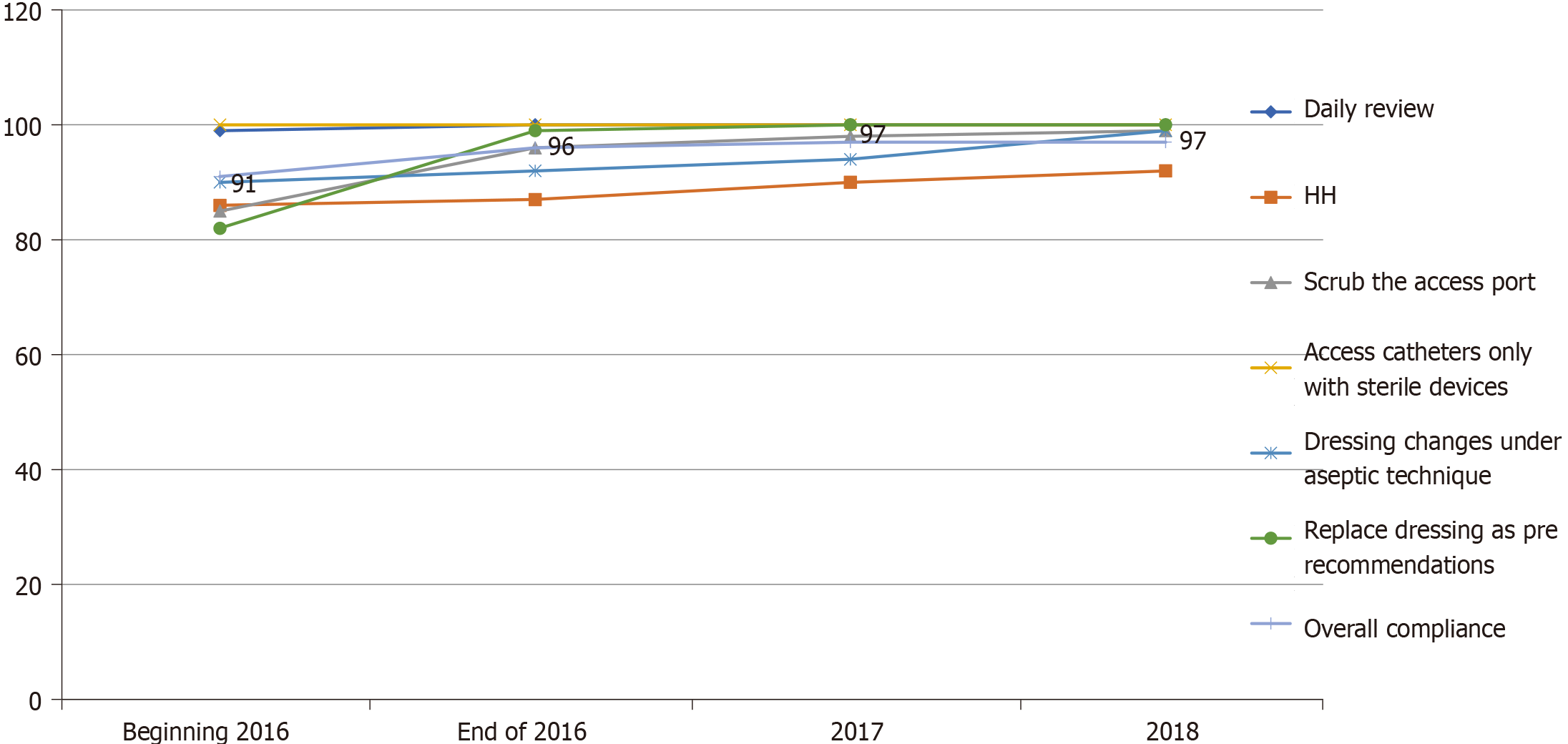
Figure 2 showed the overall compliance with the CLABSI prevention bundle for central line insertion. It showed significant improvement throughout the study period. This improvement was related to the enhancement of adherence to the optimum selection of the anatomical insertion site. Compliance was deficient (45%) at the beginning of the study in 2016 due to a lack of experience in inserting the central line in the subclavian or internal jugular by the ICU residents covering the duty. Therefore, training the ICU residents to optimize the insertion site improved adherence to the proper selection of insertion sites to reach 83% by the end of 2018. Figure 3 showed the overall compliance with the CLABSI prevention bundle for the care and maintenance of the central lines. It showed the overall improvement during the study period, predominantly the appropriate dressing replacement and hub scrub practice, which accomplished through reinforcement of the practice by the observing infection control liaison nurse.
CLABSIs result in many preventable deaths each year with a high financial cost and load on the healthcare system. Nevertheless, these infections are preventable. Therefore, implementing a prevention program is of paramount importance. After we implemented the CLABSIs prevention program, the rate of CLABSI in our ICU decreased from 4.7/1000 central-catheter days at baseline (at the beginning of implementing the CLABSI prevention program) to 1.4/1000 central catheter-days at the end of the study. This achieved rate is comparable to the international benchmark of NHSN, with its reported median CLABSI rates of 1.25/1000 central-catheter days[7]. Many previous studies showed a similar reduction of CLABSI by reinforcing the CLABSI prevention program[15-18]. For example, a promising report published by Al Abdulla illustrated a significant decline of CLABSI from 6 per 1000 central line days during 2011 to 0.3 per 1000 central line days in 2016 in ICU among one of the major teaching hospitals in Saudi Arabia[19].
The current study revealed many factors that increased the risk of CLABSI. The rate of CLABSI in our ICU increased when using femoral access. It also increased with the hospitalization duration before the ICU admission and the longer ICU admission duration before the central line's insertion. The site of insertion also affects the risk of CLABSI. The risk increased when the line insertion setting was outside the ICU, such as the Emergency Room, which commonly happened if there is a long waiting time before transferring the patient to the ICU.
The insertion site is a significant risk factor to develop CLABSI. Previously published data showed an increased risk of developing infectious complications when using femoral access, consistent with our finding[20-22]. Accordingly, we should avoid the femoral access site as much as possible to avoid increasing the rates of CLABSI and thrombotic events compared to subclavian and internal jugular sites. The subclavian site is associated with the lowest rate of CLABSI, as observed in the current study and other studies. Nevertheless, occasionally, it is difficult to use the subclavian and internal jugular sites due to coagulopathies or anatomical difficulties such as distorting anatomical features[23]. In the current study, the duration of central line insertion longer than a week was also a significant risk factor to develop CLABSI. This finding agreed with Baier et al[24], who found that central line insertion duration for more than eight days was a significant risk factor to develop CLABSI.
In the current study, the microbial profile showed a predominance of the gram-negative bacteria (56%), with a high percentage of the MDR strains. Similar data obtained from previously published studies worldwide illustrated the change in the gram-negative carriage's global tendency rather than the gram-positive. These observations were greatly accentuated in the ICU setting due to the high exposure to nosocomial microorganisms[25-29]. Addressing the bacterial profile and the prevalence of MDR bacteria causing CLABSI in patients admitted to ICUs and their antimicrobial resistance profile may help the physicians make a rapid management decision and start the most proper antibiotics until the result of bacterial culture and antibiotic sensitivity pattern becomes available.
The current study had certain limitations. We conducted the survey in a single centre in Bahrain. Consequently, the results cannot be generalized to other public or private hospital settings. We did not address the patients' clinical details, which could be a critical risk factor to develop CLABSI. However, despite the study's limitations, it can provide the clinicians with valuable data concerning the incidence rates and the prevalence of CLABSI in Bahrain, reflecting the rest Arabian Gulf region's status.
Given such a promising trend of reducing CLABSI in our ICU through reinforcement of the unit's prevention program, we believe that it is possible to achieve lower CLABSI rates. To attain such desired outcomes, we need to reinforce the ICU doctors to select the optimal site to insert the central line, avoid the femoral access, and reinforce the central line's insertion inside the ICU only by a trained ICU physician. As the microbial profile of CLABSI in our ICU showed a predominance of the gram-negative bacteria with a significant proportion of MDR organisms, we advise using broad-spectrum gram-negative coverage (in addition to gram-positive) as part of the empirical antibiotics therapy in patients with suspected CLABSI.
The central venous line is an essential component in monitoring and managing critically ill patients. Central line-associated bloodstream infection (CLABSI) are BSIs developed in patients with central venous catheters. The presence of these infections is associated with a higher risk of morbidity and mortality.
Because we do not have enough data about the rate of CLABSI and the causative organisms in the Kingdom of Bahrain, we would like to estimate the magnitude of the problem in our intensive care units (ICUs). Knowing the microbial profile of CLABSI in our ICU help proper use of the empirical antibiotics therapy in patients with suspected CLABSI.
The study aimed to define the trends of the rates of CLABSI over four years, its predicted risk factors, aetiology, and the antimicrobial susceptibility of the isolated pathogens
The study was a prospective case-control study, performed according to the guidelines of the Center for Disease Control surveillance methodology for CLABSI in patients admitted to the adult ICU and auditing the implementation of its prevention bundle.
Thirty-four CLABSI identified over the study period, giving an average CLABSI rate of 3.2/1000 central line days. The infection's time trend displayed significant reductions over time concomitantly with the CLABSI prevention bundle's reinforcement from 4.7/1000 central line days at the beginning of 2016 to 1.4/1000 central line days by 2018. The most frequently identified pathogens causing CLABSI in our ICU were Gram-negative organisms (59%). The most common offending organisms were Acinetobacter, Enterococcus, and Staphylococcus epidermidis, each of them accounted for 5 cases (15%). Multidrug-resistant organisms contributed to 56% of CLABSI. Its rate was higher when using femoral access and longer hospitalisation duration, especially in the ICU. Insertion of the central line in the non-ICU setting was another identified risk factor.
Implementing the prevention bundles reduced CLABSI significantly in our ICU. Reinforcing CLABSI prevention bundle implementation is crucial to substantially reducing the CLABSI rate in the ICU setting.
We need to study the mechanism of bacterial resistance among patients infected with CLABSI. We also need to study viral coinfection and its effects on morbidity and mortality. We should compare our data with the data from other countries to generalize the obtained results.
We thank the anonymous referees for their useful suggestions.
| 1. | Gahlot R, Nigam C, Kumar V, Yadav G, Anupurba S. Catheter-related bloodstream infections. Int J Crit Illn Inj Sci. 2014;4:162-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 230] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 2. | Mehta A, Rosenthal VD, Mehta Y, Chakravarthy M, Todi SK, Sen N, Sahu S, Gopinath R, Rodrigues C, Kapoor P, Jawali V, Chakraborty P, Raj JP, Bindhani D, Ravindra N, Hegde A, Pawar M, Venkatachalam N, Chatterjee S, Trehan N, Singhal T, Damani N. Device-associated nosocomial infection rates in intensive care units of seven Indian cities. Findings of the International Nosocomial Infection Control Consortium (INICC). J Hosp Infect. 2007;67:168-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 114] [Article Influence: 6.0] [Reference Citation Analysis (1)] |
| 3. | Al-Tawfiq JA, Amalraj A, Memish ZA. Reduction and surveillance of device-associated infections in adult intensive care units at a Saudi Arabian hospital, 2004-2011. Int J Infect Dis. 2013;17:e1207-e1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Rosenthal VD, Bijie H, Maki DG, Mehta Y, Apisarnthanarak A, Medeiros EA, Leblebicioglu H, Fisher D, Álvarez-Moreno C, Khader IA, Del Rocío González Martínez M, Cuellar LE, Navoa-Ng JA, Abouqal R, Guanche Garcell H, Mitrev Z, Pirez García MC, Hamdi A, Dueñas L, Cancel E, Gurskis V, Rasslan O, Ahmed A, Kanj SS, Ugalde OC, Mapp T, Raka L, Yuet Meng C, Thu le TA, Ghazal S, Gikas A, Narváez LP, Mejía N, Hadjieva N, Gamar Elanbya MO, Guzmán Siritt ME, Jayatilleke K; INICC members. International Nosocomial Infection Control Consortium (INICC) report, data summary of 36 countries, for 2004-2009. Am J Infect Control. 2012;40:396-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 314] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 5. | Al-Mousa HH, Omar AA, Rosenthal VD, Salama MF, Aly NY, El-Dossoky Noweir M, Rebello FM, Narciso DM, Sayed AF, Kurian A, George SM, Mohamed AM, Ramapurath RJ, Varghese ST. Device-associated infection rates, bacterial resistance, length of stay, and mortality in Kuwait: International Nosocomial Infection Consortium findings. Am J Infect Control. 2016;44:444-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 6. | Patil HV, Patil VC, Ramteerthkar MN, Kulkarni RD. Central venous catheter-related bloodstream infections in the intensive care unit. Indian J Crit Care Med. 2011;15:213-223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 7. | Chitnis AS, Edwards JR, Ricks PM, Sievert DM, Fridkin SK, Gould CV. Device-associated infection rates, device utilization, and antimicrobial resistance in long-term acute care hospitals reporting to the National Healthcare Safety Network, 2010. Infect Control Hosp Epidemiol. 2012;33:993-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Rosenthal VD, Al-Abdely HM, El-Kholy AA, AlKhawaja SAA, Leblebicioglu H, Mehta Y, Rai V, Hung NV, Kanj SS, Salama MF, Salgado-Yepez E, Elahi N, Morfin Otero R, Apisarnthanarak A, De Carvalho BM, Ider BE, Fisher D, Buenaflor MCSG, Petrov MM, Quesada-Mora AM, Zand F, Gurskis V, Anguseva T, Ikram A, Aguilar de Moros D, Duszynska W, Mejia N, Horhat FG, Belskiy V, Mioljevic V, Di Silvestre G, Furova K, Ramos-Ortiz GY, Gamar Elanbya MO, Satari HI, Gupta U, Dendane T, Raka L, Guanche-Garcell H, Hu B, Padgett D, Jayatilleke K, Ben Jaballah N, Apostolopoulou E, Prudencio Leon WE, Sepulveda-Chavez A, Telechea HM, Trotter A, Alvarez-Moreno C, Kushner-Davalos L; Remaining authors. International Nosocomial Infection Control Consortium report, data summary of 50 countries for 2010-2015: Device-associated module. Am J Infect Control. 2016;44:1495-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 239] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 9. | Balkhy HH, El-Saed A, Al-Abri SS, Alsalman J, Alansari H, Al Maskari Z, El Gammal A, Al Nasser W, AlJardani A, Althaqafi A. Rates of central line-associated bloodstream infection in tertiary care hospitals in 3 Arabian gulf countries: 6-year surveillance study. Am J Infect Control. 2017;45:e49-e51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Kaur M, Gupta V, Gombar S, Chander J, Sahoo T. Incidence, risk factors, microbiology of venous catheter associated bloodstream infections--a prospective study from a tertiary care hospital. Indian J Med Microbiol. 2015;33:248-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | National Healthcare Safety Network Patient Safety Component Manual, CDC. [cited 22 April 2021]. Available from: https://www.cdc.gov/nhsn/pdfs/pscmanual/pcsmanual_current.pdf. |
| 12. | O'Neil C, Ball K, Wood H, McMullen K, Kremer P, Jafarzadeh SR, Fraser V, Warren D. A Central Line Care Maintenance Bundle for the Prevention of Central Line-Associated Bloodstream Infection in Non-Intensive Care Unit Settings. Infect Control Hosp Epidemiol. 2016;37:692-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing: 26nd Informational Supplement M100-S26. Wayne, PA: Clinical and Laboratory Standards Institute; 2016. [cited 22 April 2021]. Available from: http://www.facm.ucl.ac.be/intranet/CLSI/CLSI-2017-M100-S27.pdf. |
| 14. | Falagas ME, Koletsi PK, Bliziotis IA. The diversity of definitions of multidrug-resistant (MDR) and pandrug-resistant (PDR) Acinetobacter baumannii and Pseudomonas aeruginosa. J Med Microbiol. 2006;55:1619-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 236] [Cited by in RCA: 310] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 15. | Khalid I, Al Salmi H, Qushmaq I, Al Hroub M, Kadri M, Qabajah MR. Itemizing the bundle: achieving and maintaining "zero" central line-associated bloodstream infection for over a year in a tertiary care hospital in Saudi Arabia. Am J Infect Control. 2013;41:1209-1213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Leblebicioglu H, Öztürk R, Rosenthal VD, Akan ÖA, Sirmatel F, Ozdemir D, Uzun C, Turgut H, Ersoz G, Koksal I, Özgültekin A, Esen S, Ulger F, Dilek A, Yilmaz H, Dikmen Y, Aygún G, Tulunay M, Oral M, Ünal N, Cengiz M, Yilmaz L, Geyik MF, Şahin A, Erdogan S, Sacar S, Sungurtekin H, Uğurcan D, Kaya A, Kuyucu N, Yýlmaz G, Kaya S, Ulusoy H, İnan A. Impact of a multidimensional infection control approach on central line-associated bloodstream infections rates in adult intensive care units of 8 cities of Turkey: findings of the International Nosocomial Infection Control Consortium (INICC). Ann Clin Microbiol Antimicrob. 2013;12:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 17. | Marschall J, Mermel LA, Fakih M, Hadaway L, Kallen A, O'Grady NP, Pettis AM, Rupp ME, Sandora T, Maragakis LL, Yokoe DS; Society for Healthcare Epidemiology of America. Strategies to prevent central line-associated bloodstream infections in acute care hospitals: 2014 update. Infect Control Hosp Epidemiol. 2014;35:753-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 322] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 18. | Mazi W, Begum Z, Abdulla D, Hesham A, Maghari S, Assiri A, Senok A. Central line-associated bloodstream infection in a trauma intensive care unit: impact of implementation of Society for Healthcare Epidemiology of America/Infectious Diseases Society of America practice guidelines. Am J Infect Control. 2014;42:865-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 19. | Al-Abdullah N. Epidemiology of Central Line-Associated Bloodstream Infection (CLABSI) Among Patients in the Intensive Care Units (ICUs) at a Teaching Hospital in Saudi Arabia from Year 2011-2016. J Intensive Crit Care. 2018;4. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Lorente L, Henry C, Martín MM, Jiménez A, Mora ML. Central venous catheter-related infection in a prospective and observational study of 2,595 catheters. Crit Care. 2005;9:R631-R635. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 204] [Cited by in RCA: 196] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 21. | Frasca D, Dahyot-Fizelier C, Mimoz O. Prevention of central venous catheter-related infection in the intensive care unit. Crit Care. 2010;14:212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 116] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 22. | Atilla A, Doğanay Z, Çelik HK, Tomak L, Günal Ö, Kılıç SS. Central line-associated bloodstream infections in the intensive care unit: importance of the care bundle. Korean J Anesthesiol. 2016;69:599-603. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Bell T, O'Grady NP. Prevention of Central Line-Associated Bloodstream Infections. Infect Dis Clin North Am. 2017;31:551-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 151] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 24. | Baier C, Linke L, Eder M, Schwab F, Chaberny IF, Vonberg RP, Ebadi E. Incidence, risk factors and healthcare costs of central line-associated nosocomial bloodstream infections in hematologic and oncologic patients. PLoS One. 2020;15:e0227772. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 74] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 25. | Zorgani A, Abofayed A, Glia A, Albarbar A, Hanish S. Prevalence of Device-associated Nosocomial Infections Caused By Gram-negative Bacteria in a Trauma Intensive Care Unit in Libya. Oman Med J. 2015;30:270-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 26. | Hidron AI, Edwards JR, Patel J, Horan TC, Sievert DM, Pollock DA, Fridkin SK; National Healthcare Safety Network Team; Participating National Healthcare Safety Network Facilities. NHSN annual update: antimicrobial-resistant pathogens associated with healthcare-associated infections: annual summary of data reported to the National Healthcare Safety Network at the Centers for Disease Control and Prevention, 2006-2007. Infect Control Hosp Epidemiol. 2008;29:996-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1483] [Cited by in RCA: 1539] [Article Influence: 90.5] [Reference Citation Analysis (0)] |
| 27. | ECDC surveillance report: Surveillance of healthcare-associated infections in Europe. [cited 22 April 2021]. Available from: http://www.ecdc.europa.eu/en/publications/Publications/120215_SUR_HAI_2007.pdf. |
| 28. | All-Union Exhibition NTTM-89: problems, pursuits, solutions. Voen Med Zh. 1989;61-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Al-Abdely HM, Alshehri AD, Rosenthal VD, Mohammed YK, Banjar W, Orellano PW, Assiri AM, Kader NMA, Enizy HAA, Mohammed DA, Al-Awadi DK, Cabato AF, Wasbourne M, Saliya R, Aromin RG, Ubalde EB, Diab HH, Alkamaly MA, Alanazi NM, Hassan Assiry IY, Molano AM, Flores Baldonado C, Al-Azhary M, Al Atawi S, Al Adwani FM, Casuyon Pahilanga AM, Nakhla R, Nair DS, Sindayen G, Malificio AA, Helali NJ, Al Dossari HB, Kelany A, Algethami AG, Yanne L, Tan A, Babu S, Abduljabbar SM, Bukhari SZ, Basri RH, Mushtaq JJ, Rushdi H, Turkistani AA, Gonzales Celiz JM, Al Raey MA, Al-Zaydani Asiri IA, Aldarani SA, Laungayan Cortez E, Demaisip NL, Aziz MR, Omer Abdul Aziz A, Al Manea B, Samy E, Al-Dalaton M, Alaliany MJ. Prospective multicentre study in intensive care units in five cities from the Kingdom of Saudi Arabia: Impact of the International Nosocomial Infection Control Consortium (INICC) multidimensional approach on rates of central line-associated bloodstream infection. J Infect Prev. 2017;18:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/Licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Critical care medicine
Country/Territory of origin: Bahrain
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ahmad S, Ewers A, Jiang M S-Editor: Wang LL L-Editor: A P-Editor: Wang LYT