Published online Nov 28, 2016. doi: 10.5320/wjr.v6.i3.69
Peer-review started: April 27, 2016
First decision: June 17, 2016
Revised: July 27, 2016
Accepted: August 17, 2016
Article in press: August 18, 2015
Published online: November 28, 2016
Processing time: 211 Days and 23.3 Hours
Transcatheter arterial chemoembolization (TACE) is an effective palliative intervention that is widely accepted for the management of hepatocellular carcinoma (HCC). Post-TACE pulmonary complications resulting in acute lung injury (ALI) or acute respiratory distress syndrome (ARDS) are rare events. Pulmonary complications after TACE are thought to be related to chemical injury subsequent to the migration of the infused ethiodized oil or chemotherapeutic agent to the lung vasculature, facilitated by arteriovenous (AV) shunts within the hyper-vascular HCC. We review herein the literature on pulmonary complications related to TACE for HCC. Post-TACE pulmonary complications have included pulmonary oil embolism, interstitial pneumonitis, chemical pneumonitis, ALI, ARDS, lipoid pneumonia, acute eosinophilic and neutrophilic pneumonia, bilious pleuritis, pulmonary abscess, pulmonary tumor embolism, and possibly pulmonary metastasis with HCC. The risk factors associated with post-TACE pulmonary complications identified in the literature include large hyper-vascular HCC with AV shunts, large-volume Lipiodol infusion, and embolization via the right inferior phrenic artery. However, the absence of known risk factors is not a guarantee against serious complications. An astute awareness of the potential post-TACE pulmonary complications should expedite appropriate therapeutic interventions and increase potential for early recovery.
Core tip: Pulmonary complications after transcatheter arterial chemoembolization (TACE) for hepatocellular carcinoma (HCC) are thought to be related to chemical injury caused by the infused ethiodized oil or chemotherapeutic agent, which can migrate to the lung vasculature via arteriovenous (AV) shunts associated with the hyper-vascular HCC. The risk factors associated with post-TACE pulmonary complications include large hyper-vascular HCC, AV shunts, large volume of Lipiodol, and embolization via the right inferior phrenic artery. However, the absence of known risk factors is not a guarantee against serious complications. Careful monitoring of patients perioperatively and an awareness of the potential post-TACE pulmonary complications should expedite early recognition and effective management of these patients.
- Citation: Nhu QM, Knowles H, Pockros PJ, Frenette CT. Pulmonary complications of transcatheter arterial chemoembolization for hepatocellular carcinoma. World J Respirol 2016; 6(3): 69-75
- URL: https://www.wjgnet.com/2218-6255/full/v6/i3/69.htm
- DOI: https://dx.doi.org/10.5320/wjr.v6.i3.69
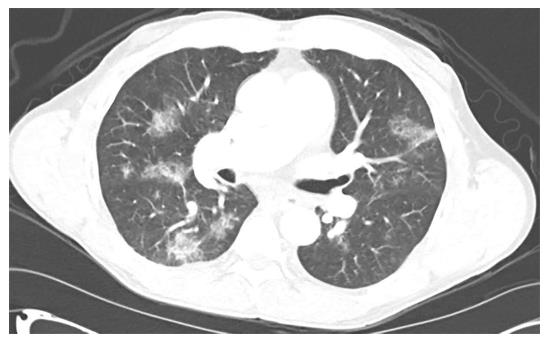
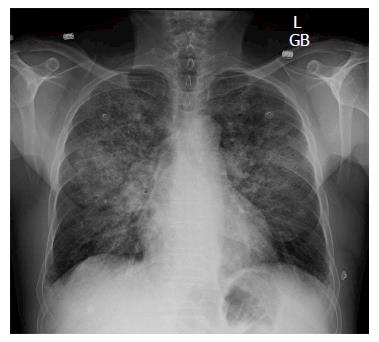
We recently reported an unexpected pulmonary complication of transcatheter arterial chemoembolization (TACE) with drug-eluting beads (DEB) for a small hepatocellular carcinoma (HCC)[1]. Briefly, a 62-year-old Caucasian male patient with hepatitis C cirrhosis, who also developed a new HCC and mild portopulmonary hypertension, underwent a DEB-TACE with LC beads (5 mL; 100-300 μm) loaded with doxorubicin (40 mg). Lipiodol (1 mL) was then infused. The patient experienced mild acute hypoxemic respiratory failure shortly after the procedure. Chest computed tomography (CT) angiogram showed multiple ground-glass opacities bilaterally (Figure 1). Chest X-ray demonstrated bilateral patchy infiltrates (Figure 2). Our patient was eventually diagnosed with post-TACE acute lung injury (ALI)/acute respiratory distress syndrome (ARDS). Methylprednisolone treatment resolved his symptoms rapidly. He subsequently underwent a successful liver transplantation as definitive treatment of his HCC, and is doing well now several years out from his surgery. As TACE gains increasing popularity, rare but potentially serious pulmonary complications may be encountered more frequently. Therefore, we review herein the literature on pulmonary complications related to TACE for HCC.
A systematic literature search was conducted using PubMed for “TACE”, “HCC”, “HCC”, “pulmonary”, “respiratory” and “lung”. Case reports and original studies were reviewed.
HCC, the most common primary hepatic malignancy, is a leading cause of cancer-related death in the world. It is expected that the incidence of HCC will continue to rise in the setting of maturation of existing hepatitis B and hepatitis C infections and the rising incidence of nonalcoholic steatohepatitis[2,3]. The treatment options for HCC include tumor resection; tumor ablation with radiofrequency, microwave, or ethanol; TACE; transarterial radioembolization; systemic small-molecule inhibitor therapy such as sorafenib; and liver transplantation. The treatment goal is either curative or palliative; however, many patients are not eligible for curative therapies, e.g., surgical resection and liver transplantation. TACE is a widely accepted palliative intervention and bridging therapy for selected patients with unresectable HCC, especially in patients awaiting liver transplantation, in whom it delays tumor growth or may even downstage HCC[4-9]. TACE improves survival in patients with unresectable HCC who have preserved liver function and no extrahepatic or vascular spread of tumor[10-12].
The rationale for TACE is based on the observation that HCC is predominantly supplied by hepatic arterial blood. In contrast, normal liver tissue is fed by the portal vein system predominantly and less so by the hepatic arterial system. The late Judah Folkman (1933-2008) crystallized the concept of angiogenesis involved during tumor growth. Hepatic tumoricentric arterialization is a multistep process that promotes a shift from venous blood supply toward arterial blood supply to tumors[13,14]. Drug delivery via the hepatic artery results in higher intratumoral drug concentration when compared to portal vein delivery[15], allowing TACE to be a tumor-selective intervention that spares normal hepatic tissue. Tumor regression was first demonstrated by hepatic artery ligation in 1966[16]. Two controlled, randomized studies and a meta-analysis in 2002 demonstrated a significant survival benefit for TACE, leading to its subsequent widespread use[10-12].
There are currently two accepted TACE techniques: Conventional TACE with an emulsion of Lipiodol-chemotherapeutic agent or DEB-TACE loaded with a chemotherapeutic agent such as doxorubicin. Lipiodol, an iodized poppy seed oil, accumulates selectively in the tumor and its neovasculature, causing a transient embolization of the HCC. The DEB come in multiple sizes, ranging from 100-300 μm to as large as 900 μm. While both conventional TACE and DEB-TACE have been shown to achieve comparable HCC tumor necrosis[17], doxorubicin DEB-TACE appears to be a relatively more effective and safe therapeutic modality for HCC when compared to conventional Lipiodol-based TACE[8,18-25]. However, TACE is not without risks. TACE can cause a post-embolization syndrome, a self-limited constellation of symptoms most notable for fever, malaise, severe nausea and vomiting, and pain in the right upper abdominal quadrant[26]. Some of the other known, but less frequent, complications related to TACE include hepatic artery injury, hepatic abscess and biloma, bacteremia and sepsis, biliary stricture, gallbladder ischemia, hepatic failure, variceal bleeding, renal failure, and pulmonary embolism[27]. Fatal pulmonary complications have been reported with smaller Tris-acryl gelatin microspheres (40 to 120 μm range)[28].
Pulmonary complications after TACE can range from mild dyspnea and mild hypoxia to ALI/ARDS and, at times, death. Post-TACE pulmonary oil embolism is a rare pulmonary complication of TACE. The current thought is that the injected oil migrates to the pulmonary vasculature via AV shunts within the hyper-vascular HCC, resulting in chemical injury that is further aggravated by the inflammatory response. Early studies with 131I-labeled Lipiodol showed that, when delivered to the hepatic artery of patients with hepatic cancer, Lipiodol predominantly concentrated in the liver, but also was detected in the lungs to a much lesser extent[29,30]. Pulmonary inflammatory reaction with bronchial pneumonia or hemorrhagic foci as a result of pulmonary oil embolism was observed in beagles infused with Lipiodol in the proper hepatic arteries[31]. In a rabbit model of ALI from ethiodized oil, hypoxia occurred early, within 24 h post exposure, followed by severe hypoxemia and extensive alveolar and interstitial inflammation, hemorrhage, and edema on days 2 to 4 after intravenous Lipiodol injection[32].
Conventional Lipiodol-based TACE and DEB-TACE both utilize chemotherapeutic agents, with doxorubicin being most commonly used, to achieve optimal therapeutic effects in HCC. Doxorubicin is an anthracycline chemotherapeutic antibiotic that inhibits DNA topoisomerase II. Classically associated with cardiotoxicity, the adverse effect of systemic doxorubicin on the lungs is a very rare occurrence[33]. However, perfusion of the lungs of healthy male foxhounds with doxorubicin caused dose-dependent damage to the pulmonary tissue[34]. Doxorubicin was reported to cause capillary leak syndrome in an adult patient and was observed to potentiate radiation pneumonitis in two pediatric patients[35,36]. Doxorubicin has also been associated with bronchiolitis obliterans organizing pneumonia[37,38]. Pegylated-liposomal doxorubicin (Doxil™) is a less toxic formulation. However, even Doxil™ caused transient and mild dyspnea, with transient relative neutropenia in patients within 1 to 5 min after infusion; symptoms resolved within 5 to 15 min after cessation of infusion[38,39]. Based on in vitro data, Skubitz et al[39] proposed that Doxil™-induced dyspnea was secondary to the transient adhesion of neutrophils in the pulmonary circulation, causing a decrease in pulmonary compliance. Taken together, Lipiodol and doxorubicin can each cause pulmonary tissue damage, and can potentially synergize and potentiate lung injury when used together in patients with HCC.
Pulmonary oil embolism complication rates in studies of patients with hepatic tumors undergoing TACE are summarized in Table 1. Post-TACE pulmonary complications have included pulmonary oil embolism, interstitial pneumonitis, chemical pneumonitis, ALI/ARDS, lipoid pneumonia, acute eosinophilic and neutrophilic pneumonia, bilious pleuritis, pulmonary abscess, pulmonary tumor embolism, and possibly pulmonary metastasis with HCC (Table 2). Proposed risk factors for developing pulmonary Lipiodol embolism include liver tumor larger than 10 cm and large Lipiodol volume of greater than 20 mL[40]. Multivariate logistic regression analysis revealed that Lipiodol dose appeared to be the primary risk factor for the development of pulmonary oil embolism[41]. Whereas the maximum safe Lipiodol dose was proposed by Chung et al[42] to be 15 to 20 mL, or approximately 0.25 mL/kg total body weight, Wu et al[41] reported that doses above 14.5 mL were associated with an increased risk for the development of pulmonary oil embolism, based on an ROC curve analysis that showed a sensitivity of 80% and a specificity of 66.3%[41,42]. Lin et al[43] reported a positive correlation (r = 0.78, P = 0.013) between Lipiodol volume and the duration of patient recovery, which was thought to be a surrogate indicator of lung injury severity.
| Ref. | Time | No. | Infusion | Pulmonary complications | Onset | Risk factors |
| Chung et al[42] | 01/1990-12/1991 | 336 patients | Iodized oil (3-40 mL) with Doxorubicin (20-60 mg) | Any pulmonary symptoms: 11 (3.27%) Pulmonary oil embolism: 6 (1.79%) Death: 1 (0.3%) | 2-5 d | Iodized oil > 20 mL |
| Tajima et al[44] | 06/1997-05/1999 | 44 patients | Lipiodol (0-10 mL) with Doxorubicin derivatives (0-50 mg) | Dyspnea or hemoptysis: 2 (4.5%) | – | Via right inferior phrenic artery |
| Sakamoto et al[45] | 1998 | 850 patients 2300 procedures | Iodized oil (2-15 mL) with drug mix, including Doxorubicin (10-30 mg) | Pulmonary oil embolism: 4 (0.17%) | – | Via right inferior phrenic artery |
| Xia et al[59] | 01/1997-02/2004 | 1348 patients 2012 procedures | Lipiodol (5-40 mL) with Chemotherapeutic agents | Pulmonary oil embolism: 1 (0.05%) | 6 h | Lipiodol 25 mL infusion |
| Wu et al[41] | 01/2005-12/2008 | 219 patients | Lipiodol (4-50 mL) with Doxorubucin (6-60 mg) | Pulmonary oil embolism: 20 (9.1%) Death: 5 (2.3%) | – | Lipiodol > 14.5 mL |
| Wu et al[60] | 01/2006-12/2006 | 89 patients | Lipiodol (4-50 mL) without or with Doxorubicin (6-60 mg) | Pulmonary oil embolism: 4 (4.5%) | 1 h-4 d | Lipiodol 20-50 mL infusion |
| Xu et al[40] | 01/2010-03/2012 | 478 patients 1026 procedures | Iodized oil (10-40 mL) with Epirubicin (20-60 mg) | Pulmonary oil embolism: 11 (2.3%) Death: 3 (0.6%) | 0 h-2 d | Tumor size > 10 cm Lipiodol > 20 mL |
| Post-TACE pulmonary complications | Ref. |
| Pulmonary oil embolism | Lin et al[43] |
| Weng et al[61] | |
| References from Table 1 | |
| Interstitial pneumonitis | Adaddin et al[57] |
| Chemical pneumonitis | Kwok et al[62] |
| ALI/ARDS | Khan et al[56] |
| Samejima et al[63] | |
| Czauderna et al[64] | |
| Hatamaru et al[47] | |
| Toro et al[65] | |
| Wu et al[60] | |
| Nhu et al[1] | |
| Lipoid pneumonia | Taupin et al[55] |
| Acute eosinophilic and neutrophilic pneumonia | Alifakioti et al[58] |
| Bilious pleuritis | Ichikawa et al[66] |
| Pulmonary abscess | Cubiella et al[67] |
| Pulmonary tumor embolism | Kwok et al[62] |
| Pulmonary metastasis with HCC | Liou et al[49] |
Post-TACE complications are increased when the right inferior phrenic artery (RIPA) is accessed for chemoembolization. Following chemoembolization via the RIPA, CT imaging showed a radiographic pulmonary complication rate of 70.5% (31 of the 44 patients): Lipiodol accumulation in the lungs in 23 patients, pulmonary consolidation in 30 patients, and pleural effusion in 18 patients[44]. Two of the 44 patients in that study developed clinical symptoms of acute dyspnea or hemoptysis. The majority of these patients, however, were reportedly asymptomatic despite the reported radiographic findings. Sakamoto et al[45] indicated a concern for a RIPA-pulmonary vasculature shunt contributing to the migration of Lipiodol or chemotherapeutic agent to the lung tissue causing ALI during TACE. This concern has been similarly shared by Bilbao et al[46] and Clark[27]. Hatamaru et al[47] reported a fatal pulmonary complication associated with TACE for HCC involving the RIPA, which was shown angiographically to contain an AV shunt.
The lungs are the most frequent sites of metastasis for HCC[48]. The prognosis of HCC patients who also have extrahepatic metastases is generally poor[48,49]. In the Uka et al[48] study of 995 consecutive patients with HCC, 151 (15.2%) patients had extrahepatic metastasis; 71 of these 151 patients (47%) had metastasis to the lungs. In a study of 230 HCC patients, Liou et al[49] reported increased pulmonary metastasis, 40 out of 156 patients (25.6%) vs 6 out of 74 patients (8.1%), in patients who underwent TACE with Lipiodol mixed with antineoplastic agents vs patients without TACE, respectively. The resulting assumption is TACE-induced tumor embolization as a means for metastasis to pulmonary tissue. Presumptive TACE-mediated lung metastasis was associated with: Solitary HCC larger than 10 cm, several to many tumors with the main one being larger than 5 cm or diffuse HCC, intrahepatic portal vein thrombosis, AV or arterioportal shunts, and the presence of incomplete HCC tumor necrosis after TACE[49]. In support of the observation by Liou et al[49], Liu et al[50] demonstrated in a human orthotopic HCC nude mouse model that occlusion of the hepatic artery resulted in tumor growth inhibition; however, there was increased potential for tumor invasion and distant metastases via a process that was associated with intratumoral hypoxia and epithelial-mesenchymal transition. In contrast, Lin et al[51] reported that transcatheter arterial embolization failed to increase the risk of pulmonary metastasis in a study of 287 patients. Further studies are necessary to determine the potential role of HCC arterial embolization in the development of lung metastasis.
The management of ALI/ARDS includes supportive care, oxygenation, and lung protective ventilation[52,53]. The management of post-TACE ALI/ARDS due to pulmonary oil embolism has been largely based on experience with the management of fat embolism syndrome[54,55]. The inflammatory response and oxidative stress involved in ARDS and chemical pneumonitis induced by Lipiodol and doxorubicin serve as the basis for intravenous steroidal treatments[56]. Successful steroid therapy has been demonstrated in several cases of post-TACE ALI/ARDS[1,43,57,58], however, treatment effectiveness remains to be demonstrated.
Post-TACE pulmonary complications in HCC patients are rare, occurring at 0.05% to 2.3% in most studies. Risk factors appear to be related to the presence of AV shunts, large hyper-vascular HCC, high volume of Lipiodol, and infusion via the RIPA. Careful evaluation of the patient’s overall health status, HCC characteristics and tumor vascularity minimizes the complication rate. However, the absence of known risk factors is not a guarantee against serious complications, as evidenced by our patient’s post-TACE ALI. Careful monitoring of the patient in the perioperative period and an astute awareness of the potential post-TACE pulmonary complications should expedite early recognition and prompt management to increase potential for good recovery in these patients.
We thank the Scripps physicians and house staff involved in the expert care of this patient. We thank Laura Nicholson, MD, PhD of the STSI for her critical review of the manuscript.
| 1. | Nhu QM, Knowles H, Pockros PJ, Frenette CT. An Unexpected Pulmonary Complication Following Transcatheter Arterial Chemoembolization of a Small Hepatocellular Carcinoma. J Clin Gastroenterol. 2016;50:524-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | El-Serag HB, Kanwal F. Epidemiology of hepatocellular carcinoma in the United States: where are we? Where do we go? Hepatology. 2014;60:1767-1775. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 475] [Cited by in RCA: 501] [Article Influence: 41.8] [Reference Citation Analysis (0)] |
| 3. | Singal AG, El-Serag HB. Hepatocellular Carcinoma From Epidemiology to Prevention: Translating Knowledge into Practice. Clin Gastroenterol Hepatol. 2015;13:2140-2151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 413] [Article Influence: 37.5] [Reference Citation Analysis (0)] |
| 4. | Wong R, Frenette C. Updates in the management of hepatocellular carcinoma. Gastroenterol Hepatol (NY). 2011;7:16-24. [PubMed] |
| 5. | Takayasu K. Transarterial chemoembolization for hepatocellular carcinoma over three decades: current progress and perspective. Jpn J Clin Oncol. 2012;42:247-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 36] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Crissien AM, Frenette C. Current management of hepatocellular carcinoma. Gastroenterol Hepatol (NY). 2014;10:153-161. [PubMed] |
| 7. | Sangro B, Salem R. Transarterial chemoembolization and radioembolization. Semin Liver Dis. 2014;34:435-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 8. | Wong R, Frenette C, Gish R. Hepatocellular carcinoma: locoregional and targeted therapies. Gastroenterol Clin North Am. 2011;40:599-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 9. | Tsochatzis EA, Fatourou EM, Triantos CK, Burroughs AK. Transarterial therapies for hepatocellular carcinoma. Recent Results Cancer Res. 2013;190:195-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734-1739. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2502] [Cited by in RCA: 2654] [Article Influence: 110.6] [Reference Citation Analysis (1)] |
| 11. | Lo CM, Ngan H, Tso WK, Liu CL, Lam CM, Poon RT, Fan ST, Wong J. Randomized controlled trial of transarterial lipiodol chemoembolization for unresectable hepatocellular carcinoma. Hepatology. 2002;35:1164-1171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1904] [Cited by in RCA: 2008] [Article Influence: 83.7] [Reference Citation Analysis (2)] |
| 12. | Cammà C, Schepis F, Orlando A, Albanese M, Shahied L, Trevisani F, Andreone P, Craxì A, Cottone M. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology. 2002;224:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 674] [Cited by in RCA: 622] [Article Influence: 25.9] [Reference Citation Analysis (1)] |
| 13. | Park YN, Yang CP, Fernandez GJ, Cubukcu O, Thung SN, Theise ND. Neoangiogenesis and sinusoidal “capillarization” in dysplastic nodules of the liver. Am J Surg Pathol. 1998;22:656-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 204] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 14. | Dezso K, Bugyik E, Papp V, László V, Döme B, Tóvári J, Tímár J, Nagy P, Paku S. Development of arterial blood supply in experimental liver metastases. Am J Pathol. 2009;175:835-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 15. | Sigurdson ER, Ridge JA, Kemeny N, Daly JM. Tumor and liver drug uptake following hepatic artery and portal vein infusion. J Clin Oncol. 1987;5:1836-1840. [PubMed] |
| 16. | Mori W, Masuda M, Miyanaga T. Hepatic artery ligation and tumor necrosis in the liver. Surgery. 1966;59:359-363. [PubMed] |
| 17. | Frenette CT, Osorio RC, Stark J, Fok B, Boktour MR, Guy J, Rhee J, Osorio RW. Conventional TACE and drug-eluting bead TACE as locoregional therapy before orthotopic liver transplantation: comparison of explant pathologic response. Transplantation. 2014;98:781-787. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Lopez RR, Pan SH, Lois JF, McMonigle ME, Hoffman AL, Sher LS, Lugo D, Makowka L. Transarterial chemoembolization is a safe treatment for unresectable hepatic malignancies. Am Surg. 1997;63:923-926. [PubMed] |
| 19. | Martin R, Geller D, Espat J, Kooby D, Sellars M, Goldstein R, Imagawa D, Scoggins C. Safety and efficacy of trans arterial chemoembolization with drug-eluting beads in hepatocellular cancer: a systematic review. Hepatogastroenterology. 2012;59:255-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 23] [Reference Citation Analysis (0)] |
| 20. | Recchia F, Passalacqua G, Filauri P, Doddi M, Boscarato P, Candeloro G, Necozione S, Desideri G, Rea S. Chemoembolization of unresectable hepatocellular carcinoma: Decreased toxicity with slow-release doxorubicineluting beads compared with lipiodol. Oncol Rep. 2012;27:1377-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 21. | Vogl TJ, Lammer J, Lencioni R, Malagari K, Watkinson A, Pilleul F, Denys A, Lee C. Liver, gastrointestinal, and cardiac toxicity in intermediate hepatocellular carcinoma treated with PRECISION TACE with drug-eluting beads: results from the PRECISION V randomized trial. AJR Am J Roentgenol. 2011;197:W562-W570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 146] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 22. | Dhanasekaran R, Kooby DA, Staley CA, Kauh JS, Khanna V, Kim HS. Comparison of conventional transarterial chemoembolization (TACE) and chemoembolization with doxorubicin drug eluting beads (DEB) for unresectable hepatocelluar carcinoma (HCC). J Surg Oncol. 2010;101:476-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 167] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 23. | Martin RC, Rustein L, Pérez Enguix D, Palmero J, Carvalheiro V, Urbano J, Valdata A, Kralj I, Bosnjakovic P, Tatum C. Hepatic arterial infusion of doxorubicin-loaded microsphere for treatment of hepatocellular cancer: a multi-institutional registry. J Am Coll Surg. 2011;213:493-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Skowasch M, Schneider J, Otto G, Weinmann A, Woerns MA, Dueber C, Pitton MB. Midterm follow-up after DC-BEAD™-TACE of hepatocellular carcinoma (HCC). Eur J Radiol. 2012;81:3857-3861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Song MJ, Park CH, Kim JD, Kim HY, Bae SH, Choi JY, Yoon SK, Chun HJ, Choi BG, Lee HG. Drug-eluting bead loaded with doxorubicin versus conventional Lipiodol-based transarterial chemoembolization in the treatment of hepatocellular carcinoma: a case-control study of Asian patients. Eur J Gastroenterol Hepatol. 2011;23:521-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 26. | Dhand S, Gupta R. Hepatic transcatheter arterial chemoembolization complicated by postembolization syndrome. Semin Intervent Radiol. 2011;28:207-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 27. | Clark TW. Complications of hepatic chemoembolization. Semin Intervent Radiol. 2006;23:119-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 28. | Brown KT. Fatal pulmonary complications after arterial embolization with 40-120- micro m tris-acryl gelatin microspheres. J Vasc Interv Radiol. 2004;15:197-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 29. | Raoul JL, Bourguet P, Bretagne JF, Duvauferrier R, Coornaert S, Darnault P, Ramée A, Herry JY, Gastard J. Hepatic artery injection of I-131-labeled lipiodol. Part I. Biodistribution study results in patients with hepatocellular carcinoma and liver metastases. Radiology. 1988;168:541-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 91] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 30. | Nakajo M, Kobayashi H, Shimabukuro K, Shirono K, Sakata H, Taguchi M, Uchiyama N, Sonoda T, Shinohara S. Biodistribution and in vivo kinetics of iodine-131 lipiodol infused via the hepatic artery of patients with hepatic cancer. J Nucl Med. 1988;29:1066-1077. [PubMed] |
| 31. | Kishi K, Sonomura T, Satoh M, Nishida N, Terada M, Shioyama Y, Yamada R. Acute toxicity of lipiodol infusion into the hepatic arteries of dogs. Invest Radiol. 1994;29:882-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 32. | Silvestri RC, Huseby JS, Rughani I, Thorning D, Culver BH. Respiratory distress syndrome from lymphangiography contrast medium. Am Rev Respir Dis. 1980;122:543-549. [PubMed] |
| 33. | Vahid B, Marik PE. Infiltrative lung diseases: complications of novel antineoplastic agents in patients with hematological malignancies. Can Respir J. 2008;15:211-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Minchin RF, Johnston MR, Schuller HM, Aiken MA, Boyd MR. Pulmonary toxicity of doxorubicin administered by in situ isolated lung perfusion in dogs. Cancer. 1988;61:1320-1325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 35. | Krzesiński P, Wierzbowski R, Gielerak G, Hałka J, Matysiak O, Smurzyński P. Impedance cardiography in the diagnosis of capillary leak syndrome caused by doxorubicin therapy in a patient with myeloma multiplex. Cardiol J. 2010;17:88-91. [PubMed] |
| 36. | Ma LD, Taylor GA, Wharam MD, Wiley JM. “Recall” pneumonitis: adriamycin potentiation of radiation pneumonitis in two children. Radiology. 1993;187:465-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 31] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 37. | Jacobs C, Slade M, Lavery B. Doxorubicin and BOOP. A possible near fatal association. Clin Oncol (R Coll Radiol). 2002;14:262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 38. | Vahid B, Marik PE. Pulmonary complications of novel antineoplastic agents for solid tumors. Chest. 2008;133:528-538. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 171] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 39. | Skubitz KM, Skubitz AP. Mechanism of transient dyspnea induced by pegylated-liposomal doxorubicin (Doxil). Anticancer Drugs. 1998;9:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 40. | Xu H, Yang R, Wang X, Zhu X, Chen H. Symptomatic pulmonary lipiodol embolism after transarterial chemoembolization for hepatic malignant tumor: clinical presentation and chest imaging findings. Chin Med J (Engl). 2014;127:675-679. [PubMed] |
| 41. | Wu GC, Chan ED, Chou YC, Yu CY, Hsieh TY, Hsieh CB, Chian CF, Ke FC, Dai YL, Su WL. Risk factors for the development of pulmonary oil embolism after transcatheter arterial chemoembolization of hepatic tumors. Anticancer Drugs. 2014;25:976-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 42. | Chung JW, Park JH, Im JG, Han JK, Han MC. Pulmonary oil embolism after transcatheter oily chemoembolization of hepatocellular carcinoma. Radiology. 1993;187:689-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 101] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 43. | Lin MT, Kuo PH. Pulmonary lipiodol embolism after transcatheter arterial chemoembolization for hepatocellular carcinoma. JRSM Short Rep. 2010;1:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 44. | Tajima T, Honda H, Kuroiwa T, Yabuuchi H, Okafuji T, Yosimitsu K, Irie H, Aibe H, Masuda K. Pulmonary complications after hepatic artery chemoembolization or infusion via the inferior phrenic artery for primary liver cancer. J Vasc Interv Radiol. 2002;13:893-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | Sakamoto I, Aso N, Nagaoki K, Matsuoka Y, Uetani M, Ashizawa K, Iwanaga S, Mori M, Morikawa M, Fukuda T. Complications associated with transcatheter arterial embolization for hepatic tumors. Radiographics. 1998;18:605-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 168] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 46. | Bilbao JI, Martínez-Cuesta A, Urtasun F, Cosín O. Complications of embolization. Semin Intervent Radiol. 2006;23:126-142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 47. | Hatamaru K, Azuma S, Akamatsu T, Seta T, Urai S, Uenoyama Y, Yamashita Y, Ono K. Pulmonary embolism after arterial chemoembolization for hepatocellular carcinoma: an autopsy case report. World J Gastroenterol. 2015;21:1344-1348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 8] [Cited by in RCA: 14] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 48. | Uka K, Aikata H, Takaki S, Shirakawa H, Jeong SC, Yamashina K, Hiramatsu A, Kodama H, Takahashi S, Chayama K. Clinical features and prognosis of patients with extrahepatic metastases from hepatocellular carcinoma. World J Gastroenterol. 2007;13:414-420. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 272] [Cited by in RCA: 353] [Article Influence: 18.6] [Reference Citation Analysis (1)] |
| 49. | Liou TC, Shih SC, Kao CR, Chou SY, Lin SC, Wang HY. Pulmonary metastasis of hepatocellular carcinoma associated with transarterial chemoembolization. J Hepatol. 1995;23:563-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 50. | Liu L, Ren ZG, Shen Y, Zhu XD, Zhang W, Xiong W, Qin Y, Tang ZY. Influence of hepatic artery occlusion on tumor growth and metastatic potential in a human orthotopic hepatoma nude mouse model: relevance of epithelial-mesenchymal transition. Cancer Sci. 2010;101:120-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 51. | Lin SC, Shih SC, Kao CR, Chou SY. Transcatheter arterial embolization treatment in patients with hepatocellular carcinoma and risk of pulmonary metastasis. World J Gastroenterol. 2003;9:1208-1211. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 52. | Janz DR, Ware LB. Approach to the patient with the acute respiratory distress syndrome. Clin Chest Med. 2014;35:685-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 53. | Modrykamien AM, Gupta P. The acute respiratory distress syndrome. Proc (Bayl Univ Med Cent). 2015;28:163-171. [PubMed] |
| 54. | Habashi NM, Andrews PL, Scalea TM. Therapeutic aspects of fat embolism syndrome. Injury. 2006;37 Suppl 4:S68-S73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 55. | Taupin D, Mukherjee V, Nathavitharana R, Green DA, Fridman D. Lipiodol embolism following transarterial chemoembolization: an atypical case. Crit Care Med. 2014;42:e481-e484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 56. | Khan I, Vasudevan V, Nallagatla S, Arjomand F, Ali R. Acute lung injury following transcatheter hepatic arterial chemoembolization of doxorubicin-loaded LC beads in a patient with hepatocellular carcinoma. Lung India. 2012;29:169-172. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 57. | Aladdin M, Ilyas M. Chemoembolization of hepatocellular carcinoma with drug-eluting beads complicated by interstitial pneumonitis. Semin Intervent Radiol. 2011;28:218-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 58. | Alifakioti D, Daccord C, Lachenal Y, Fitting JW. Acute eosinophilic and neutrophilic pneumonia following transarterial chemoembolization with drug-eluting beads loaded with doxorubicin for hepatocellular carcinoma: a case report. Respiration. 2014;88:426-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 59. | Xia J, Ren Z, Ye S, Sharma D, Lin Z, Gan Y, Chen Y, Ge N, Ma Z, Wu Z. Study of severe and rare complications of transarterial chemoembolization (TACE) for liver cancer. Eur J Radiol. 2006;59:407-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 122] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 60. | Wu GC, Perng WC, Chen CW, Chian CF, Peng CK, Su WL. Acute respiratory distress syndrome after transcatheter arterial chemoembolization of hepatocellular carcinomas. Am J Med Sci. 2009;338:357-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 61. | Weng MT, Chen CH. Education and Imaging. Hepatobiliary and pancreatic: pulmonary emboli after transcatheter arterial chemoembolization for hepatocellular carcinoma. J Gastroenterol Hepatol. 2010;25:1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 62. | Kwok PC, Lam TW, Lam CL, Lai AK, Lo HY, Chan SC. Rare pulmonary complications after transarterial chemoembolisation for hepatocellular carcinoma: two case reports. Hong Kong Med J. 2003;9:457-460. [PubMed] |
| 63. | Samejima M, Tamura S, Kodama T, Yuuki Y, Takasaki J, Sekiva R, Koga Y, Watanabe K. [Pulmonary complication following intra-arterial infusion of lipiodol-adriamycin emulsion for hepatocellular carcinoma, report of a case]. Nihon Igaku Hoshasen Gakkai Zasshi. 1990;50:24-28. [PubMed] |
| 64. | Czauderna P, Zbrzezniak G, Narozanski W, Sznurkowska K, Skoczylas-Stoba B, Stoba C. Pulmonary embolism: a fatal complication of arterial chemoembolization for advanced hepatocellular carcinoma. J Pediatr Surg. 2005;40:1647-1650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 65. | Toro A, Bertino G, Arcerito MC, Mannnino M, Ardiri A, Patane’ D, Di Carlo I. A lethal complication after transarterial chemoembolization with drug-eluting beads for hepatocellular carcinoma. Case Rep Surg. 2015;2015:873601. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 66. | Ichikawa T, Yamada T, Takagi H, Abe T, Ito H, Sakurai S, Nagamine T, Mori M. Transcatheter arterial embolization-induced bilious pleuritis in a patient with hepatocellular carcinoma. J Gastroenterol. 1997;32:405-409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 67. | Cubiella J, Sans M, Llovet JM, Bustamante J, Ferrer A, Caballeria J, Rodés J. Pulmonary abscess as a complication of transarterial embolization of multinodular hepatocellular carcinoma. Am J Gastroenterol. 1997;92:1942-1943. [PubMed] |
Manuscript source: Invited manuscript
Specialty type: Respiratory system
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): E
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Eghtesad B, Kim JH, Molinari M, Zhang ZM S- Editor: Ji FF L- Editor: A E- Editor: Li D