Published online Dec 9, 2025. doi: 10.5409/wjcp.v14.i4.109874
Revised: July 1, 2025
Accepted: September 10, 2025
Published online: December 9, 2025
Processing time: 160 Days and 22.4 Hours
Rett syndrome is a monogenic X-linked dominant condition that affects 1/(10000-15000) girls due to de novo mutations in the methyl-CpG binding protein 2 (MECP2) gene mapped to chromosome Xq28. The disease-causing gene was iden
In this study, we report an unusual and rare clinical presentation of Rett syn
This case, the first reported instance of Rett syndrome in Sudan, is of significant interest. The patient carries both the MECP2 gene mutation and the chromosome 15q22-qter deletion, which may explain the autistic behavior with atypical presentation of Rett syndrome. This report expands the genetic diversity of Rett syndrome, demonstrating how co-occurring 15q22-qter deletions can reshape MECP2-associated phenotypes in Rett syndrome.
Core Tip: This report presents a rare atypical case of Rett syndrome with a novel concomitant of a pathogenic methyl-CpG binding protein 2 p.S134F mutation and 15q22-qter karyotype from Sudan. The patient displayed atypical Rett syndrome phenotype, including significant growth regression and pronounced autistic behaviors, stereotypic hand-flapping and chest-pounding, and absence seizures. The terminal 15q22-qter deletion may disrupt critical neurodevelopmental loci (such as ubiquitin-protein ligase E3A and insulin-like growth factor 1 receptor), which could exacerbate autism and growth failure while potentially reducing susceptibility to seizures. This case expands the phenotypic and genotypic heterogeneity of Rett syndrome, enhances our understanding of how synchronous genomic alterations modulate methyl-CpG binding protein 2-related phenotypes, and underscores the imperative for equitable genetic diagnostics in underrepresented populations.
- Citation: Fadl-Elmula I, Abdel-Raheem SY, Khalid R. Atypical case of Rett syndrome with concurrent MECP2 gene mutation and del(15)(q22qter) karyotype: A case report and review of literature. World J Clin Pediatr 2025; 14(4): 109874
- URL: https://www.wjgnet.com/2219-2808/full/v14/i4/109874.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v14.i4.109874
Rett syndrome is a rare neurogenetic disorder that predominantly affects girls. It is characterized by a period of normal development followed by a significant progressive decline in motor skills, communication abilities, and purposeful hand movements[1]. The syndrome has an estimated prevalence of approximately 1 in 10000 to 15000 female births[2]. The disorder is primarily caused by a mutation in the methyl-CpG-binding protein 2 (MECP2) gene on chromosome Xq28. Mutations in the MECP2 gene disrupt neuronal function, leading to hallmark features of Rett syndrome, including seizures, motor deficits, neurogenic apneas, and speech delay[3,4]. While neurologic symptoms indeed constitute the most apparent aspects of Rett syndrome, these patients may also exhibit significant non-neurologic pathologies such as osteopenia, scoliosis, gastrointestinal dysfunction, and a general growth deficit[5].
Recent studies have highlighted the importance of global representation in understanding the phenotypic and genetic heterogeneity of Rett syndrome[2]. This highlights the need for enhanced documentation from underrepresented populations, particularly those from Africa and the Middle East. This will increase our ability to fully appreciate how environmental, ethnic, and genetic factors influence disease expression and outcomes. Previous studies observed maternal duplications of 15q11-q13 inherited in 1%-3% of children with autism spectrum disorder (ASD), suggesting that an abnormal dosage of the gene within this region, such as ubiquitin-protein ligase E3A (UBE3A) and gamma-ami
In this report, we present the first confirmed case of Rett syndrome in Sudan, as determined through cytogenetic analysis of the patient’s lymphocytes and complete sequencing of the MECP2 gene. The unique phenotypic findings, particularly the autistic features and absence of seizures, suggest a genetic interplay between MECP2 (p.S134F) and the 15q22-qter deletion, which may contribute to the unusual phenotypic spectrum of Rett syndrome observed in this report. Additionally, this case arose incidentally during routine clinical and genetic evaluation of a patient presenting with developmental regression and stereotypical behaviors, contributes uniquely to the understanding of Rett syndrome’s genetic and phenotypic diversity within underrepresented populations, such as Sudan, where genomic research and neurodevelopmental disorder reporting remain sparse, addressing geographical and genetic gaps in the current literature.
A 12-year-old female originally from North Sudan, ethnically from Rofeen (an Arabic origin tribe), was brought by her family complaining of abnormal walking, abnormal hand movement, loss of speech, and mental retardation for ten years.
The history revealed a gradual onset of the disease at the age of 2 years when the patient started to show abnormal walking and hand movement, loss of speech and acquired language, and regression in social communication. She re
Patient went through normal milestones till the age of 2 years.
She was the third offspring from a consanguineous marriage, as her parents were second-degree relatives. She was an outcome of an uneventful pregnancy, terminated by vaginal delivery at home. There was no family history of similar conditions, dysmorphic features, or other neurological illnesses.
On examination, she was conscious and attentive to her surroundings, and her vital signs were normal. She had stunted growth with preadolescent Tanner stage 1. Her body weight was 15 kg, and her height was 118 cm, both were below the 3rd centile. She had proportionated short stature with an upper/Lower segment ratio of 1:1. Although she had micro
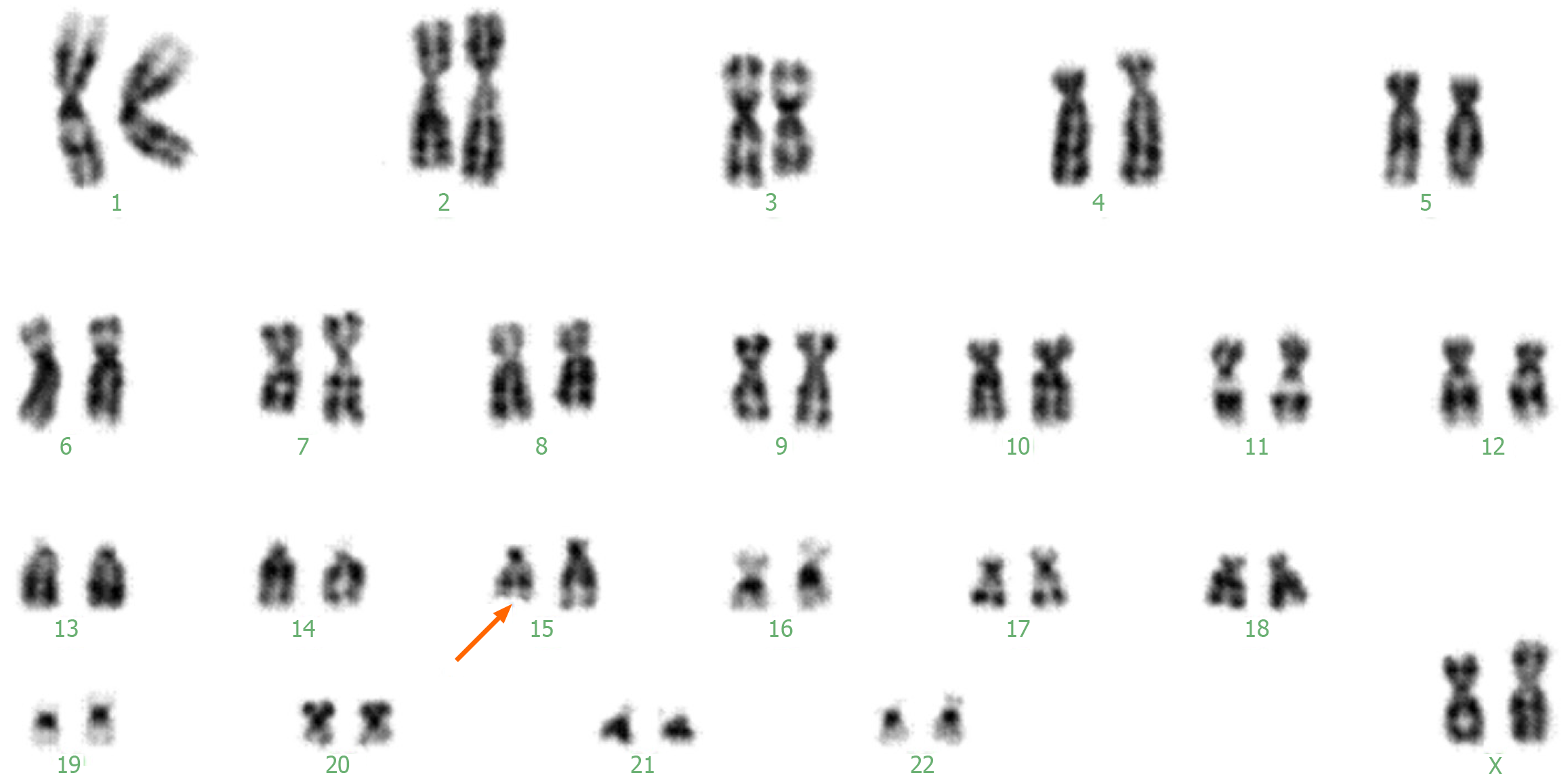
Investigations revealed normal hematological and urine analyses, liver and kidney function tests, and negative metabolic screening tests. Cytogenetic analysis revealed an abnormal female karyotype 46,XX, del(15)(q22qter) (Figure 2). MECP2 gene sequencing revealed a change cytosine > thymine at nucleotide 401, resulting in phenylalanine replacing a serine at amino acid position 134.
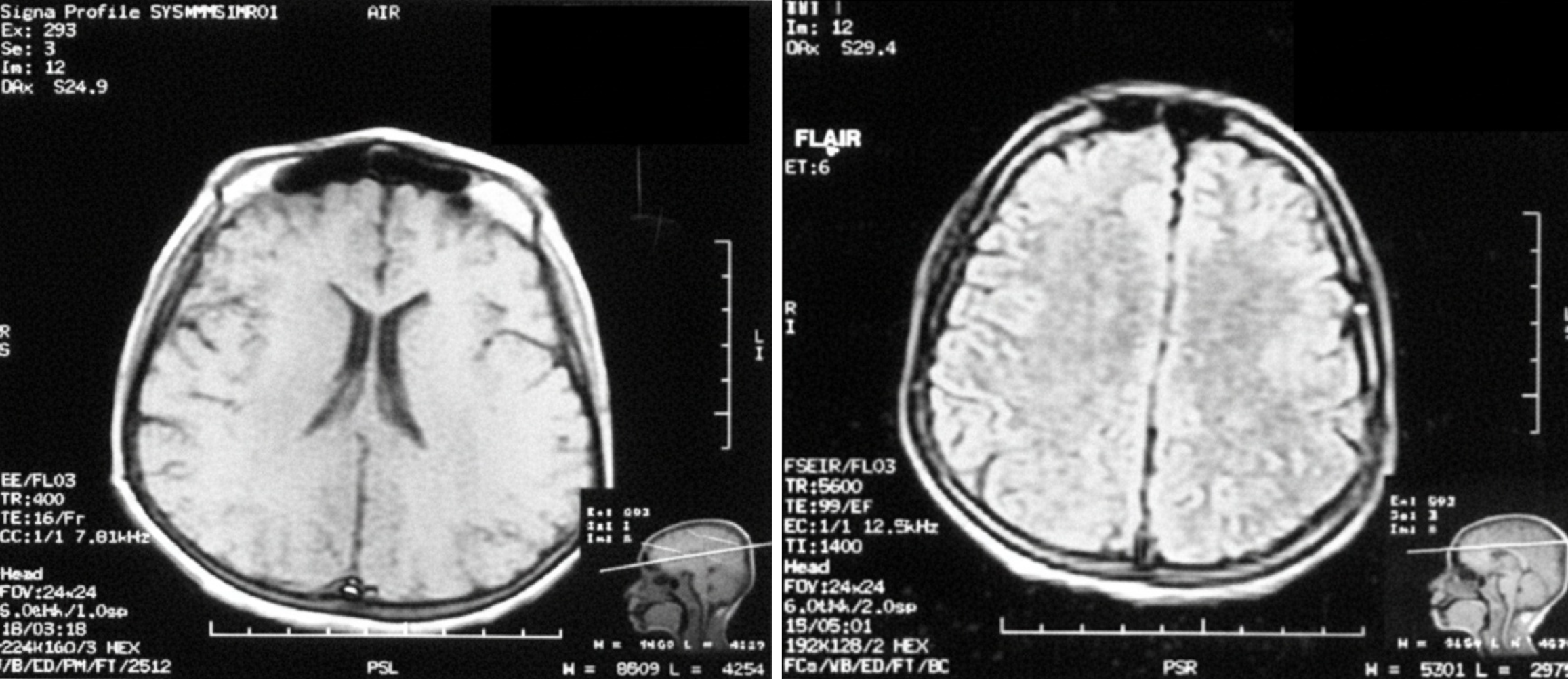
Both magnetic resonance imaging and electroencephalography results were normal (Figure 3).
Accordingly, a diagnosis of Rett syndrome was established.
After profound counseling, the patient was referred to a pediatric neurologist to supervise her management plan.
Regular visits were arranged to the multidisciplinary team at the tertiary hospital collaboratively to optimize patient care.
This case report details a 12-year-old girl diagnosed with Rett syndrome who carries both a pathogenic MECP2 mutation (p.S134F) and a deletion on chromosome 15q22-qter. She presents a combination of classic and atypical features. The patient demonstrated a typical Rett syndrome medical history characterized by normal development during the first 6 months to 18 months, followed by a regression in acquired skills, growth retardation, loss of speech, gait abnormalities, and intellectual disability[12]. While her developmental regression, speech loss, and gait issues correspond with classic months[13]. Her lack of seizures, hypotonia, and dysmorphic signs, along with noticeable autistic behaviors (such as repetitive hand flapping and chest pounding), indicate a potential phenotypic alteration linked to the 15q22-qter deletion.
In addition to neurological symptoms, Rett syndrome is recognized as a multisystem disorder that can present with growth failure characteristics, including short stature and growth stagnation, as observed in this patient. These features may relate to MECP2’s function in regulating mitochondrial activity and bone health. Recent studies emphasize the highly variable clinical pictures among patients carrying MECP2 variants, extending to males and mosaic individuals, further complicating genotype-phenotype correlations[14,15]. The existence of a terminal 15q22-qter deletion may worsen somatic comorbidities due to disrupted imprinting[16,17], where complex interactions between multiple genetic variants can lead to syndromic autism presentations[18].
Approximately 50 cases of 15q22-q24 deletion have been documented globally, sharing several characteristics such as hypotonia, feeding issues, and global developmental delays. These cases also exhibit similar dysmorphic traits, including a flat face, flat nasal bridge, epicanthic fold, micrognathia, microphthalmia, and minor skeletal and urogenital abnor
This case represents the first instance of a terminal 15q22-qter deletion and is among the few globally that involve a combination of genetic abnormalities. This may clarify the unusual movement patterns previously overlooked in Rett syndrome. However, some studies have noted cases of Rett syndrome with interstitial deletion or duplication of 15q11q22 that exhibit Prader-Willi-like features[20]. In contrast, our case displays a deletion karyotype of 15q22-qter and does not show this overlap, indicating different gene involvement and the typical dysmorphism and hypotonia associated with Rett syndrome syndrome[21]. The deletion at 15q22-qter may worsen features of Rett syndrome, likely by affecting neurodevelopmental genes (UBE3A, cytoplasmic FMR1 interacting protein 1) linked to ASD[22], which accounts for her ongoing autistic behaviors, differing from the temporary ASD-like traits observed in typical Rett syndrome[4].
The loss of insulin-like growth factor 1 receptor (15q26.3) could also aggravate growth failure by disrupting somatic growth regulation[23]. Furthermore, MECP2 is recognized for its role in regulating chromatin structure, while UBE3A (on 15q) manages synaptic protein degradation; their joint disruption may enhance neurodevelopmental deficits, suggesting potential epigenetic influences[24]. Prior research has highlighted common features between Rett syndrome and An
Our research highlights the crucial role of comprehensive genetic testing, encompassing chromosomal analysis, for individuals with neurodevelopmental disorders, notably when their clinical presentation deviates from the typical phenotype. It also highlights the need for greater awareness and research on Rett syndrome in underrepresented groups, such as Sudan, where access to advanced diagnostic resources may be restricted. Documenting cases from various populations enriches our understanding of Rett syndrome’s genetic and phenotypic diversity and supports initiatives to enhance global health equity[28]. In summary, this case report contributes to the growing body of research on the genetic diversity of Rett syndrome. It highlights the influence of additional genetic factors on its clinical manifestation. Further studies are needed to elucidate the molecular mechanisms underlying the interaction between MECP2 gene mutations and chromosomal abnormalities, as well as their impact on disease progression and outcomes. In instances involving 15q22-qter, the potential disruption of the UBE3A gene suggests possibilities for tailored therapy through UBE3A-targeted treatments (e.g., antisense oligonucleotides), which could alleviate ASD-related symptoms[29]. While insulin-like growth factor-1 supplementation may help resolve growth issues[30].
This case highlights a novel genetic interplay between the MECP2 (p.S134F) mutation and the 15q22-qter deletion, ex
| 1. | Henriksen MW, Breck H, Sejersted Y, Diseth T, von Tetzchner S, Paus B, Skjeldal OH. Genetic and clinical variations in a Norwegian sample diagnosed with Rett syndrome. Brain Dev. 2020;42:484-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Petriti U, Dudman DC, Scosyrev E, Lopez-Leon S. Global prevalence of Rett syndrome: systematic review and meta-analysis. Syst Rev. 2023;12:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 3. | Amir RE, Van den Veyver IB, Wan M, Tran CQ, Francke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3531] [Cited by in RCA: 3627] [Article Influence: 134.3] [Reference Citation Analysis (12)] |
| 4. | Gold WA, Percy AK, Neul JL, Cobb SR, Pozzo-Miller L, Issar JK, Ben-Zeev B, Vignoli A, Kaufmann WE. Rett syndrome. Nat Rev Dis Primers. 2024;10:84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 25] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 5. | Cronk JC, Derecki NC, Litvak V, Kipnis J. Unexpected cellular players in Rett syndrome pathology. Neurobiol Dis. 2016;92:64-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Mishra A, Prabha PK, Singla R, Kaur G, Sharma AR, Joshi R, Suroy B, Medhi B. Epigenetic Interface of Autism Spectrum Disorders (ASDs): Implications of Chromosome 15q11-q13 Segment. ACS Chem Neurosci. 2022;13:1684-1696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 7. | Nicholls RD. Genomic imprinting and candidate genes in the Prader-Willi and Angelman syndromes. Curr Opin Genet Dev. 1993;3:445-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 8. | Matsumoto A, Kumagai T, Miura K, Miyazaki S, Hayakawa C, Yamanaka T. Epilepsy in Angelman syndrome associated with chromosome 15q deletion. Epilepsia. 1992;33:1083-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Longo I, Russo L, Meloni I, Ricci I, Ariani F, Pescucci C, Giordano CT, Canitano R, Hayek G, Zappella M, Neri G, Renieri A, Gurrieri F. Three Rett patients with both MECP2 mutation and 15q11-13 rearrangements. Eur J Hum Genet. 2004;12:682-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Watson P, Black G, Ramsden S, Barrow M, Super M, Kerr B, Clayton-Smith J. Angelman syndrome phenotype associated with mutations in MECP2, a gene encoding a methyl CpG binding protein. J Med Genet. 2001;38:224-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 149] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Depienne C, Moreno-De-Luca D, Heron D, Bouteiller D, Gennetier A, Delorme R, Chaste P, Siffroi JP, Chantot-Bastaraud S, Benyahia B, Trouillard O, Nygren G, Kopp S, Johansson M, Rastam M, Burglen L, Leguern E, Verloes A, Leboyer M, Brice A, Gillberg C, Betancur C. Screening for genomic rearrangements and methylation abnormalities of the 15q11-q13 region in autism spectrum disorders. Biol Psychiatry. 2009;66:349-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 109] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 12. | Ellaway C, Christodoulou J. Rett syndrome: clinical characteristics and recent genetic advances. Disabil Rehabil. 2001;23:98-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Smeets EE, Townend GS, Curfs LMG. Rett syndrome and developmental regression. Neurosci Biobehav Rev. 2019;104:100-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Lötjönen J, Kurra V, Laivuori H, Bjelogrlić N. Broadening the Phenotype Spectrum of MECP2 Variants in Men. Mol Genet Genomic Med. 2025;13:e70056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Wen Y, Wang J, Zhang Q, Yang X, Wei L, Bao X. MECP2 germline mosaicism plays an important part in the inheritance of Rett syndrome: a study of MECP2 germline mosaicism in males. BMC Med. 2023;21:155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 16. | Pepe G, Coco R, Corica D, Luppino G, Morabito LA, Lugarà C, Abbate T, Zirilli G, Aversa T, Stagi S, Wasniewska M. Endocrine disorders in Rett syndrome: a systematic review of the literature. Front Endocrinol (Lausanne). 2024;15:1477227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 17. | Siegel MS, Smith WE. Psychiatric features in children with genetic syndromes: toward functional phenotypes. Pediatr Clin North Am. 2011;58:833-864. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Genovese AC, Butler MG. Behavioral and Psychiatric Disorders in Syndromic Autism. Brain Sci. 2024;14:343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 19. | Cushman LJ, Torres-Martinez W, Cherry AM, Manning MA, Abdul-Rahman O, Anderson CE, Punnett HH, Thurston VC, Sweeney D, Vance GH. A report of three patients with an interstitial deletion of chromosome 15q24. Am J Med Genet A. 2005;137:65-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 31] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 20. | Huppke P, Laccone F, Krämer N, Engel W, Hanefeld F. Rett syndrome: analysis of MECP2 and clinical characterization of 31 patients. Hum Mol Genet. 2000;9:1369-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 159] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 21. | Tang X, Kim J, Zhou L, Wengert E, Zhang L, Wu Z, Carromeu C, Muotri AR, Marchetto MC, Gage FH, Chen G. KCC2 rescues functional deficits in human neurons derived from patients with Rett syndrome. Proc Natl Acad Sci U S A. 2016;113:751-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 169] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 22. | Butler MG, Rafi SK, Manzardo AM. High-resolution chromosome ideogram representation of currently recognized genes for autism spectrum disorders. Int J Mol Sci. 2015;16:6464-6495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 53] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 23. | Walenkamp MJ, Wit JM. Single gene mutations causing SGA. Best Pract Res Clin Endocrinol Metab. 2008;22:433-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 24. | Krishnan V, Stoppel DC, Nong Y, Johnson MA, Nadler MJ, Ozkaynak E, Teng BL, Nagakura I, Mohammad F, Silva MA, Peterson S, Cruz TJ, Kasper EM, Arnaout R, Anderson MP. Autism gene Ube3a and seizures impair sociability by repressing VTA Cbln1. Nature. 2017;543:507-512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 100] [Cited by in RCA: 121] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 25. | Jedele KB. The overlapping spectrum of rett and angelman syndromes: a clinical review. Semin Pediatr Neurol. 2007;14:108-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 26. | Veenstra-VanderWeele J, Cook EH, King BH, Zarevics P, Cherubini M, Walton-Bowen K, Bear MF, Wang PP, Carpenter RL. Arbaclofen in Children and Adolescents with Autism Spectrum Disorder: A Randomized, Controlled, Phase 2 Trial. Neuropsychopharmacology. 2017;42:1390-1398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 117] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 27. | Young DJ, Bebbington A, Anderson A, Ravine D, Ellaway C, Kulkarni A, de Klerk N, Kaufmann WE, Leonard H. The diagnosis of autism in a female: could it be Rett syndrome? Eur J Pediatr. 2008;167:661-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 52] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 28. | Ruderman A. Population diversity and equity in the genomic era: going global to return to the local. J Community Genet. 2023;14:519-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 29. | Visser JC, Rommelse NN, Greven CU, Buitelaar JK. Autism spectrum disorder and attention-deficit/hyperactivity disorder in early childhood: A review of unique and shared characteristics and developmental antecedents. Neurosci Biobehav Rev. 2016;65:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 103] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 30. | Haider KH. Priming mesenchymal stem cells to develop "super stem cells". World J Stem Cells. 2024;16:623-640. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/