Published online Feb 8, 2017. doi: 10.5409/wjcp.v6.i1.34
Peer-review started: July 14, 2016
First decision: September 12, 2016
Revised: October 14, 2016
Accepted: November 1, 2016
Article in press: November 2, 2016
Published online: February 8, 2017
Processing time: 210 Days and 13.4 Hours
To determine if a standardized asthma severity scoring system (PASS) was associated with the time spent on continuous albuterol and length of stay in the pediatric intensive care unit (PICU).
This is a single center, retrospective chart review study at a major children’s hospital in an urban location. To qualify for this study, participants must have been admitted to the PICU with a diagnosis of status asthmaticus. There were a total of 188 participants between the ages of two and nineteen, excluding patients receiving antibiotics for pneumonia. PASS was calculated upon PICU admission. Subjects were put into one of three categories based on PASS: ≤ 7 (mild), 8-11 (moderate), and ≥ 12 (severe). The groups were compared based on different variables, including length of continuous albuterol and PICU stay.
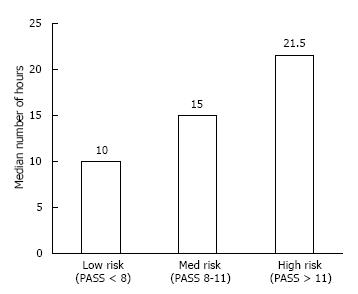
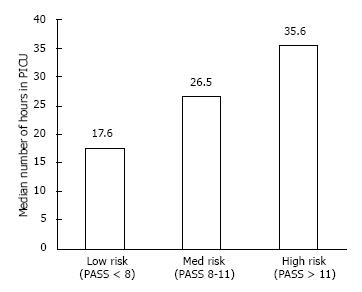
The age distribution across all groups was similar. The median length of continuous albuterol was longest in the severe group with a duration of 21.5 h (11.5-27.5), compared to 15 (7.75-23.75) and 10 (5-15) in the moderate and mild groups, respectively (P = 0.001). The length of stay was longest in the severe group, with a stay of 35.6 h (22-49) compared to 26.5 (17-30) and 17.6 (12-29) in the moderate and mild groups, respectively (P = 0.001).
A higher PASS is associated with a longer time on continuous albuterol, an increased likelihood to require noninvasive ventilation, and a longer stay in the ICU. This may help safely distribute asthmatics to lower and higher levels of care in the future.
Core tip: This is a single center retrospective study designed to determine whether or not the pediatric asthma severity score was associated with critical care interventions. It was found that patients with a higher (more severe) severity score were more likely to require continuous albuterol for longer (P = 0.001) and were more likely to have a longer length of pediatric intensive care unit stay compared to those with less severe scores (P = 0.001). It was also determined that patients with a higher severity score were more likely to require other critical care interventions, including noninvasive positive pressure ventilation and amiodarone. This may help safely distribute asthmatics to higher and lower levels of care in the future.
- Citation: Maue DK, Krupp N, Rowan CM. Pediatric asthma severity score is associated with critical care interventions. World J Clin Pediatr 2017; 6(1): 34-39
- URL: https://www.wjgnet.com/2219-2808/full/v6/i1/34.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v6.i1.34
Asthma is the one of the most common chronic pediatric medical condition in the United States[1,2] and accounts for approximately 150000 hospitalizations per year[3,4]. It is estimated that upwards of 30% of pediatric emergency department visits due to asthma result in hospitalization[5]. A portion of these patients are admitted to the pediatric intensive care unit (PICU), although the rate of ICU admission varies widely by institution. A recent study in New Jersey showed that while admission rates for status asthmaticus are overall on the decline, there has been an increase in ICU admission for these patients from 4% in 1992 to 35% in 2006, which also led to an increase in cost to treat patients with status asthmaticus from 6.6 million in 1992 to 9.5 million in 2006[6]. PICU admissions are very stressful for families[7] and are expensive. PICU beds can be limited, particularly during respiratory seasons. Hospital systems across the nation are making difficult decisions to triage those who truly are in need of critical care services, to optimize ICU bed availability for the sickest patients[8]. In making initial bed assignment decisions, however it is prudent to ensure that patients who are admitted to floor level care are unlikely to clinically decompensate after admission, thus requiring later ICU transfer.
Asthma severity scores have been developed, validated, and used in various settings of pediatric care. The PASS (Pediatric Asthma Severity Score) was developed and validated in the early 2000’s[9,10]. In at least one study, the PASS was superior to spirometry at predicting the need for further treatment[10]. Severity scores have generally been used in the emergency department to help determine whether a patient requires hospital admission or can be safely discharged home[9-13]. However, there are no published studies to date that examine the effectiveness of a pediatric asthma scoring system in triage to ICU vs ward treatment.
The aim of this study is to determine whether the PASS is associated the length of continuous albuterol usage, and higher level respiratory support that often requires PICU level care. The hypothesis is that PASS symptom stratification (mild, moderate, or severe respiratory compromise) can be used to help identify patients most likely to require critical care interventions.
Approval for this study was obtained from the Indiana University institutional review board prior to data collection. This is a single center, retrospective chart review of patients admitted to the PICU from July 2012-June 2013 with an admitting diagnosis of status asthmaticus. Inclusion criteria were age two through nineteen years, admitted to Riley Hospital for Children PICU via the emergency department or direct admission to the PICU. Patients transferred from the ward or receiving antibiotics for bacterial pneumonia were excluded. The data was obtained from the electronic medical record system. Patients were identified based on those admitted to the PICU with the primary diagnosis code for status asthmaticus. Data points collected include: Demographics, medical history, admitting diagnosis, medications for asthma care (inpatient and outpatient), vital signs, level of respiratory support, clinical assessments by respiratory therapy, nursing and physicians, times of ICU admission, transfer, and medication changes. It was also noted whether patients were already followed in the Riley Hospital High Risk Asthma Clinic prior to admission, which is an intensive outpatient management program. Prior to data analysis, a time of greater than or less than 6 h of continuous albuterol was determined to be a collection point. This time frame was chosen as it seemed to be a reasonable time frame to attempt weaning of continuous albuterol in the emergency department or ward setting.
PASS scores were calculated retrospectively at admission to the PICU, at the end of continuous bronchodilator treatment, and at the first interval bronchodilator treatment. The PASS calculation can be found in Table 1. This was accomplished by reviewing nursing vitals and respiratory therapy (RT) documentation. The first set of vitals and RT documentation after arrival to the PICU was used to calculate the admission score. The total length of time on continuous albuterol was calculated from charting on the medication administration record. The total length of time in the PICU was calculated from the first set of vitals charted by the PICU nurse to the time transfer orders were written. Groups were assigned as follows based on clinical guidelines utilized at other institutions for bronchodilator weaning: mild respiratory compromise were defined as PASS ≤ 7; moderate respiratory compromise PASS 8-11; severe respiratory compromise PASS ≥ 12[14].
| Score | 1 | 2 | 3 |
| Respiratory rate | |||
| 2 to 3 yr | ≤ 34 | 35 to 39 | ≥ 40 |
| 4 to 5 yr | ≤ 30 | 31 to 35 | ≥ 36 |
| 6 to 12 yr | ≤ 26 | 27 to 30 | ≥ 31 |
| Older than 12 yr | ≤ 23 | 24 to 27 | ≥ 28 |
| Oxygen | > 90% on room air | 85%-90% on room air | < 85% on room air |
| requirements | |||
| Auscultation | Normal breath sounds or end-expiratory wheeze only | Expiratory wheezing | Inspiratory and expiratory wheezing or diminished breath sounds |
| Retractions | ≤ One site | Two sites | ≥ Three sites |
| Dyspnea | Speaks in sentences, coos and babbles | Speaks in partial sentences, short cry | Speaks in single words/short phrases/grunting |
Descriptive statistics using medians and interquartile ranges were calculated for continuous variables. Comparison of continuous variables between risk groups was done using Kruskal-Wallis test. Categorical variables were compared using χ2 or fisher exact test where appropriate. Statistical significance was set at a value of 0.05. We used Statistical Package of the Social Science (SPSS) Statistical software for Windows, Version 20.0 (SPSS Inc., Chicago, IL, United States) and Microsoft Office Excel (Microsoft Corporation, Redmond, WA).
A total of 188 subjects with the admission diagnosis of status asthmaticus were included in the study. The mean age at admission was 7.2 ± 4.0 years (range 2-19 years). African American race and male gender accounted for the majority of the study population. Additional demographic information can be found in Table 2.
| Characteristic | Subgroup | Total n (%) | Mild n (%) | Moderate n (%) | Severe n (%) | P value |
| Gender | Male | 112 (59.6) | 26 (65.0) | 68 (58.1) | 18 (58.1) | 0.18 |
| Female | 76 (40.4) | 14 (35.0) | 49 (41.9) | 13 (41.9) | ||
| Race/ethnicity | Caucasian | 44 (23.4) | 5 (12.5) | 29 (24.8) | 10 (32.3) | 0.18 |
| African American | 130 (69.1) | 33 (82.5) | 79 (67.5) | 18 (58.1) | ||
| Hispanic | 10 (5.3) | 2 (5.0) | 7 (6.0) | 1 (3.2) | ||
| Other | 4 (2.1) | 0 (0) | 2 (1.7) | 2 (6.4) | ||
| Asthma history | Uses controller medication | 137 (72.9) | 35 (87.5) | 78 (66.7) | 24 (77.4) | 0.03 |
| Patient in high risk asthma clinic | 61 (32.4) | 16 (40.0) | 33 (28.2) | 12 (38.7) | 0.25 |
In total, there were 40 subjects that fell into the mild respiratory compromise group, 117 in the moderate group, and 31 in the severe group.
The degree of respiratory support required for each patient was documented, specifically high flow nasal cannula, noninvasive positive pressure ventilation (NIPPV), intubation, and extracorporeal membrane oxygenation (ECMO). In total, 6 subjects required high flow nasal cannula, 6 required NIPPV, 1 was intubated, and none were on ECMO. The breakdown of the number in each group that required each form of respiratory support is found in Table 3. Not surprisingly, those in the severe group were more likely to receive NIPPV.
| Medication | Total received all groups (%) | Mild compromise number received (%) | Moderate compromise number received (%) | Severe compromise number received (%) | P value |
| Systemic steroids | 187 (99.5) | 40 (100) | 116 (99.1) | 31 (100) | 0.737 |
| Magnesium sulfate | 126 (67.0) | 26 (65) | 77 (65.8) | 23 (74.1) | 0.646 |
| Aminophylline | 4 (2.1) | 0 | 0 | 4 (12.9) | < 0.001 |
| Terbutaline | 4 (2.1) | 0 | 2 (1.7) | 2 (6.5) | 0.155 |
| Scheduled ipratropium | 146 (77.7) | 21 (55.3) | 100 (86.2) | 25 (83.3) | < 0.001 |
| Continuous albuterol > 6 h | 139 (73.9) | 23 (57.5) | 88 (75.2) | 28 (90.3) | 0.007 |
Medication use for the 188 patients as a whole and per group is displayed in Table 3. The severe respiratory compromise group was more likely to receive continuous albuterol for more than 6 h as well as aminophylline. The medium and high risk groups were more likely to receive scheduled ipratropium. There was no significant difference among the groups with terbutaline or magnesium sulfate. The difference is shown below in Table 4.
| Respiratory support | Mild | Moderate | Severe | Pearson χ2 |
| High flow nasal cannula | 1 (2.6) | 4 (3.4) | 1 (3.2) | 0.96 |
| Noninvasive positive pressure ventilation | 0 | 2 (1.7) | 4 (12.9) | 0.003 |
| Invasive mechanical ventilation | 0 | 0 | 1(3.2) | 0.078 |
The moderate and severe group was on continuous albuterol longer. The mild group received continuous albuterol for a median of 10 h (IQR 5-15), moderate group for 15 h (IQR 7.75-23.75) and the severe group for 21.5 h (IQR 11.5 – 27.5) (P = 0.001). The difference among the groups is shown in Figure 1.
The severe group was in the Pediatric ICU for a longer period of time. The mild group had a median length of stay of 17.6 h (IQR 13-29), the moderate group 26.5 h (17.3-39.4), and the severe group 35.6 h (22.2-49.6) (P = 0.001). The difference among the groups is demonstrated in Figure 2.
To our knowledge, this is the first study that associates an asthma clinical severity score with PICU interventions and outcomes. Our high number of patients at a large pediatric hospital, recent study period, and common standard practices for status asthmaticus make the results generalizable to many children’s hospitals in the United States.
The most reassuring result of our study is the significant difference among severity groups with regards to length of time on continuous albuterol and length of PICU stay. Based on these results, a patient’s admission PASS could help clinicians predict how long a patient could need ICU resources, and their risk of requiring intensive pharmacologic treatment such as aminophylline. Particularly in times of high utilization of critical care resources, the ability to identify those that will not require continuous albuterol for extended periods of time and have a lower risk of requiring higher levels of respiratory support can be an exceedingly useful triage tool. Since the mild respiratory compromise group was less likely to receive prolonged continuous albuterol, it is feasible that these patients could be weaned to intermittent albuterol in the emergency room in a reasonable amount of time. The ability to objectively identify these patients during times of limited PICU bed availability can help ensure those that are likely to have the highest need for critical care obtain the resources first. This will not only improve patient safety but assist with patient flow through the emergency department. It may also assist accepting hospitals in triage of referred patients from outside facilities.
In this study, 99.5% of patients received systemic steroids, and there was no significant difference among the groups. It is also clear that magnesium sulfate is often used in asthmatics that are deemed critically ill, requiring PICU admission. Regardless of PASS score, there was no difference in this intervention when comparing severity groups. It is not particularly surprising that we did not see a significant difference in the use of most of medications among the different groups. Magnesium sulfate is commonly used at many institutions, including ours, in both the emergency department as well as the PICU. It has been shown to reduce bronchoconstriction and has been shown to help avoid hospitalization. The toxicity rates are also relatively low so it is considered safe to use[15].
There was a significant difference with aminophylline, with patients in the severe group more likely to receive it. At our institution, aminophylline use is restricted to the ICU setting, due to the potential for significant toxicity, and is reserved for patients with persistent respiratory distress despite other modalities. The fact that aminophylline use was seen only in the severe group base on PASS speaks to the congruence of the PASS to more subjective estimation of clinical status. The fact that the moderate group (PASS 8-11) had no aminophylline use at all during their clinical course is notable, in that the patients in this severity category did not decompensate to the point of requiring this particular intervention. Admittedly, there were only a total of four patients in the study that received aminophylline so it is difficult to make generalizations based on this data. The data on aminophylline is mixed with a recent study showing that, while it did improve lung function at six hours, there was no reduction in number of nebulized treatments as well as being inconclusive on whether or not it reduced length of stay or complications such as mechanical ventilation. There is also significant toxicity associated with aminophylline[16,17]. Therefore, most providers reserve it for patients who have a severe case when they are not responding to traditional treatments.
It is interesting that our study did not show any significant difference among the groups in terms of heated humidified high flow nasal cannula (HHFNC). This may be reflective of the trend to use HHFNC more commonly, even on general wards. It may also be due to the fact many patients on HHFNC were on it prior to arrival to the PICU, thus improving their work of breathing and improving their PASS. Recent studies have shown that when used correctly, HHFNC is safe to use on a general pediatric ward as long as there is a Pediatric ICU bed available should worsening respiratory failure or other complications ensue[18].
There are a few limitations with this study, mostly related to the retrospective nature of the study. Data sources to identify admission and discharge times and to calculate the PASS were consistent among all patients, which may mitigate some of the retrospective limitation. Another limitation is the lack of data surrounding PASS scores at arrival to the emergency department. As PASS is not a standard part of emergency department care at our institution, there was not enough detail in the retrospective charts to obtain this information uniformly for all patients. It is possible that some patients could have initially had a more severe PASS and then with treatment could have improved substantially before arriving to the PICU. Future studies will work to address this.
Our study finds that PASS ranges are associated with the use of critical care interventions. Patients that fall into the severe range were more likely to require a longer time on continuous albuterol as well as a longer time in the PICU. They were also more likely to require NIPPV as well as other medications typically reserved for severe status asthmaticus. Future prospective studies investigating PASS ranges as a triage tool would be needed to fully understand its utility in patients admitted to the hospital with asthma.
Asthma is the most common chronic condition of childhood and status asthmaticus is a very frequent admitting diagnosis in the pediatric intensive care unit (PICU). These patients come to the PICU because it can be very serious and life threatening, and some patients are sicker than others. At the authors’ institution, the decision of whether or not to increase or decrease support is based on subjective exam of the patient. In previous studies, asthma severity scores have been studied in the ER as to whether or not they can predict if a patient needs to be admitted or safely discharged. In their study, we look at whether or not an asthma severity score (PASS, or Pediatric Asthma Severity Score) is predictive of the need for critical care services, specifically length of time on continuous albuterol and length of time in the PICU.
There have been studies in the past looking at asthma severity scores and patient outcomes. Previous studies have mainly looked at using these scores in the emergency department and predicting whether or not a patient needs to be admitted or could be discharged. Healthcare is changing and many institutions are striving to standardize care, and using these scores could be another way to do so.
To their knowledge, this is the first study that has studied whether or not a patient’s asthma severity score (in this case, PASS) can be predictive of their need for critical care interventions. Their study found that if a patient fell into the severe group, he/she was more likely to be on continuous albuterol for longer and need the PICU longer. These patients were also more likely to require noninvasive positive pressure ventilation and be on aminophylline.
This study could be applied in the future in multiple ways. It could be used to help PICU physicians predict which patients will need more intensive treatment or could safely have treatment de-escalated. It could also potentially be used to decide whether or not a patient needs PICU admission or could be safely treated on a general floor.
PASS: Pediatric asthma severity score - a score that is determined by different variables (oxygen requirement, respiratory rate, work of breathing, retractions, auscultation) that “scores” how severe an asthma exacerbation is at that point in time.
The paper is well-written.
| 1. | Moorman JE, Rudd RA, Johnson CA, King M, Minor P, Bailey C, Scalia MR, Akinbami LJ. National surveillance for asthma--United States, 1980-2004. MMWR Surveill Summ. 2007;56:1-54. [PubMed] |
| 2. | Bisgaard H, Szefler S. Prevalence of asthma-like symptoms in young children. Pediatr Pulmonol. 2007;42:723-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 204] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 3. | Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States, 1980-2007. Pediatrics. 2009;123 Suppl 3:S131-S145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 629] [Cited by in RCA: 624] [Article Influence: 36.7] [Reference Citation Analysis (0)] |
| 4. | Hasegawa K, Tsugawa Y, Brown DF, Camargo CA. Childhood asthma hospitalizations in the United States, 2000-2009. J Pediatr. 2013;163:1127-33.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 103] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 5. | Lougheed MD, Garvey N, Chapman KR, Cicutto L, Dales R, Day AG, Hopman WM, Lam M, Sears MR, Szpiro K. The Ontario Asthma Regional Variation Study: emergency department visit rates and the relation to hospitalization rates. Chest. 2006;129:909-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 85] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Hartman ME, Linde-Zwirble WT, Angus DC, Watson RS. Trends in admissions for pediatric status asthmaticus in New Jersey over a 15-year period. Pediatrics. 2010;126:e904-e911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 64] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 7. | Ubel PA, Arnold RM. The unbearable rightness of bedside rationing. Physician duties in a climate of cost containment. Arch Intern Med. 1995;155:1837-1842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Bood K. Coping with critical illness: the child in the ICU. Nurs Crit Care. 1996;1:221-224. [PubMed] |
| 9. | Gorelick M, Scribano PV, Stevens MW, Schultz T, Shults J. Predicting need for hospitalization in acute pediatric asthma. Pediatr Emerg Care. 2008;24:735-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Gorelick MH, Stevens MW, Schultz TR, Scribano PV. Performance of a novel clinical score, the Pediatric Asthma Severity Score (PASS), in the evaluation of acute asthma. Acad Emerg Med. 2004;11:10-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Alnaji F, Zemek R, Barrowman N, Plint A. PRAM score as predictor of pediatric asthma hospitalization. Acad Emerg Med. 2014;21:872-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Farion KJ, Wilk S, Michalowski W, O’Sullivan D, Sayyad-Shirabad J. Comparing predictions made by a prediction model, clinical score, and physicians: pediatric asthma exacerbations in the emergency department. Appl Clin Inform. 2013;4:376-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Arnold DH, Gebretsadik T, Hartert TV. Spirometry and PRAM severity score changes during pediatric acute asthma exacerbation treatment in a pediatric emergency department. J Asthma. 2013;50:204-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 14. | Children’s Health Care of Colorado. Asthma Clinical Care Guidelines, 2014. Available from: http://www.childrenscolorado.org. |
| 15. | Rowe BH, Bretzlaff JA, Bourdon C, Bota GW, Camargo CA. Intravenous magnesium sulfate treatment for acute asthma in the emergency department: a systematic review of the literature. Ann Emerg Med. 2000;36:181-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 91] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 16. | Dalabih AR, Bondi SA, Harris ZL, Saville BR, Wang W, Arnold DH. Aminophylline infusion for status asthmaticus in the pediatric critical care unit setting is independently associated with increased length of stay and time for symptom improvement. Pulm Pharmacol Ther. 2014;27:57-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Mitra A, Bassler D, Goodman K, Lasserson TJ, Ducharme FM. Intravenous aminophylline for acute severe asthma in children over two years receiving inhaled bronchodilators. Cochrane Database Syst Rev. 2005;CD001276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 44] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 18. | Hutchings FA, Hilliard TN, Davis PJ. Heated humidified high-flow nasal cannula therapy in children. Arch Dis Child. 2015;100:571-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Pediatrics
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Al-Biltagi M, Pereira-Vega A, Wang HY S- Editor: Qiu S L- Editor: A E- Editor: Lu YJ