Published online Mar 28, 2015. doi: 10.5412/wjsp.v5.i1.155
Peer-review started: October 9, 2014
First decision: November 14, 2014
Revised: December 4, 2014
Accepted: February 4, 2015
Article in press: February 9, 2015
Published online: March 28, 2015
Processing time: 175 Days and 13.6 Hours
AIM: To determine short and long-term outcomes following operative management of acute diverticulitis in immunosuppressed (IMS) compared to immunocompetent (IMC) patients.
METHODS: PRISMA guidelines were followed in conducting this systematic review. We searched PubMed (1946 to present), OVID MEDLINE(R) In-Process and Other Non-Indexed Citations, OVID MEDLINE(R) Daily and OVID MEDLINE(R) (1946 to present), EMBASE on OVID platform (1947 to present), CINAHL on EBSCO platform (1981 to present), and Cochrane Library using a systematic search strategy. There were no restrictions on publication date and language. We systematically reviewed all published cohort comparative studies, case-control studies, and randomized controlled trials that reported outcomes on operative management of acute episode of colonic diverticulitis in IMS in comparison to IMC patients.
RESULTS: Seven hundred and fifty-five thousand five hundred and eighty-three patients were included in this systematic review; of which 1478 were IMS and 754105 were IMC patients. Of the nine studies included there was one prospective cohort, seven retrospective cohorts, one retrospective case-control study, and no randomized controlled trials. With the exception of solid organ transplant patients, IMS patients appeared to be older than IMC when they presented with an acute episode of diverticulitis. IMS patients presented with more severe acute diverticulitis and more insidious onset of symptoms than IMC patients. In the emergency setting, peritonitis was the main indication for operative intervention in both IMS and IMC patients. IMS patients were more likely to undergo Hartmann’s procedure and less likely to undergo reconstructive procedures compared to IMC patients. Furthermore, IMS patients had higher morbidity and mortality rates in the emergency setting compared to IMC patients. In the elective settings, it appeared that reconstruction with primary anastomosis with or without a diverting loop stoma is the procedure of choice in the IMS patients and carried minimal morbidity and mortality equivalent to IMC patients.
CONCLUSION: Emergency operations for diverticulitis in IMS compared to IMC patients have higher morbidity and mortality, whereas, in the elective setting both groups have comparable outcomes.
Core tip: Immunosuppressed (IMS) patients present with more severe episodes of diverticulitis compared to immunocompetent patients and are at increased risk of an emergency operation. However, IMS patients have a vague disease presentation with insidious onset. The postoperative morbidity and mortality following emergency operations for diverticulitis is worse in the IMS patient population, whereas, in the elective setting, the morbidity and mortality is comparable to the general population.
- Citation: Al-Khamis A, Abou Khalil J, Torabi N, Demian M, Kezouh A, Gordon PH, Boutros M. Operative management of acute diverticulitis in immunosuppressed compared to immunocompetent patients: A systematic review. World J Surg Proced 2015; 5(1): 155-166
- URL: https://www.wjgnet.com/2219-2832/full/v5/i1/155.htm
- DOI: https://dx.doi.org/10.5412/wjsp.v5.i1.155
Acute diverticulitis is an increasingly common problem in Western countries and is managed non-operatively in most cases[1]. However, some cases do require operative intervention. As the indications for immunosuppressant medications continue to expand, and an increasing number of patients are immunosuppressed (IMS), the management of colonic diverticulitis in this patient population has become increasingly relevant. The appropriate time and type of management for colonic diverticulitis in the IMS remains a topic of controversy. IMS patients are thought to have a higher incidence of diverticulitis, more virulent disease, and more complicated recurrences than the immunocompetent (IMC) population. In turn, authors have suggested that IMS patients may require more aggressive operative management[2-5], including an elective sigmoid resection after a single episode of uncomplicated diverticulitis[6,7]. However, these recommendations are based on anecdotal experience or on single center retrospective studies. One qualitative systematic review[8] reported high morbidity and mortality in kidney transplant recipients and patients on chronic corticosteroid therapy with acute diverticulitis. The objective of our study was to determine the post-operative morbidity, mortality and long-term outcomes following an acute episode of colonic diverticulitis in IMS compared to IMC patients in the emergency and elective operative settings.
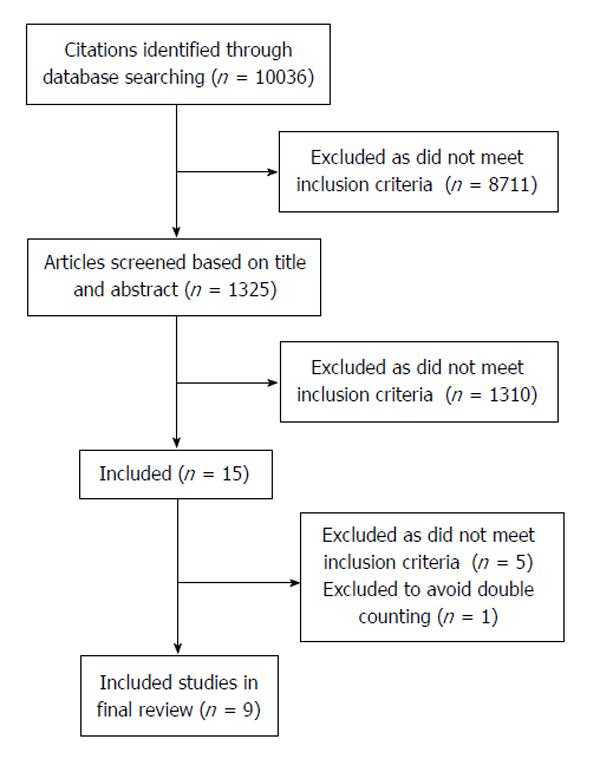
Type of studies: All studies reporting on peri-operative outcomes following acute colonic diverticulitis with a comparative study design that included IMS and IMC populations were assessed for inclusion. Study designs such as randomized controlled trials, cohort comparative studies, or case control studies were included, whereas case series, case reports, and clinical guidelines were excluded (Figure 1).
In the literature, various clinical, radiological and/or pathological findings were used to determine the diagnosis of acute diverticulitis. For this review, we relied on the individual studies’ inclusion criteria to determine the diagnosis of acute diverticulitis. We included all studies that investigated colonic diverticulitis without excluding studies that had participants with ascending, transverse or descending colon diverticulitis.
Type of participants: Participants were considered IMS if one of the following conditions were met: (1) the patient was a solid organ transplant (SOT) (heart, liver, kidney, lung, and/or pancreas) recipient; (2) the patient was taking immunosuppressive medications; or (3) the patient was receiving chemotherapy for a concurrent extracolonic malignant neoplasm.
Type of intervention: Patients who underwent a procedure requiring general anesthesia in the operating room were considered as receiving operative intervention. All participants who were managed operatively for acute diverticulitis were considered eligible for inclusion. Studies, which did not include outcomes on operative management, were excluded.
Type of outcomes measured: In order to be included in the review, studies had to provide data on at least one of the following postoperative outcomes: mortality, postoperative complications, length of hospital stay (LOS), stoma closure rate, quality of life (QoL), or cost.
PRISMA guidelines were followed in conducting this systematic review. We searched PubMed (1946 to present), OVID MEDLINE(R) In-Process and Other Non-Indexed Citations, OVID MEDLINE(R) Daily and OVID MEDLINE(R) (1946 to present), EMBASE on OVID platform (1947 to present), CINAHL on EBSCO platform (1981 to present), and Cochrane Library on August 12, 2013 using a systematic search strategy. The search was designed and carried out by (Torabi N), a librarian at McGill University. Individual strategies were developed for each database to accommodate for difference between subject headings and syntax among different databases. There were no restrictions on publication date and language. The final MEDLINE search strategy is provided in Table 1. In addition, we searched Clinicaltrials.gov to find possible clinical trials related to the research topic. Citation tracking (backward and forward) of selected studies using SCOPUS were conducted to locate any potentially relevant articles that had not been obtained in the original search. Abstracts were reviewed and relevant studies were identified. The identified studies were downloaded into EndNote 7.1X (Thomson Reuters, Philadelphia, PA), and duplicates were deleted. We also searched all registered clinical trials on clinicaltrials.gov and conference proceedings retrieved via EMBASE. We sent emails or letters to authors of abstracts published as podium presentations or posters that we deemed potential for inclusion, requesting information on unpublished data and ongoing studies. We also searched the bibliographies of all included studies and review papers to identify other potentially suitable studies.
| 1 Colonic diverticulitis.mp. or diverticulitis, colonic/ |
| 2 Colonic diverticulosis.mp. or diverticulosis, colonic/ |
| 3 Colonic diverticulum.mp. or diverticulum, colon/ |
| 4 Colonic diverticula.mp. |
| 5 (Colon diverticulosis or colon diverticulitis or colon diverticula or colon diverticulum).mp. |
| 6 Diverticulitis/su [Surgery] |
| 7 (Diverticulosis or diverticulitis).mp. |
| 8 1 or 2 or 3 or 4 or 5 or 6 or 7 |
| 9 HIV infections/or acquired immunodeficiency syndrome/or sexually transmitted diseases, viral/ |
| 10 Immunologic deficiency syndromes/ |
| 11 HIV infections.ab,ti. |
| 12 “HIV/aids”.ab,ti. |
| 13 Aids positive.ab,ti. |
| 14 HIV positive.ab,ti. |
| 15 Chemoprevention/or chemoradiotherapy/or chemotherapy, adjuvant/ |
| 16 Chemotherapy.mp. |
| 17 Neutropenia/or Neutropenia.mp. or febrile neutropenia/or chemotherapy-induced febrile neutropenia/ |
| 18 Corticosteroid.ab,ti. |
| 19 Steroid.ab,ti. |
| 20 Radiation oncology/mt (methods) |
| 21 Radiation/ae, th (Adverse Effects, Therapy) |
| 22 Exp organ transplantation |
| 23 Organ transplanta.ab,ti. |
| 24 [(Heart or Kidney or Liver or Pancreas or Lung) adj transplanta].ab,ti. |
| 25 Immunodeficienta.ab,ti. |
| 26 (Solid adj3 transplant).ab,ti. |
| 27 Lymphocyte depletion.mp. or lymphocyte depletion/ |
| 28 Graft enhancement, immunologic/ |
| 29 Graft enhancement.mp. |
| 30. Desensitization, immunologic/ |
| 31 Hyposensitization therapy.mp. |
| 32 (Anti-Rejection Therapa or Antirejection Therapa).mp. |
| 33 Immunosuppressa.mp. or immunosuppressive agents/ |
| 34 Immunocompromised host.mp. or immunocompromised host |
| 35 Immunocompromised.mp. |
| 36 Exp immune tolerance/ |
| 37 Immunosuppression.mp. or Immunosuppression/ |
| 38 6-mercaptopurine.mp. or 6-Mercaptopurine/ |
| 39 Methotrexate.mp. or methotrexate |
| 40 Methylprednisolone/or methyl-prednisolone.mp./ |
| 41 Basiliximab.mp. |
| 42 Mycophenolate.mp. |
| 43 Mycophenolic acid.mp. or mycophenolic acid |
| 44 Copaxone.mp. |
| 45 Exp prednisolone/ |
| 46 Cyclophosphamide/or ifosfamide/ |
| 47 Cyclophosphamide.mp. |
| 48 Prednisone.mp. or prednisone/ |
| 49 Cyclosporine.mp. or cyclosporine/ |
| 50 Remicade.mp. |
| 51 Daclizumab.mp. |
| 52 Sirolimus.mp. or Sirolimus/ |
| 53 Dexamethasone.mp. or exp Dexamethasone/ |
| 54 Tacrolimus.mp. or tacrolimus/ |
| 55 Interferons.ab,ti. |
| 56 humira.mp. |
| 57 Imuran.mp. or azathioprine/ |
| 58 CellCept.mp. |
| 59 Infliximab.mp. |
| 60. Etanercept.mp. |
| 61 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 or 39 or 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 |
| 62 Postoperative complications/or surgical wound dehiscence/or |
| surgical wound infection/ |
| 63 Perioperativeoutcomea.mp. |
| 64 Prognosisa/or treatment outcome/or treatment failure/ |
| 65 Peri-operative outcomes.mp. |
| 66 Perioperative period.mp. or exp perioperative period/ |
| 67 Postoperative outcomes.mp. |
| 68 Sepsis.mp. or exp sepsis/ |
| 69 Septicemiaa.mp. |
| 70 Pyemiaa.mp. |
| 71 Exp patient acuity/ |
| 72 Failure to rescue.mp. |
| 73 (Surgical adj2 infectiona).mp. |
| 74 (Surgery adj5 infectiona).mp. |
| 75 Anastomosis, surgical/or anastomosis leak.mp |
| 76 Length of stay.mp. or “length of stay”/ |
| 77 Mortality/or “cause of death”/or survival rate/ |
| 78 (Mortality or surgery).ab,ti. |
| 79 Colectomy.mp. or colectomy/ |
| 80 (Hartmann's or Hartmanns or Hartmann).ab,ti. |
| 81 Laparotomy.mp. or laparotomy/ |
| 82 Bowel resection.mp. |
| 83 Colostomy.mp. or colostomy/ |
| 84 Ileostomy.mp. or ileostomy/ |
| 85 Anterior resection.mp. |
| 86 Colon resection.mp. |
| 87 Recurrencea/ |
| 88 Recurrence.ab,ti. |
| 89 Acute kidney injury.mp. or acute kidney injury/ |
| 90 Acute renal failure.mp. |
| 91 Complications.ab,ti. |
| 92 Implications.ab,ti. |
| 93 62 or 63 or 64 or 65 or 66 or 67 or 68 or 69 or 70 or 71 or 72 or 73 or 74 or 75 or 76 or 77 or 78 or 79 or 80 or 81 or 82 or 83 or 84 or 85 or 86 or 87 or 88 or 89 or 90 or 91 or 92 |
| 94 Immunocompetent.mp. |
| 95 Immunocompetence.mp. or immunocompetence/ |
| 96 (Immune adj competenca).mp. |
| 97 (Immuno adj competenca).mp. |
| 98 Immunocompetency.ab,ti. |
| 99 (Nonimmunocompromised or nonimmunocompromized).ab,ti. |
| 100 (Non adj immunocompromi?ed).ab,ti. |
| 101 (Immunologica adj Competence).ab,ti. |
| 102 (Immune adj system).ab,ti. |
| 103 (Control or comparison or compare or groups or normal or different or difference).ab,ti. |
| 104 Comparative studies.ab,pt,ti. |
| 105 94 or 95 or 96 or 97 or 98 or 99 or 100 or 101 or 102 or 103 or 104 |
| 106 8 and 61 and 93 and 105 |
| 107 (Case Reports or Practice Guideline or Guideline or case study).pt. |
| 108 Case series.ab,ti. |
| 109 Case report.ab,ti. |
| 110 107 or 108 or 109 |
| 111 106 not 110 |
Selection of the studies: Two authors (Al-Khamis A/Abou Khalil J) independently examined the titles and abstracts of the articles identified in the searches as reporting potential relevant studies. From this initial assessment, we obtained full versions of all potential relevant articles. Any disagreements were resolved by a third author (Boutros M).
Data extraction and management: Data were extracted into data extraction forms by two authors (Al-Khamis A and Abou Khalil J). Any disagreements were resolved by a third author (Boutros M). For publications reporting data in more than one paper, both papers were obtained for full review, however data was extracted only from the most complete publication.
Using the search strategy specified in Table 1, 10036 citations were identified. The citations were reviewed by two reviewers (Al-Khamis A and Abou Khalil J), and 8711 citations were excluded because they did not include patients with acute colonic diverticulitis or did not include IMS patients. One thousand three hundred and twenty-five titles and abstracts were reviewed by the two reviewers, and 1310 were excluded because they were case reports, case series, review articles, clinical guidelines, or because the studies reported on medical management of acute diverticulitis or did not include peri-operative outcomes following operative management of acute diverticulitis. Fifteen full papers were reviewed by both reviewers, and 6 papers were excluded because of data duplication (1 paper) or non-comparative methodology. Thus, nine articles met inclusion criteria and were included in this review (Figure 1). Of the nine included studies, one study had a prospective cohort comparative design, seven studies used a retrospective comparative cohort design, and one study was a retrospective case-control study (Table 2). There were no randomized controlled trials.
| Ref. | Year | Country | Study design | No. of patients1 | Total n | Follow-up period (mo) | |
| IMS | IMC | ||||||
| Canter et al[9] | 1970 | United States | Retrospective | 11 | 38 | 49 | NR |
| Perkins et al[10] | 1984 | United States | Retrospective | 10 | 31 | 41 | NR |
| Tyau et al[11] | 1991 | United States | Retrospective | 23 | 55 | 78 | NR |
| Hesterberg et al[15] | 1994 | Germany | Retrospective | 12 | 80 | 92 | NR |
| Qasabian et al[16] | 2004 | Australia | Retrospective | 8 | 16 | 24 | Mean 57 (SD NR) |
| Reshef et al[1] | 2012 | United States | Case control | 51 | 51 | 102 | NR |
| Biondo et al[13] | 2012 | Spain | Prospective | 61 | 254 | 315 | Mean 81.62 ± 67.62 SD |
| Halabi et al[12] | 2013 | United States | Retrospective | 1249 | 753517 | 754766 | NR |
| Golda et al[14] | 2014 | Spain | Retrospective | 53 | 63 | 116 | NR |
| Total | 1478 | 754105 | 755583 | ||||
The included studies were published between 1970 and 2014. Five studies were from centers in the United States[1,9-12], two from Spain[13,14], one from Germany[15], and one from Australia[16]. All studies were published in English except Hesterberg et al[15], which was published in German.
The total number of patients who were managed operatively in the included studies was 755583 patients, of those, 1478 were IMS and 754105 were IMC (Table 2). The follow-up period was not reported in most studies; however in the two studies reporting the length of follow-up, the mean was 81[13] and 57[16] mo.
Three studies limited the IMS group to SOT patients[1,12,17], while four other studies[10,13-15] included SOT among other causes of immunosuppression in the IMS group. Canter et al[9] only included patients on long-term steroids in their IMS group (Table 3). Definition of immunosuppressants listed in each article is included in Table 3.
| Ref. | Definition |
| Canter et al[9] | Long-term steroid use |
| Perkins et al[10] | Renal transplant |
| Glomerulonephritis on steroids | |
| Lymphoma | |
| Long-term steroid use | |
| Tyau et al[11] | Long-term steroid use |
| Concurrent extracolonic malignant neoplasm/chemotherapy | |
| Malnutrition | |
| Uremia | |
| Hesterberg et al[15] | Long-term steroid use |
| Concurrent extracolonic malignant neoplasm/chemotherapy | |
| Azathioprine | |
| Iatrogenic leucopenia | |
| Qasabian et al[16] | Heart and lung transplant |
| Biondo et al[13] | Concurrent history of immunosuppressant |
| Solid organ transplant | |
| Concurrent extracolonic malignant neoplasm | |
| Emphysema | |
| Concurrent extracolonic malignant neoplasm/chemotherapy | |
| Collagen vascular disease, arthritis | |
| Chronic pulmonary fibrosis | |
| Congenital or acquired immunodeficiency syndromes | |
| End stage renal failure (hemodialysis) | |
| Reshef et al[1] | Liver transplant |
| Heart transplant | |
| Lung transplant | |
| Renal transplant | |
| Halabi et al[12] | Renal transplant |
| Golda et al[14] | Concurrent history of immunosuppressant |
| Long-term steroid use | |
| Concurrent extracolonic malignant neoplasm | |
| End stage renal failure (hemodialysis, peridialysis) |
The age range of patients who presented with acute diverticulitis was between 37 to 80 years old in the IMS and between 37 to 77 years old in the IMC groups (Table 4). Biondo et al[13] compared IMS to IMC at presentation and reported that the IMS group had significantly worse American Society of Anesthesiologists (ASA) scores and were significantly older (mean age of 68.4 vs 61 years in IMC patients, P < 0.00). Golda et al[14] also reported their IMS to be older and have worse ASA scores. Qasabian et al[16] also observed that the IMS population was significantly older than their IMC group. On the other hand, in the studies including mainly SOT patients in the IMS group[1,12,15], IMS patients were younger than the IMC patients.
| Ref. | IMS (yr) | IMC (yr) |
| Canter et al[9] | 60 | 58 |
| Perkins et al[10] | NR (37-83) | 64 (37-93) |
| Tyau et al[11] | 64 ± 12.9 SD | 59.1 ± 14.7 |
| Hesterberg et al[15] | 63 (38-90) | NR |
| Qasabian et al[16] | 54 (41-69) | 66 (45-91) |
| Reshef et al[1] | 55.9 ± 9.3 SD | 62.3 ± 11.3 SD |
| Biondo et al[13] | 68.4 ± 11.7 SD | 61 ± 15.1 SD |
| Halabi et al[12] | 59 (51-67) | 65 (55-77) |
| Golda et al[14] | 68.5 ± 10.6 SD | 59.7 ± 16.4 SD |
Reshef et al[1] matched cases to controls with regard to timing of operation, ASA status, gender, cardiac and pulmonary comorbidities, diabetes status, and type of operative procedure, so these preoperative comorbidities could not be assessed. As with the other publications on SOT patients, the IMS group in this case matched study was significantly younger.
Halabi et al[12] reported IMS patients were more anemic, more likely to have chronic obstructive pulmonary disease, chronic liver disease, peripheral vascular disease, congestive heart failure, and hypertension, more likely to be smokers, diabetic, obese, and female, and had worse comorbidity scores.
Overall, in the included studies, it appears that IMS patients tend to be older than the general population when they present with an episode of acute diverticulitis, except in the SOT population, who are younger than the general population at the time of presentation. Previous studies have reported SOT patients to be relatively young compared to general population when they present with acute diverticulitis[18,19].
Clinical presentation at the time of presentation with an acute episode of diverticulitis was only described in 4 of the 9 included studies[9,10,13,14]. Biondo et al[13] found that IMS patients had significantly more severe acute first (de novo) episodes of diverticulitis (defined as diverticulitis with abscess or perforation and/or high Hinchey peritonitis grade) compared to IMC patients. They attributed a significantly higher emergency operation rate in the IMS group compared to the IMC group (31.3% vs 21%, P = 0.004) to this significant difference in clinical presentation.
Golda et al[14] also reported a more severe disease presentation in the IMS compare to the IMC group, though it was not clear if IMS had previous episodes of diverticulitis. They also reported no difference in Hinchey peritonitis grade between the two groups. However, they found that the mean peritonitis severity score, a scoring system that allows stratification of patients according to mortality risk, was significantly higher in the IMS compared to the IMC group; 11.1 ± 1.3 SD vs 8.1 ± 1.7 SD, (P < 0.001) respectively.
Perkins et al[10] described a difference in clinical presentation between the IMS and IMC patients. IMS patients were less likely to present with abdominal pain and tenderness on clinical examination, while they were more likely to present with fever and hypotension compared to IMC patients. Canter et al[9] were the only study to look at the relationship between location of the perforation and immune status, and found no significant difference.
Overall, two studies found that in the emergency setting, IMS patients presented with more severe episodes of acute diverticulitis. Furthermore, one study highlighted that the insidious presentation with atypical symptoms and signs in IMS patients along with a more severe disease makes the IMS population much more challenging than IMC patients[10]. Thus, when IMS patients present with vague abdominal symptoms, fever or hypotension, the evaluating surgeon should have high level of suspicion for an acute abdominal process such as diverticulitis.
The indication for operative management in patients with complications of diverticulitis was specified in six studies[1,9-11,14,15], while the indications for operative management in the remainder of patients was not clearly specified in any study.
The most frequently reported indication for operative approach in the emergency setting in the IMS group was peritonitis and it was reported in 5 studies[1,9-11,15]. The other frequently reported indication for operative approach was abscess and it was reported in four studies[9-11,15]. Further indications for operative intervention included fistula[10,11] and bowel obstruction[1,10].
Three studies[9-11] reported the indication for operative management in the IMC patients. The most common reported indications for operative management in this group were peritonitis and fistula formation, both reported by three studies[9-11]. Other indications for operative management in the IMC patients included abscess[10,11] and recurrence[10].
Summing all included studies, it appears that peritonitis and perforation followed by intra-abdominal abscess are the main indications for operative management in both IMS and IMC patients. Tyau et al[11] specifically examined the difference in diverticular perforation rate as the indication for surgery in IMS and IMC patients, and found that IMS patients have a significantly higher rate of diverticular perforations requiring surgery (42.5% vs 14.2%, P < 0.05). In addition, we observed that fistula formation was reported more frequently as an indication for operative management in the IMC compared to the IMS group. This late complication of diverticulitis, which was more frequently reported in IMC patients, may be attributed to the ability of IMC patients to have more walled off and localized perforation rather than a free perforation.
Four studies included data on the operative approach[1,9,10,13]. Three studies[9,10,13] only included laparotomies, while Reshef et al[1] reported that 10% of operations were performed laparoscopically.
The choice of operative procedure in the emergency setting was reported in eight studies (Table 5). In each of these studies, the choice of operative intervention was based on the surgeon’s preference and experience rather than institutional protocols. The most common emergency operation performed in the IMS group was Hartmann’s procedure (HP), followed by resection and primary anastomosis (RPA) with a diverting loop stoma (DLS). The most common emergency operation in the IMC patients was also HP, however HP was far less frequent in IMC compared to IMS patients. The second most common operative intervention in the IMC population was RPA with DLS, similar to IMS patients but far more frequently. We also noted that RPA without diversion was rarely performed, however it was more frequently reported in IMC patients. Biondo et al[13] and Golda et al[14] both individually reported that IMS patients underwent significantly more HP and less RPA with or without DLS than IMC patients. On the other hand, Tyau et al[11] and Reshef et al[1] found no significant difference. Overall, from the data in the included studies, we found that in the emergency settings, IMS patients are more likely to undergo HP than a reconstructive procedure.
| Ref. | Emergency | Elective | ||||||||||||
| Immune status(IMS/IMC) | Total n | Stoma anddrainage | HP | RPA | RPA with DLS | Subtotal colectomywith anastomosis | Subtotal colectomywith ileostomy | Drainageonly | Unknown | Total n | RPA | RPA with DLS | Unknown | |
| Canter et al[9] | IMS | 9 | 8 | 12 | 0 | 2 | 2 | 0 | ||||||
| IMC | 22 | NR | NR | 22 | 16 | NR | 16 | |||||||
| Perkins et al[10] | IMS | 101 | 5 | 3 | 0 | 1 | 0 | |||||||
| IMC | 31 | 11 | 7 | 12 | 1 | 0 | ||||||||
| Tyau et al[11] | IMS | 23 | 4 | 17 | 1 | 1 | 0 | 0 | ||||||
| IMC | 55 | 8 | 27 | 14 | 3 | 3 | 0 | |||||||
| Hesterberg et al[15] | IMS | 8 | 1 | 5 | 1 | 1 | 0 | 4 | 4 | 0 | ||||
| IMC | 36 | NR | NR | NR | NR | 36 | 44 | NR | 44 | |||||
| Qasabian et al[16] | IMS | 8 | 6 | 2 | 0 | |||||||||
| IMC | 16 | 13 | 3 | |||||||||||
| Reshef et al[1] | IMS | 37 | 1 | 28 | 0 | 8 | 0 | 14 | 5 | 9 | 0 | |||
| IMC | 37 | 0 | 28 | 1 | 8 | 0 | 14 | NR | NR | 14 | ||||
| Biondo et al[13] | IMS | 57 | 37 | 11 | 3 | 1 | 5 | 4 | ||||||
| IMC | 1822 | 56 | 97 | 5 | 3 | 21 | 72 | |||||||
| Halabi et al[12] | IMS | |||||||||||||
| IMC | ||||||||||||||
| Golda et al[14] | IMS | 53 | 42 | 2 | 9 | 0 | ||||||||
| IMC | 63 | 15 | 39 | 9 | 0 | |||||||||
| Total n | IMS | 205 | 25 | 132 | 4 | 32 | 3 | 1 | 2 | 5 | 20 | 11 | 9 | 0 |
| IMC | 442 | 32 | 133 | 66 | 120 | 5 | 3 | 4 | 79 | 132 | NR | NR | 67 | |
HP has been historically and still considered to be a life-saving procedure at the time of an acute severe attack of diverticulitis. However, in the general population, this operation is notably associated with a high permanent stoma rate[20] and complication rate for reversal[20]. Given the more difficult post-operative recovery in IMS compared to IMC, the observed high morbidity rate following emergency surgery in this review is expected.
Only three studies included elective operations for diverticulitis (Table 6). Resection and primary anastomosis with or without a protective ileostomy was the only procedure performed in IMS patients. Elective operative procedures were not described for any IMC patients in the included studies.
| Ref. | Immune status(IMS/IMC) | Total n | Mortalityn (%) | Morbidityn (%) | Anastomoticleak n (%) | Abdominal collection/Abscess n (%) | Wound infectionn (%) | Sepsisn (%) | Reoperationn (%) | Post op bleedn (%) | Post op ileusn (%) | Readmissionn (%) |
| Canter et al[9] | IMS | 2 | 0 | |||||||||
| IMC | 16 | NR | ||||||||||
| Hesterberg et al[15] | IMS | 4 | 0 | |||||||||
| IMC | 44 | 0 | ||||||||||
| Qasabian et al[16] | ||||||||||||
| 1Reshef et al[1] | IMS | 14 | 0 | 4 (29) | 1 (7) | 0 | 3 (20) | 1 (7) | 0 | 1 (7) | 4 (24) | |
| IMC | 14 | 0 | 4 (29) | 1 (7) | 1 (7) | 2 (13) | 2 (14) | 1 (7) | 1 (7) | 2 (14) | ||
| 2Biondo et al[13] | IMS | 4 | 0 | 4 (100) | 1 (25) | 1 (25) | 2 (50) | |||||
| IMC | 72 | 0 | 4 (5) | 0 | 3 (4) | 1 (1) | ||||||
| Halabi et al[12] | IMS | 471 | 3 (0.6) | |||||||||
| IMC | 404623 | 4856 (1) |
Overall morbidity following emergency operations for diverticulitis in the IMS patients was 65% compared to 40% in IMC patients. Individual complications following emergency operations for acute diverticulitis were reported in four studies (Table 7). The most common reported complication in the IMS patients was wound infection followed by sepsis and intra-abdominal collections. Other complications in this group included postoperative ileus and cardiopulmonary complications. Of the seven studies including SOT patients, Hesterberg et al[15] were the only authors that reported on graft rejection following emergency operations for diverticulitis. They reported this complication in one of the five SOT recipients included in their study. In the IMC population, wound infection was the most commonly reported complication. Other postoperative complications in the IMC group are listed in Table 7.
| Ref. | Immunestatus(IMS/IMC) | Totaln | Mortalityn (%) | Morbidityn (%)2 | Anastomoticleak n (%) | Abdominalcollection/Abscess n (%) | Woundinfectionn (%) | Sepsisn (%) | Post-opileusn (%) | Post-opbleedn (%) | Renalfailuren (%) | Reoperationn (%) | Arrythmias/cardiacdecompensationn (%) | Pulmonaryinfection/Insufficient n (%) | Othersn (%) |
| Canter et al[9] | IMS | 9 | 3 (33) | 3 (33) | 3 (33) | ||||||||||
| IMC | 22 | NR | NR | 0 | |||||||||||
| 1Perkins et al[10] | IMS | 10 | 1 (10) | 7 (70) | 3 (30) | 4 (40) | 5 (50) | ||||||||
| IMC | 31 | 0 | NR | 2 (7) | 5 (16) | 1 (3) | |||||||||
| Tyau et al[11] | IMS | 23 | 9 (39) | 15 (65) | |||||||||||
| IMC | 55 | 1 (2) | 13 (24) | ||||||||||||
| Hesterberg et al[15] | IMS | 8 | 1 (13) | ||||||||||||
| IMC | 36 | NR | |||||||||||||
| Qasabian et al[16] | |||||||||||||||
| Reshef et al[1] | IMS | 37 | 7 (19) | 19 (51) | 1 (3) | 2 (5) | 3 (8) | 3 (8) | 4 (11) | 1 (3) | 2 (5) | DVT; IMS, 3 IMC, 2 UTI; IMS, 2 Readmission; IMS, 5 IMC, 4 | |||
| IMC | 37 | 0 | 9 (24) | 1 (3) | 3 (8) | 5 (14) | 2 (5) | 1 (3) | 0 | 2 (5) | |||||
| Biondo et al[13] | IMS | 57 | 19 (33) | ||||||||||||
| IMC | 182 | 29 (16) | |||||||||||||
| Halabi et al[12] | IMS | 778 | 57 (0.7) | ||||||||||||
| IMC | 348894 | 17130 (5) | |||||||||||||
| Golda et al[14] | IMS | 53 | 14 (26) | 42 (79) | 1 (2) | 11 (21) | 22 (42) | 13 (25) | 10 (19) | 0 | 7 (13) | 16 (30) | 9 (17) | 11 (21) | |
| IMC | 63 | 4 (6) | 40 (64) | 3 (5) | 4 (6) | 30 (48) | 7 (11) | 8 (13) | 1 (2)3 | 5 (8) | 5 (8) | 2 (3) | 7 (11) | ||
| Total | IMS | 975 | 111/ 975 (11) | 86/132 (65) | 5 | 16 | 29 | 18 | 13 | 4 | 7 | 16 | 10 | 13 | |
| IMC | 349320 | 17164/ 349320 (5) | 62/155 (40) | 4 | 9 | 40 | 8 | 10 | 2 | 5 | 5 | 2 | 9 |
Perkins et al[10] examined the relationship between the choice of operative intervention and the occurrence of postoperative complications and identified more complications in the IMS patients compared to the IMC patients when drainage and colostomy was performed, but not when resection and colostomy was performed.
In summary, all studies reported a higher complication rate in IMS patients following operations in the emergency setting compared to IMC patients in the same setting. This increased morbidity rate is likely due to several factors. Firstly, IMS patients tend to present with more insidious disease onset that may result in significant delays in diagnosis. Secondly, this group of patients tends to have more significant comorbidities reflected by worse ASA classification. Thirdly, the IMS state itself is associated with a significant deficiency to mount a response against infection, and an inherent detrimental effect on the ability for tissue to heal following an operation[21,22].
In addition, it appeared that potentially life-threatening complications including sepsis, intra-abdominal collections and cardiopulmonary complications were more common in IMS compared to IMC patients. We did not observe differences in the distribution of other major complications between the IMS and IMC populations. Wound infections, postoperative ileus, postoperative bleed and renal complications appeared to be comparable in both populations.
Two studies examined postoperative morbidity after elective operations in patients with previous history of acute diverticulitis (Table 6). The sample size for the IMS and IMC subgroups that underwent elective operations in both these studies was small. The rate of anastomotic leak, wound infection and reoperation were the most common reported complications and appeared equivalent in IMS and IMC patients. Other postoperative complications are listed in Table 6. Data regarding complications following elective surgery in the IMS compared to the IMC population are lacking. However, from the available literature, it does not appear that there is any significant difference in complication rate between IMS and IMC groups following elective operations for diverticulitis, as both appear low.
Following emergency surgery for diverticulitis, the mortality rate ranged from 1% to 39% in the IMS groups and 0 to 16% in the IMC groups. The mean mortality of all included studies was 11% for IMS patients and 5% for IMC patients, respectively.
All studies, but three[10,15,16] reported significantly higher mortality in the IMS compared to IMC patients. Two studies reported zero mortality in the IMC cohorts[1,10]. The majority of studies did not report the cause of mortality. Only two studies[10,11] included a description regarding the cause of death. Perkins et al[10] hypothesized that one case of death in an IMS patient in their study was due to a delay in diagnosis with resultant sepsis and intraoperative death. Tyau et al[11] reported that nearly all deaths in their study were secondary to sepsis.
Subgroup analysis of the data in Golda et al[14] showed significantly higher mortality associated with HP compared to reconstructive procedures. As this is a retrospective study, this finding is likely confounded by significantly worse disease in the patients who underwent HP compared to RPA.
Mortality following elective operations for diverticular disease patients was reported in five studies (Table 6). Three of the four studies reported no mortality in both IMS and IMC patients following elective resections, while one study reported no statistically significant difference in mortality between IMS and IMC patients[12] (0.6% vs 1% respectively, P = 0.14).
Data regarding mortality in IMS patients following elective operations was scarce. However, it appears that mortality in elective operations for diverticular disease in the IMS population is comparably low to IMC patients.
Length of hospital stay was poorly reported in the included studies (Table 8). Only two studies included LOS following operations in the emergency setting[1,14]. Golda et al[14] found that IMS patients had significantly longer hospital stay compared to IMC patients following emergency operations (mean days 24.8 ± 25.2 SD vs 15.5 ± 10.5 SD, P = 0.002). Reshef et al[1] also found similar trends though it was not statistically significant (mean 19 d IMS vs 13 d IMC, P = 0.1).
Similarly, only two studies reported LOS following elective resections for diverticulitis[1,13]. Both studies observed a trend towards longer hospital stay in the IMS compared to IMC patients.
From the limited existing data, it appears that IMS patients tend to stay longer, especially following emergency operations for diverticular disease.
Stoma closure: Only one study compared stoma closure and complication rates in IMS and IMC patients[1]. They found that there was no significant difference in the interval between stoma creation and stoma closure in IMS and IMC patients (5.4 mo ± 2.9 SD vs 6.1 mo ± 3.4 SD respectively, P = 0.23). Furthermore, permanent stoma rates were similar between IMS and IMC patients (7 vs 8 patients, P = 0.7). Moreover, postoperative morbidity after all types of stoma closure was similar (16% IMS vs 17% IMC patients, P = 1). Another study reported that three of the 12 IMS patients eventually underwent stoma closure[15]. As this data represents a small sample size, it is difficult to draw any conclusions. Furthermore, it is known that Hartmann’s reversal is associated with a far greater complication rate compared to ileostomy closure. Larger studies, which make this distinction, will shed more light on the complications following stoma closure in IMS and IMC patients, particularly following Hartmann’s reversal.
QoL: No studies reported data about QoL following emergency or elective operations in the IMS compared to IMC patient populations.
Though an increasingly important outcome, cost was not a reported outcome in any of the included studies.
Though the inclusion criteria for this systematic review were patients who underwent an operation for acute diverticulitis, few of the included studies also commented on non-operative management. As there is increasing interest in this treatment option, we have summarized the available literature.
Three studies reported data on some aspect of their non-operative management of acute diverticulitis in IMS patients[10,11,13]. Tyau et al[11] reported that they used non-operative management more frequently in IMC (67%) compared to IMS patients (42.5%). The severity of diverticulitis and the presence of complications secondary to diverticulitis were not reported for this subset of patients. In 1984, Perkins et al[10] reported that none of their IMS patients had successful medical therapy compared to 76% of the IMC group. Again, the severity of diverticulitis and the presence of complications secondary to diverticulitis were not reported for this subset of patients.
Biondo et al[13] was the first study to examine the risk of recurrence necessitating emergency operations in IMS patients following successful non-operative management of diverticulitis. After excluding patients who had an operation during or after the first episode, 107 IMS patients and 657 IMC patients were prospectively followed for recurrence. There was no significant difference in overall recurrence rate between the IMS and IMC patients (21.5% IMS vs 20.5%, respectively, P = 0.82). They also observed that a severe first episode (defined as abscess or perforation) in the IMS group was associated with a higher recurrence rate, and shorter interval to the first episode of recurrence of acute diverticulitis (median 3.3 mo in IMS vs 9 mo in IMC, P = 0.01). However, there was no significant difference in the rate of emergency operation for recurrence (only 17.4% IMS patients vs 15% IMC patients, P = 0.77). The mean follow up for IMS and IMC patients was 82 and 65 mo respectively. As in the previously mentioned studies, Biondo et al[13] also reported that IMC patients were more often treated with non-operative management compared to IMS patients.
Overall, it appears that IMS patients are less likely to be managed non-operatively compared to IMC patients. Though based on a small subgroup, Biondo et al[13] observed that IMS patients who are successfully managed non-operatively following a severe episode of diverticulitis are not at increased risk of emergency operations for future recurrences.
To date, this is the only systematic review comparing outcomes of operative management in IMS and IMC patients in both elective and emergency settings. Overall, we observed a worse disease severity for IMS compared to IMC patients with acute diverticulitis. Furthermore, IMS patients were more likely to fail non-operative management, undergo a HP, require a longer hospitalization, suffer complications or die following emergency operative management.
In this systematic review, we observed a higher morbidity and mortality rate following emergency surgery in the IMS compared to the IMC population. On the other hand, it appears that the morbidity and mortality associated with elective operations for both groups are low and comparable. This beckons the question whether IMS patients should be routinely offered an elective resection following a first episode of diverticulitis in order to avoid an emergency surgery. Interestingly, Biondo et al[13] report a similar rate of emergency operations for recurrence in IMS and IMC patients. Therefore, it seems that IMS patients are not at higher risk of recurrence requiring emergency surgery, but the morbidity and mortality for recurrence managed operatively is not known and may be significantly higher than in IMC patients.
Despite a rigorous and inclusive search methodology, the collected available literature regarding diverticulitis in the IMS population mainly included retrospective studies with a small number of patients, from a single institution, and lacked any randomized controlled trials. In an attempt to reduce the risk of bias and heterogeneity, we only included comparative cohorts and case control studies and excluded all case series, case reports, and clinical guidelines. Nonetheless, the studies available for inclusion were mostly retrospective, without clearly specified a priori sample size/power calculations and had missing data. Thus, our results are fraught with the limitations of the original data, including information and recall bias. Furthermore, this systematic review is based on populations from the developed world where advanced peri-operative support is readily available; thus these results may not be generalizable to less developed hospital systems. Larger, multi-institutional prospective studies are required to address the optimal timing and indication for operative intervention following an episode of acute diverticulitis in this challenging population.
Acute diverticulitis is a common problem in western societies and is managed non-operatively in most cases. The appropriate type and timing of management in immunosuppressed (IMS) patients remains a topic of controversy. Some authors have suggested that IMS patients may require more aggressive operative management, including an elective colonic surgical resection after a single episode of acute diverticulitis. However, these recommendations are based on anecdotal experience or on single center retrospective studies.
The current research goal is to investigate outcomes following operative management of colonic diverticulitis in IMS compared to immunocompetent (IMC) patients who present with a history of acute diverticulitis in both emergency and elective settings.
As the indications for immunosuppressant medications continue to expand, and an increasing number of patients are IMS, the appropriate type and time of management of acute diverticulitis in this patient population has become increasingly relevant. IMS patients are thought to have a higher incidence of diverticulitis, more virulent disease, and more complicated recurrences than the IMC patients. To date there is scarcity of data on the outcomes following operative management of colonic diverticulitis in IMS patients. In an attempt to produce a robust review article, the authors conducted an exhaustive systematic search of the literature and included the best available conducted comparative studies to form the basis of our findings. They observed that IMS patients who underwent a colectomy for acute diverticulitis in the emergency setting were more likely to present with severe disease, fail non-operative management, undergo salvage surgical procedures, stay longer in hospital, have more complications and to die compared to IMC patients. However, in following a colectomy for acute diverticulitis in the elective setting, the authors observed that IMS patients have less complications and a lower risk of death, that is comparable to IMC patients. This beckons the question whether IMS patients should be routinely offered an elective resection following a first episode of diverticulitis in the emergency setting in order to avoid an emergency surgery in subsequent attacks. Larger, multi-institutional prospective studies are required to address the actual incidence of recurrence in the IMS population, and optimal timing and indication for operative intervention following an episode of acute diverticulitis in this challenging population, as most current studies are limited by a retrospective design and limited sample size.
Emergency operations for diverticulitis in IMS compared to IMC patients are associated with increased morbidity and mortality, whereas; in the elective setting both groups have similar outcomes. These findings shed a light on whether elective surgical colon resection should be offered to IMS patients following successful non-operative management of an acute episode of diverticulitis. Elective resection of the diseased colon segment will spare these patients the increased risk of complications and death associated with emergency operation.
Acute diverticulitis, refers to acute inflammation of colonic diverticulosis. Diverticulosis, which commonly occurs in the sigmoid segment of colon, is outpocketing of colonic mucosa and submucosa through weaknesses in the colon wall. IMS patients are those who have undergone a solid organ transplant such as lung/heart/liver/kidney and pancreatic transplants, or patients on immunosuppressive medications such as steroids or chemotherapy. IMC patients are patients from the general population who are not on immunosuppressive medications.
This manuscript seems to include the largest series on this topic. The authors reviewed several large studies and conducted a meta-analysis of the topic. They addressed several aspects, including demographic data, clinical presentation, indication and choice of operation, post-operative morbidity and mortality, length of hospital stay, long-term outcome and non-operative management. The analysis is detailed. Despite the limitations of the available literature, the results are reliable. The limitations of the study are inevitable and acceptable.
| 1. | Reshef A, Stocchi L, Kiran RP, Flechner S, Budev M, Quintini C, Remzi FH. Case-matched comparison of perioperative outcomes after surgical treatment of sigmoid diverticulitis in solid organ transplant recipients versus immunocompetent patients. Colorectal Dis. 2012;14:1546-1552. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 2. | Ambrosetti P, Robert JH, Witzig JA, Mirescu D, Mathey P, Borst F, Rohner A. Acute left colonic diverticulitis in young patients. J Am Coll Surg. 1994;179:156-160. [PubMed] |
| 3. | Anderson DN, Driver CP, Davidson AI, Keenan RA. Diverticular disease in patients under 50 years of age. J R Coll Surg Edinb. 1997;42:102-104. [PubMed] |
| 4. | Pourfarziani V, Mousavi-Nayeeni SM, Ghaheri H, Assari S, Saadat SH, Panahi F, Noorbala MH, Vasei A, Norouzi AR, Simforoosh N. The outcome of diverticulosis in kidney recipients with polycystic kidney disease. Transplant Proc. 2007;39:1054-1056. [PubMed] |
| 5. | Mueller XM, Tevaearai HT, Stumpe F, Hurni M, Ruchat P, Fischer AP, Seydoux C, Goy JJ, von Segesser LK. Gastrointestinal disease following heart transplantation. World J Surg. 1999;23:650-655; discussion 655-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.3] [Reference Citation Analysis (1)] |
| 6. | McCune TR, Nylander WA, Van Buren DH, Richie RE, MacDonell RC, Johnson HK, Shull H, Cate CK, Helderman JH. Colonic screening prior to renal transplantation and its impact on post-transplant colonic complications. Clin Transplant. 1992;6:91-96. [PubMed] |
| 7. | Rafferty J, Shellito P, Hyman NH, Buie WD. Practice parameters for sigmoid diverticulitis. Dis Colon Rectum. 2006;49:939-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 463] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 8. | Hwang SS, Cannom RR, Abbas MA, Etzioni D. Diverticulitis in transplant patients and patients on chronic corticosteroid therapy: a systematic review. Dis Colon Rectum. 2010;53:1699-1707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Canter JW, Shorb PE. Acute perforation of colonic diverticula associated with prolonged adrenocorticosteroid therapy. Am J Surg. 1971;121:46-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 65] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Perkins JD, Shield CF, Chang FC, Farha GJ. Acute diverticulitis. Comparison of treatment in immunocompromised and nonimmunocompromised patients. Am J Surg. 1984;148:745-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 63] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 11. | Tyau ES, Prystowsky JB, Joehl RJ, Nahrwold DL. Acute diverticulitis. A complicated problem in the immunocompromised patient. Arch Surg. 1991;126:855-858; discussion 858-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 95] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 12. | Halabi WJ, Jafari MD, Nguyen VQ, Carmichael JC, Mills S, Pigazzi A, Stamos MJ, Foster CE. Colorectal surgery in kidney transplant recipients: a decade of trends and outcomes in the United States. Am Surg. 2013;79:1026-1033. [PubMed] |
| 13. | Biondo S, Borao JL, Kreisler E, Golda T, Millan M, Frago R, Fraccalvieri D, Guardiola J, Jaurrieta E. Recurrence and virulence of colonic diverticulitis in immunocompromised patients. Am J Surg. 2012;204:172-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 75] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 14. | Golda T, Kreisler E, Mercader C, Frago R, Trenti L, Biondo S. Emergency surgery for perforated diverticulitis in the immunosuppressed patient. Colorectal Dis. 2014;16:723-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 15. | Hesterberg R, Müller F, Schmidt WU, Möslein G, Lammers B. [Sigmoid diverticulitis in immunosuppressive drug therapy]. Chirurg. 1994;65:873-876. [PubMed] |
| 16. | Qasabian RA, Meagher AP, Lee R, Dore GJ, Keogh A. Severe diverticulitis after heart, lung, and heart-lung transplantation. J Heart Lung Transplant. 2004;23:845-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Andeweg CS, Mulder IM, Felt-Bersma RJ, Verbon A, van der Wilt GJ, van Goor H, Lange JF, Stoker J, Boermeester MA, Bleichrodt RP. Guidelines of diagnostics and treatment of acute left-sided colonic diverticulitis. Dig Surg. 2013;30:278-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 18. | McAdams-Demarco MA, Grams ME, Hall EC, Coresh J, Segev DL. Early hospital readmission after kidney transplantation: patient and center-level associations. Am J Transplant. 2012;12:3283-3288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 19. | Veroux M, Grosso G, Corona D, Mistretta A, Giaquinta A, Giuffrida G, Sinagra N, Veroux P. Age is an important predictor of kidney transplantation outcome. Nephrol Dial Transplant. 2012;27:1663-1671. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 74] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 20. | Lin FL, Boutros M, Da Silva GM, Weiss EG, Lu XR, Wexner SD. Hartmann reversal: obesity adversely impacts outcome. Dis Colon Rectum. 2013;56:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 21. | Morris CR, Harvey IM, Stebbings WS, Speakman CT, Kennedy HJ, Hart AR. Anti-inflammatory drugs, analgesics and the risk of perforated colonic diverticular disease. Br J Surg. 2003;90:1267-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 59] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Cryer B. Nonsteroidal anti-inflammatory drug gastrointestinal toxicity. Curr Opin Gastroenterol. 1999;15:473-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
P- Reviewer: Barreto S, Chiu CC S- Editor: Tian YL L- Editor: A E- Editor: Liu SQ
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/