Published online Mar 9, 2024. doi: 10.5409/wjcp.v13.i1.88912
Peer-review started: October 14, 2023
First decision: December 23, 2023
Revised: January 3, 2024
Accepted: February 18, 2024
Article in press: February 18, 2024
Published online: March 9, 2024
Processing time: 144 Days and 9.1 Hours
Lung damage in systemic juvenile arthritis (sJIA) is one of the contemporary topics in pediatric rheumatology. Several previous studies showed the severe course and fatal outcomes in some patients. The information about interstitial lung disease (ILD) in the sJIA is scarce and limited to a total of 100 cases.
To describe the features of sJIA patients with ILD in detail.
In the present retrospective cohort study, information about 5 patients less than 18-years-old with sJIA and ILD were included. The diagnosis of sJIA was made according to the current 2004 and new provisional International League of Associations for Rheumatology criteria 2019. ILD was diagnosed with chest computed tomography with the exclusion of other possible reasons for concurrent lung involvement. Macrophage activation syndrome (MAS) was diagnosed with HLH-2004 and 2016 EULAR/ACR/PRINTO Classification Criteria and hScores were calculated during the lung involvement.
The onset age of sJIA ranged from 1 year to 10 years. The time interval before ILD ranged from 1 mo to 3 years. The disease course was characterized by the prevalence of the systemic features above articular involvement, intensive rash (100%), persistent and very active MAS (hScore range: 194-220) with transaminitis (100%), and respiratory symptoms (100%). Only 3 patients (60%) developed a clubbing phenomenon. All patients (100%) had pleural effusion and 4 patients (80%) had pericardial effusion at the disease onset. Two patients (40%) developed pulmonary arterial hypertension. Infusion-related reactions to tocilizumab were observed in 3 (60%) of the patients. One patient with trisomy 21 had a fatal disease course. Half of the remaining patients had sJIA remission and 2 patients had improvement. Lung disease improved in 3 patients (75%), but 1 of them had initial deterioration of lung involvement. One patient who has not achieved the sJIA remission had the progressed course of ILD. No cases of hyper-eosinophilia were noted. Four patients (80%) received canakinumab and one (20%) tocilizumab at the last follow-up visit.
ILD is a severe life-threatening complication of sJIA that may affect children of different ages with different time intervals since the disease onset. Extensive rash, serositis (especially pleuritis), full-blown MAS with transaminitis, lymphopenia, trisomy 21, eosinophilia, and biologic infusion reaction are the main predictors of ILD. The following studies are needed to find the predictors, pathogenesis, and treatment options, for preventing and treating the ILD in sJIA patients.
Core Tip: We evaluated 5 patients with systemic juvenile arthritis and interstitial lung disease. This is an ultra-rare, unrecognized, life-threatening and potentially fatal complication of systemic juvenile arthritis. This complication is usually associated with early onset age, systemic features of the disease, especially with pleuritis, severe and long-term macrophage activation syndrome, lymphopenia, trisomy 21 syndrome, and biologic anaphylaxis. The recognition of these symptoms can help in early suspicion of this severe complication.
- Citation: Belozerov KE, Solomatina NM, Isupova EA, Kuznetsova AA, Kostik MM. Systemic juvenile idiopathic arthritis–associated lung disease: A retrospective cohort study. World J Clin Pediatr 2024; 13(1): 88912
- URL: https://www.wjgnet.com/2219-2808/full/v13/i1/88912.htm
- DOI: https://dx.doi.org/10.5409/wjcp.v13.i1.88912
Juvenile idiopathic arthritis with systemic onset (sJIA) is the most life-threatening form of JIA due to macrophage activation syndrome (MAS) and internal organ involvement[1,2]. The lung disease is a rare, severe, potentially fatal manifestation of sJIA. Its prevalence has grown in the last 20 years from single cases at the beginning of 2000 to 5% nowadays[1]. Lung involvement in sJIA includes pulmonary arterial hypertension (PAH), interstitial lung disease (ILD), presenting with pulmonary alveolar proteinosis, and lipoid pneumonia[1,2]. Patients may have a combination of ILD and PAH. The mechanisms of lung involvement in sJIA are still unclear. It is known that hyperproduction of interleukin (IL) 1, IL-18, and interferon (IFN) γ pathway signaling are the main key points of the pathogenesis of lung involvement in sJIA. Several risk factors, associated with lung involvement in sJIA were proposed: onset age < 2 years, prevalence of systemic features, chronic or recurrent or poor controlled MAS, persistent and progressed lymphopenia, anaphylaxis to IL-6 and IL blockers, trisomy on 21 chromosomes[3]. The outcomes of the patients with sJIA with lung diseases (sJIA-LDs) are extremely serious. In the first case series of 25 patients published by Kimura et al[4], 68% died in 8.8±11.4 mo after the lung involvement appeared. Several recent studies showed better outcomes with a mortality rate near 4.6% which is 7.5 times more than in sJIA patients without lung involvement[5]. There are no approved pathogenic medications for the treatment of lung involvement in sJIA patients. Treatment with IFN-γ direct blocker (emapalumab), indirect blockers (JAK-inhibitors), and anti-IL-18 blockers (IL-18 binding protein) seems to be promising but requires approval[6-8]. Additional treatment options might include corticosteroids (glucocorticosteroids), anti-IL-1 and anti-IL-6 biologics, cyclosporine A and tacrolimus, mofetil mycophenolate, intravenous immunoglobulin, and PAH for specific treatment to control the pulmonary blood pressure and oxygen supplementation[1,2,9]. Children with sJIA and chronic lung involvement are more susceptible to lung infections and require specific prophylaxis[4].
The Information about patients with lung involvement is scarce and related to patients whose chronic lung disease has already been diagnosed.
Our study aimed to describe the patients with sJIA-LD with a focus on the initial clinical and laboratory features.
In the present retrospective cohort study, we included available information about 5 pediatric patients (onset age < 18 years) with sJIA-LD. The diagnosis of sJIA was made according to the current 2004[10] and new provisional International League of Associations for Rheumatology (ILAR) criteria 2019[11]. If the patient did not fit one of the major criteria he/she was diagnosed with sJIA-like disease (probable”/“possible” sJIA).
ILD was diagnosed with chest computed tomography and the exclusion of other possible reasons for concurrent lung involvement.
MAS was diagnosed with HLH-2004[12] and 2016 EULAR/ACR/PRINTO Classification Criteria for Macrophage Activation Syndrome Complicating Systemic Juvenile Idiopathic Arthritis[13] and hScore was calculated during the lung involvement[14].
The sample size was not calculated initially. We included all available cases in our center. We used only descriptive statistics (quantitative and categorical data).
Diagnosis of sJIA was established in all patients. Two patients did not meet the current ILAR criteria in 2004, because patients 1 and 2 did not have arthritis at the onset. All patients corresponded to new provisional criteria for sJIA 2019. Patient 2 developed severe polyarthritis 2 years after the disease onset. The correspondence of the patients to current (2004) and new provisional (2019) ILAR criteria is shown in Table 1. Serositis was presented by pericarditis in 3 cases (patients 1, 2, and 4), pleurisy in 4 cases (patients 1,2,3 and 4 ), and peritonitis in patient 2. One patient (patient 5) developed leucopenia at onset due to MAS. The demographic characteristics of patients are in Table 2.
| No. ID | Major criteria | Minor criteria | Overall correspondence ILAR2004 criteria | Overall correspondence ILAR2019 criteria | |||||
| Fever | Erythematous rash | Arthritis | Lymphadenopathy/hepatomegaly/splenomegaly | Serositis | Arthralgia | Leukocytosis as/mm3 | |||
| 1 | Yes | Yes | No | Yes | Yes | No | 53.300 | No | Yes |
| 2 | Yes | Yes | No | Yes | Yes | Yes | 15.100 | No | Yes |
| 3 | Yes | Yes | Yes | Yes | Yes | Yes | 47.200 | Yes | Yes |
| 4 | Yes | Yes | Yes | Yes | Yes | Yes | 30,820 | Yes | Yes |
| 5 | Yes | Yes | Yes | Yes | Yes | No | 2.300 | Yes | Yes |
| No. | Sex | Age of onset in yr | Age of last follow-up visit in yr | Time to sJIA-LD | Concomitant disease |
| 1 | Male | 1 | 10 | 3 yr | -- |
| 2 | Female | 2 | 11 | 3 yr | -- |
| 3 | Female | 10 | 17 | 1 month | -- |
| 4 | Male | 2 | 11 | 4 months | Atopic dermatitis |
| 5 | Female | 2 | 7 | 4 yr | Trisomy 21 syndrome |
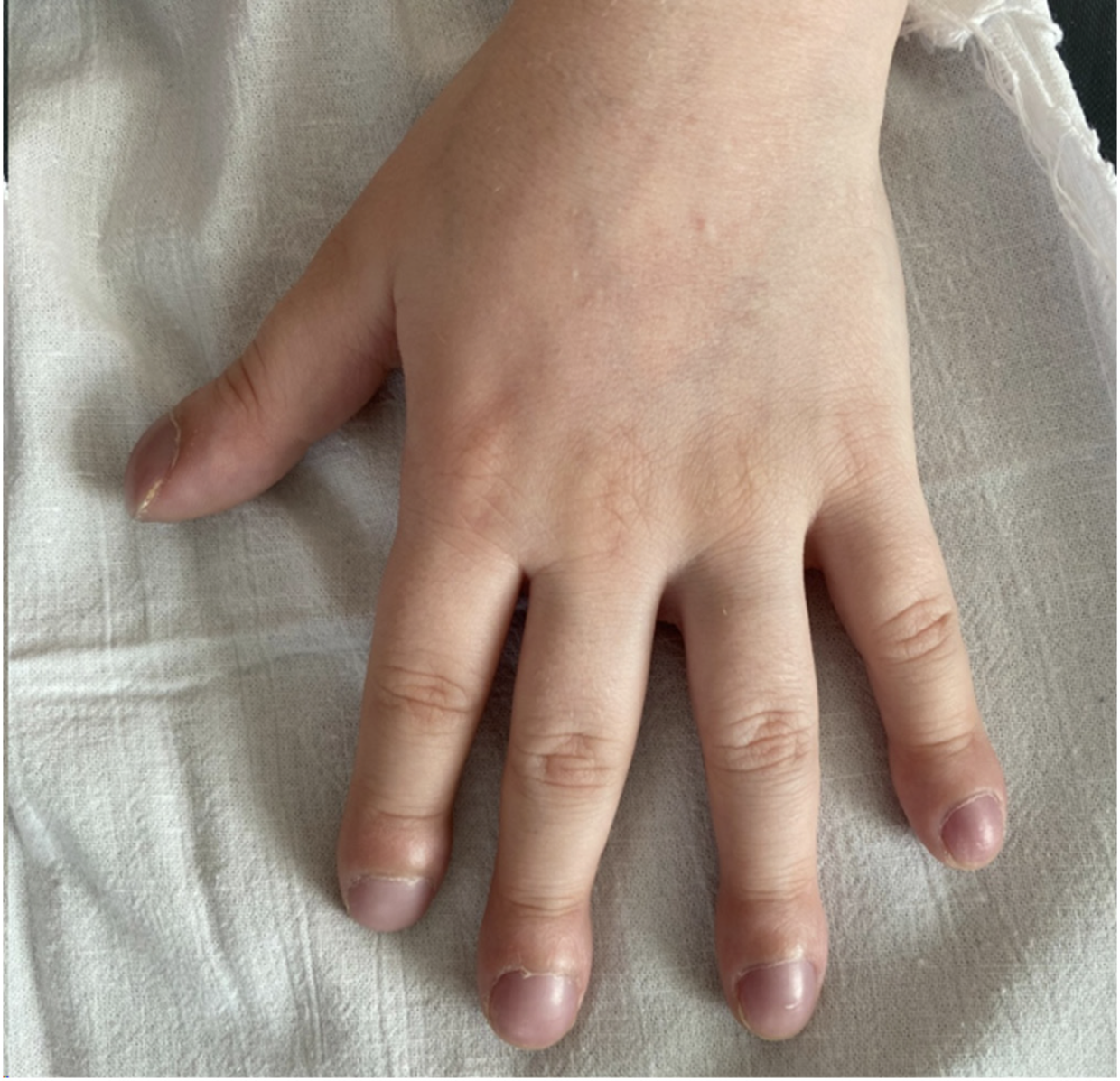
All patients had dyspnea, but only 1 patient had a cough (patient 2). Clubbing (Figure 1) of the fingers was in 3 (60%) patients. Respiratory failure was diagnosed in 4 (80%) patients. They were admitted to the Intensive Care Unit for respiratory support.
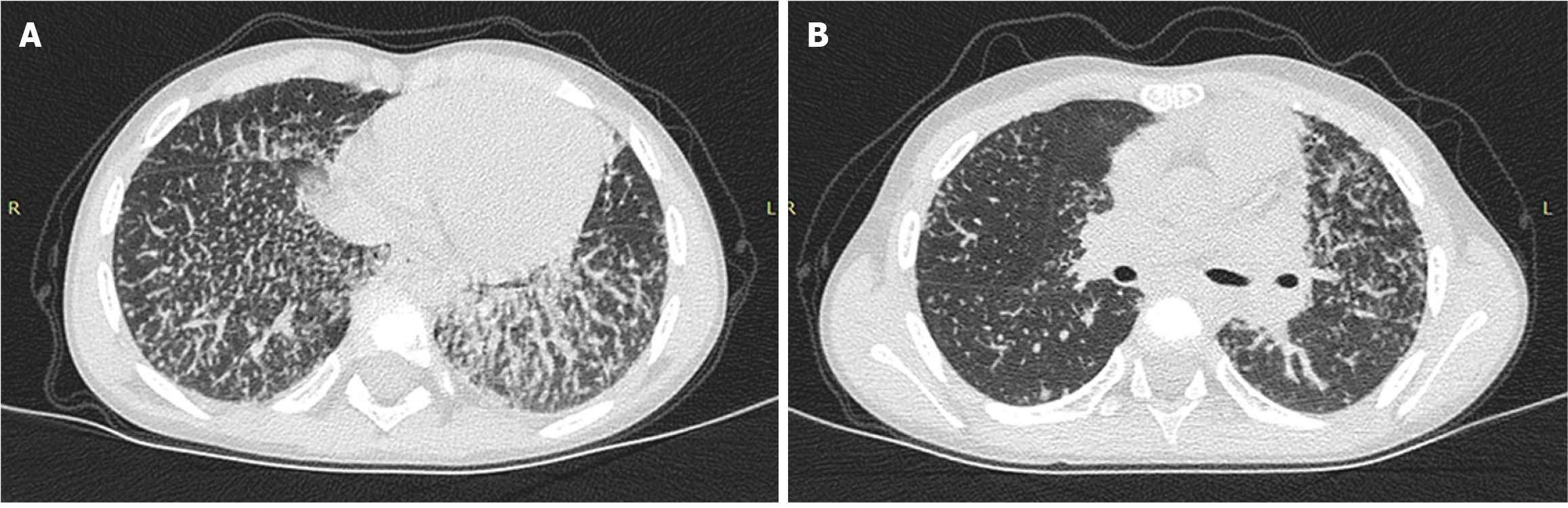
In 2 cases, lung disease was diagnosed at the sJIA onset (patients 3 and 4) and in 3 cases, lung disease developed later in patients 1, 2, and 5 (Figure 2).
Two patients developed PAH, patient 1 had persistent PAH and required PAH-specific treatment, and patient 2 had temporary PAH at the lung disease onset and this was successfully resolved in 1 mo after high-dose systemic glucocorticosteroid treatment.
All patients have met the above mentioned MAS criteria. Severe full-blown MAS had all 5 (100%) patients at the onset with a score range of 194-220 points. All patients had persistent/relapsed courses of MAS. In all cases, ILD was detected in patients with features of MAS. Interestingly, MAS was more aggressive and hardly controlled in patients with early onset (patients 1 and 2) and patients with trisomy 21 syndrome (patient 5).
We observed the risk factors which were previously described[1,3]. Infusion reaction on tocilizumab had 3 (60%) patients. Trisomy 21 syndrome had 1 patient (Patient 5). Four patients developed sJIA at the age of 2 years or younger, and patient 3 developed sJIA at the age of 10 years. All patients had severe MAS.
All patients received corticosteroids. High doses of intravenous corticosteroids were received at the onset and with a major flare, including MAS. Inhalational corticosteroids (budesonide and fluticasone) were used in 1 case with lipoid pneumonia. All 5 patients have experienced tocilizumab treatment, and as we have already pointed out, infusion reaction was diagnosed in 3 cases (patients 1, 2, and 4). In 4 of 5 (80%) cases, tocilizumab was changed to canakinumab; abatacept was added to canakinumab therapy in patient 1. Patient 1 with PAH has received sildenafil with positive dynamic and stabilization in PAH.
The outcomes of our cases were different. Patient 5 with trisomy 21 (Down Syndrome) had a fatal outcome. The female developed a flare of sJIA with respiratory and heart failure. Two patients (patients 2 and 3) achieved sJIA remission with the improvement of ILD, but patient 2 initially had deterioration followed by improvement. Two patients had incomplete sJIA remission (patients 1 and 4) with ILD improvement in patient 4, but patient 1, despite the combination treatment of canakinumab and abatacept has not achieved ILD improvement. His PAH is under the control of sildenafil. Patients with early onset had more severe ILD. Demographic characteristics, clinical with ILAR criteria, radiological features, and treatment outcomes are in Tables 1-4.
| No. | Rash | Hepatitis | Lymphadenopathy | Cough | Dyspnea | Clubbing | Respiratory failure | Infusion reaction on TCZ | PAH | MAS | hScorein points | Heart involvement | X-ray, CT, or MRI or US findings | Eosinophils as × 109/L |
| 1 | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | 220 | Pericarditis | ILD, pleurisy | 0.63 |
| 2 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 209 | Myocarditis, Pericarditis | Interstitial lung disease with intralobular foci, pleurisy | 0.19 |
| 3 | Yes | Yes | No | No | Yes | No | No | No | No | Yes | 224 | N | Alveolitis, diffuse focal lesions, pleurisy | 0.29 |
| 4 | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes | 194 | Pericarditis | ILD, atelectasis, pleurisy | 0.4 |
| 5 | Yes | Yes | Yes | No | Yes | No | Yes | No | No | Yes | 220 | Heart Failure | ILD, pleurisy | 0.12 |
| No. | First biologic | Biologic at the ILD onset | Final therapy | Respiratory symptoms at the last follow-up visit | Dose reduction of non-biologic DMARDs | Dose reduction of BA | Discontinuation of GCS therapy | The outcome of sJIA-LD | The outcome of sJIA |
| 1 | TCZ | TCZ | CAN + ABT + CsA + GCS + SDF | No | No | No | No | Progression | Improvement |
| 2 | TCM | CAN | CAN + MMF + inhGS | No | Yes | No | Yes | Progression with the following improvement | Remission |
| 3 | CAN | CAN | TCZ + CsA | No | No | No | Yes | Improvement | Remission |
| 4 | TCZ | TCZ | CAN + MMF | No | No | No | Yes | Improvement | Improvement |
| 5 | TCZ | TCZ | CAN + GCS + IVIG | - | - | - | No | Death | Death |
sJIA is an autoinflammatory disease that is characterized by fever, rash, arthritis, and damage to other organs[1].
MAS is a life-threatening complication in children with sJIA, related to the hyperproduction of proinflammatory cytokines, especially: IL-1, IL-6, IL-18, IFN-γ[2,9,15,16]. sJIA-LD is a troupe of nosology that is characterized by chronic lung disease in patients with sJIA[1] Now, it is clear, that lung involvement in sJIA patients is associated with persistent systemic inflammation, especially with MAS[1-3].
Unfortunately, typical respiratory symptoms at the beginning of the disease are usually absent or poorly expressed, and because of this, sJIA-LD occurs unexpectedly in many patients. For example, the cough was present in 33%-43%, tachypnea in 33%-38%, auscultative changes in the lungs in 30%, while hypoxemia was already registered in 43% of patients, and symptomatic PAH in 30%[1,3].
Sometimes, the main clinical symptoms indicating lung lesions are distal phalangeal dilation or the so-called clubbing symptom (61%) and erythema of the distal phalanges (34%).
Despite the diagnosis of sJIA, patients with lung involvement had unusual clinical presentations such as an itchy rash (56%), eosinophilia (37%), and unexplained intense abdominal pain (16%)[3].
In our group, patient 2 had a severe sJIA flare with aseptic peritonitis that required diagnostic surgery 1 year before the lung involvement.
Another important feature is the development of a hypersensitivity reaction (anaphylaxis) to 2-3 injections of tocilizumab in many children with JIA and lung damage[1,3,9]. The estimated probability of a hypersensitivity reaction during treatment with tocilizumab is up to 9.1%[17-19]. Three (60%) of our patients had a tocilizumab anaphylaxis. Hypersensitivity to biological agents was found to be a risk factor for ILD[1,3].
Lymphopenia (< 60% of the lower normal limit for age) was detected in sJIA patients with lung involvement. This could not be explained by the current MAS and was found in 42%. The combination of hyperferritinemia and severe lymphopenia serves as a marker of the risk of lung involvement in patients with sJIA[3]. Another important laboratory symptom is eosinophilia, associated with ILD in sJIA patients[3].
Pulmonary alveolar proteinosis is a poorly studied disease manifested by the accumulation of lipid substances in the alveoli due to ineffective excretion of lipid substances by macrophages[20]. Macrophage dysfunction in sJIA-LD is not associated with congenital defects of macrophages, as in primary lung disease[1,20-22]. Patients with MAS have a highly active systemic inflammation that contributes to macrophage differentiation disturbances[1]. Similar cytokine transmission pathways in MAS and sJIA-LD explain the close similarity between both conditions. Several cytokines, such as IFN-γ and IL-18 are now the focus of MAS pathogenesis[6,23].
The persistence of high levels of IL-18 in patients with sJIA receiving сanakinumab may explain the development of lung damage in children being in remission under the biological treatment[24].
In IL-18-dependent diseases, specific therapy with IL-18-binding protein is required, since other treatments may be ineffective[7].
It is known that the lungs are the main source of physiological production of IL-1β and IL-6. These proinflammatory cytokines, as well as the levels of the endogenous antagonist of IL-1 receptors, are higher in children under the age of 4 years, which may explain the higher frequency of ILD in younger children[25-28].
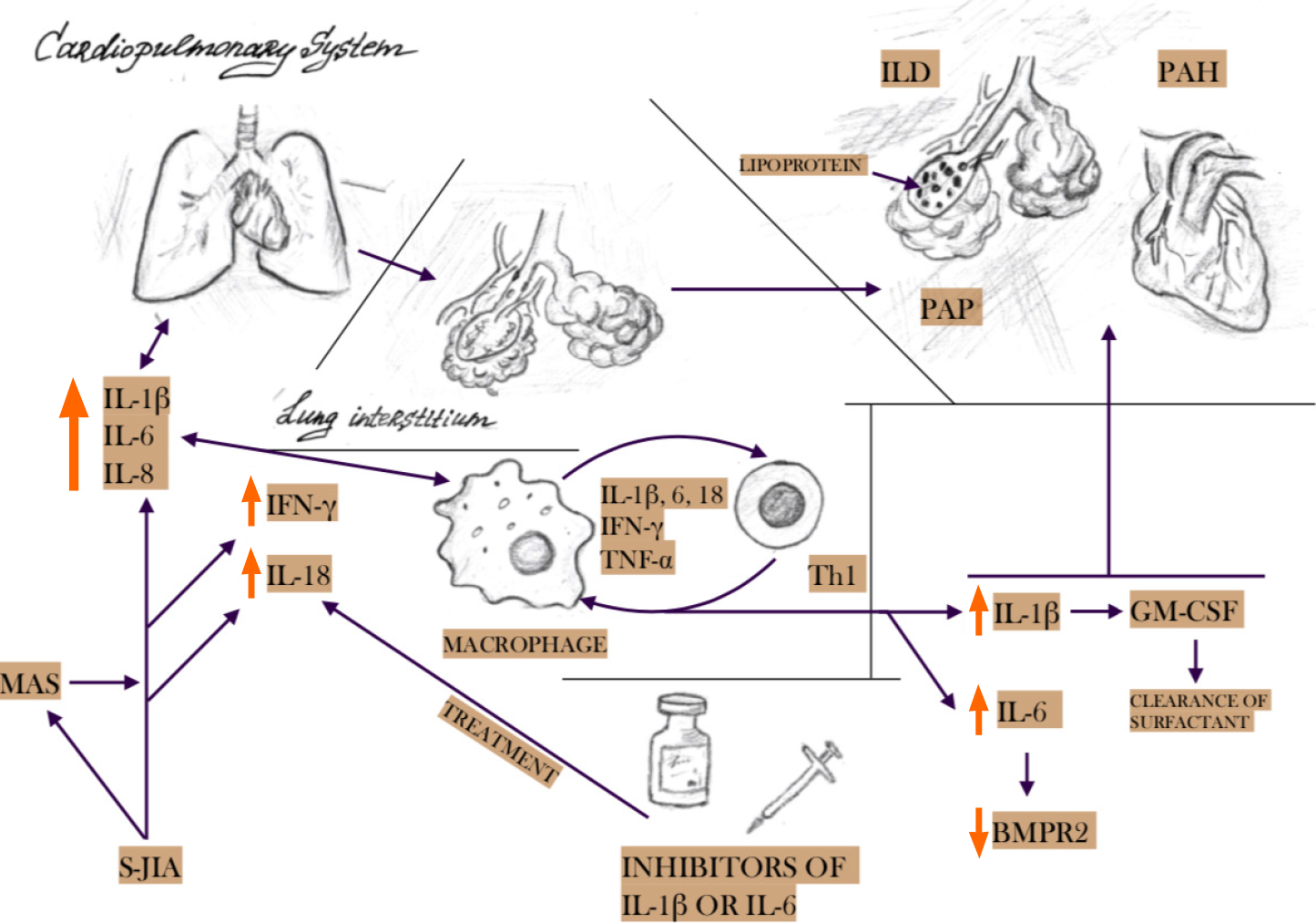
IL-1β, IL-6, and IFN-γ are the main cytokines involved in the pathogenesis of sJIA and MAS[1]. The same cytokines play a key role in lung tissue damage, in particular, due to activation and/or dysfunction of macrophages in the pulmonary interstitium[20-22]. Hyperinfection and increased regulation of innate immunity lead to an increase in the production of IL-1β, which stimulates the levels of granulocyte-macrophage colony-stimulating factor, as well as hyperproduction of surfactant and its accumulation in tissues and impaired clearance. Elevated levels of IL-6 inhibit the production of type II bone morphogenetic protein receptors, which control cell growth and differentiation. IL-18, associated with the IFN-γ signaling pathway, is also associated with severe forms of MAS and ILD in patients with sJIA. The level of this cytokine remains elevated, despite the control of systemic inflammation by IL-1 or IL-6 blockade. This may explain lung damage in patients with sJIA who are in remission with IL-1 and IL-6 blockade[6,23,24]. Chronic lung inflammation with accumulation of surfactant and lipoproteins in the alveoli leads to interstitial pulmonary fibrosis, decreased elasticity of the pulmonary artery with the formation of pulmonary hypertension[1]. A brief pathogenesis of lung damage in sJIA is shown in Figure 3.
The pathogenesis of PAH is a result of systemic inflammation with proinflammatory cytokine disbalance. It’s known, that the low expression of BMPR2 (bone morphogenic protein receptor type II) associated with potential endothelial dysfunction and PAH, in turn, one of the central cytokines in the pathogenesis of systemic arthritis (IL-6) in vitro BMPR reduced its activity[29-31].
In clinical practice, radiological methods are often used to diagnose lung lesions. sJIA-LD is characterized by compaction/infiltration of lung tissue, thickening of the interlobular septa, and damage to the peripheral parts of several lobes, mainly basal, para mediastinal, or anterior parts of the upper lobes in combination with the symptom of frosted glass, as well as the detection of enlarged lymph nodes with an increased density in CT of the chest with contrast[1,3].
The most alarming problem of sJIA-LD is the high mortality and a short life expectancy because of the development of lung damage. According to available data, 68% (n = 17) of patients died after 8.8 ± 11.4 mo from the onset of lung damage[4]. Unfortunately, mortality was about 40 times higher in the group of people with sJIA-LD[3]. In males, hypoxia at the beginning of lung damage, and neutrophilia in bronchial lavage (> 10 times higher) were considered the main predictors of death[3,31].
In managing children with ILD, a multidisciplinary approach is required with the participation of specialists in various fields, including a rheumatologist, pulmonologist, infectious disease specialist, rehabilitation specialist, psychologist, transplant surgery, as well as comprehensive laboratory and instrumental support, including, in particular, spirometry, pulse-oximetry, assessment of diffusion ability lung, computed tomography of the chest, echocardiography with assessment of pressure in the pulmonary artery, electrocardiography, assessment of sJIA and MAS laboratory activity. Sometimes, with chronic progressive hypoxemia, lung transplantation is the only method that can prolong the patient’s life. Knowledge of the pathogenesis of this condition is important for the formation of potential prediction markers, targeted therapy, and prognosis. The following studies are needed to find the predictors, pathogenesis, and treatment options, for preventing and treating the ILD in sJIA patients.
The main limitations of this study are related to the retrospective analysis and the very small sample size. The authors could not influence the treatment and could not if the treatment chosen in the past could influence the development of the complication and its severity or not. The absence of molecular studies decreased the value of this study.
ILD is a severe life-threatening complication of sJIA that may affect children of different ages with different time intervals since the disease onset. Extensive rash, serositis (especially pleuritis), full-blown MAS with transaminitis, lymphopenia, trisomy 21, eosinophilia, and biologic infusion reaction are the main predictors of ILD.
Chronic lung involvement is an ultra-rare, unrecognized, poorly understood condition in children with systemic juvenile idiopathic arthritis.
To describe this ultra-rare complication and disease course in children with systemic juvenile idiopathic arthritis with interstitial lung involvement.
The clinical and laboratory data of these patients are not well-diagnosed. The number of patients is nearly a hundred.
The clinical, radiological, and laboratory features were described in detail. The H score was applied to these patients for the first time.
The main clinical features of the disease are associated with early onset, chronic course of macrophage activation syndrome, pleuritis at onset, protracted lymphopenia, eosinophilia, and anaphylaxis drug-reaction on biologics.
This life-threatening complication is associated with chronic, persistent macrophage activation syndrome, drug-associated anaphylaxis similar to DRESS syndrome.
The future collection of information on these patients requires the following study of the features of the macrophage activation syndrome (cytokine profile, interferon signatures), and new target drugs are needed.
| 1. | Schulert GS, Yasin S, Carey B, Chalk C, Do T, Schapiro AH, Husami A, Watts A, Brunner HI, Huggins J, Mellins ED, Morgan EM, Ting T, Trapnell BC, Wikenheiser-Brokamp KA, Towe C, Grom AA. Systemic Juvenile Idiopathic Arthritis-Associated Lung Disease: Characterization and Risk Factors. Arthritis Rheumatol. 2019;71:1943-1954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 164] [Article Influence: 23.4] [Reference Citation Analysis (1)] |
| 2. | Minoia F, Davì S, Horne A, Demirkaya E, Bovis F, Li C, Lehmberg K, Weitzman S, Insalaco A, Wouters C, Shenoi S, Espada G, Ozen S, Anton J, Khubchandani R, Russo R, Pal P, Kasapcopur O, Miettunen P, Maritsi D, Merino R, Shakoory B, Alessio M, Chasnyk V, Sanner H, Gao YJ, Huasong Z, Kitoh T, Avcin T, Fischbach M, Frosch M, Grom A, Huber A, Jelusic M, Sawhney S, Uziel Y, Ruperto N, Martini A, Cron RQ, Ravelli A; Pediatric Rheumatology International Trials Organization; Childhood Arthritis and Rheumatology Research Alliance; Pediatric Rheumatology Collaborative Study Group; Histiocyte Society. Clinical features, treatment, and outcome of macrophage activation syndrome complicating systemic juvenile idiopathic arthritis: a multinational, multicenter study of 362 patients. Arthritis Rheumatol. 2014;66:3160-3169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 320] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 3. | Saper VE, Chen G, Deutsch GH, Guillerman RP, Birgmeier J, Jagadeesh K, Canna S, Schulert G, Deterding R, Xu J, Leung AN, Bouzoubaa L, Abulaban K, Baszis K, Behrens EM, Birmingham J, Casey A, Cidon M, Cron RQ, De A, De Benedetti F, Ferguson I, Fishman MP, Goodman SI, Graham TB, Grom AA, Haines K, Hazen M, Henderson LA, Ho A, Ibarra M, Inman CJ, Jerath R, Khawaja K, Kingsbury DJ, Klein-Gitelman M, Lai K, Lapidus S, Lin C, Lin J, Liptzin DR, Milojevic D, Mombourquette J, Onel K, Ozen S, Perez M, Phillippi K, Prahalad S, Radhakrishna S, Reinhardt A, Riskalla M, Rosenwasser N, Roth J, Schneider R, Schonenberg-Meinema D, Shenoi S, Smith JA, Sönmez HE, Stoll ML, Towe C, Vargas SO, Vehe RK, Young LR, Yang J, Desai T, Balise R, Lu Y, Tian L, Bejerano G, Davis MM, Khatri P, Mellins ED; Childhood Arthritis and Rheumatology Research Alliance Registry Investigators. Emergent high fatality lung disease in systemic juvenile arthritis. Ann Rheum Dis. 2019;78:1722-1731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 159] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 4. | Kimura Y, Weiss JE, Haroldson KL, Lee T, Punaro M, Oliveira S, Rabinovich E, Riebschleger M, Antón J, Blier PR, Gerloni V, Hazen MM, Kessler E, Onel K, Passo MH, Rennebohm RM, Wallace CA, Woo P, Wulffraat N; Childhood Arthritis Rheumatology Research Alliance Carra Net Investigators. Pulmonary hypertension and other potentially fatal pulmonary complications in systemic juvenile idiopathic arthritis. Arthritis Care Res (Hoboken). 2013;65:745-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 125] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 5. | Proceedings of the 25th European Paediatric Rheumatology Congress (pReS 2018). Pediatr Rheumatol. 2018;16:52. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 6. | Put K, Avau A, Brisse E, Mitera T, Put S, Proost P, Bader-Meunier B, Westhovens R, Van den Eynde BJ, Orabona C, Fallarino F, De Somer L, Tousseyn T, Quartier P, Wouters C, Matthys P. Cytokines in systemic juvenile idiopathic arthritis and haemophagocytic lymphohistiocytosis: tipping the balance between interleukin-18 and interferon-γ. Rheumatology (Oxford). 2015;54:1507-1517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 120] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 7. | Canna SW, Girard C, Malle L, de Jesus A, Romberg N, Kelsen J, Surrey LF, Russo P, Sleight A, Schiffrin E, Gabay C, Goldbach-Mansky R, Behrens EM. Life-threatening NLRC4-associated hyperinflammation successfully treated with IL-18 inhibition. J Allergy Clin Immunol. 2017;139:1698-1701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 276] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 8. | Mistry P, Reid J, Pouliquen I, McHugh S, Abberley L, DeWall S, Taylor A, Tong X, Rocha Del Cura M, McKie E. Safety, tolerability, pharmacokinetics, and pharmacodynamics of single-dose antiinterleukin- 18 mAb GSK1070806 in healthy and obese subjects. Int J Clin Pharmacol Ther. 2014;52:867-879. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Schulert GS, Grom AA. Pathogenesis of macrophage activation syndrome and potential for cytokine- directed therapies. Annu Rev Med. 2015;66:145-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 299] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 10. | Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, He X, Maldonado-Cocco J, Orozco-Alcala J, Prieur AM, Suarez-Almazor ME, Woo P; International League of Associations for Rheumatology. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31:390-392. [PubMed] |
| 11. | Martini A, Ravelli A, Avcin T, Beresford MW, Burgos-Vargas R, Cuttica R, Ilowite NT, Khubchandani R, Laxer RM, Lovell DJ, Petty RE, Wallace CA, Wulffraat NM, Pistorio A, Ruperto N; Pediatric Rheumatology International Trials Organization (PRINTO). Toward New Classification Criteria for Juvenile Idiopathic Arthritis: First Steps, Pediatric Rheumatology International Trials Organization International Consensus. J Rheumatol. 2019;46:190-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 431] [Cited by in RCA: 331] [Article Influence: 47.3] [Reference Citation Analysis (0)] |
| 12. | Henter JI, Horne A, Aricó M, Egeler RM, Filipovich AH, Imashuku S, Ladisch S, McClain K, Webb D, Winiarski J, Janka G. HLH-2004: Diagnostic and therapeutic guidelines for hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2007;48:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3075] [Cited by in RCA: 3783] [Article Influence: 199.1] [Reference Citation Analysis (1)] |
| 13. | Ravelli A, Minoia F, Davì S, Horne A, Bovis F, Pistorio A, Aricò M, Avcin T, Behrens EM, De Benedetti F, Filipovic L, Grom AA, Henter JI, Ilowite NT, Jordan MB, Khubchandani R, Kitoh T, Lehmberg K, Lovell DJ, Miettunen P, Nichols KE, Ozen S, Pachlopnik Schmid J, Ramanan AV, Russo R, Schneider R, Sterba G, Uziel Y, Wallace C, Wouters C, Wulffraat N, Demirkaya E, Brunner HI, Martini A, Ruperto N, Cron RQ; Paediatric Rheumatology International Trials Organisation; Childhood Arthritis and Rheumatology Research Alliance; Pediatric Rheumatology Collaborative Study Group; Histiocyte Society. 2016 Classification Criteria for Macrophage Activation Syndrome Complicating Systemic Juvenile Idiopathic Arthritis: A European League Against Rheumatism/American College of Rheumatology/Paediatric Rheumatology International Trials Organisation Collaborative Initiative. Arthritis Rheumatol. 2016;68:566-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 384] [Article Influence: 38.4] [Reference Citation Analysis (0)] |
| 14. | Fardet L, hScore for Reactive Hemophagocytic Syndrome [cited 4 October 2023]. In MDCalc. Available from: https://www.mdcalc.com/calc/10089/hscore-reactive-hemophagocytic-syndrome. |
| 15. | Vastert SJ, Prakken BJ. Paediatric rheumatic disease: Diagnosing macrophage activation syndrome in systemic JIA. Nat Rev Rheumatol. 2014;10:640-642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Yasin S, Schulert GS. Systemic juvenile idiopathic arthritis and macrophage activation syndrome: update on pathogenesis and treatment. Curr Opin Rheumatol. 2018;30:514-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 17. | Rabinovitch M. Molecular pathogenesis of pulmonary arterial hypertension. J Clin Invest. 2008;118:2372-2379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 277] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 18. | Yokota S, Itoh Y, Morio T, Origasa H, Sumitomo N, Tomobe M, Tanaka K, Minota S. Tocilizumab in systemic juvenile idiopathic arthritis in a real-world clinical setting: results from 1 year of postmarketing surveillance follow-up of 417 patients in Japan. Ann Rheum Dis. 2016;75:1654-1660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 83] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 19. | Kostik MM, Isupova EA, Chikova IA, Dubko MF, Masalova VV, Snegireva LS, Kalashnikova OV, Chasnyk VG. Reasons for inactive disease and flare in systemic onset juvenile idiopathic arthritis patients during tocilizumab treatment. Clin Exp Rheumatol. 2018;36:335-341. [PubMed] |
| 20. | Kostik MM, Isupova EA, Rumyantseva MV, Garipova NT, Gharabaghtsyan MM, Krasnogorskaya OL, Paneyakh MB, Rodionovskaya SR, Chikova IА, Masalova VV, Likhacheva TS. Interstitial lung disease in patients with juvenile arthritis with systemic onset: a description of a series of clinical cases with bibliographical review. Pediatria NA GN Speransky. 2020;99:125-136. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Seymour JF, Presneill JJ. Pulmonary alveolar proteinosis: progress in the first 44 years. Am J Respir Crit Care Med. 2002;166:215-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 445] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 22. | Uchida K, Nakata K, Carey B, Chalk C, Suzuki T, Sakagami T, Koch DE, Stevens C, Inoue Y, Yamada Y, Trapnell BC. Standardized serum GM-CSF autoantibody testing for the routine clinical diagnosis of autoimmune pulmonary alveolar proteinosis. J Immunol Methods. 2014;402:57-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 85] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 23. | Bracaglia C, de Graaf K, Pires Marafon D, Guilhot F, Ferlin W, Prencipe G, Caiello I, Davì S, Schulert G, Ravelli A, Grom AA, de Min C, De Benedetti F. Elevated circulating levels of interferon-γ and interferon-γ-induced chemokines characterise patients with macrophage activation syndrome complicating systemic juvenile idiopathic arthritis. Ann Rheum Dis. 2017;76:166-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 240] [Cited by in RCA: 236] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 24. | Brachat AH, Grom AA, Wulffraat N, Brunner HI, Quartier P, Brik R, McCann L, Ozdogan H, Rutkowska-Sak L, Schneider R, Gerloni V, Harel L, Terreri M, Houghton K, Joos R, Kingsbury D, Lopez-Benitez JM, Bek S, Schumacher M, Valentin MA, Gram H, Abrams K, Martini A, Lovell DJ, Nirmala NR, Ruperto N; Pediatric Rheumatology International Trials Organization (PRINTO) and the Pediatric Rheumatology Collaborative Study Group (PRCSG). Early changes in gene expression and inflammatory proteins in systemic juvenile idiopathic arthritis patients on canakinumab therapy. Arthritis Res Ther. 2017;19:13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 25. | GTEx Consortium. Human genomics. The Genotype-Tissue Expression (GTEx) pilot analysis: multitissue gene regulation in humans. Science. 2015;348:648-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4162] [Cited by in RCA: 3964] [Article Influence: 360.4] [Reference Citation Analysis (0)] |
| 26. | Sack U, Burkhardt U, Borte M, Schädlich H, Berg K, Emmrich F. Age-dependent levels of select immunological mediators in sera of healthy children. Clin Diagn Lab Immunol. 1998;5:28-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 79] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 27. | Iosef C, Alastalo TP, Hou Y, Chen C, Adams ES, Lyu SC, Cornfield DN, Alvira CM. Inhibiting NF-κB in the developing lung disrupts angiogenesis and alveolarization. Am J Physiol Lung Cell Mol Physiol. 2012;302:L1023-L1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 99] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 28. | De Benedetti F, Brunner HI, Ruperto N, Kenwright A, Wright S, Calvo I, Cuttica R, Ravelli A, Schneider R, Woo P, Wouters C, Xavier R, Zemel L, Baildam E, Burgos-Vargas R, Dolezalova P, Garay SM, Merino R, Joos R, Grom A, Wulffraat N, Zuber Z, Zulian F, Lovell D, Martini A; PRINTO; PRCSG. Randomized trial of tocilizumab in systemic juvenile idiopathic arthritis. N Engl J Med. 2012;367:2385-2395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 608] [Cited by in RCA: 632] [Article Influence: 45.1] [Reference Citation Analysis (1)] |
| 29. | Humbert M, Deng Z, Simonneau G, Barst RJ, Sitbon O, Wolf M, Cuervo N, Moore KJ, Hodge SE, Knowles JA, Morse JH. BMPR2 germline mutations in pulmonary hypertension associated with fenfluramine derivatives. Eur Respir J. 2002;20:518-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 131] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 30. | Deng Z, Morse JH, Slager SL, Cuervo N, Moore KJ, Venetos G, Kalachikov S, Cayanis E, Fischer SG, Barst RJ, Hodge SE, Knowles JA. Familial primary pulmonary hypertension (gene PPH1) is caused by mutations in the bone morphogenetic protein receptor-II gene. Am J Hum Genet. 2000;67:737-744. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 847] [Cited by in RCA: 786] [Article Influence: 30.2] [Reference Citation Analysis (0)] |
| 31. | Meyer KC, Raghu G, Baughman RP, Brown KK, Costabel U, du Bois RM, Drent M, Haslam PL, Kim DS, Nagai S, Rottoli P, Saltini C, Selman M, Strange C, Wood B; American Thoracic Society Committee on BAL in Interstitial Lung Disease. An official American Thoracic Society clinical practice guideline: the clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med. 2012;185:1004-1014. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 603] [Cited by in RCA: 774] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Rheumatology
Country/Territory of origin: Russia
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Sharma D, India; Zhai J, China S-Editor: Liu JH L-Editor: Filipodia P-Editor: Zhang XD