Published online Dec 9, 2025. doi: 10.5409/wjcp.v14.i4.106219
Revised: April 21, 2025
Accepted: June 7, 2025
Published online: December 9, 2025
Processing time: 254 Days and 13.7 Hours
Gestational alloimmune liver disease (GALD), previously known as neonatal hemochromatosis, is a rare antenatal immune condition in which maternal anti
Core Tip: Gestational alloimmune liver disease is a rare neonatal hepatic condition with clinical manifestations ranging from benign presentations to fulminant liver failure. Curative treatments include intravenous immunoglobulin (IVIG) and double-volume plasma exchange; liver transplantation may be necessary in severe cases. The recurrence rate in subsequent preg
- Citation: Helali N, Gagnon H, Álvarez F. Gestational alloimmune liver disease reconsidered: Advocating for a new nomenclature and enhanced diagnosis accuracy. World J Clin Pediatr 2025; 14(4): 106219
- URL: https://www.wjgnet.com/2219-2808/full/v14/i4/106219.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v14.i4.106219
Gestational alloimmune liver disease (GALD), previously referred to as neonatal hemochromatosis (NH), is a rare antenatal and neonatal allo-immune disease. It is the primary cause of neonatal liver failure and has a broader spectrum than previously thought, covering from mild to severe liver damage[1,2]. The pathophysiology of GALD is characterized by the production of maternal antibodies [immunoglobulin G (IgG)] damaging the fetal hepatocytes in response to fetal antigens. In opposition to initial belief, the iron accumulation in the liver and extrahepatic tissues sparing the reticuloendothelial system, is probably associated to the liver injury and is not the primary mechanism, therefore explaining the appellation change from NH to GALD[3,4].
Practioner’s main goal is to obtain a prompt diagnosis, through liver biopsy, iron status and salivary glands biopsy[1,5]. A timely initiation of effective treatment is also required to improve survival with native liver as delayed diagnosis can be associated with a liver transplant and death[5].
Management should address both immediate newborn treatment and future pregnancies. Recurrence can be as high as 90% and can be prevented with intravenous immunoglobulin (IVIG) therapy[4].
Given the limited official practice recommendation on GALDs diagnosis and treatment strategies, and limited data on outcomes for neonates and subsequent pregnancies, we present here a cohort of patients diagnosed in our gastroenterology unit of the CHU Sainte-Justine in the last ten years, to illustrate the variability and complexity of diagnosis and treatment of this disease. In addition, we conducted a literature review of related GALD articles to present the etiopathogeneses, clinical presentation, signs and symptoms, diagnosis, treatment and prognosis of those patients.
A female newborn was delivered at 39 weeks of gestation to a 26-year-old mother gravida 2, para 2, following an uneventful pregnancy. The Apgar score was 9 at 1 minute, 5 minutes, and 10 minutes, and birth weight was within the normal range.
Shortly after birth, the infant developed hypoglycemia and thrombocytopenia, which was attributed to platelet alloimmunization, confirmed by the presence of positive anti-human leukocyte antigen antibodies. During her hospitalization in the neonatal intensive care unit (NICU), she exhibited severe coagulopathy, requiring repeated transfusions of fresh frozen plasma (FFP) to maintain an international normalized ratio (INR) below 5. Additionally, she developed hyperammonemia and showed signs of portal hypertension.
A portosystemic shunt embolization was performed on day 14 of life due to portal hypertension, but this intervention led to worsening hyperammonemia and progressive splenomegaly. Laboratory investigations revealed elevated transferrin saturation.
A salivary gland biopsy performed on day 18 showed no evidence of iron overload. Then, a liver biopsy performed on day 21 demonstrated fibrosis, significant remodeling of the hepatic parenchyma with only two remaining portal tracts, and iron overload. Findings were consistent with GALD.
The patient underwent a successful liver transplant at the age of 3 months from a cadaveric donor. Now, at 8 years old, she has normal liver function with no major complications apart from mild signs of portal hypertension secondary to a portal vein stenosis. Her growth and development are within normal parameters.
A female infant born from parents of Haitian origin. The mother was 34 years old with a history of five pregnancies, including three live births and two miscarriages. The pregnancy was complicated by oligohydramnios and asymmetric intrauterine growth restriction.
At birth, the infant presented with persistent hypoglycemia, requiring continuous enteral nutrition. Laboratory tests showed normal alanine aminotransferase (ALT) levels, cholestasis with normal gamma-glutamyl transferase levels, a normal INR, and thrombocytopenia. Ferritinemia was markedly elevated (4103 μg/L), raising suspicion of GALD. Liver ultrasound revealed a patent ductus venosus.
Further investigations included magnetic resonance imaging (MRI), which showed no evidence of hepatic or ex
A female newborn was delivered at 33 weeks of gestation due to placental abruption to a 31-year-old mother, gravida 2, para 1 (G2P1), with a history of miscarriage. Prenatal ultrasounds showed no evidence of oligohydramnios or growth restriction. The Apgar scores were 2, 6, and 7. Birth asphyxia was diagnosed by the neonatology team.
At birth, the infant presented with hepatomegaly, with the liver palpable 4 cm below the costal margin and hypoglycemia. Laboratory investigations revealed cholestasis, mild elevation of ALT, elevated INR, hypoalbuminemia, and renal failure, most likely secondary to hepatorenal syndrome. Coagulopathy was not improved by repeated doses of vitamin K and required FFP transfusions.
MRI showed no hepatic or extrahepatic siderosis. Salivary gland biopsies revealed no iron deposits. Liver biopsy confirmed siderosis and fibrosis, consistent with a diagnosis of GALD. Immunohistochemical staining for C5b-9 was negative.
The patient received two doses of IVIG with a favorable outcome, without requiring double-volume exchange transfusion or liver transplantation. At the last follow-up at two years of age, growth and liver function were normal, as was liver elastography (FibroScan). However, liver ultrasound showed a heterogeneous liver texture.
The mother had a subsequent pregnancy at 33 years old and received IVIG at 14 weeks of gestation, followed by weekly infusions until 37 weeks. No side effects were noted. She gave birth to a healthy boy with normal liver function.
A male newborn was born at 34 weeks of gestation to a 32-year-old mother (G2P1), with a history of one miscarriage. Delivery was induced due to maternal N-methyl-D-aspartate receptor encephalitis, diagnosed at 30 weeks of gestation, which did not improve with corticosteroids and IVIG. Antenatal ultrasound revealed intrauterine growth restriction.
At birth, the infant was admitted to the NICU for severe hypoglycemia, requiring high glucose infusion through total parenteral nutrition. He developed severe coagulopathy, requiring twice-daily transfusions of FFP. Laboratory tests showed cholestasis, hypoalbuminemia, thrombocytopenia, and an elevated ferritin level of 2515 μg/L.
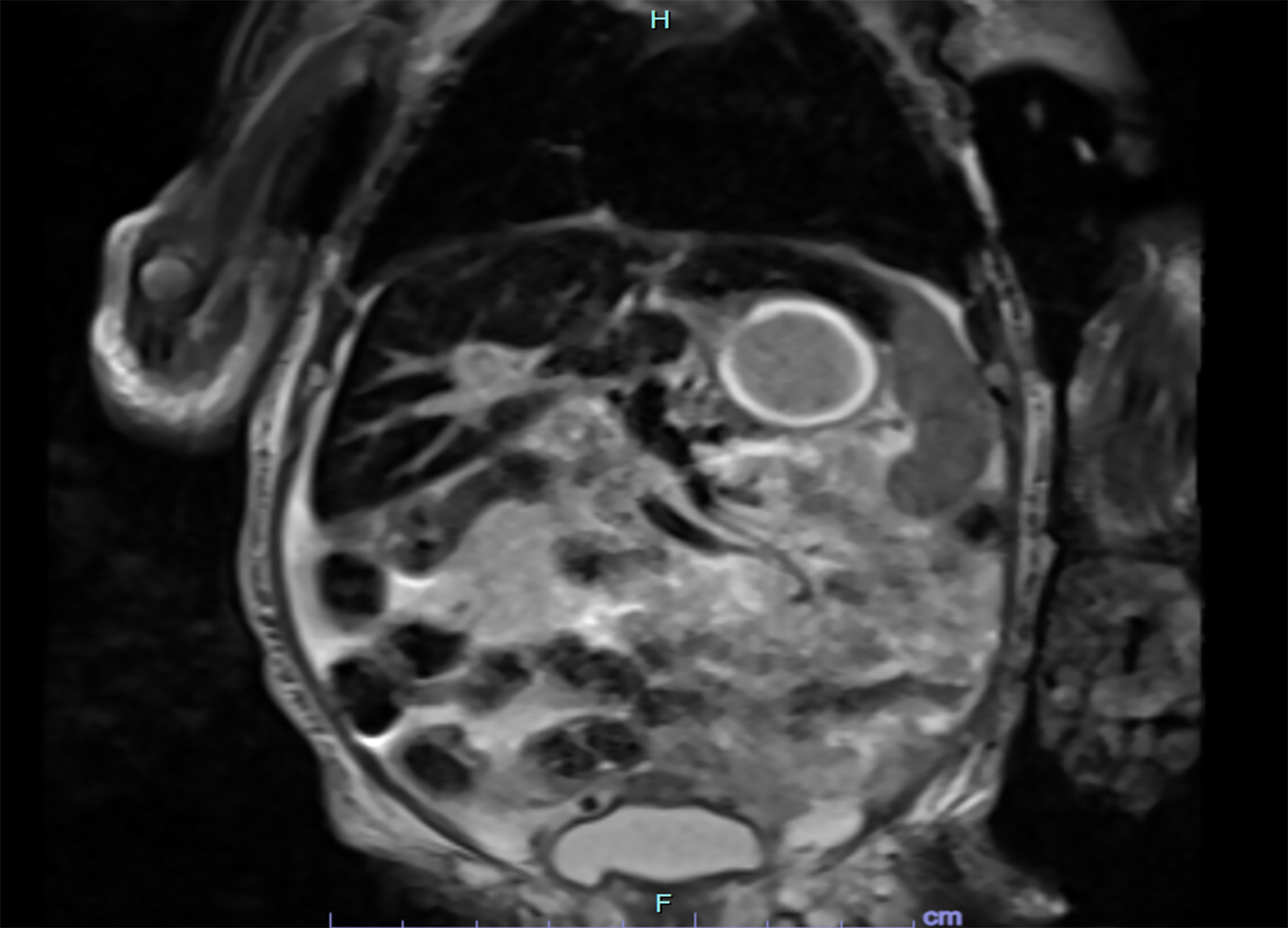
Liver ultrasound demonstrated a patent ductus venosus, which required embolization, and a heterogeneous liver. MRI confirmed hepatic siderosis (Figure 1), while salivary gland biopsy was normal.
Despite receiving three doses of IVIG and undergoing double-volume exchange transfusion, the patient showed no improvement. He was listed for liver transplantation on day 16 of life but unfortunately succumbed to a cerebral hemorrhage.
Autopsy confirmed the diagnosis of GALD with evidence of hepatic and pancreatic siderosis.
The mother had a subsequent pregnancy, and she received IVIG weekly from 18 weeks of gestation. She gave birth to a healthy child. Table 1 summarizes the characteristics of each patient.
| Case 1 | Case 2 | Case 3 | Case 4 | |
| Mothers’ characteristics | 26-year-old, gravida 2, para 2 | 34-year-old, gravida 5, para 3A2 | 31-year-old, gravida 2, para 1A1 | 32-year-old, gravida 2, para 0A1, N-methyl-D-aspartate encephalitis at 30 gestation weeks |
| Antenatal findings | None | Oligoamnios, asymmetric intrauterine growth restriction | None | Intrauterine growth restriction |
| Term of birth (weeks of gestation) | 39 | 37 | 33 | 34 |
| Preterm birth cause | N/A | N/A | Placental abruption | Maternal encephalitis |
| Major clinical presentation | Hypoglycemia | Hypoglycemia | Hypoglycemia, birth asphyxia, hepatorenal syndrome, hepatomegaly | Hypoglycemia |
| Laboratory findings | Thrombocytopenia, coagulopathy, hyperammonemia, iron saturation level 86% | Thrombocytopenia, cholestasis, high ferritin (4103 μg/L) | Thrombocytopenia, cholestasis, coagulopathy, hypoalbuminemia, high ferritin (3347 μg/L), iron saturation level 88% | Thrombocytopenia, cholestasis, coagulopathy, hypoalbuminemia, high ferritin (2515 μg/L) |
| Liver ultrasound findings | Portosystemic shunt: Patent ductus venosus | Portosystemic shunt: Patent ductus venosus | Heterogenous liver | Heterogenous liver, portosystemic shunt: Patent ductus venosus |
| Magnetic resonance imaging findings | Not performed | No hepatic or extrahepatic siderosis | No hepatic siderosis. Nonspecific for GALD | Hepatic siderosis |
| Salivary glands pathology | No iron deposits | No iron deposits | No iron deposits (Prussian blue) | No iron deposits |
| Liver pathology | Fibrosis, remodeling of the hepatic parenchyma, iron overload. Conclusion: GALD confirmed | Portal fibrosis, giant hepatocytes, siderosis. Conclusion: Nonspecific hepatitis | Membrane attack complex negative, fibrosis, siderosis. Conclusion: GALD confirmed | On autopsy: Fibrosis, siderosis +++, siderosis of pancreas |
| Treatments | Embolization of porto-systemic shunt and liver transplantation | None | Two doses of IVIG | Three doses of IVIG. Double volume exchange transfusion. Listed for liver transplantation |
| Outcomes | Normal liver function and normal growth | Spontaneous amelioration of liver function. Now: Normal liver function | Now: Normal liver function | Dead from cerebral hemorrhage |
| Subsequent pregnancy | No | No | Yes | Yes |
| Antenatal IVIG | N/A | N/A | Yes (14-16-18-19 weeks then every week) | Yes |
| Outcome for subsequent pregnancy | N/A | N/A | Healthy child | Healthy child |
GALD is the leading treatable cause of liver failure in newborns[2]. However, it presents with a broad-spectrum of clinical manifestations. NH, is the most common, but not the only, presentation of GALD, this explains why the appellation NH has been replaced by GALD[1]. The incidence of GALD is estimated to be 4 per 10000 live births, in the United States, according to Kasko et al[6].
GALD is attributed to the transplacental transfer of maternal IgG antibodies, which induce fetal hepatocyte injury through the membrane attack complex (complement component 5Cb-9 complex)[3]. The specific fetal liver antigen targeted by these antibodies remains unknown, despite extensive research efforts[7].
Destruction of fetal hepatocytes leads to dysregulation of iron homeostasis, resulting in siderosis of hepatic and extrahepatic tissues[4]. Additionally, it contributes to congenital collapse of liver lobules and acute liver failure with or without iron overload[4]. Depressed hepcidin expression in fetal liver disrupts placental iron regulation[8]. Specifically, the absence of hepcidin reduces negative feedback on ferroportin, resulting in increased transplacental iron flux[1]. Moreover, decreased transferrin gene expression, results in an excess of non-transferrin-bound iron (NTBI)[1]. Siderosis occurs in tissues capable of absorbing NTBI but unable to expel it[8].
The onset of GALD manifestation ranges from 18 weeks of gestation to three months of age[9]. Most often children present within hours of birth[9]. GALD should be considered in cases of unexplained stillbirth, fetal loss and early postnatal death, particularly in the context of severe liver disease[1]. In these cases, autopsy can be valuable in diagnosing GALD and may guide the decision to administer IVIG in subsequent pregnancies.
As observed in our cases, prenatal signs of GALD, including oligohydramnios, fetal growth restriction, hepatomegaly and fetal hydrops can be observed from 18 weeks of gestation[3,10]. Additional fetal ultrasound findings may include ascites, abnormalities of the liver and spleen[3]. Staicu et al[3] and Sciard et al[11] reported that in fetuses who present these features, MRI can aid in the diagnosis of GALD, after excluding placental vascular pathology and infectious fetopathies. Prenatal MRI typically reveals hepatic atrophy and significant iron overload in both hepatic and extrahepatic tissues[3,11].
In our cohort, we presented patients with a range of GALD severity, from benign, self-resolving case to patient who required liver transplantation or had fatal outcome. The clinical spectrum of GALD is broad, spanning from asymp
Coagulopathy with a high INR with or without hemorrhagic manifestations as well as recurrent hypoglycemia are frequent manifestations. These manifestations are followed by progressive edema, hypoalbuminemia, cholestasis and renal impairment[17]. They typically appear within the first few hours to days after birth, due to the antenatal onset of the disease[17].
Laboratory findings in GALD, beyond cholestasis and features of acute liver failure, typically include mild elevation of aminotransferase levels, rarely exceeding 100 UI/L[17]. Ferritin (> 800 ng/mL), serum iron, and transferrin saturation (> 70%) are elevated, while transferrin levels are decreased[12,18]. Although ferritin is a sensitive marker for NH, it lacks specificity[1].
Alpha foeto-protein levels may be markedly elevated, ranging from 100000 ng/mL to 600000 ng/mL[1]. Thrombocytopenia is frequently observed[6].
Doppler ultrasound commonly demonstrates a patent ductus venosus in affected neonates[4].
The diagnosis relies on identification of extrahepatic iron overload sparing the reticuloendothelial system[5], in the context of severe liver disease, with or without iron overload[1]. T2-weighted MRI typically reveals iron deposition in the brain, pancreas and liver[5]. Iron staining of salivary gland tissue using Perls’ Prussian blue may aid in diagnosis[1]. However, salivatory gland biopsies can be negative in 30% of cases[12].
Histopathological findings in the liver include hepatocyte loss, siderosis, giant cells and pseudoacinar transformation[1]. Panlobular parenchymal colapse and regenerative nodules may also be observed[1].
A definitive diagnosis requires immunohistochemical detection of the membrane attack complex (C5b-9) in hepatocytes and giant cells[1,5]. However, this technique is not widely available[12]. Absence of these finding does not allow to rule out the diagnosis of GALD[17].
The current standard of care for GALD is IVIG therapy, with or without exchange transfusions, achieving a survival rate of approximately 75% without the need for liver transplantation[17]. Feldman and Whittington[1] recommend administering a single dose of IVIG (1 g/kg) to any infant presenting with liver failure when GALD is suspected. Following diagnosis confirmation, a second IVIG dose should be administered, accompanied by a double volume exchange transfusion[19,20]. Normalization of INR can take up to 6 weeks[19].
Liver transplantation remains the definitive treatment for infants with acute liver failure who do not respond to medical therapy[9]. In our cohort one patient was successfully transplanted. Due to the overall severity of illness and the small size of affected infants, liver transplantation can be particularly challenging[9].
Neonates often receive left lateral segment graft. ABO-incompatible liver transplantation is a viable option, as newborns have not yet developed blood group sensitization[9]. However, liver transplantation in the group of age 0 day to 90 days is associated with prolonged hospitalizations, extended intensive care units stays, longer mechanical ventilation and high reoperation rates, compared to older children[21].
Survival rates of liver transplant in infants with GALD were reported to be 35% in the cohort studied by Rand et al[19], while Taylor et al[8] observed a 43% survival rate among infant transplanted. In contrast, Kelgeri et al[22] reported a 100% survival rate in their cohort of GALD-transplanted infants.
The recurrence risk of GALD in subsequent pregnancies is estimated to be as high as 90%, irrespective of paternal changes[4,10]. This risk can be significantly reduced through antenatal administration of IVIG starting at 14 weeks, 16 weeks, 18 weeks of gestation and continuing weekly until birth. The effectiveness of IVIG in preventing adverse outcome has been well established[8,23].
Whitington et al[23] reported a 94% of healthy offspings in treated pregnancies compared to 30% in untreated ones (P < 0.0001). A review of 73 pregnancies treated with antenatal IVIG confirmed that all resulted in live births, with no infant requiring liver transplantation[24]. Beyond reducing the frequency of affected live-born infants, antenatal IVIG has also been shown to lower stillbirth rates[23].
Baruteau et al[16] documented an 8/8 survival rate among newborns of treated mothers, compared to 0/9 among untreated cases. Of the eight surviving infants, five exhibited mild, transient liver disease[16].
While IVIG therapy is generally well tolerated, mild side effects have been reported, including headaches, rash, shivering, tinnitus and burn sensation at the injection site[16]. More severe side effects, such as pancytopenia, have also been described, as reported by Sarker et al[25], with a few additional cases documented in the literature[26].
GALD must be considered in any newborn with coagulopathy or signs of liver failure. Prompt laboratory and radiological investigations are essentials to ensure early diagnosis and timely intervention. GALD is a serious condition that can have life-threatening consequences potentially leading to liver transplant or death if not promptly diagnosed and treated. Careful management of future pregnancies is crucial for optimizing outcomes. Antenatal IVIG prophylaxis, administered to mothers starting at 14 weeks of gestation, has demonstrated excellent results, significantly improving outcomes for the unborn child. Further research is needed to enhance our understanding of this rare disease and develop more effective therapeutic strategies.
The authors would like to thank the members of the gastroenterology unit at the Sainte Justine Center for their valuable support in the management of the reported cases. We also acknowledge the contributions of the radiology and pathology departments for their expertise and collaboration.
| 1. | Feldman AG, Whitington PF. Neonatal hemochromatosis. J Clin Exp Hepatol. 2013;3:313-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 86] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 2. | European Association for the Study of the Liver; Clinical practice guidelines panel; Wendon J, Panel members, Cordoba J, Dhawan A, Larsen FS, Manns M, Samuel D, Simpson KJ, Yaron I; EASL Governing Board representative; Bernardi M. EASL Clinical Practical Guidelines on the management of acute (fulminant) liver failure. J Hepatol. 2017;66:1047-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 669] [Article Influence: 74.3] [Reference Citation Analysis (1)] |
| 3. | Staicu A, Popa-Stanila R, Albu C, Chira A, Constantin R, Boitor-Borza D, Surcel M, Rotar IC, Cruciat G, Muresan D. Neonatal Hemochromatosis: Systematic Review of Prenatal Ultrasound Findings-Is There a Place for MRI in the Diagnostic Process? J Clin Med. 2023;12:2679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 4. | Lopriore E, Mearin ML, Oepkes D, Devlieger R, Whitington PF. Neonatal hemochromatosis: management, outcome, and prevention. Prenat Diagn. 2013;33:1221-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Di Giorgio A, Bartolini E, Calvo PL, Cananzi M, Cirillo F, Della Corte C, Dionisi-Vici C, Indolfi G, Iorio R, Maggiore G, Mandato C, Nebbia G, Nicastro E, Pinon M, Ranucci G, Sciveres M, Vajro P, D'Antiga L. Diagnostic Approach to Acute Liver Failure in Children: A Position Paper by the SIGENP Liver Disease Working Group. Dig Liver Dis. 2021;53:545-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 6. | Kasko O, Klose E, Rama G, Newberry D, Jnah A. Gestational Alloimmune Liver Disease: A Case Study. Neonatal Netw. 2018;37:271-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Rieneck K, Rasmussen KK, Schoof EM, Clausen FB, Holze H, Bergholt T, Jørgensen MH, Christensen VB, Almaas R, Jordal PL, Locard-Paulet M, Runager K, Nielsen LK, Schlotmann BC, Weischenfeldt JL, Jensen LJ, Dziegiel MH. Hunting for the elusive target antigen in gestational alloimmune liver disease (GALD). PLoS One. 2023;18:e0286432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 8. | Taylor SA, Kelly S, Alonso EM, Whitington PF. The Effects of Gestational Alloimmune Liver Disease on Fetal and Infant Morbidity and Mortality. J Pediatr. 2018;196:123-128.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Larson-Nath C, Vitola BE. Neonatal Acute Liver Failure. Clin Perinatol. 2020;47:25-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Zermano S, Novak A, Vogrig E, Parisi N, Driul L. GALD: new diagnostic tip for early diagnosis - a case report and literature review. Front Reprod Health. 2023;5:1077304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 11. | Sciard C, Collardeau-Frachon S, Atallah A, Combourieu D, Massardier J, Heissat S, Gaucherand P, Guibaud L, Massoud M. Prenatal imaging features suggestive of liver gestational allo immune disease. J Gynecol Obstet Hum Reprod. 2019;48:61-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Costaguta A, Alvarez F. [The new paradigm of neonatal hemochromatosis: fetal alloimmune hepatitis]. Arch Argent Pediatr. 2012;110:237-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Casas-Alba D, Clotet J, Inarejos EJ, Jou C, Fons C, Molera C. Broadening the spectrum of neonatal hemochromatosis. J Matern Fetal Neonatal Med. 2020;33:1024-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 14. | Yeh PJ, Huang SF, Chiang MC, Wang CJ, Lai MW. Efficacy of Intravenous Immunoglobulin/Exchange Transfusion Therapy on Gestational Alloimmune Liver Disease. Front Pediatr. 2021;9:680730. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Mulzer LM, Reutter H, Jüngert J, Knisely AS, Schmid M, Hoerning A, Morhart P. Premature birth associated with a favorable course in gestational alloimmune liver disease (GALD): A case report. Front Pediatr. 2023;11:1104530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 16. | Baruteau J, Heissat S, Broué P, Collardeau-Frachon S, Bouvier R, Fabre M, Debiec H, Ronco P, Uzan M, Narcy P, Cordier MP, Lachaux A, Lamireau T, Elleau C, Filet JP, Mitanchez D, Dupuy MP, Salaün JF, Odent S, Davison J, Debray D, Guigonis V. Transient neonatal liver disease after maternal antenatal intravenous Ig infusions in gestational alloimmune liver disease associated with neonatal haemochromatosis. J Pediatr Gastroenterol Nutr. 2014;59:629-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Roos Mariano da Rocha C, Rostirola Guedes R, Kieling CO, Rossato Adami M, Cerski CT, Gonçalves Vieira SM. Neonatal Liver Failure and Congenital Cirrhosis due to Gestational Alloimmune Liver Disease: A Case Report and Literature Review. Case Rep Pediatr. 2017;2017:7432859. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 18. | Bonilla S, Prozialeck JD, Malladi P, Pan X, Yu S, Melin-Aldana H, Whitington PF. Neonatal iron overload and tissue siderosis due to gestational alloimmune liver disease. J Hepatol. 2012;56:1351-1355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Rand EB, Karpen SJ, Kelly S, Mack CL, Malatack JJ, Sokol RJ, Whitington PF. Treatment of neonatal hemochromatosis with exchange transfusion and intravenous immunoglobulin. J Pediatr. 2009;155:566-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 91] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 20. | Taylor SA, Whitington PF. Neonatal acute liver failure. Liver Transpl. 2016;22:677-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 76] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 21. | Sundaram SS, Alonso EM, Anand R; Study of Pediatric Liver Transplantation Research Group. Outcomes after liver transplantation in young infants. J Pediatr Gastroenterol Nutr. 2008;47:486-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Kelgeri C, Kanthimathinathan HK, Couper M, Alnagar A, Biradar V, Sharif K, Hartley J, Mirza D, Gupte GL. Etiology, Characteristics, and Outcomes of Neonatal Liver Failure: Lessons Learned Over the Last 3 Decades. J Pediatr. 2024;275:114245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 23. | Whitington PF, Kelly S, Taylor SA, Nóbrega S, Schreiber RA, Sokal EM, Hibbard JU. Antenatal Treatment with Intravenous Immunoglobulin to Prevent Gestational Alloimmune Liver Disease: Comparative Effectiveness of 14-Week versus 18-Week Initiation. Fetal Diagn Ther. 2018;43:218-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 24. | Regan S, Kao C, Chambers T, Chandra S. Antenatal intravenous immunoglobulin treatment for gestational alloimmune liver disease: A systematic review. J Obstet Gynaecol Ca. 2019;41:731-732. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 25. | Sarker M, DeBolt C, Strong N. Intravenous immunoglobulin induced pancytopenia while preventing development of gestational alloimmune liver disease: A case report. Case Rep Womens Health. 2022;35:e00422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Herrmann A, Samelson-Jones BJ, Brake S, Samelson R. IVIG-Associated Maternal Pancytopenia during Treatment for Neonatal Alloimmune Thrombocytopenia. AJP Rep. 2017;7:e197-e200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/