Published online Dec 9, 2025. doi: 10.5409/wjcp.v14.i4.107075
Revised: April 24, 2025
Accepted: June 10, 2025
Published online: December 9, 2025
Processing time: 231 Days and 7.5 Hours
Spigelian hernia (SH), a protrusion of intra-abdominal contents through a defect in the semilunar line (Spigelian fascia) of the abdominal wall, is extremely rare in the pediatric population. Fewer than 100 cases of pediatric SH have been reported in the literature since the first description in 1939. Pediatric SH is often congenital and may present with non-specific symptoms, making diagnosis challenging. Notably, about one-quarter to one-third of reported pediatric cases are associated with ipsilateral undescended testis, an association sometimes termed the "Spige
To systematically review all reported cases of Spigelian hernia in children and identify its diagnostic and surgical features.
A comprehensive literature search was performed (1939 through 2023) using PubMed and other databases for all publications on Spigelian (semilunar line) hernias in children. Both English and non-English articles were included. Case reports, case series, and relevant reviews were analyzed. Data extracted included patient demographics, hernia side/location, clinical features, imaging and intraoperative findings, coexisting conditions (particularly cryptorchidism), management (open vs laparoscopic repair), and outcomes.
A total of approximately 90 pediatric SH cases from 44 publications were identified. The median age at pre
Spigelian hernia in children is a rare but clinically important entity that should be considered in cases of unex
Core Tip: Spigelian hernia in children is an exceptionally rare and often misdiagnosed condition due to its transient nature and non-specific clinical presentation. This review provides the most comprehensive analysis to date, incorporating historical perspectives, anatomical insights, and modern diagnostic and surgical approaches. Ultrasound remains the primary diagnostic tool, while laparoscopy is emerging as an effective surgical option, particularly in cases with localization challenges. Increased awareness among pediatric surgeons and radiologists can enhance early detection, reduce complications, and improve patient outcomes.
- Citation: Shchapov NF, Kulikov DV, Viborniy MI, Bullikh PV, Keshishian ES, Degtyarev AS. Spigelian hernia in children: A systematic review. World J Clin Pediatr 2025; 14(4): 107075
- URL: https://www.wjgnet.com/2219-2808/full/v14/i4/107075.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v14.i4.107075
Spigelian hernia (SH) is a rare but clinically important form of abdominal wall herniation, especially in the pediatric population[1-5]. Characterized by protrusion through the semilunar line — the lateral border of the rectus abdominis — this condition poses diagnostic and therapeutic challenges due to its atypical location and variable presentation[1,4-6]. In children, SHs are often congenital and may go undetected until symptoms arise, sometimes as an emergency with incarceration or as an incidental finding during surgery for other conditions.
Despite nearly a century since the first pediatric cases were described[7], the condition remains underrecognized. The literature consists predominantly of isolated case reports and small series, with limited attempts at synthesizing data to guide clinical decision-making. The association between SH and undescended testis (UDT) has been noted in several reports[8-10], yet its frequency, anatomical basis, and practical implications remain poorly defined. Similarly, the role of laparoscopy in diagnosis and treatment, while growing, lacks robust analysis across case data.
Given these gaps, a comprehensive synthesis of existing evidence is urgently needed. This systematic review compiles and analyzes all accessible pediatric cases of SH published to date, with a focus on anatomical features, clinical pre
We conducted a systematic literature review to identify all reported cases of SH in the pediatric population. A comprehensive search was performed across multiple international and regional scientific databases, including PubMed, Scopus, Web of Science, MEDLINE, CyberLeninka, the Russian Science Citation Index, and the Central Scientific Medical Library. Search terms included combinations of "Spigelian hernia", "hernia of the semilunar line", "pediatric", "children", "congenital", "cryptorchidism", and "undescended testis". There were no restrictions on language or publication date. Articles in English, Russian, and other accessible languages were included if sufficient clinical data could be extracted. Additionally, we conducted manual searches of bibliographies, institutional archives, and historical literature to identify rare or non-indexed publications.
We included any publication (case report, case series, or review) that documented one or more cases of SH in patients aged 0-18 years. To focus on clinical details, only reports providing individual patient-level information (age, sex, clinical findings, management, and outcomes) were considered "full cases" for data analysis. Publications that did not provide original case details (for example, general review articles without new cases, or papers hypothesizing about SH without case data) were included only for background or anatomical discussions but not in the primary case analysis. Duplicate reports of the same case (if identified) were excluded. Specifically, three cases were excluded from the analysis: A report of surgical treatment of an intermuscular lipoma[11] and another of a misclassified inguinal hernia[12], both of which mimicked a SH clinically, as well as a case of ectopic testis located in the anterior abdominal wall without a true fascial defect at the semilunar line[13].
From each included case report or series, we extracted the following data: Patient demographics (age at diagnosis or surgery, sex), clinical presentation (symptoms, presence of an abdominal wall mass, any incarceration or strangulation event), diagnostic methods used [physical exam findings, imaging such as ultrasound, computed tomography (CT) or magnetic resonance imaging (MRI)], hernia characteristics (side/location of hernia, contents of hernia sac, relation to the inferior epigastric vessels), associated anomalies or syndromic features (particularly the presence of ipsilateral UDT or other congenital anomalies), surgical treatment (open vs laparoscopic approach, timing of repair, any special surgical maneuvers such as orchiopexy or use of mesh), and outcomes (intraoperative findings, complications, recurrence on follow-up, and any mortality). Two reviewers (from our author group) independently screened the literature and extracted data, resolving any discrepancies by consensus.
Given the rarity of the condition, a quantitative meta-analysis was not feasible; instead, we summarized the data descriptively. We tabulated the key characteristics of all pediatric SH cases and calculated simple proportions (for example percentage male, percentage with certain features). For continuous data such as age, we report mean and standard deviation and range. To explore the proposed association between Spigelian hernia and undescended testis, we specifically analyzed the subset of cases involving cryptorchidism, looking for patterns in anatomical findings (such as absence of the inguinal canal or gubernaculum as noted by authors of those cases). The findings of this systematic review are reported in accordance with PRISMA guidelines for comprehensive literature reviews.
Our search identified a total of 59 relevant publications on pediatric SH. Of these, 42 articles provided sufficient individual case data and were included in the detailed analysis of clinical cases. The remaining publications consisted of literature reviews or discussions of SH (including anatomical studies) without new case data; these were used to inform background and anatomical considerations. From the 42 case-bearing reports, we compiled 96 unique pediatric cases of SH spanning the period from 1935 to 2023. This represents the most comprehensive collection of pediatric SH cases to date, covering nearly 90 years of literature[1,5]. A summary of the reviewed publications and their clinical characteristics is presented in Table 1.
| Ref. | Age | Sex | Side | Associated pathology | Contents of the hernia | Procedure | Peculiarities |
| Scopinaro[7], 1935 | 6 days | Male | Left | N/O | Strangulated hernia - died | ||
| Landry[42], 1956 | 14 year | Male | Left | Sigmoid, omentum | Open | Trauma | |
| Hurlbut and Moseley[37], 1967 | 8 year | Male | Right | Colon | Open | Trauma | |
| Graivier and Alfieri[43], 1970 | 10 month | Male | Bilateral | Umbilical & bilateral inguinal hernia | Open | ||
| Herbert and Turner[44], 1973 | 7 year | Male | Left | Trauma | |||
| Constantino et al[45], 1974 | 8 year | Male | Left | Strangulated hernia | |||
| Houlihan[46], 1976 | 15 year | Male | Bilateral | Peptic ulcer of the stomach | |||
| Graivier et al[40], 1978 | 9 month | Male | Left | Inguinal hernia | Open | ||
| Podkamenev et al[47], 1986 | 10 month | Male | Right | Small intestine | Open | Strangulated hernia | |
| Bar-maor and Sweed[27], 1989 | 3 month | Male | Left | Conginental diaphragmatic hernia | Open | ||
| 5 year | Female | Right | N/O | Trauma | |||
| Grechanyĭ and Gruminskiĭ[48], 1990 | 9 month | Male | Right | Caecum, ascending colon and ileum | Open | Strangulated hernia | |
| Azuma et al[49], 1992 | 2 month | Female | Left | Meningocele | Open | ||
| Pul and Pul[1], 1994 | 18 month | Male | Right | Small intestine | Open | ||
| 2,5 month | Male | Right | Omentum | Open | |||
| Komura et al[16], 1994 | 6 month | Female | Right | Neuroblastoma | Open | ||
| 8 month | Female | Right | Neuroblastoma | N/O | |||
| Walton and Bass[28], 1995 | 7 month | Female | Left | Ovary | Open | ||
| 0 days | Female | Left | Conginental diaphragmatic hernia | N/O | |||
| Silberstein et al[35], 1996 | 2 month | Male | Left | Small intestine | Open | ||
| 4.5 month | Male | Right | Small intestine | Open | |||
| Beimanova[50], 1999 | 11 year | Male | Right | Small intestine, omentum | Open | Strangulated hernia | |
| Prokopenkoand Koval’chuk[51], 1999 | 8 year | Male | Right | Small intestine | Open | Trauma | |
| Al-Salem[2], 2000 | 3 month | Male | Left | Толстая кишка | Open | ||
| 7 days | Male | Left | Skeletal anomalies | Open | |||
| Bychkov et al[52], 2000 | 14 year | Male | Right | Caecum, appendix | Open | Strangulated hernia | |
| Losanoff et al[23], 2002 | 12 year | Male | Right | Omentum | Open | Strangulated hernia | |
| White[53], 2002 | 30 days | Female | Right | Bilateral inguinal hernia | Small intestine | Open | |
| Levy et al[25], 2003 | 30 days | Male | Bilateral | Small intestine | Open | Strangulated hernia | |
| 35 days | Male | Left | Small intestine | Open | |||
| Raveenthiran[8], 2005 | 55 days | Male | Right | Anal atresia, umbilical & inguinal hernia | Colon | Open | |
| Vaos et al[3], 2005 | 20 month | Male | Left | Small intestine, omentum | Open | Strangulated hernia | |
| Torres de Aguirre et al[54], 2005 | 26 days | Male | Right | Small intestine | Open | Strangulated hernia | |
| 40 days | Male | Bilateral | Small intestine | Open | |||
| O'Sullivan et al[55], 2006 | 4 month | Male | Left | Hypospadia | Open | ||
| Aksu et al[19], 2008 | 4 year | Female | Bilateral | Skeletal anomalies | Small intestine | Open | |
| Christianakis et al[6], 2009 | 6 year | Male | Left | Omentum | Open | Strangulated hernia | |
| Fascetti-Leon et al[56], 2010 | 0 days | Male | Bilateral | Skin aplasia | Open | ||
| Rushfeldt et al[30], 2010 | 16 days | Male | Right | Small intestine | Open | ||
| Lopez et al[57], 2010 | 14 year | Male | Left | Fat & vessels | Lap | Trauma | |
| Singal et al[58], 2011 | 3 year | Male | Right | Open | |||
| 3 month | Male | Left | Hypospadia | Open | |||
| Thakur et al[14], 2013 | 9 year | Male | Right | Small intestine | Open | Trauma | |
| Spinelli et al[4], 2014 | 14 year | Female | Right | Omentum | Open | ||
| Vega-Mata et al[29], 2015 | 13 year | Male | Right | Common bile duct stenosis | Lap | ||
| Sinopidis et al[20], 2018 | 6 month | Male | Left | Bilateral inguinal hernia | Small intestine | Open | |
| Khan and Islam[32], 2018 | 9 month | Male | Right | Lap-ass | |||
| Sengar et al[34], 2018 | 4 year | Female | Right | Fat & vessels | Open | ||
| 4 year | Male | Left | Small intestine | Open | |||
| 30 days | Female | Left | Omentum | Open | |||
| 30 days | Male | Right | Umbilical, lumbar hernia | N/O | |||
| Deshmukh et al[33], 2019 | 11 month | Male | Left | Lap | |||
| Rashi[39], 2019 | 18 month | Male | Right | Lap | |||
| Taha et al[5], 2021 | 50 days | Male | Right | Small intestine | Open | ||
| Kropilak and Sawaya[40], 2022 | N/A | N/A | Open | Trauma | |||
| Shchapov et al[25], 2022 | 6 month | Female | Right | Hydronephrosis | Lap | Strangulated hernia |
Among the 96 pediatric SH cases, male patients were the majority, with 73 boys (76% of cases with known sex) and 22 girls (23%); one case did not specify the child’s sex. The age at presentation or surgery ranged from newborn (including a few cases detected in the neonatal period) up to 17 years old, with a mean age of approximately 3.6 ± 4.0 years. Over half of the reported cases presented in infancy or early childhood (under 2 years of age), though a significant number were diagnosed in older children as well. In many instances, the hernia was noted to be congenital in origin – indeed, in the pediatric population, SHs are generally considered a congenital defect of the abdominal wall[1,2]. Only a small fraction of cases was attributed to trauma or other acquired causes. We identified 9 cases (9.4%) where a traumatic etiology was clearly described – these typically involved blunt abdominal injury leading to a sudden hernia appearance, such as bicycle handlebar impacts or falls[14,15]. The vast majority (about 90%) had no history of trauma and were presumed to result from an inherent fascial defect present from birth[16,17].
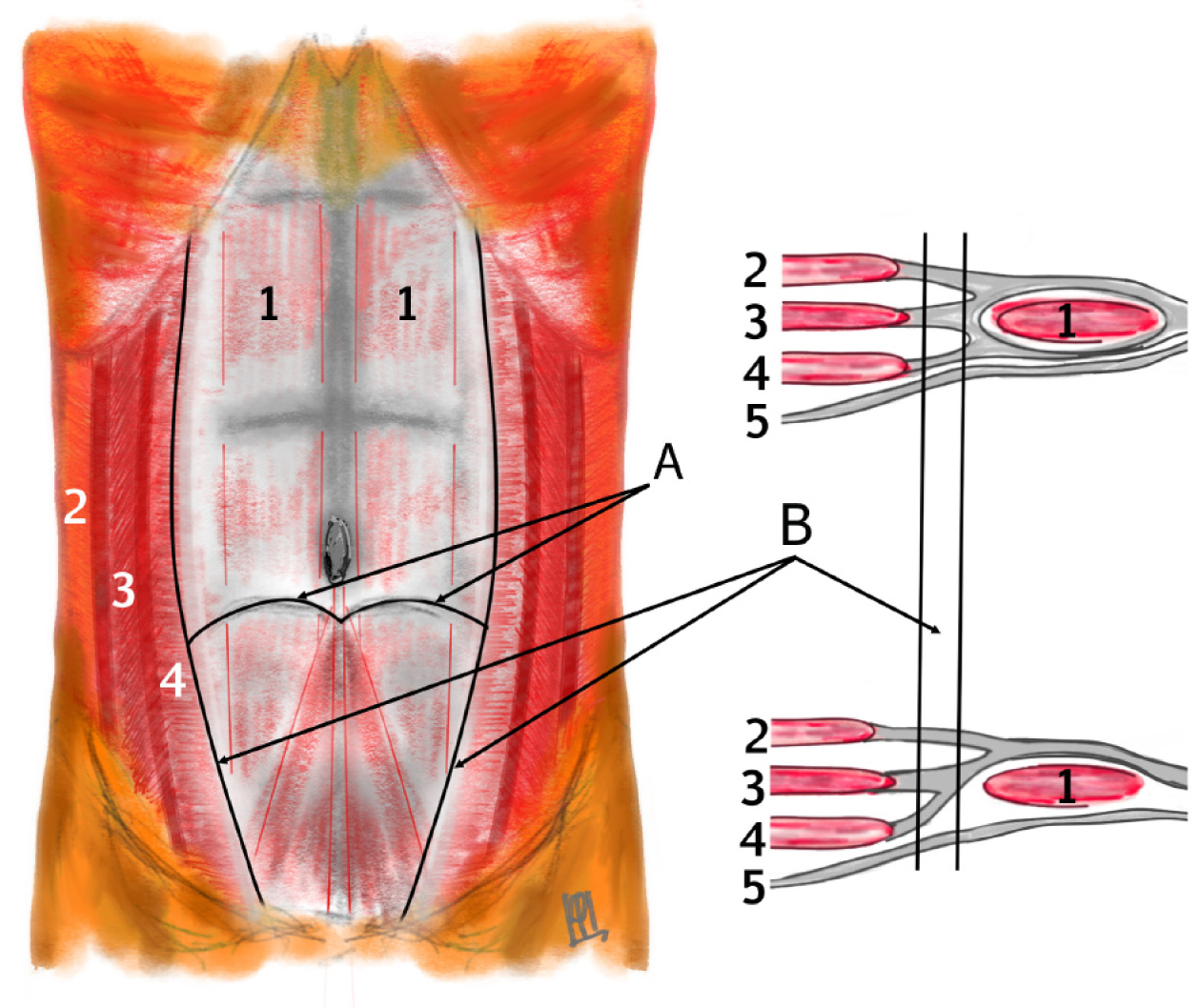
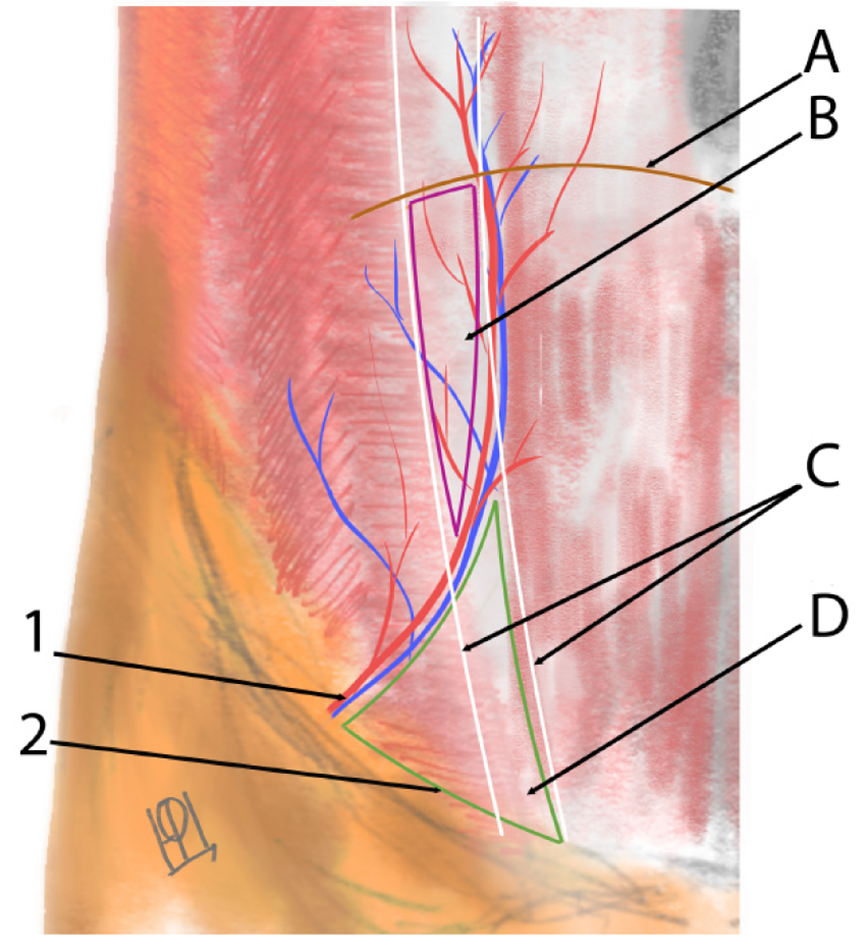
The anatomical location of the hernia along the semilunar line below the level of the arcuate line (semicircular line of Douglas), where the transversus abdominis aponeurosis transitions and the posterior rectus sheath is deficient (Figure 1)[18]. In our compiled data, the lateralization of hernias was roughly balanced, with a slight right-side predominance among unilateral hernias. Specifically, of the total cases, about 55% were right-sided and 45% left-sided when considering only unilateral occurrences. A notable portion of children (approximately 10%-15% of cases) presented with bilateral SHs, meaning defects on both sides of the abdominal wall[19,20]. Some bilateral cases were synchronous (both hernias detected around the same time), while in other instances the contralateral hernia was discovered incidentally during surgery or on follow-up. Overall, the distribution of hernia laterality can be summarized approximately as right: Left: Bilateral approximately equal to 3:2.6:1, consistent with prior observations[5]. The position of the hernia relative to the inferior epigastric vessels was noted when imaging or surgical descriptions were available: The majority of pediatric SH defects are located above and lateral to the inferior epigastric vessels (the "classical" Spigelian hernia location), though a minority occur below these vessels in the Hesselbach’s tringle (Figure 2)[3,21]. These latter cases represent the so-called "low Spigelian hernia", which can clinically mimic an inguinal hernia[6,12]. Indeed, a few children in the literature were initially misdiagnosed with incarcerated inguinal hernias when in fact the hernia defect was in the Spigelian fascia[6].
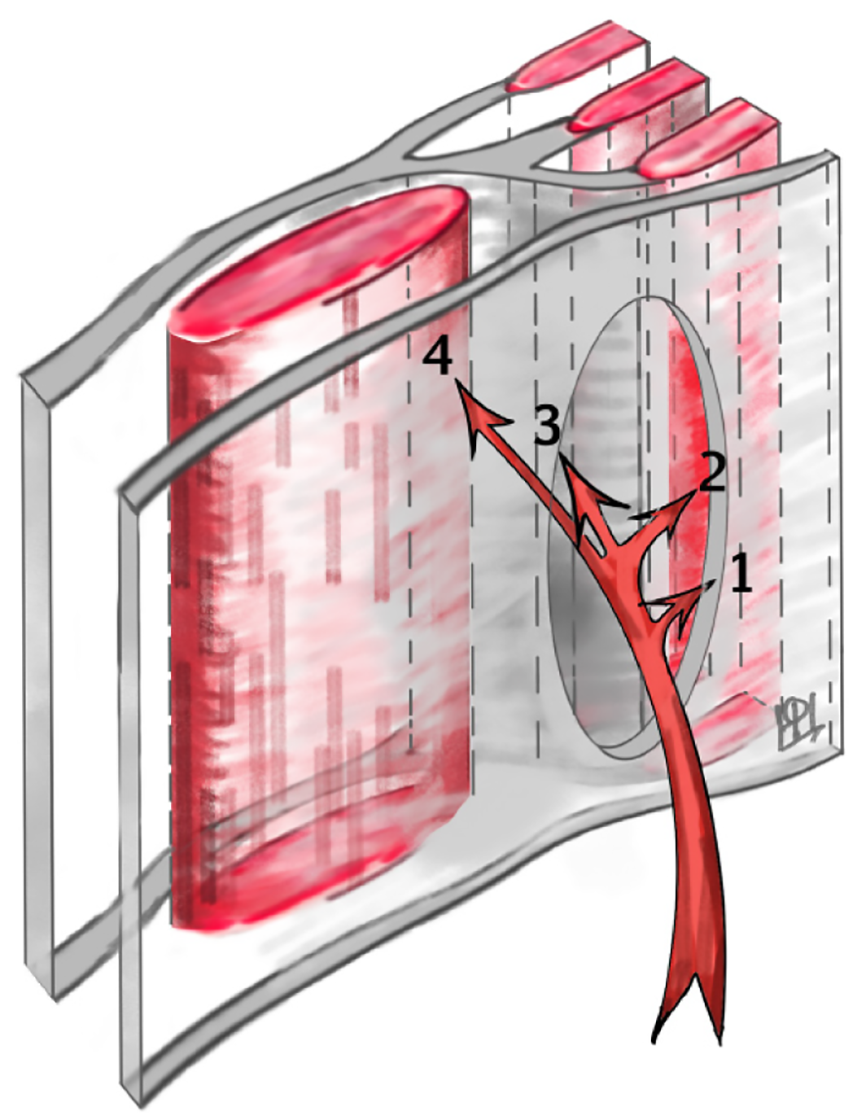
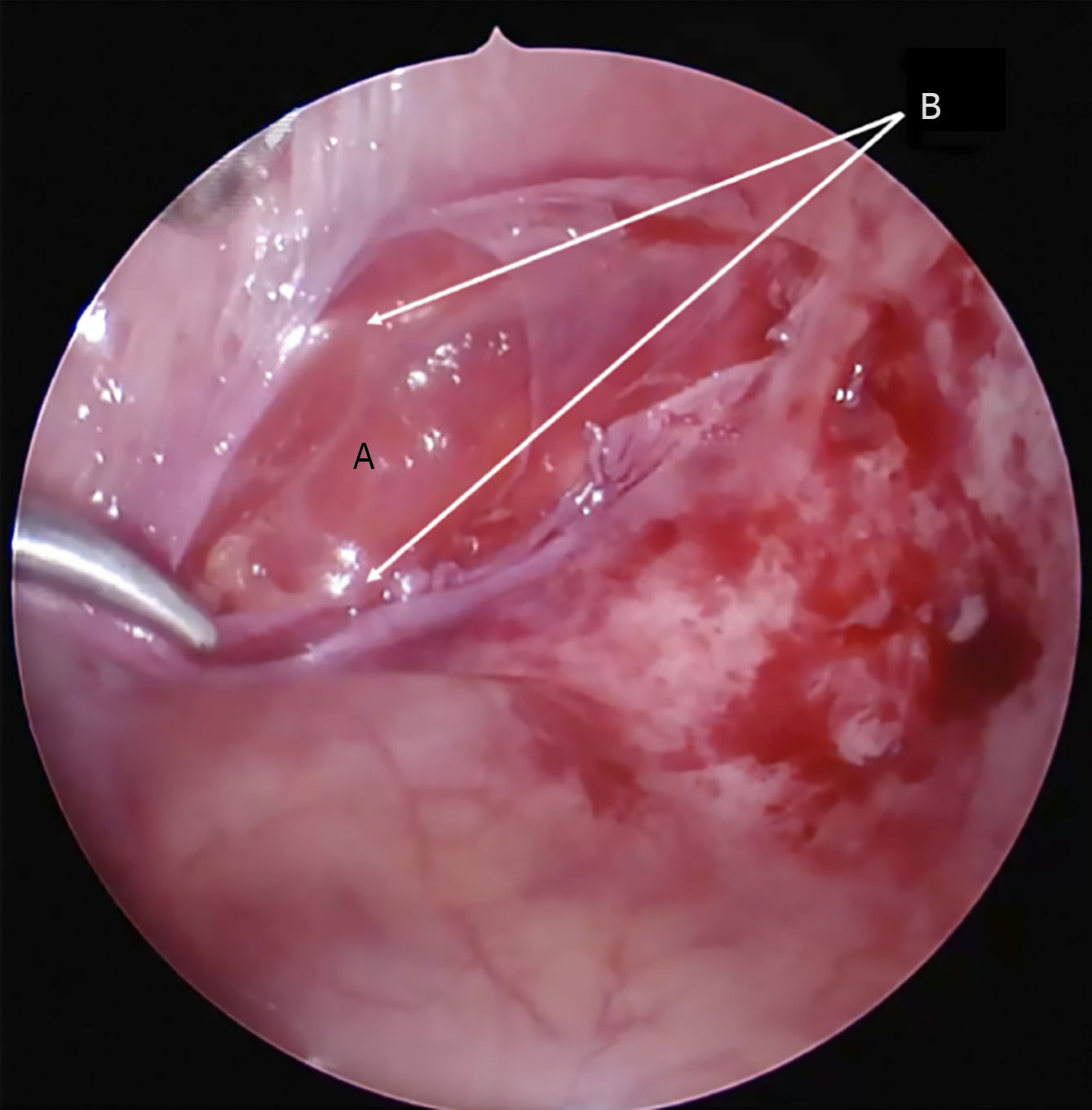
Intraoperatively, contents within the hernial sac (aside from an UDT, when present) were observed in 32 patients (33%). The most common content was a loop of small intestine (18 cases), with other reported contents including omental tissue, the dome of the cecum with the appendix, segments of the colon (such as portions of the ascending or sigmoid colon), an ovary, or preperitoneal fat with associated vasculature. Additionally, Komura et al[16] reported histopathological evidence of atrophy and fatty infiltration in the transversus abdominis and internal oblique muscles adjacent to the hernia site. These muscular changes suggest that the hernia sac can dissect through the weakened muscle layers rather than through a discrete fascial defect. In some instances, the sac may lie beneath the external oblique aponeurosis or even extend beyond it into the subcutaneous tissue (Figure 3).
Symptoms and signs: The clinical presentation of SH in children is often subtle and can be highly variable, contributing to diagnostic delays. A palpable mass on the anterior abdominal wall was the most commonly reported finding, present in the majority of cases where the child was examined during a protrusion episode[1,2]. Typically, this mass is located along the lateral edge of the rectus abdominis muscle, anywhere from just above the level of the umbilicus down to the level of the inguinal region. Parents or clinicians often noticed a soft bulge that comes and goes, becoming more prominent when the child cries, strains, or coughs, and disappearing when the child is calm or lying flat[15]. This intermittent nature was especially noted in infants – in several reports, caregivers described a transient lump that could reduce spontaneously or with gentle pressure[1]. In older children who could report symptoms, localized pain was a frequent complaint: Many described mild discomfort in the lower quadrant or lateral abdominal area, sometimes exacerbated by standing or physical activity and relieved by rest[21]. Adolescents with SH, for instance, have presented with chronic intermittent pain and only a subtle bulge[4].
Importantly, episodes of acute pain with signs of bowel obstruction have occurred in this population when the hernia became incarcerated. About 11% of the collected cases experienced strangulation of the hernia contents at presentation (based on our literature analysis), manifesting as a surgical emergency[5]. Signs of strangulation or incarceration in infants included inconsolable crying, tender firm mass at the semilunar line that does not reduce, abdominal distension, bilious vomiting, and in some cases, features of intestinal obstruction such as feeding intolerance or absence of bowel movements[3,8]. Several authors have noted indirect or nonspecific symptoms that should raise suspicion of SH: Episodic irritability, colicky abdominal pain, unexplained fussiness, or feeding difficulties in an infant that resolve and recur[1,8]. Some reported cases had bloating, bilious vomiting, or alternating diarrhea and constipation in a toddler, which initially led clinicians to suspect gastrointestinal disorders before the true cause was found[5]. It has been emphasized that any child with recurrent, unexplained abdominal pain should undergo a careful examination for a possible SH, even if a mass is not obvious[22]. In our review, laboratory tests were generally unremarkable in these patients – inflammatory markers were not elevated unless bowel strangulation had progressed to necrosis, and routine bloodwork was typically normal[1,8].
One distinctive feature of pediatric SH is the transient nature of the herniation. Unlike in adults, where an abdominal wall hernia tends to produce a consistently palpable protrusion, in children the hernia sac (which is essentially peritoneum) can slip in and out of the fascial defect. Prior to being "fixed" by adhesions or incarceration, the herniating loop or organ may only bulge intermittently. Several authors have described this as a "vanishing bulge" phenomenon[2,21]. For this reason, the physical exam can be misleadingly normal if the child is relaxed or the hernia has reduced spontaneously. It is recommended to examine young infants both in calm states and during crying or straining[1]. If the initial examination is negative but clinical suspicion persists (for example, due to episodic pain), repeating the exam at a different time or having the parents photograph the lump when it appears can be helpful[22]. Indeed, one report noted that a careful re-examination was needed to finally palpate a tiny fascial gap after an infant had been seen multiple times for colic[1].
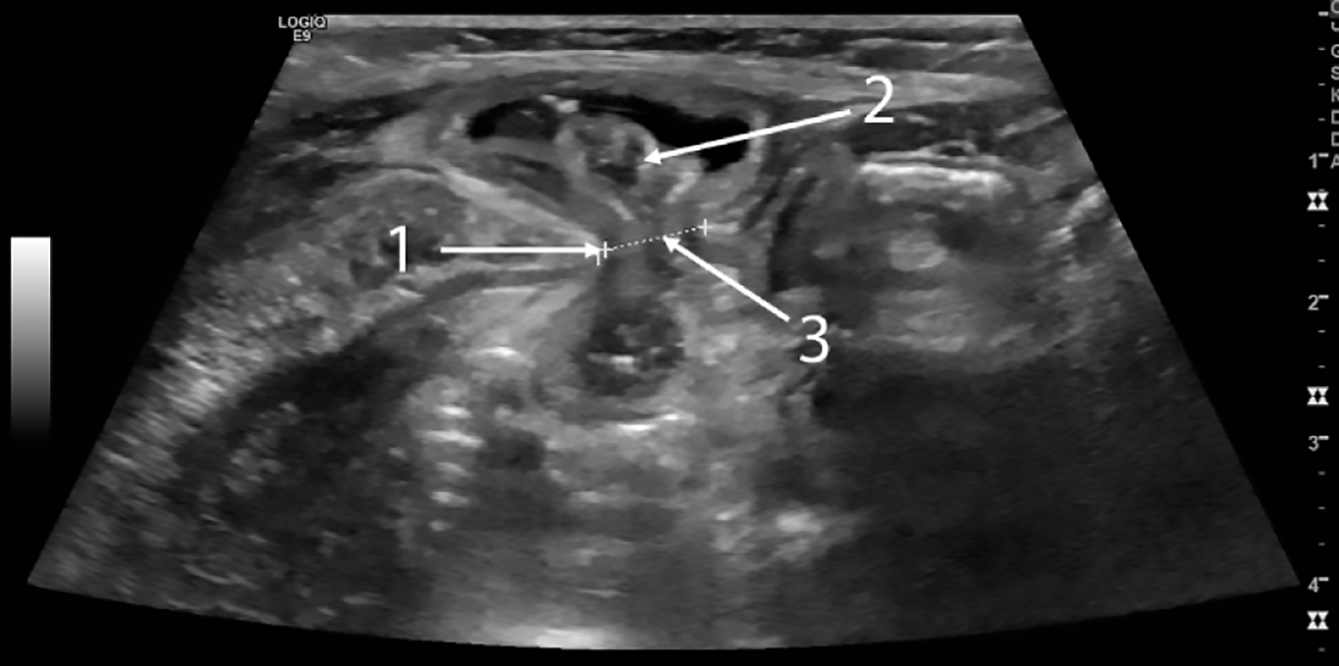
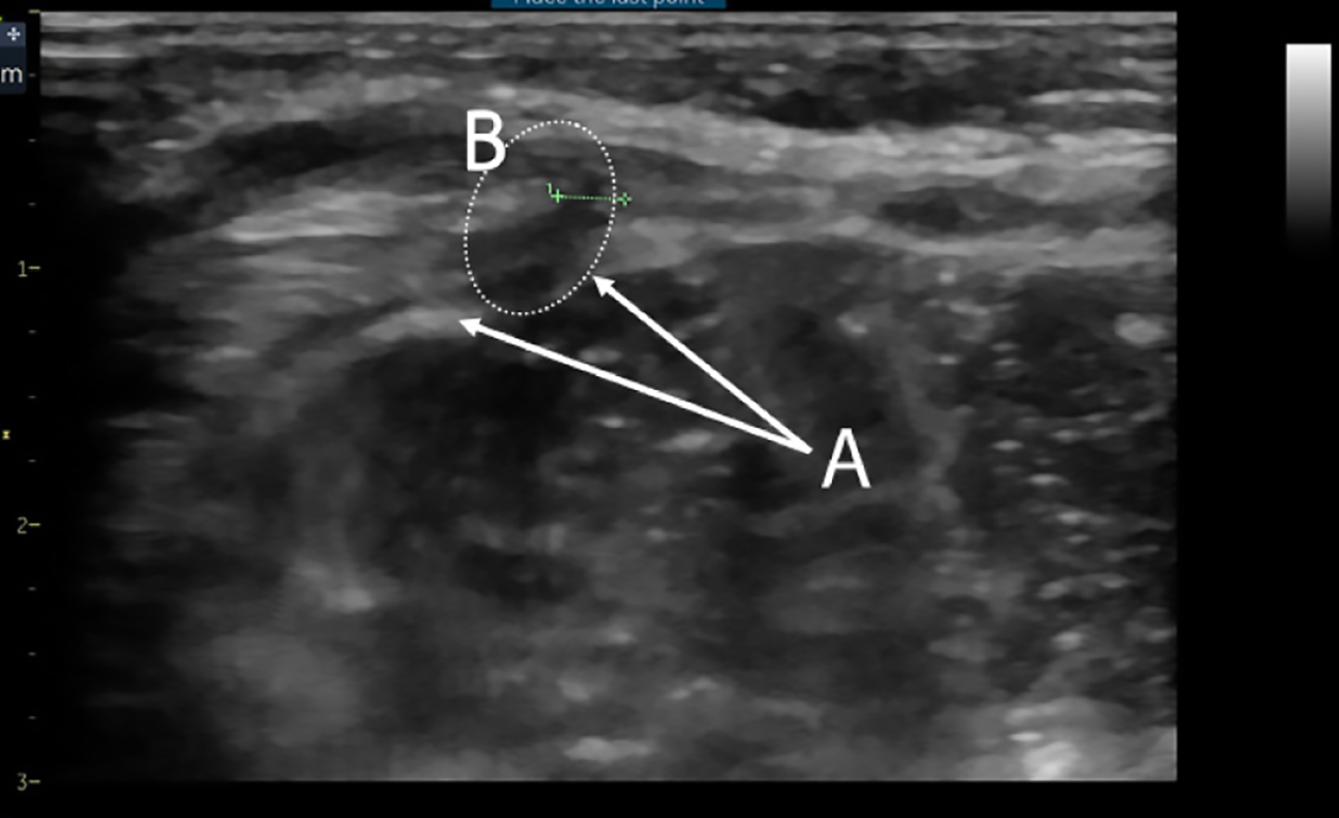
Imaging and diagnosis: Given the subtle exam findings, imaging studies play a crucial role in diagnosing SH in children. Across the cases, ultrasonography emerged as the most commonly used and most useful diagnostic modality[23,24]. Ultrasound is considered the gold standard initial investigation for suspected SH in pediatric patients[5]. High-resolution ultrasound can visualize the abdominal wall layers and detect defects in the fascia. In cases where the hernia is not externally obvious, sonographers have identified discontinuity in the transversus abdominis muscle and transversalis fascia with a small amount of fat or bowel protruding, confirming the diagnosis (Figure 4)[25]. For example, Levy et al[26] reported a neonatal SH in which ultrasound clearly showed a loop of bowel herniating through a defect near the semilunar line, concurrently visualizing the ipsilateral UDT in the hernia sac. Even when the hernia sac is empty at rest, ultrasonography may show subtle signs: A focal area of muscle discontinuity or thinning, often with a hypoechoic (dark) gap in the normally echogenic fascial line, and sometimes a localized area of altered echotexture in the adjacent muscle due to the intermittent passage of abdominal contents (Figure 5)[25]. Some authors describe this as a "window" or weak spot-on ultrasound, which correlates with the site of the defect[24]. Dynamic ultrasound, performed while increasing intra-abdominal pressure (crying or Valsalva in older kids), can catch the hernia protruding in real time.
Other imaging modalities were employed less frequently. CT scans have diagnosed pediatric SH, especially in ambiguous cases or when ultrasound results were inconclusive[15]. CT can delineate the muscular defect and any herniated organ with high precision; however, due to radiation concerns in children, CT is not first-line and was usually done only when the presentation mimicked other acute conditions (for example, appendicitis) or when the hernia was discovered incidentally on a scan for something else[12]. MRI is rarely indicated but has been used in a few instances, particularly if other anomalies were being evaluated simultaneously[9]. In one teenage patient, an MRI helped confirm a SH containing fat, distinguishing it from a possible soft tissue tumor[4]. The key to radiologic diagnosis is that the interpreter must specifically look for the abdominal wall defect; several reports caution that failure to recognize the fascia opening on imaging can lead to missing the diagnosis[1]. In summary, a combination of a high index of suspicion and appropriate imaging (ultrasound being the modality of choice) is usually required to confirm pediatric SH.
One objective of this review was to catalog other anomalies or conditions associated with SH in children. Excluding cryptorchidism (which we address separately as a syndrome), there were 19 cases (20% of the total) in which an additional congenital anomaly or noteworthy co-morbidity was present. The most frequent co-occurring conditions were other types of hernias in the abdominal region, suggesting a generalized weakness of fascial structures in some patients. Specifically, a number of children had concurrent inguinal hernias (either simultaneously or at some point in their course)[20], and a few had umbilical hernias as well[9]. There was even one infant reported with both bilateral inguinal hernias and a SH, all presenting in early infancy[20]. Such cases underscore that multiple hernia defects can coexist, and a thorough examination of the entire abdominal wall is warranted once one defect is found.
Beyond hernias, reported anomalies included two cases of congenital diaphragmatic hernia (CDH) occurring in tandem with SH. In one neonate, the CDH was repaired soon after birth, and the SH (which had been noted on imaging) was electively repaired later, once the child stabilized[27]. Another infant’s SH actually resolved on its own during recovery from CDH repair, suggesting that the abdominal wall defect may have closed as the infant grew[28]. One child had skeletal anomalies –multiple skeletal deformities and fibular aplasia, hinting at a broader developmental disturbance in mesodermal tissues[2]. Other rare associations included genitourinary anomalies like hypospadias reported in two cases, and hydronephrosis in one infant[25]. Additionally, one case involved an anorectal malformation (anal atresia) alongside the SH[8], and in one case common bile duct stenosis was present[29]. Perhaps most intriguing was the observation of neonatal tumors in two cases: Komura et al[16] reported that in their series of three infants with SH, two of the infants also had a mediastinal neuroblastoma on the same side as the hernia. This raised questions about whether increased intra-abdominal pressure from the tumor or some embryologic linkage could be at play. However, no causal relationship between neuroblastoma and SH has been proven. These assorted associations do not point to a single syndrome (except for the cryptorchidism, discussed below), but they do suggest that some children with SH have a broader predisposition to defects in the body wall or mesodermal-derived structures.
One of the most striking associations in pediatric SH is its link with ipsilateral UDT in males. Our review confirms that this combination is relatively common: Out of the 73 boys in our series, 34 (47%) had an UDT on the same side as the hernia. In all such cases, the testis was found within the hernia sac, rather than along the normal inguinal canal route. This clinico-anatomical entity has been termed Spigelian Hernia-Undescended Testis syndrome, or Spigelian-cryptorchidism syndrome, by various authors[8,30]. The SH-UDT association was first highlighted by Raveenthiran[8] in 2005, who postulated it might represent a distinct syndrome wherein the developing testis fails to descend normally because the hernia defect offers an abnormal exit path. Since then, multiple case reports and small series have supported the idea that this is more than coincidence[2,9,31].
From the 34 cases we gathered, several consistent patterns emerge (Table 2). Almost all were younger than 2 years at presentation, with many diagnosed in infancy (several at neonatal age). The SH in these patients was invariably on the same side as the UDT (most commonly left side in some series, although our compiled cases did not show a strong left/right dominance specific to the syndrome). Intraoperative findings often described an absent or underdeveloped inguinal canal on the affected side – in other words, the normal pathway through which the testis would descend into the scrotum was not formed[5,30]. We found documentation in 24 cases regarding the inguinal canal or/and gubernaculum. In 16 cases, the inguinal canal was explicitly noted to be absent or closed, whereas in only one case was a normal inguinal canal reportedly present (the remaining had no comment, likely not assessed or not visualized)[9,30]. Similarly, the gubernaculum testis was reported as missing or abnormally attached in 13 out of 24 cases where this was assessed, the gubernaculum was absent, and the testis was instead attached to the hernia sac with one side. In one case, the gu
| Ref. | Year | Age | Side | Contents of the hernia | Inguinal canal | Gubernaculum | Procedure | Complication |
| Graivier et al[41], 1978 | 6 months | Right | Testicle | Open with orchidopexy | ||||
| 9 months | Left | Testicle | Open with orchidopexy | |||||
| Podkamenev et al[47], 1986 | 10 months | Right | Testicle, small intestine | Open with orchidopexy and bowel resection | Strangulated hernia | |||
| Grechanyĭ and Gruminskiĭ[48], 1990 | 9 months | Right | Testicle, colon & small intestine | Absent | Open with orchidopexy | Strangulated hernia | ||
| Pul and Pul[1], 1994 | 18 months | Right | Testicle, small intestine | Open with orchidopexy | ||||
| Silberstein et al[35], 1996 | 70 days | Left | Testicle, small intestine | Absent | Open with orchidopexy | |||
| 4.5 months | Right | Testicle, small intestine | Absent | Absent | Open with orchidopexy | |||
| Ostlie and Zerella[59] | 1998 | 5 month | Right | Testicle | Absent | Absent | Open with orchidopexy | |
| Al-Salem[2] | 2000 | 3 months | Left | Testicle, colon | Absent | Absent | Open with orchidopexy | |
| 7 days | Left | Testicle | Open with orchidopexy | |||||
| Levy et al[26] | 2003 | 30 days | Bilateral | r – testicle, small intestine, l - testicle | Open with orchidopexy | |||
| 35 days | Left | Testicle, small intestine | Open with orchidopexy | |||||
| Raveenthiran[8] | 2005 | 55 days | Right | Testicle, colon | Absent | Open with orchidopexy | ||
| Torres de Aguirre et al[54] | 2005 | 26 days | Right | Testicle, small intestine | Absent | Absent | Open with orchidopexy | Strangulated hernia |
| 40 days | Bilateral | r – testicle, small intestine, l – Testicle | Open with orchidopexy | |||||
| Durham and Ricketts[36] | 2006 | 8 months | Left | Testicle | Absent | Absent | Open with orchidopexy | Scrotal abscess |
| 13 months | Bilateral | Testicle | Absent | Absent | Open with orchidopexy | |||
| 14 months | Bilateral | Testicle | Absent | Absent | Open with orchidopexy | |||
| 2 months | Right1 | Testicle | Absent | Absent | Open with orchidopexy | |||
| Rushfeldt et al[30] | 2010 | 16 days | Right | Testicle, small intestine | Absent | Open with orchidopexy | ||
| Singal[58] | 2011 | 3 year | Right | Testicle | Open with orchidopexy | |||
| 3 months | Left | Testicle | Open with orchidopexy | |||||
| Bilici et al[31] | 2012 | 6 months | Bilateral | Testicle | Open with orchidopexy | |||
| 1 year | Right | Testicle | Absent | Absent | Open with orchidopexy | |||
| 2 years | Left | Testicle | Open with orchidopexy | |||||
| 5 years | Left | Testicle | Open with orchidopexy | |||||
| Inan et al[60], 2012 | 20 days | Right | Testicle | Absent | Absent | Open with orchidopexy | Scrotal abscess & testicular atrophy | |
| Khan and Islam[32] | 2018 | 9 months | Right | Testicle | Absent | Present | Lap-ass with orchidopexy | |
| Sengar et al[34] | 2018 | 2, 3 years | Right | Testicle | Open with orchidopexy | |||
| 2 years | Right | Testicle | Open with orchidopexy | |||||
| 1, 6 years | Right | Testicle | Open with orchidopexy | |||||
| 6 days | Left | Testicle | Open with orchidopexy | |||||
| Deshmukh et al[33] | 2019 | 11 months | Left | Testicle | Lap with orchidopexy | |||
| Rashi[39] | 2019 | 18 months | Right | Testicle | Present | Lap with orchidopexy | ||
| Taha et al[5] | 2021 | 50 days | Right | Testicle, small intestine | Open with orchidopexy | Testicular retaraction & atrophy |
The prevailing theory explaining the SH-UDT syndrome is that a congenital defect in the transversalis fascia (such as the SH) occurs early in fetal development[8,30]. Normally, the testis descends from near the kidney down into the scrotum between approximately 24-28 weeks’ gestation, guided by the gubernaculum through the inguinal canal. If the inguinal canal fails to develop (perhaps as a result of the same developmental mishap that produced the Spigelian defect), the testis has no defined path to the scrotum. It may then remain in the abdomen. Some authors suggest that the presence of the SH itself may draw the testis toward it or at least provide a point of herniation that "distracts" from inguinal canal formation[30]. Our findings support that many of these infants indeed lack an inguinal canal and have the testis abnormally sited. In statistical terms, we observed a positive association but not a perfect correlation – in fact, about half of male SH patients did not have cryptorchidism, and conversely, a typical UDT in a boy usually does not imply a SH (cryptorchidism is much more common on its own). Thus, this syndrome likely represents a subset of cryptorchidism cases with a specific concomitant fascia defect.
Clinically, the recognition of SH-UDT syndrome is important because it changes the management. In a baby boy with an UDT that cannot be felt in the groin, one might not suspect a hernia initially; however, if there is also a noticeable swelling on the lateral side of the abs, the two should be connected. Conversely, if an infant is diagnosed with a SH, the examining surgeon should always check the scrotum for the presence of the testicle on that side[2,5]. If the scrotum is empty, imaging (ultrasound) can be used to see if the testis is perhaps within the hernia sac or in the abdomen. In our review, some cases were first identified due to the combination of an empty hemiscrotum and an abdominal wall lump, prompting a laparoscopic exploration that found both issues[32,33]. Overall, our synthesis supports the notion that SH with ipsilateral UDT is a real, syndromic condition, albeit with a spectrum of presentations. Nearly half of pediatric SH cases in males involve this syndrome, yet the conditions are not entirely interdependent[9,21]. Further discussion of management considerations for this syndrome is provided in the Discussion section.
Conservative vs surgical management: Due to the significant risk of incarceration, the consensus in the literature is that all SH in children should be treated surgically once identified[2,23]. In our compiled series of 96 cases, 91 children (95%) underwent surgical repair of the hernia. Only five patients did not receive an operation. In three of those, the authors reported that the hernia appeared to resolve spontaneously on follow-up[15,16,27,28]. One child was being observed and had not yet reached surgery at the time of report[34]. Unfortunately, the remaining case was a tragic exception – one infant died due to complications of an incarcerated SH, representing the only mortality in the literature directly attributed to pediatric SH (this occurred because the diagnosis was missed initially and strangulation led to bowel necrosis)[7]. This fatality underscores the importance of early recognition and intervention.
There were a few instances of deliberate delay in surgical repair under special circumstances. For example, in one report an infant with a large congenital diaphragmatic hernia had an accompanying SH; surgeons chose to fix the life-threatening diaphragmatic defect first and postponed the SH repair to a later date to reduce operative time and stress on the baby[28]. In another case, a premature infant had both bilateral inguinal hernias and a SH; the inguinal hernias were repaired at 2 months of age, while the SH was left until the child was a bit older and stronger, in order to shorten anesthesia time and avoid exacerbating the infant’s respiratory issues[20]. In one case, a SH was detected in the early postoperative period after nephroureterectomy complicated by significant intraoperative blood loss, and therefore a decision was made to delay reoperation. The hernia was repaired 2 months later[25]. These examples show that in the setting of multiple comorbid conditions, the surgical team may triage which problem to address first. However, such delays are only chosen if the SH is reducible and not acutely dangerous; the child must be closely monitored in the interim for any signs of incarceration. One innovative approach to temporizing an incarcerated hernia without immediate surgery was described by Levy et al[26] and Shchapov et al[25]: They successfully performed an ultrasound-guided manual reduction of a strangulated SH in a neonate, buying time to schedule a controlled surgery rather than an emergency operation. Such non-operative reduction techniques are seldom reported, but they can be considered in an emergency if immediate surgery is high risk. Nevertheless, definitive surgical correction is indicated as soon as feasible in essentially all cases.
Surgical techniques – open repair: Traditional open hernia repair was by far the most common approach in the lite
After addressing contents, the hernia defect repair (herniorrhaphy) was typically done by suturing the edges of the fascial gap. Nearly all reports describe using the child’s own tissues to close the defect, given the small size of the hole and the excellent healing capacity in children[23]. Non-absorbable sutures (such as polypropylene or silk) placed as interrupted figure-of-eight stitches are commonly used to ensure a strong closure of the transversalis fascia and internal oblique aponeurosis[2,37]. In some cases, absorbable sutures (like polydioxanone) were used, especially if the child was very small, to avoid a long-term foreign body[4]. Only four cases in our review mentioned using a mesh to reinforce the repair – these were older children or cases where the defect was larger than usual[36]. The use of synthetic mesh is generally avoided in young children due to risk of infection and because most pediatric hernias can be closed primarily; however, if a SH were found in an older adolescent with a sizable defect or in a reoperative scenario, mesh reinforcement might be considered by some surgeons[38]. After closing the fascial defect, the external oblique muscle/aponeurosis is usually left intact or reapproximated if it was cut. Excision of the sac is performed in many cases to prevent future cyst formation, except when the sac is very thin or fused with contents (then it may be left in situ after reduction).
In cases of the SH-UDT syndrome, orchidopexy is done in conjunction with hernia repair. The UDT, once freed from attachments, is mobilized. If an inguinal canal is absent, surgeons will often create a new pathway to bring the testis into the scrotum[36]. The testis is then fixed in the scrotum (often via a scrotal incision or by a trans-septal suture) as per standard orchidopexy techniques.
Laparoscopic repair: The advent of minimally invasive surgery has gradually extended to pediatric hernia repairs, including a few SH cases. In our entire review, only 5 children (approximately 5% of cases) underwent a purely laparoscopic repair of their SH. Additionally, we identified one hybrid case where laparoscopy was used for diagnosis and to assist in orchidopexy, but the fascial defect was closed via a small open incision[32]. The limited number of laparoscopic cases is due to the rarity of the condition and the fact that many reports predated modern laparoscopic techniques. However, interest in laparoscopy for SH has increased over the past decade[29,34].
The laparoscopic cases included two adolescent patients (ages 13 and 14) with isolated SH who had their hernia defect repaired minimally-invasively[4,29]. In these, trocars were placed intra-abdominally and the defect was either sutured laparoscopically or covered with a small mesh patch from inside. The other three laparoscopic cases were infants or toddlers with the SH-UDT syndrome[32,33,39]. For instance, Deshmukh et al[33] reported a total laparoscopic repair in a 15-month-old boy with SH and UDT: They performed a laparoscopic orchidopexy and simultaneously sutured the hernia defect internally using stitches passed through the abdominal wall. Rashi et al[39] similarly treated a 1-year-old boy with a laparoscopic approach. The youngest reported patient to undergo laparoscopic SH repair is our own case – a 6-month-old infant girl – making our case the fifth known laparoscopic pediatric SH repair and the earliest age achieved[25]. In all laparoscopic approaches, the transperitoneal route was used (entering the abdominal cavity with trocars) as opposed to an extraperitoneal laparoscopy, because the small working space in infants makes extraperitoneal endoscopic repair quite challenging.
Surgeons who advocate for laparoscopy cite several potential advantages: First, the ability to inspect the abdominal wall from the inside helps in locating a small Spigelian defect that might be hard to pinpoint externally[25]. This is especially useful when the hernia is not obvious on the surface – under laparoscopy, one can see the bulge of preperitoneal fat or bowel coming through the fascia and directly visualize the hernia orifice. In our infant case, for example, laparoscopy allowed us to identify the defect quickly by using the inferior epigastric vessels as landmarks for the semilunar line, whereas externally the exact spot was elusive[25]. Second, laparoscopic repair is achieved with very small incisions (trocar sites), potentially resulting in less post-operative pain and a better cosmetic outcome than an open incision[39]. Third, for cryptorchidism cases, laparoscopy permits a combined one-stage procedure: The testis can be mobilized under vision and brought into the scrotum, avoiding a larger groin incision[32,33]. Additionally, if there is any doubt about contralateral anomalies (like a contralateral hernia), laparoscopy allows examination of the opposite side and the entire peritoneal cavity.
On the other hand, authors have raised some concerns and contraindications for laparoscopy in pediatric SH. One issue is that in an incarcerated or strangulated hernia, laparoscopic manipulation might be difficult or risky – dense adhesions or a tightly trapped bowel loop could favor an open approach for safer reduction[3]. Another concern specific to the syndrome cases is the challenge of handling the ectopic testis and spermatic cord laparoscopically – freeing a testis that is fused to a hernia sac or embedded in the abdominal wall may be more straightforward via an open incision, where delicate structures can be directly palpated and dissected[5,30]. Ensuring a robust closure of the fascia is also critical; while laparoscopic suturing is feasible, some surgeons worry about the learning curve and whether the repair is as solid as an open one, especially in a small infant[5]. In summary, open surgery remains the standard in pediatric SH, but laparoscopy is a promising option in select cases – typically when the hernia is elective, the surgeon is experienced in pediatric minimally invasive techniques, and when a combined procedure can be efficiently accomplished with the scope.
Outcomes: The outcomes after repair of SH in children are generally excellent. Among the 91 operated cases, there were no reported recurrences of the hernia during the follow-up periods documented. Follow-up duration varied widely – many reports followed the child for only a few months post-op to confirm healing, while some had multi-year follow-ups[4,23]. In the aggregate, only 23 patients had clearly documented long-term follow-up beyond 2 years (average follow-up approximately 2.6 years among those)[23]. Even in those, no recurrence was noted. Despite the short follow-ups in many cases, these data suggest that a properly performed hernia repair (with or without orchidopexy) in a child is durable. Losanoff et al[23] recommended prolonged surveillance especially if novel techniques are used, to ensure the repair method is sound.
Regarding postoperative complications, the hernia repairs themselves did not appear to cause major issues. There were zero reports of wound infections or mesh infections in the series, and no reports of postoperative bowel obstruction or other abdominal complications attributable to the hernia surgery. The only complications identified were related to the testis in the syndrome cases. We found three cases where the child who had an orchidopexy as part of SH repair later developed problems: Two developed a scrotal abscess[10,36] and ultimately the affected testis underwent atrophy[5]. These were infants in whom perhaps the testicular blood supply was compromised either by the initial hernia incarceration or by tension during the orchidopexy. Testicular atrophy is a known risk in cryptorchidism surgery, especially if the testis is high and requires extensive mobilization. The rate of this complication in SH-UDT syndrome is not well-defined given the small numbers; Taha et al[5] highlighted this occurrence in their analysis and stressed gentle handling and adequate collateral vessel development for orchidopexy success. Apart from those, no child had recurrence of cryptorchidism or hernia. There were also no developmental delays or growth issues attributed to the condition or surgery. Cosmetically, children healed with minimal scarring, particularly when laparoscopy or small transverse incisions were used[4].
In summary, surgical management of pediatric SH – either open or laparoscopic – is safe and effective, with a nearly 100% cure rate and very low complication profile when performed in a timely fashion. The most significant determinant of outcome is timeliness of intervention; delayed diagnosis can lead to strangulation, which carries the risk of bowel resection or even death, whereas early elective repair avoids such catastrophes[8].
Pediatric SH is fundamentally a congenital defect of the abdominal wall, in contrast to adult SH which more often have acquired or degenerative etiologies[2,21]. The findings of this review strongly support a developmental basis: The majority of cases had no history of precipitating factors, and often other congenital anomalies were present, indicating an inborn origin. The anatomical weak point responsible for SH is the transversus abdominis aponeurosis and transversalis fascia along the semilunar line. Pioneering work by Zimmerman et al[17] shed light on the morphology of this area – in about 20% of examined specimens, they found natural weak spots or fenestrations in the transversalis fascia even without a hernia present. These weak spots are thought to be areas where the connective tissue fibers are loosely interwoven or deficient, potentially along paths of perforating vessels. In the Spigelian region, branches of the inferior epigastric vessels and nerves penetrate the abdominal wall, which might create small hiatuses in the fascia[20]. It has been hypothesized that a congenital malformation or incomplete fusion of the fascia at this site leads to a predisposition for hernia formation[1,37]. Our review of pediatric cases noted that, indeed, surgeons frequently commented on the appearance of the fascia: Terms like "defect in the transversalis fascia with attenuated edges" or an "area of aplasia in the internal oblique aponeurosis" were used[1,37]. In our own laparoscopic observation, the fascia around the hernia was thin and almost translucent, reminiscent of congenital linea alba hernias in babies where small openings occur between decussating fibers (Figure 6). These findings resonate with Zimmermann’s description and suggest that SH in children might represent an extreme manifestation of these normal fascial weak areas[17,25].
Traumatic vs congenital etiology: Although rare, trauma can cause SHs in children, which raises the question of whether those are fundamentally different from congenital cases. Our review identified 9 traumatic cases, mostly due to blunt force from bicycle or motorcycle handlebar injuries – a well-known mechanism for abdominal wall hernias in pediatrics[15,40]. In such injuries, the sudden pressure can rip the muscle and fascia, creating an acute hernia. Interestingly, these traumatic SHs often had no other intra-abdominal injury, and in some, the hernia actually closed spontaneously during healing[27]. The capacity for spontaneous closure suggests that when a normal abdominal wall is acutely torn, the body’s repair mechanisms (scar formation) can mend the defect if the child is kept at relative rest and if the defect is small. This is quite unlike congenital cases, which will not "heal" on their own because they represent a developmental lack of tissue that persists. If we examine the hypothesis of traumatic origin of SH in adults through the lens of pediatric experience, it raises certain doubts. There is no clear evidence suggesting that the frequency of anterior abdominal wall trauma is significantly higher in adults compared to children. Moreover, given the presence of multiple anatomically predisposed weak zones in the abdominal wall, it is unlikely that elevated intra-abdominal pressure would preferentially lead to rupture at the level of the transversalis fascia. A more plausible explanation is that many adult SHs may in fact represent congenital defects that went unrecognized in childhood. Over time, with episodes of increased intra-abdominal pressure—such as during physical exertion—these latent defects may permit visceral protrusion, eventually becoming clinically apparent. In such cases, the patient may notice the swelling, or the hernia may present acutely with incar
From a developmental standpoint, it’s noteworthy that some pediatric SH patients have multiple fascial defects. This hints at a generalized developmental weakness of the abdominal wall fascia in certain individuals[9]. There may be genetic factors or embryological events that predispose these children to hernias in various locations. However, given the rarity of SH, no specific genetic or syndromic cause has been identified. Each case thus seems sporadic, possibly related to isolated errors in mesodermal development or collagen formation localized to the semilunar line region.
Diagnosing a SH in a child can be challenging due to the intermittent nature of the hernia and the nonspecific symptoms. Our review underscores several important clinical pearls. First, the presence of a reducible lump along the lateral rectus margin in an infant or child-especially one that enlarges with crying or straining-should prompt consideration of SH[2,21]. In practice, such a lump might not be as obvious as the more familiar inguinal or umbilical hernias, because it can lie under intact external oblique muscle. As noted by multiple authors and confirmed in our case analyses, marking the location of the bulge on the skin before surgery is very helpful[2,41]. Both Al-Salem[2] and Graivier et al[41] recounted instances where under anesthesia the hernia became difficult to find; they advocate marking the site when the child is upright or crying preoperatively. In our experience, we had similar difficulty palpating the defect after reduction in the operation room, which is why we opted for laparoscopic visualization. Thus, surgeons should be prepared for a bit of a "treasure hunt" when operating-knowing the anatomic landmarks (semilunar line intersection with arcuate line, and location relative to inferior epigastric vessels) can guide the search[25].
The symptomatology ranges from completely asymptomatic to acute abdomen. This wide spectrum can delay diagnosis, as milder symptoms may be attributed to common pediatric issues like infant colic, gastroenteritis, or constipation. One critical lesson is that repetition of clinical exam is necessary. A single examination might miss a small SH, whereas repeated exams or an exam during symptoms might reveal it[22]. We recommend that if a high suspicion exists (for instance, a combination of abdominal pain with an empty hemiscrotum in a boy, or a localized area of tenderness laterally), the child should be evaluated multiple times. Pediatricians and pediatric surgeons should keep SH in mind as a differential diagnosis for episodic abdominal pain or atypical hernia presentations.
The role of ultrasound in diagnosis cannot be overemphasized. Many of the cases in literature were confirmed by ultrasound[24,26]. It is a readily available, non-invasive tool that can be done even at bedside. Radiologists should be informed of the suspicion so they can target the semilunar line region. Our review indicates ultrasound has a high yield if performed by an experienced operator: Signs include a defect in the abdominal wall (sometimes only seen when pressure is applied or the child cries), and herniation of preperitoneal fat or bowel through that defect[26]. There are documented ultrasound images showing a loop of intestine popping in and out of the lateral abdomen – a diagnostic image when captured[25,26]. When the hernia is reduced, ultrasound might only show a subtle discontinuity or localized distortion of muscle layers[25]. These subtleties highlight why a high index of suspicion and perhaps repeat ultrasound at different times might be needed if initial imaging is negative but suspicion persists.
Another advanced diagnostic tip is the use of laparoscopy for diagnosis. In one report, a 9-month-old with suspected SH and cryptorchidism underwent a diagnostic laparoscopy to confirm the hernia’s presence and to locate the testis, which was then reduced and fixed[32]. Diagnostic laparoscopy is minimally invasive and can definitively establish the diagnosis while simultaneously allowing for treatment in the same session. We would advocate considering diagnostic laparoscopy in cases where imaging is equivocal yet clinical suspicion remains high, as it provides a direct visual confirmation.
The strong association between SH and UDT in infant boys has significant implications. Practically, it means that pediatric surgeons and urologists should collaborate when this syndrome is encountered[21]. The presence of an UDT often is what brings the child to attention (e.g., a non-palpable testis during a routine check-up), and if an ultrasound or exam then finds a SH, the management plan must address both issues. Our review found that nearly half of all male SH cases involved cryptorchidism, which is far above the approximately 1%-4% baseline rate of cryptorchidism in full-term male infants[21]. This clearly indicates a linkage, supporting previous literature that labeled it a distinct syndrome[8,30].
From a management perspective, recognizing this syndrome prompts simultaneous treatment of both issues. The surgical approach needs to ensure both hernia closure and a successful orchidopexy. As our results showed, this can be done open or laparoscopically. The outcomes for the hernia part are excellent, but the outcomes for the testis can sometimes be suboptimal (with a small risk of atrophy). Surgeons have debated the timing of surgery for SH-UDT syndrome. Some have recommended waiting until around 1 year of age to do a combined repair, especially if the hernia is not causing symptoms, reasoning that very young infants have higher anesthesia risks and that orchidopexy at around 1 year is standard for isolated cryptorchidism anyway[36]. On the other hand, waiting carries the risk that the hernia could incarcerate and potentially strangulate bowel or damage the testis. There is also evidence in cryptorchidism research that earlier orchidopexy (by 6 months of age) might improve testicular outcomes[21]. Given this, many now lean toward earlier intervention for the SH-UDT syndrome, provided the infant can tolerate surgery, to avoid incarceration events[5]. Each case should be individualized: For example, a premature infant might wait a bit until medically stable, whereas a robust 3-6 month old with a large hernia might benefit from prompt repair.
In counseling parents, it’s important to explain that their child effectively has two problems that need fixing at once. The concept of a "syndrome" helps here: It conveys that these are linked birth defects rather than random bad luck. Parents should also be made aware of the slight chance of testicular loss or atrophy, which, while uncommon, has occurred in a few cases[5]. Long-term follow-up for testicular growth and fertility potential is prudent for these patients as they reach puberty, although no issues have been reported so far in the limited follow-ups available.
In conclusion on this point, the SH-UDT syndrome is a fascinating example of how a localized abdominal wall defect can be part of a broader developmental anomaly. Further research, perhaps in the form of multicenter registries of these cases, could illuminate whether there's a specific genetic or embryologic trigger. For now, awareness and prompt combined surgical management are the main takeaways.
The treatment of SH in children is decisively surgical, and our findings affirm that outcome success is nearly 100% when managed appropriately. An overarching principle is that once diagnosed (or strongly suspected), surgery should not be significantly delayed because of the risk of incarceration. We saw that strangulation occurred in about 11% of cases overall – a substantial risk when compared to more common hernias (for instance, pediatric inguinal hernias have an incarceration risk around 12%-17% in infants, somewhat similar)[23]. However, SHs pose an additional threat: The hernia ring (defect) is often rigid and narrow, with sharp fascial margins that can constrict the herniated viscus tightly[3,8]. Some authors have likened it to a femoral hernia in adults, which is known for a high strangulation rate due to its stiff boundaries[1,2,37]. Indeed, delays in treating pediatric SH have led to necrotic bowel requiring resection. Therefore, early elective repair is advised as soon as the diagnosis is confirmed or even if it remains strongly presumed.
The choice of surgical approach – open vs laparoscopic-is influenced by patient age, surgeon expertise, and whether emergency conditions exist. Our review clearly shows that open repair via a small incision is the time-tested and prevalent method, with excellent results[1,2]. Open surgery offers direct tactile feedback, which is helpful for locating a tiny defect, and it is relatively straightforward to perform, often taking under 30 minutes in experienced hands[36]. The scar is usually small (2-4 cm) and can be placed in a skin crease or along Langer’s lines to heal cosmetically. For these reasons, open repair remains the standard of care, especially in resource settings where advanced laparoscopy for infants might not be available.
However, the emerging reports of laparoscopic repairs suggest some scenarios where minimal access surgery has advantages. One scenario is when the hernia is not easily palpable or visible externally – laparoscopy can avoid a larger dissection in searching for it[25]. Another is in the context of concomitant procedures: If a child needs a diagnostic laparoscopy for an UDT or to rule out other intra-abdominal issues, it is logical to continue and fix the hernia laparoscopically during the same session[32,33]. Also, in bilateral hernia cases, a laparoscopic approach might allow both sides to be repaired through the same port sites, whereas open would need two separate incisions.
Our case, along with a handful of others, demonstrates that even in infants, laparoscopy can be performed safely by skilled pediatric surgeons[25,39]. The learning curve and equipment availability are considerations; not all centers may be equipped for 3-mm infant laparoscopic instruments. Additionally, if an SH is discovered intraoperatively, the surgeon might simply fix it open at that moment rather than reposition for laparoscopy. So, practicality plays a role.
In reviewing outcomes, it appears that whether open or laparoscopic, recurrence rates are essentially zero when proper technique is used. Children’s tissues heal rapidly and strongly, and the defect sizes are small, which likely contributes to the success of primary sutured repairs[23]. Our literature search did not find a single confirmed recurrence of a pediatric SH after repair. While follow-up in some cases was short, many had at least 1-2 years of observation which is usually enough to catch a recurrence if it were to happen.
Postoperative complications were minimal. Aside from the orchidopexy-related issues in the syndrome cases, no major complications were reported. A few children had superficial seromas or minor wound issues, but these resolved without incident[4]. The lack of infections, even when non-absorbable sutures or mesh were used, is reassuring and likely due to the well-vascularized muscle tissue and good surgical technique in small incisions. The two cases of scrotal abscess after orchidopexy[5] point to the need for vigilance in wound care and possibly prophylactic antibiotics when moving a testis that has been in an unusual position. Testicular atrophy in those cases could result from either infection or intrinsic damage from being strangulated in the hernia initially; it’s difficult to say. Nonetheless, even in those cases, the hernia repairs themselves stayed intact.
An interesting point raised by some authors[25,28] is the concept of spontaneous closure of SH under certain conditions, which challenges the notion that surgery is absolutely always required. In one documented case, as mentioned, a neonatal SH closed after a diaphragmatic hernia repair[28]. We also noted cases where minor traumatic SH in children healed without surgery. These instances suggest that if the abdominal wall is given a chance to scar and strengthen, a small defect could seal itself. However, relying on spontaneous closure is risky in congenital cases – nearly all congenital SH will enlarge or incarcerate over time if untreated[8]. Possibly, the rare spontaneous closures occurred in scenarios where the defect was exceptionally small and the patient’s condition facilitated healing. For now, non-operative management is not standard for congenital SH, except maybe in an unusual circumstance where surgery must be deferred. Even then, those patients should get the hernia repaired once it’s safe to do so.
Finally, our review highlights that long-term prognosis is excellent after repair. Children go on to have normal growth and activity. The only long-term consideration might be in those who lost a testis or have one testis – they would need monitoring for fertility and hormonal function later, but that pertains more to the cryptorchidism aspect than the hernia itself.
In summary, this systematic review provides a comprehensive overview of SH in children, consolidating nearly a century’s worth of case reports into a clearer picture. Key conclusions include:
Spigelian hernia in children is a rare, primarily congenital condition that often presents in infancy or early childhood. Fewer than 100 cases have been reported, highlighting the importance of recognizing even subtle signs.
Clinical vigilance is crucial: Pediatric SH can present with an intermittent bulge and mild symptoms or as acute strangulation. A high index of suspicion in any child with intermittent abdominal pain or an atypical abdominal wall mass can lead to timely diagnosis. Ultrasound is the diagnostic modality of choice and is highly effective when focused on the semilunar line region.
Nearly half of male patients have the SH-UDT syndrome, an association that requires coordinated surgical management. Understanding this syndrome’s anatomy (often absent inguinal canal/gubernaculum) helps in planning orchidopexy and hernia repair. This syndrome supports a developmental link between abdominal wall formation and testicular descent.
Surgical repair is definitively curative. Open surgical techniques have an excellent track record with essentially zero recurrences when properly executed. Laparoscopic repair is feasible and advantageous in select cases (especially older children or concurrent cryptorchidism), offering good visualization and minimal invasiveness, though it should be employed by experienced surgeons.
Outcomes for the child are overwhelmingly positive. Complications are scarce; the most serious risk – strangulation of bowel – is preventable with early surgery. When treated electively, children recover quickly and resume normal lives without restriction. Long-term follow-up suggests durable repairs, though continued observation is recommended in literature to detect any late issues.
Looking forward, because SH in children is so uncommon, it would benefit from multi-institutional collaboration. Creating a registry or at least consistently reporting new cases will further our understanding. Particular areas where questions remain include the precise embryology of the SH-UDT syndrome (why exactly does it happen in some and not others?), the optimal timing for intervention in infant cases balancing anesthesia risk and incarceration risk, and the potential role of minimally invasive approaches as technology and surgeon experience advance.
In conclusion, SH in the pediatric population, though rare, should be on the radar of clinicians dealing with abdominal complaints in children. Awareness of its hallmark signs, its frequent pairing with UDT, and the effective treatment options can lead to prompt diagnosis and cure. As this review has compiled, the collective experience worldwide emphasizes that early recognition and surgical management of pediatric SH result in excellent outcomes, preventing morbidity and even mortality from this elusive condition. Further research and case accumulation will continue to refine the care for these patients, but even with the knowledge we have today, we can confidently manage SH in children with a high expectation of success.
| 1. | Pul N, Pul M. Spigelian hernia in children--report of two cases and review of the literature. Yonsei Med J. 1994;35:101-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Al-Salem AH. Congenital spigelian hernia and cryptorchidism: cause or coincidence? Pediatr Surg Int. 2000;16:433-436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Vaos G, Gardikis S, Zavras N. Strangulated low Spigelian hernia in children: report of two cases. Pediatr Surg Int. 2005;21:736-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Spinelli C, Strambi S, Pucci V, Liserre J, Spinelli G, Palombo C. Spigelian hernia in a 14-year-old girl: a case report and review of the literature. European J Pediatr Surg Rep. 2014;2:58-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 5. | Taha A, Algethami NE, AlQurashi R, Alnemari AK. Outcome of Orchidopexy in Spigelian Hernia-Undescended Testis Syndrome. Cureus. 2021;13:e13714. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 6. | Christianakis E, Paschalidis N, Filippou G, Rizos S, Smailis D, Filippou D. Low Spigelian hernia in a 6-year-old boy presenting as an incarcerated inguinal hernia: a case report. J Med Case Rep. 2009;3:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Raveenthiran V. Congenital Spigelian hernia with cryptorchidism: probably a new syndrome. Hernia. 2005;9:378-380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Patoulias I, Rahmani E, Patoulias D. Congenital Spigelian hernia and ipsilateral cryptorchidism: a new syndrome? Folia Med Cracov. 2019;59:71-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 10. | Vega Y, Zequeira J, Delgado A, Lugo-Vicente H. Spigelian hernia in children: case report and literature review. Bol Asoc Med P R. 2010;102:62-64. [PubMed] |
| 11. | Saha M. Intermuscular lipoma in a 4-year-old child presenting like Spigelian hernia. J Indian Assoc Pediatr Surg. 2015;20:189-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 12. | Mohta A, Gupta CR. Inguinal hernia masquerading as a Spigelian hernia in a child. Hernia. 2009;13:327-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Alaqeel SM, Hakeem AH, Almaary JO. Testicular Ectopia in a Child's Anterior Abdominal Wall: A Case Report and Literature Review. Am J Case Rep. 2020;21:e927495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 14. | Thakur SK, Gupta S, Goel S. Traumatic spigelian hernia due to handlebar injury in a child: a case report and review of literature. Indian J Surg. 2013;75:404-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Litton K, Izzidien AY, Hussien O, Vali A. Conservative management of a traumatic abdominal wall hernia after a bicycle handlebar injury (case report and literature review). J Pediatr Surg. 2008;43:e31-e32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 16. | Komura J, Yano H, Uchida M, Shima I. Pediatric spigelian hernia: reports of three cases. Surg Today. 1994;24:1081-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Zimmerman LM, Anson BJ, Morgan EH, McVay CB. Ventral hernia due to normal banding of the abdominal muscles. Surg Gynecol Obstet. 1944;78:535-540. |
| 18. | Spiegel A. De humani corporis fabrica libri decem, Francofurti: Impensis & Caelo Matthaei Meriani, 1632: 137. |
| 19. | Aksu B, Temizöz O, Inan M, Gençhellaç H, Başaran UN. Bilateral spigelian hernia concomitant with multiple skeletal anomalies and fibular aplasia in a child. Eur J Pediatr Surg. 2008;18:205-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 20. | Sinopidis X, Panagidis A, Alexopoulos V, Karatza A, Georgiou G. Congenital Spigelian Hernia Combined with Bilateral Inguinal Hernias. Balkan Med J. 2018;35:402-403. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 21. | Jones BC, Hutson JM. The syndrome of Spigelian hernia and cryptorchidism: a review of paediatric literature. J Pediatr Surg. 2015;50:325-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 22. | Jarvis PA, Seltzer MH. Pediatric Spigelian hernia: a case report. J Pediatr Surg. 1977;12:609-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | Losanoff JE, Richman BW, Jones JW. Spigelian hernia in a child: case report and review of the literature. Hernia. 2002;6:191-193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Moles Morenilla L, Gómez Rubio D, Sánchez Blanco JM, Galindo Galindo A, Recio Moyano O, Brox Jiménez A. [The new congenital Spigelian hernia and cryptorchidism syndrome. Analysis of 16 cases]. Cir Esp. 2008;84:154-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 25. | Shchapov NF, Vyborniy MI, Kulikov DV, Bullikh PV, Degtyarev AS, Elagin DA. Spigelian hernia in children: a case report and meta-analysis of the literature. Russ J Pediatr Surg. 2023;27:304-316. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 26. | Levy G, Nagar H, Blachar A, Ben-Sira L, Kessler A. Pre-operative sonographic diagnosis of incarcerated neonatal Spigelian hernia containing the testis. Pediatr Radiol. 2003;33:407-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Bar-Maor J, Sweed Y. Spigelian hernia in children, two cases of unusual etiology. Pediatr Surg Int. 1989;4:357-359. [DOI] [Full Text] |
| 28. | Walton JM, Bass JA. Spigelian hernias in infants: report of two cases. Can J Surg. 1995;38:95-97. [PubMed] |
| 29. | Vega-Mata N, Vázquez-Estevez JJ, Montalvo-Ávalos C, Raposo-Rodríguez L. [Abordaje laparoscópico de una hernia de Spiegel en edad pediátrica. Revisión de la literatura]. Cir Cir. 2019;87:101-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 30. | Rushfeldt C, Oltmanns G, Vonen B. Spigelian-cryptorchidism syndrome: a case report and discussion of the basic elements in a possibly new congenital syndrome. Pediatr Surg Int. 2010;26:939-942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 31. | Bilici S, Güneş M, Göksu M, Melek M, Pirinçci N. Undescended testis accompanying congenital Spigelian hernia: is it a reason, a result, or a new syndrome? Eur J Pediatr Surg. 2012;22:157-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 32. | Khan FA, Islam S. Laparoscopic Repair of Congenital Lumbar Hernia in a 4 Week Old Infant. APSP J Case Rep. 2018;9:6. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 33. | Deshmukh SS, Kothari PR, Gupta AR, Dikshit VB, Patil P, Kekre GA, Deshpande A, Kulkarni AA, Hukeri A. Total laparoscopic repair of Spigelian hernia with undescended testis. J Minim Access Surg. 2019;15:265-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Sengar M, Mohta A, Neogi S, Gupta A, Viswanathan V. Spigelian hernia in children: low versus classical. J Pediatr Surg. 2018;53:2346-2348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 35. | Silberstein PA, Kern IB, Shi EC. Congenital spigelian hernia with cryptorchidism. J Pediatr Surg. 1996;31:1208-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 36. | Durham MM, Ricketts RR. Congenital spigelian hernias and cryptorchidism. J Pediatr Surg. 2006;41:1814-1817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 37. | Hurlbut HJ, Moseley T. Spigelian hernia in a child. South Med J. 1967;60:602 passim. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 38. | Kılıç MÖ, Değirmencioğlu G, Dener C. A rare case of Spigelian hernia combined with direct and indirect inguinal hernias. Turk J Surg. 2017;33:40-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 39. | Rashi, Kumar A, Sinha A, Kumar B, Sambedna. Undescended testis with Spigelian hernia: A rare association treated laparoscopically. Arch Int Surg. 2019;9:13. [DOI] [Full Text] |
| 40. | Kropilak AD, Sawaya DE. Traumatic Spigelian Hernia in a Pediatric Patient Following a Bicycle Injury. Am Surg. 2022;88:1933-1935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 41. | Graivier L, Bernstein D, RuBane CF. Lateral ventral (spigelian) hernias in infants and children. Surgery. 1978;83:288-290. [PubMed] |
| 42. | Landry RM. Traumatic hernia. Am J Surg. 1956;91:301-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 43. | Graivier L, Alfieri AL. Bilateral Spigelian hernias in infancy. Am J Surg. 1970;120:817-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 44. | Herbert RJ, Turner FW. Traumatic abdominal wall hernia in a 7-year-old child. J Pediatr Surg. 1973;8:975-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 45. | Constantino L, Contestabile D, Rocca E. Strangulated Spigelian hernia in a child. Riv Chir Pediatr. 1974;16:236-243. |
| 46. | Houlihan TJ. A review of Spigelian hernias. Am J Surg. 1976;131:734-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 47. | Podkamenev VV, Uman' NV, Moroz VM. [Strangulated spigelian hernia in a child]. Klin Khir (1962). 1986;72. [PubMed] |
| 48. | Grechanyĭ VP, Gruminskiĭ VS. [Incarcerated hernia of the semilunar line in a child]. Vestn Khir Im I I Grek. 1990;144:87. [PubMed] |
| 49. | Azuma T, Nakamura S, Hatakeyama G, Nagahara N, Nakamura T, Yonekura T, Kashiwai A, Kawata H, Tsuji H. A spigelian hernia in an infant. Osaka City Med J. 1992;38:155-160. [PubMed] |
| 50. | Beimanova SM. Strangulated hernia of Spigeli line in a child. Healthcare (Minsk). 1999;8:56. |
| 51. | Prokopenko UD, Koval’chuk ES. Spigelian hernias in children. Pediatr Surg. 1999;2:52. |
| 52. | Bychkov VA, Butov VS, Gerasimova SYu, Al-kadi KM. Complicated course of Spigelian hernia in a 14-year-old boy. Pediatr Surg. 2000;2:52-53. |
| 53. | White JJ. Concomitant Spigelian and inguinal hernias in a neonate. J Pediatr Surg. 2002;37:659-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 54. | Torres de Aguirre A, Cabello Laureano R, García Valles C, Garrido Morales M, García Merino F, Martínez Caro A. [Spigelian hernia: two cases associated to cryptorchidism]. Cir Pediatr. 2005;18:99-100. [PubMed] |
| 55. | O’Sullivan O, Bannon C, Clyne O, Flood H. Hypospadias associated undescended testis in a Spigelian hernia. Ir J Med Sci. 2006;175:77-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 56. | Fascetti-Leon F, Gobbi D, Gamba P, Cecchetto G. Neonatal bilateral spigelian hernia associated with undescended testes and scalp aplasia cutis. Eur J Pediatr Surg. 2010;20:123-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 57. | Lopez R, King S, Maoate K, Beasley S. Trauma may cause Spigelian herniae in children. ANZ J Surg. 2010;80:663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 58. | Singal AK, Ravikumar VR, Kadam V, Jain V. Undescended testis in Spigelian hernia–a report of 2 cases and review of the literature. Eur J Pediatr Surg. 2011;21:194-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 59. | Ostlie DJ, Zerella JT. Undescended testicle associated with spigelian hernia. J Pediatr Surg. 1998;33:1426-1428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 60. | Inan M, Basaran UN, Aksu B, Dortdogan Z, Dereli M. Congenital Spigelian hernia associated with undescended testis. World J Pediatr. 2012;8:185-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/