Published online Mar 28, 2015. doi: 10.5412/wjsp.v5.i1.147
Peer-review started: December 4, 2014
First decision: December 12, 2014
Revised: December 23, 2014
Accepted: January 9, 2015
Article in press: January 12, 2015
Published online: March 28, 2015
Processing time: 119 Days and 10.9 Hours
AIM: To study the feasibility and oncological outcomes following laparoscopic total mesorectal excision (LTME) in patients who have received Neo-adjuvant long course chemo-radiotherapy (LCRT).
METHODS: A protocol driven systematic review of published literature was undertaken to assess the feasibility and oncological outcomes following LTME in patients receiving LCRT. The feasibility was assessed using peri-operative outcomes and short term results. The oncological outcomes were assessed using local recurrence, disease free survival and overall survival.
RESULTS: Only 8 studies-1 randomized controlled trial, 4 Case Matched/Controlled Studies and 3 Case Series were identified matching the search criteria. The conversion rate was low (1.2% to 28.1%), anastomotic leak rates were similar to open total mesorectal excision (0%-4.1% vs 0%-8.3%). Only 3 studies reported on local recurrence rates (5.2%-7.6%) at median 34 mo follow-up. A single study described disease free survival and overall survival at 3 years as 78.8% and 92.1% respectively.
CONCLUSION: LTME following LCRT is feasible in experienced hands, with acceptable short term surgical outcomes and with the usual benefits associated with minimally invasive procedures. The long term oncological outcomes of LTME after LCRT appear to be comparable to open procedures but need further investigation.
Core tip: Laparoscopic total mesorectal excision (LTME) following long course chemo-radiotherapy (LCRT) is feasible in experienced hands, with acceptable short term surgical outcomes and with the usual benefits associated with minimally invasive procedures. The long term oncological outcomes of LTME after LCRT appear to be comparable to open procedures but need further investigation.
- Citation: Dhruva Rao PK, Nair MS, Haray PN. Feasibility and oncological outcomes of laparoscopic rectal resection following neo-adjuvant chemo-radiotherapy: A systematic review. World J Surg Proced 2015; 5(1): 147-154
- URL: https://www.wjgnet.com/2219-2832/full/v5/i1/147.htm
- DOI: https://dx.doi.org/10.5412/wjsp.v5.i1.147
Total Mesorectal Excision using an open approach (OTME) is now accepted as the gold standard for treatment rectal cancer[1]. In recent years, since the medical research council United Kingdom trial, neo-adjuvant long course chemo-radiotherapy is being routinely used as a part of treatment of locally advanced mid and low rectal cancers[2]. Laparoscopic rectal resection has been shown to have superior short term outcomes compared to open resections. However, long term oncological results are still debated[3]. In addition, it is generally accepted that laparoscopic low rectal resection and Abdomino-perineal resections (APR) are technically challenging[4].
Most trials comparing laparoscopic and open resections for rectal cancer suggest that laparoscopic rectal resections are technically feasible however, short and long term outcomes in this group are difficult to determine[5,6]. Also, laparoscopic total mesorectal excision (LTME) following neo-adjuvant chemo-radiotherapy (LCRT) is oncologically and technically challenging due to tissue fibrosis and scarring[7].
This systematic review addresses the feasibility and outcome of laparoscopic rectal resection following neo-adjuvant chemo-radiotherapy. There is no level 1 evidence addressing this and to the best of our knowledge there is no structured review of the published literature on this topic.
A systematic review of literature was performed as per the protocol described below to address the issue of feasibility of laparoscopic TME following neo-adjuvant chemo-radiotherapy. PubMed, Cochrane, Embase, OVID, and CINAHL were searched for articles published between Jan 2004 to June 2014 using the search criteria as described in Table 1.
| Search strategy |
| 1 Rectal adenocarcinoma - tracked to MeSH to include all subheadings and combining with OR and clicking the Explode box; limit to English language and Humans - no time limits selected |
| 2 Surgery - tracked to MeSH to include all subheadings and combining with OR and clicking the Explode box; limit to English language and Humans - no time limits selected |
| 3 Laparoscopy - tracked to MeSH to include all subheadings and combining with OR and clicking Explode box; limit to English language and Humans - no time limits selected |
| 4 Minimally invasive surgery - tracked to MeSH to include all subheadings and combining with OR and clicking Explode box; limit to English language and Humans - no time limits selected |
| 5 Anterior Resection - Keyword search only (not linked to MeSH headings) |
| 6 Neo-adjuvant chemo-radiotherapy |
| 7 Proctectomy - Keyword search only (not linked to MeSH headings) |
| 8 Total Mesorectal Excision - Keyword search only (not linked to MESH headings) |
| 9 Combine 1 and 2 and 5 and 6 and 7 and 8 |
| 10 Combine 1 and 3 and 4 and 5 and 6 and 7 and 8 |
The keywords for search were laparoscopy, minimally invasive surgery, open, rectum, cancer, abdomino-perineal resection, anterior resection, colorectal neoplasms, rectal neoplasms, rectal adenocarcinoma, rectal cancer, neo-adjuvant chemo-radiotherapy, proctectomy, and total mesorectal excision. Search was done as free text words and in their variable combinations.
The retrieved results were screened by two authors (Dhruva Rao PK and Nair MS) using the title and abstracts against the inclusion and exclusion criteria as described below. Any studies that did not have published abstracts were excluded. Full text articles of potentially relevant studies were obtained and assessed independently by two authors (Dhruva Rao PK and Nair MS) considering the inclusion and exclusion criteria for review. All references of all guideline articles and review articles were searched to identify any potential articles not already identified. Disagreements were resolved through discussion and by involving the third author (Haray PN).
Randomized studies comparing open and laparoscopic rectal resection following neo-adjuvant chemo-radiotherapy for rectal adenocarcinoma; Case matched series comparing LTME with OTME following neo-adjuvant chemo-radiotherapy for rectal adenocarcinoma; Case control studies comparing LTME with OTME following neo-adjuvant chemo-radiotherapy for rectal adenocarcinoma; Case series with > 20 patients from tertiary centres; Published in English language; Feasibility studies of laparoscopic rectal resections for cancer including historical control cohorts.
Study groups were not clearly defined; Studies in whom the “cancer” group cannot be separated; Studies comparing resections performed for benign indications only; Studies including local resections (trans-anal endoscopic microsurgery, trans-anal excision) but the major resection group cannot be separated; the outcomes of interest defined below were not reported or it was impossible to determine them from the published results; the surgical procedures were performed by surgical trainees or by surgeons during the learning curve for laparoscopic or conventional rectal surgery.
A structured proforma was used for data extraction for the patients undergoing laparoscopic resection after neo-adjuvant long course chemo-radiotherapy only. No attempt was made to contact the authors of studies if inadequate amount of information was available and such studies were excluded.
We have assessed 2 sets of outcomes.
For feasibility assessment, we have considered estimated blood loss, ureteral injuries, other collateral injuries, overall peri-operative morbidity, length of hospital stay, anastomotic leakage, intra-abdominal abscess, urinary retention, postoperative ileus, 30 d mortality. We have also assessed circumferential/radial resection margin (CRM) and lymph node harvest.
For oncological outcome assessment, we have considered loco-regional recurrence, metachronous distant metastasis, disease free survival (DFS) and overall survival.
Prior to pooled analysis, the studies must pass 2 assessments of heterogeneity - qualitative and quantitative[8]. Qualitative assessment is based on 4 key concepts of study design (Patients, Interventions, Outcomes and Study Types). If studies are deemed heterogeneous on this assessment, it is inappropriate to proceed to quantitative assessment using statistical tests such as χ2 test or Cochrane Q, etc.[8]. In this review the studies were deemed heterogeneous based on the above mentioned qualitative criteria and so we did not proceed to statistical analysis.
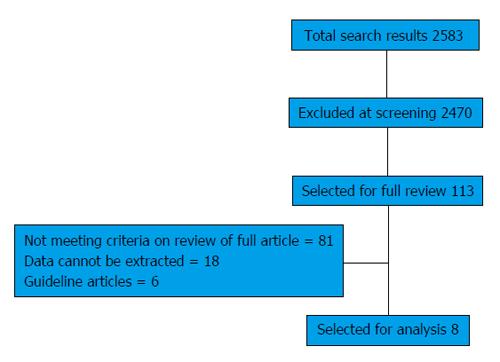
The initial search identified 2583 studies (Figure 1). Two thousand four hundred and seventy were excluded after initial screening of titles and abstracts. The remaining 113 studies were critically reviewed using the full article. Of these, 26 articles met the inclusion criteria and reviewed in detail. However, data relevant to this review could be extracted from only 8 studies (Table 2). Table 3 summarizes the 18 studies from which adequate extraction of appropriate data was not possible.
| Ref. | Year | Country | Type of study | Total No. of patients | Patients Lap | Patients open |
| Kang et al[9] | 2010 | South Korea | RCT | 340 | 170 | 170 |
| Kusano et al[11] | 2014 | Japan | Case control Study | 33 | 19 | 14 |
| Hu et al[14] | 2013 | China | Case control Study | 137 | 51 | 86 |
| Seshadri et al[12] | 2011 | India | Case control Study | 144 | 72 | 72 |
| Denoya et al[15] | 2009 | United States | Case matched series | 64 | 32 | 32 |
| 1Saklani et al[13] | 2013 | South Korea | Case series | 64 | 64 | NA |
| 1Denost et al[10] | 2011 | France | Case series | 292 | 292 | NA |
| Motson et al[7] | 2011 | United Kingdom | Case series | 26 | 26 | NA |
| Ref. | Year | Country | Type of study | Percent having LCRT in Lap group |
| van der Pas et al[5] | 2013 | The Netherlands | RCT1 | 59 |
| Lujan et al[6] | 2009 | Spain | RCT | 72.3 |
| Lujan et al[16] | 2013 | Spain | Case Control | 58.1 |
| McKay et al[17] | 2012 | Australia | Case Control | 48.8 |
| Laurent et al[18] | 2011 | France | Case Control | 93.6 |
| Patel et al[19] | 2011 | United States | Case Matched | 50 |
| Li et al[20] | 2011 | China | Case Control | 34.5 |
| Kellokumpu et al[21] | 2011 | Finland | Case Control | 34 |
| Greenblatt et al[22] | 2011 | United States | Case Control | 31.6 |
| da Luz Moreira et al[23] | 2011 | United States | Case Matched | 33 |
| Baik et al[24] | 2010 | United States | Case Matched | 79.6 |
| Westerholm et al[25] | 2012 | Canada | Case Series | 7.4 |
| Jefferies et al[26] | 2011 | United Kingdom | Case Series | 43.8 |
| Glancy et al[27] | 2011 | United Kingdom | Case Series | 8 |
| Lam et al[28] | 2010 | Belgium | Case Series | 56.7 |
| Sartori et al[29] | 2010 | Italy | Case Series | 39.1 |
| Cheung et al[30] | 2010 | Hong Kong | Case Series | 21.5 |
| Park et al[31] | 2010 | South Korea | Case Series | 8.1 |
The selected publications included a combination of randomized controlled trial (RCT) and non RCT. Qualitative assessment of the studies revealed: (1) Type of studies identified were clearly heterogeneous (Table 2); (2) Patient selection criteria for LCRT were different in the different studies (Table 4); and (3) The LCRT regimen patients received was also different (Table 4).
| Ref. | Staging imaging | Criteria for LCRT | Chemo agent | Rad dose/duration |
| Kang et al[9] | CT, MRI, ERUS | cT3N0-2 M0 Mid/low rectal cancer | I/V 5FU + leucovorin or oral capecitabine | 50.4 Gy over 5.5 wk (tumour boost used) |
| Kusano et al[11] | CT, MRI | T3N0-3M02 | Different protocols | Total dose = 45 Gy/duration not reported |
| Hu et al[14] | CT, MRI, ERUS | Stage 2/3 tumours | Capecitabine and oxaliplatin | 50 Gy over 5 wk |
| 1Seshadri et al[12] | CT | T2/T3 N+, T4 excluded | Mitomycin and 5FU | Total dose = 50 Gy/duration not reported |
| Denoya et al[15] | CT, MRI, ERUS | T3/4 or N+ disease | 5FU or Xeloda | Total dose = 50.4 Gy/duration not reported |
| Saklani et al[13] | NR | T3/4 or N+ disease | 5FU | Total dose = 50.4 Gy/duration not reported |
| Denost et al[10] | CT, MRI, ERUS | T3/4 = 265 (90.8%), T1/2 = 27 (9.2%) | I/V 5FU and leucovorin | 45 Gy over 5 wk |
| Motson et al[7] | CT, MRI | T3/4 N+ + involved/ threatened CRM | 5FU or Uftoral | 45/50 Gy over 5 wk (3/4 fields) |
Thus the studies were heterogeneous in terms of Study Design, Patient Groups and Interventions. Due to this heterogeneity, a pooled analysis or meta-analysis was considered inappropriate and hence was not carried out.
Of the 8 studies included, one was a RCT and 4 were case controlled studies or case matched series. The number of patients in the Laparoscopic group in the selected studies range from 19 to 292. The only RCT (COREAN trial[9]) that we have been able to identify had 170 patients in the study arm. The study with largest number of patients with LTME following LCRT is from France with 292 patients[10].
The patient characteristics of all the studies are shown in Table 5. As can be seen from the table, they were mid or low rectal tumours. The APR rates varied from 11.2% to 89%. All studies had reported the imaging modalities and selection criteria for LCRT with the type and dose of chemo and radiotherapy (Table 4). There was wide heterogeneity in the type, dose and duration of LCRT among the studies.
| Ref. | Age (yr) | Laparoscopic Group | Open Group | BMI | Distance from Anal Verge (cm) | Laparoscopic Group | Open Group | |||||||
| Lap | Open | Men | Women | Men | Women | Lap | Open | Lap | Open | AR | APR | AR | APR | |
| 1Kang et al[9] | 57.8 (11.1) | 59.1 (9.9) | 64.7% | 35.3% | 64.7% | 35.3% | 24.1 (3.2) | 24.1 (3.2) | 5.6 (2.3) | 5.3 (2.5) | 151 (88.8%) | 19 (11.2%) | 146 (85.9%) | 24 (14.1%) |
| Kusano et al[11] | 58 (32-82) | 55 (39-73) | 15 (78.9%) | 4 (21.1%) | 8 (57.1%) | 6 (42.9%) | ≤ 25 = 14 (73.7%) > 25 = 5 (26.3%) | ≤ 25 = 9 (64.3%) >25 = 5 (35.7%) | 2 (0-50) | 3.7 (0-10) | 11 (57.9%) | 8 (42.1%) | 4 (28.6%) | 10 (71.4%) |
| Hu et al[14] | 55 (35-78) | 55 (29-82) | 34 (66.7%) | 17 (33.3%) | 56 (65.1%) | 30 (34.9%) | 23.4 (16-31.2) | 24.2 (16.3-36.2) | ≤ 5 = 33 (64.7%) > 5 = 18 (35.3%) | ≤ 5 = 54 (62.8%) > 5 = 32 (37.2%) | 32 (62.7%) | 18 (35.3%) | 36 (41.9%) | 44 (51.2%) |
| Seshadri et al[12] | 48 (22-73) | 48 (19-71) | 47 (65%) | 25 (35%) | 45 (62%) | 27 (38%) | 21 (15-33) | 22 (14-38) | 3 (0-8) | 3 (0-10) | 8 (11%) | 64 (89%) | 8 (11%) | 64 (89%) |
| 1Denoya et al[15] | 56.3 | 57.1 | 19 (59.4%) | 13 (40.6%) | 18 (56.3%) | 14 (43.7%) | 25 | 26.4 | 4.1 | 4.6 | 24 (75%) | 8 (25%) | 24 (75%) | 8 (25%) |
| Denost et al[10] | 65 (20-85) | NA | 179 (61.3%) | 113 (38.7%) | NA | NA | 25 (16-39) | NA | < 5 = 175 (59.9%) > 5 = 117 (40.1%) | NA | NR | NR | NA | NA |
| Motson et al[7] | 63 (39-81) | NA | 21 (80.8%) | 5 (19.2%) | NA | NA | NR | NA | < 5 = 11 (42.3%) > 5 = 15 (57.7%) | NA | 16 (61.5%) | 10 (38.5%) | NA | NA |
Table 6 reports the peri-operative course. The interval between LCRT and surgery was reported by all except by one study[11] with the median minimum and maximum intervals being 6 and 8 wk respectively. The reported conversion rates from laparoscopic to open operations ranged from 1.2% to 28.1%. In the Laparoscopic arm, three of the eight identified studies reported a median estimated blood loss of 200 mL.
| Ref. | Interval to surgery | Conversion | Estimated blood loss | Intra-op injury | Diversion stoma | |||
| Lap | Open | Lap | Open | Lap | Open | |||
| 1Kang et al[9] | 26-8 wk | 1.2% | Median - 200 mL | Median - 217.5 mL | Yes1 | Yes1 | 91.4% | 88.4% |
| Kusano et al[11] | NR | NR | < 200 mL = 47.4% > 200 = 52.6% | < 200 = 92.9% > 200 = 7.1% | NR | NR | NR | NR |
| Hu et al[14] | Mean 53 d (28-105 d) | 5.9% | Mean 204.7 (80-1000 mL) | Mean 352.5 (100-1200) mL | No | Ureteric injury = 1.2% | NR | NR |
| Seshadri et al[12] | Median 8 (4-36) wk | 4.1% | Median 200 (100-600) mL | Med 400 (150-1500) mL | NR | NR | NR | NR |
| Denoya et al[15] | Mean 6.5 wk | 28.1% | Mean 313 ± 443 | Mean 279 ± 229 | NR | NR | 75% | 75% |
| Denost et al[10] | 26 wk | 18.8% | NR | NA | NR | NA | 81.2% | NA |
| Motson et al[7] | Median 11 wk | 11.5% | NR | NA | NR | NA | 75% | NA |
While only two studies reported intra operative complications (Table 6), all studies have reported post-operative complications (Table 7). In the studies where comparative data was available, the laparoscopic group had a low anastomotic leak rate compared to the open group (0%-4% vs 0%-8.3% respectively). The COREAN trial reported a higher leak rate for LTME vs OTME (1.2% vs 0% respectively). However, 2 case series reported anastomotic leak rates of 12.7%[10] and 18.7%[7]. Interestingly these had higher conversion rates as well (18.8%[10] and 11.5%[7] respectively). Pelvic abscess was also less in laparoscopic group compared to the open group (0%-10.5% vs 0.6%-14.2%). Post-operative ileus was less in Laparoscopic group (0%-10% vs 1.2%-12.9%). Post-operative voiding difficulty varied from 2%-10% in laparoscopic group compared to 2.3%-7.1% in open group.
| Ref. | Anastomotic leak (%) | Pelvic abscess (%) | Post-op Ileus (%) | Acute voiding difficulty (%) | Stoma complications (%) | |||||
| Lap | Open | Lap | Open | Lap | Open | Lap | Open | Lap | Open | |
| Kang et al[9] | 1.2 | 0 | 0 | 0.6 | 10 | 12.9 | 10 | 4.1 | 0.6 | 0 |
| Kusano et al[11] | 0 | 7.1 | 10.5 | 14.2 | 5.2 | 7.1 | 0 | 7.1 | NR | NR |
| 2Hu et al[14] | 3.1 | 8.3 | 0 | 1.2 | 0 | 1.2 | 1.2 | 2.3 | 0 | 2 |
| Seshadri et al[12] | 4.1 | 8.3 | NR | NR | NR | NR | 11 | 7 | NR | NR |
| Denoya et al[15] | NR | NR | NR | NR | 5 | 5 | NR | NR | NR | NR |
| Denost et al[10] | 12.7 | NA | NR | NA | NR | NA | NR | NA | NR | NA |
| 1Motson et al[7] | 18.7 | NA | NR | NA | NR | NA | 15.4 | NA | NR | NA |
The short term outcomes are summarized in Table 8. All except 2 studies have reported post-operative length of stay with the median stay ranging between 8-24 d for the laparoscopic group and 9-35 for the open group. Only 2 case series[7,10] reported procedure related mortality (0.3%-3.8%).
| Ref. | Post-op length of stay | 30 d mortality (%) | Length of follow-up | Local recurrence | ||
| Lap | Open | Lap | Open | |||
| Kang et al[9] | 8 (7-12) | 9 (8-12) | NR | 3 mo | NA | NA |
| Kusano et al[11] | 24 (14-92) | 35 (14-70) | NR | Median 39 mo | 1 (5.2%) | 3 (21.4%) |
| Hu et al[14] | 10 (6-34) | 16 (6-44) | NR | Short term outcomes only | NA | NA |
| Seshadri et al[12] | 12 (6-45) | 15 (10-50) | None | Short term outcomes only | NA | NA |
| 1Denoya et al[15] | 6.1 ± 2.4 | 7.6 ± 2.3 | NR | Short term outcomes only | NA | NA |
| Denost et al[10] | NR | NA | 0.3 | NR | NR | NA |
| Motson et al[7] | 8 (5-17) | NA | 3.8 | Median 34 mo | 2 (7.6%) | NA |
| Saklani et al[13] | NR | NA | NR | Median 36 mo | 4 (6.3%) | NA |
The markers of surgical quality are reported in Table 9. Only the COREAN trial[9] reported the TME quality with 72.4% of the resection as complete. One study[12] defined the CRM positivity as 2 mm while the others used the standard 1 mm measurement. The CRM positivity in the laparoscopic group was 1.3% to 2.9% and that for the open group was 3.5%-9.7%. The numbers of lymph nodes harvested did not differ between laparoscopic and open groups.
| Ref. | CRM positivity | Lymph node harvest1 | ||
| Lap | Open | Lap | Open | |
| Kang et al[9] | 2.9% | 4.1% | 17 (12-22) | 18 (13-24) |
| 3Kusano et al[11] | NR | NR | < 12 = 73.7% > 12 = 26.3% | < 12 = 64.3% > 12 = 35.7% |
| Hu et al[14] | 1.9% | 3.5% | 12 (2-20) | 11 (1-25) |
| 4Seshadri et al[12] | 1.3% | 9.7% | 7 (1-24) | 7 (1-25) |
| Denoya et al[15] | Yes5 | Yes5 | 19 ± 92 | 19 ± 92 |
| Denost et al[10] | NR | NA | NR | NA |
| 6Motson et al[7] | Yes6 | NA | 5 (0-14) | NA |
Only 3 studies reported a follow up period of 34 mo or above (Table 8). The rest had data on short term follow up with one study not reporting any follow up data. Local recurrence was reported as 5.2%-7.6% in the laparoscopic groups after 34 mo of follow up. Only one study reported comparative local recurrence between the laparoscopic and open groups (5.2% for laparoscopic vs 21.4% for open)[11]. Only one study[13] reported disease free survival and overall survival at 3 years (78.8% and 92.1 % respectively).
The studies were heterogeneous. In spite of this, the reported short term outcomes for LTME were not inferior to OTME. Available data shows LTME offers the same short term advantages in outcomes like estimated blood loss, other collateral injuries, overall intra-operative morbidity, post-operative length of stay, intra-abdominal abscess and post-operative ileus even after LCRT. Short term surrogate measures of oncologic parameters are at least equal to the open procedure.
LCRT makes the normal anatomical planes within the pelvis challenging due to tissue fibrosis and scarring. The tissue planes can be more difficult to follow compared with non-irradiated cases[7].
The magnified view of operative field and the improving technology with efficient energy devices in addition to meticulous attention to haemostasis to maintain good views during LMTE are factors that help reduce the blood loss as reflected in the reported estimated blood loss of these studies. Pelvic abscess was also less in laparoscopic group compared to open. This may be due to the fact that the blood loss is less with consequent less postoperative haematoma, etc.
Irradiation causes fibrosis and ischaemia[10] and increases the thickness of the rectal wall making a safe rectal division by stapling devices technically more difficult[10]. It is also thought to increase the risk of anastomotic leak. However, the reported anastomotic leak rate in LTME was generally low. One study[7] reported a higher leak rate (18.7%) but this is probably due to low number of patients in this study.
The surrogate markers of oncological outcome like lymph node harvest, positivity of CRM margins with LTME were comparable and not inferior to both contemporaneous open procedures as well as historically reported data.
The only RCT identified, the COREAN trial[9], randomised 340 patients after LCRT to LTME or OTME. It observed no difference between CRM positivity, macroscopic quality of the total mesorectal excision, number of harvested lymph nodes or perioperative morbidity between the two groups[9]. The short term benefits were better in LTME. This trial demonstrated LTME after LCRT was safe in the hands of experienced surgeons (participating surgeons had a median experience of 75 LTMEs). Although this trial was not sufficiently powered to address survival outcomes (one of the limitations of this trial), the long term outcome from COREAN trial is expected to shed more light on the oncological effectiveness of LTME in this group of rectal cancer patients.
The other end-points of this review were the local recurrence rates, and DFS. These results are based on case controlled study or data from experienced tertiary centres. The rate of local recurrence varied from 5.2% to 7.6% in the LTME group. Only one study[11] reported comparative data for OTME (21.4%). Only one study reported DFS of 78.8% in LTME after 3 years of follow up. Unfortunately this did not report on a similar figure for OTME[13].
We identified 18 other studies which had a subgroup of patients who underwent LCRT followed by laparoscopic rectal resection. However, insufficient data were included for relevant data extraction and analysis. An analysis of the raw data from these published studies may provide interim results quicker. However, such an exercise would require the co-operation of various authors from around the world to contribute their data to help create an international registry for analysis: this is unlikely to be feasible retrospectively. Hence, a prospective, multicentre randomised trial recruiting patients from appropriate centres and adequately powered to address survival outcomes is needed to answer the question of oncological effectiveness.
Although there is paucity of published data on the rates of local and distant disease recurrence (Disease Free Survival) following LTME after LCRT, available data shows LTME following LCRT is not inferior to open TME with the inherent advantages of Laparoscopic surgery.
LTME is feasible in experienced hands, with acceptable short term surgical outcomes and with the usual benefits associated with minimally invasive procedures. The long term oncological outcomes of LTME after LCRT appear to be comparable to open procedures but need further investigation probably with a well-designed adequately powered multicentre trial.
Laparoscopic total mesorectal excision (LTME) has been shown to be feasible with acceptable short and intermediate term results in management of rectal cancers. However, the increasing use of neo-adjuvant long course chemo-radiotherapy (LCRT), and the resultant increased fibrosis and alterations to the tissue planes has increased the challenges of the LTME. To the authors’ knowledge, there is no level 1 evidence to support its use.
Over the recent years, numerous publications addressing this area of rectal cancer management have been published. The authors aimed to conduct a systematic review of the published literature to inform future practice.
This systematic review has shown that LTME in patients undergoing LCRT is feasible with acceptable short and intermediate term surgical and oncological outcomes in experienced hands. It has also identified the need for a sufficiently powered RCT to address this issue. In the interim, this study which has assimilated and analysed the raw data from various publications could provide useful information on the subject.
This review lends support to the practice of LTME in experienced centres within the multimodal approach to rectal cancer. However, long term outcomes (as in all oncological treatments) need to be continuously monitored.
Total mesorectal excision (TME): this is the gold standard surgical technique for the management of mid to low rectal cancers and involves the complete removal of the rectum and mesorectal tissue. As it is traditionally performed by an open approach, it can also be called open TME. Laparoscopic TME: Using the laparoscopic approach to perform TME. LCRT: Use of pre-operative course of radiotherapy with potentiating chemotherapy (neo-adjuvant treatment) over a few weeks, usually a 5 wk cycle. Following the chemo-radiation, surgery in the form of TME is performed after a delay of several weeks. The aim is to shrink the tumour or “sterilize” the circumferential resection margin.
This review addresses a very interesting and timely clinical issue.
| 1. | Enker WE. Total mesorectal excision--the new golden standard of surgery for rectal cancer. Ann Med. 1997;29:127-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 158] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 2. | Foster JD, Jones EL, Falk S, Cooper EJ, Francis NK. Timing of surgery after long-course neoadjuvant chemoradiotherapy for rectal cancer: a systematic review of the literature. Dis Colon Rectum. 2013;56:921-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 3. | Shussman N, Wexner SD. Current status of laparoscopy for the treatment of rectal cancer. World J Gastroenterol. 2014;20:15125-15134. [PubMed] |
| 4. | Lee JK, Delaney CP, Lipman JM. Current state of the art in laparoscopic colorectal surgery for cancer: Update on the multi-centric international trials. Ann Surg Innov Res. 2012;6:5. [PubMed] |
| 5. | van der Pas MH, Haglind E, Cuesta MA, Fürst A, Lacy AM, Hop WC, Bonjer HJ. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013;14:210-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1030] [Cited by in RCA: 1246] [Article Influence: 95.8] [Reference Citation Analysis (1)] |
| 6. | Lujan J, Valero G, Hernandez Q, Sanchez A, Frutos MD, Parrilla P. Randomized clinical trial comparing laparoscopic and open surgery in patients with rectal cancer. Br J Surg. 2009;96:982-989. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 300] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 7. | Motson RW, Khan JS, Arulampalam TH, Austin RC, Lacey N, Sizer B. Laparoscopic total mesorectal excision following long course chemoradiotherapy for locally advanced rectal cancer. Surg Endosc. 2011;25:1753-1760. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Hatala R, Keitz S, Wyer P, Guyatt G. Tips for learners of evidence-based medicine: 4. Assessing heterogeneity of primary studies in systematic reviews and whether to combine their results. CMAJ. 2005;172:661-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 116] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 9. | Kang SB, Park JW, Jeong SY, Nam BH, Choi HS, Kim DW, Lim SB, Lee TG, Kim DY, Kim JS. Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol. 2010;11:637-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 687] [Cited by in RCA: 766] [Article Influence: 47.9] [Reference Citation Analysis (0)] |
| 10. | Denost Q, Laurent C, Paumet T, Quintane L, Martenot M, Rullier E. Laparoscopic surgery for rectal cancer: preoperative radiochemotherapy versus surgery alone. Surg Endosc. 2012;26:1878-1883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Kusano T, Inomata M, Hiratsuka T, Akagi T, Ueda Y, Tojigamori M, Shiroshita H, Etoh T, Shiraishi N, Kitano S. A comparison of laparoscopic and open surgery following pre-operative chemoradiation therapy for locally advanced lower rectal cancer. Jpn J Clin Oncol. 2014;44:305-310. [PubMed] |
| 12. | Seshadri RA, Srinivasan A, Tapkire R, Swaminathan R. Laparoscopic versus open surgery for rectal cancer after neoadjuvant chemoradiation: a matched case-control study of short-term outcomes. Surg Endosc. 2012;26:154-161. [PubMed] |
| 13. | Saklani AP, Lim DR, Hur H, Min BS, Baik SH, Lee KY, Kim NK. Robotic versus laparoscopic surgery for mid-low rectal cancer after neoadjuvant chemoradiation therapy: comparison of oncologic outcomes. Int J Colorectal Dis. 2013;28:1689-1698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Hu JJ, Liang JW, Wang Z, Zhang XM, Zhou HT, Hou HR, Zhou ZX. Short-term outcomes of laparoscopically assisted surgery for rectal cancer following neoadjuvant chemoradiotherapy: a single-center experience. J Surg Res. 2014;187:438-444. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Denoya P, Wang H, Sands D, Nogueras J, Weiss E, Wexner SD. Short-term outcomes of laparoscopic total mesorectal excision following neoadjuvant chemoradiotherapy. Surg Endosc. 2010;24:933-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 16. | Lujan J, Valero G, Biondo S, Espin E, Parrilla P, Ortiz H. Laparoscopic versus open surgery for rectal cancer: results of a prospective multicentre analysis of 4,970 patients. Surg Endosc. 2013;27:295-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 17. | McKay GD, Morgan MJ, Wong SK, Gatenby AH, Fulham SB, Ahmed KW, Toh JW, Hanna M, Hitos K. Improved short-term outcomes of laparoscopic versus open resection for colon and rectal cancer in an area health service: a multicenter study. Dis Colon Rectum. 2012;55:42-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 18. | Laurent C, Paumet T, Leblanc F, Denost Q, Rullier E. Intersphincteric resection for low rectal cancer: laparoscopic vs open surgery approach. Colorectal Dis. 2012;14:35-41; discussion 42-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Patel CB, Ragupathi M, Ramos-Valadez DI, Haas EM. A three-arm (laparoscopic, hand-assisted, and robotic) matched-case analysis of intraoperative and postoperative outcomes in minimally invasive colorectal surgery. Dis Colon Rectum. 2011;54:144-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Li S, Chi P, Lin H, Lu X, Huang Y. Long-term outcomes of laparoscopic surgery versus open resection for middle and lower rectal cancer: an NTCLES study. Surg Endosc. 2011;25:3175-3182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Kellokumpu IH, Kairaluoma MI, Nuorva KP, Kautiainen HJ, Jantunen IT. Short- and long-term outcome following laparoscopic versus open resection for carcinoma of the rectum in the multimodal setting. Dis Colon Rectum. 2012;55:854-863. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 22. | Greenblatt DY, Rajamanickam V, Pugely AJ, Heise CP, Foley EF, Kennedy GD. Short-term outcomes after laparoscopic-assisted proctectomy for rectal cancer: results from the ACS NSQIP. J Am Coll Surg. 2011;212:844-854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 79] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 23. | da Luz Moreira A, Mor I, Geisler DP, Remzi FH, Kiran RP. Laparoscopic resection for rectal cancer: a case-matched study. Surg Endosc. 2011;25:278-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 24. | Baik SH, Gincherman M, Mutch MG, Birnbaum EH, Fleshman JW. Laparoscopic vs open resection for patients with rectal cancer: comparison of perioperative outcomes and long-term survival. Dis Colon Rectum. 2011;54:6-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 25. | Westerholm J, Garcia-Osogobio S, Farrokhyar F, Cadeddu M, Anvari M. Midterm outcomes of laparoscopic surgery for rectal cancer. Surg Innov. 2012;19:81-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 26. | Jefferies MT, Evans MD, Hilton J, Chandrasekaran TV, Beynon J, Khot U. Oncological outcome after laparoscopic abdominoperineal excision of the rectum. Colorectal Dis. 2012;14:967-971. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Glancy DG, Chaudhray BN, Greenslade GL, Dixon AR. Laparoscopic total mesorectal excision can be performed on a nonselective basis in patients with rectal cancer with excellent medium-term results. Colorectal Dis. 2012;14:453-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 28. | Lam HD, Stefano M, Tran-Ba T, Tinton N, Cambier E, Navez B. Laparoscopic versus open techniques in rectal cancer surgery: a retrospective analysis of 121 sphincter-saving procedures in a single institution. Surg Endosc. 2011;25:454-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Sartori CA, Dal Pozzo A, Franzato B, Balduino M, Sartori A, Baiocchi GL. Laparoscopic total mesorectal excision for rectal cancer: experience of a single center with a series of 174 patients. Surg Endosc. 2011;25:508-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 30. | Cheung HY, Ng KH, Leung AL, Chung CC, Yau KK, Li MK. Laparoscopic sphincter-preserving total mesorectal excision: 10-year report. Colorectal Dis. 2011;13:627-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 31. | Park JS, Choi GS, Lim KH, Jang YS, Jun SH. Robotic-assisted versus laparoscopic surgery for low rectal cancer: case-matched analysis of short-term outcomes. Ann Surg Oncol. 2010;17:3195-3202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 185] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
P- Reviewer: Lorenzon L, Morino M, Wang SK S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/