Published online Mar 28, 2015. doi: 10.5320/wjr.v5.i1.40
Peer-review started: September 26, 2014
First decision: November 19, 2014
Revised: December 6, 2014
Accepted: December 18, 2014
Article in press: December 20, 2014
Published online: March 28, 2015
Processing time: 180 Days and 3.4 Hours
The tumor suppressor gene p53 regulates a wide range of cellular processes including cell cycle progression, proliferation, apoptosis and tissue development and remodeling. Lung cell apoptosis and tissue remodeling have critical roles in many lung diseases. Abnormal proliferation or resistance to apoptosis of lung cells will lead to structural changes of many lung tissues, including the pulmonary vascular wall, small airways and lung parenchyma. Among the many lung diseases caused by vascular cell apoptosis and tissue remodeling are chronic obstructive pulmonary disease, bronchial asthma and pulmonary arterial hypertension. Recent advances in biology and medicine have provided new insights and have resulted in new therapeutic strategies for tissue remodeling in human and animal models. This review is focused on lung disease susceptibility associated with the p53 pathway and describes molecular mechanisms upstream and downstream of p53 in lung tissue remodeling. Improved understanding of structural changes associated with pulmonary vascular remodeling and lung cell apoptosis induced by the p53 pathway may new provide therapeutic targets.
Core tip: The activated p53 protein and its associated pathway play a pivotal role in tissue remodeling in chronic obstructive pulmonary disease, asthma and pulmonary hypertension. p53 protein regulates numerous genes and proteins associated with cell cycle arrest and apoptosis. In response to oxidative stress or hypoxia, p53 can become stabilized and activate signal transduction towards lung tissue remodeling and functional loss.
- Citation: Mizuno S, Bogaard HJ, Ishizaki T, Toga H. Role of p53 in lung tissue remodeling. World J Respirol 2015; 5(1): 40-46
- URL: https://www.wjgnet.com/2218-6255/full/v5/i1/40.htm
- DOI: https://dx.doi.org/10.5320/wjr.v5.i1.40
The p53 tumor suppressor protein regulates a number of cellular functions such as cell cycle arrest, gene transcription, and apoptosis in response to DNA damage[1,2]. p53 was first identified in 1979 as an oncogene[3], and later study revealed that the gene is a tumor suppressor in nature[4]. p53 is a regulator of transcription which will activate the target gene transcription. Generally, p53 binds as a tetramer to the promoter regions of target genes, and the most known about p53 target genes including p21, a cyclin-dependent kinase inhibitor, mouse double minute 2 homolog (MDM2), p53 up-regulated modulator of apoptosis (Puma), and Bcl-2-associated X protein (Bax), which are known as apoptosis inducers[5].
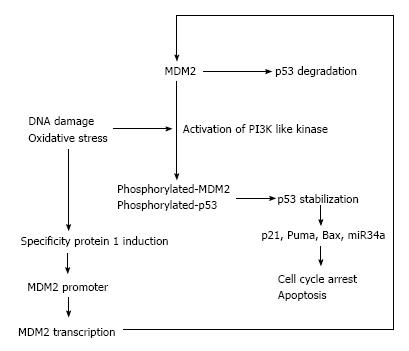
In normal cells, the p53 expression is at very low levels, which is controlled by a negative feedback loop between MDM2 and p53. MDM2 up-regulates p53 transcription activity directly, and leads to p53 nuclear export and proteasome mediated degradation. As such, elevation of p53 up-regulates MDM2 and subsequently, p53 is down-regulated[5]. DNA damage and oxidative stress are the main stimuli of p53 protein expression. Oxidative and genotoxic stress activate PI3 kinase pathways at DNA break sites and these kinases phosphorylate MDM2 and p53, leading to p53 stabilization and activation of p53 pathways[6-8]. MDM2 expression is also regulated by specificity protein 1 (Sp1) that binds to GC-rich motifs of many promoters and is involved in many cellular processes, including cell differentiation, cell growth, apoptosis, immune responses and DNA damage[9]. Sp1 is induced by oxidative stress and regulates expression of vascular endothelial growth factor A (VEGF-A)[10], which plays a critical role in pulmonary vascular remodeling and emphysema[11,12].
In addition to inhibiting proliferation, p53 may promote differentiation. The role of p53 as a tumor suppressor is generally attributed to a stop in proliferation of precancerous cells through the induction of cell-cycle arrest or apoptosis, but p53 also has essential functions in embryonic development and differentiation control[13]. Most of the biological and physiological functions of p53 have been examined by genetically modified mice. Because of p53’s critical effects on cell proliferation and apoptosis, p53 deficient mice were estimated to have severe developmental disturbances. Interestingly, the p53 deficient mice appeared with no birth defects, however, most of the mutant mice largely developed tumors such as lymphomas around the age of 6 mo[13,14]. This implies that basal levels of p53 expression may regulate normal cell growth and development, and that a further reduction in p53 has no significant effects on p53-dependent pathways[15]. Another possibility is that when p53 is lacking, its function in tissue development is substituted by the function of p63 and p73[16]. Conversely, higher expressed p53 induced by genotoxic stress, could play a critical role in cell growth and development.
In recent years, microRNAs (miRNAs) which were modulated by p53 was found by several groups[17,18]. miRNAs, known as non-coding RNA that down-regulate gene expression by bind to target mRNAs at the region of complementary sequences in the code or the 3’-untranslated sequences. Several miRNAs are involved in the regulation of proliferation, differentiation and apoptosis of the cells[19,20]. miR34a is the best-studied miRNA induced by p53, and its expression is highly associated with apoptosis and cell cycle control in cancer cells[21]. We have previously reported that miRNA34a is also related to the expression of hypoxia-inducible factor-1 alpha (HIF-1α) both in the lung tissues from animal and human[22-24], which means miRNA regulated by p53 is also involving in the lung tissue remodeling via p53 pathway. The present article will continue with a review of in vitro and in vivo studies on lung tissue remodeling especially focused on the p53 pathways (Figure 1). In addition, we will discuss recently published strategies designed to identify and characterize novel function of p53.
Chronic obstructive pulmonary disease (COPD) is a heterogeneous and multi-component disease comprising of a combination of small airways disease and parenchyma destruction, and is characterized by airflow limitation. The two characteristic features of COPD are chronic bronchitis and emphysema and each is associated with a specific physiopathology and set of symptoms[25]. In the clinical situation, the presence of emphysema can be expected on the basis of medical history, physical examination and pulmonary function tests, but since emphysema is defined as a structural pulmonary abnormality, its presence can only be confirmed by CT imaging or lung histology[26].
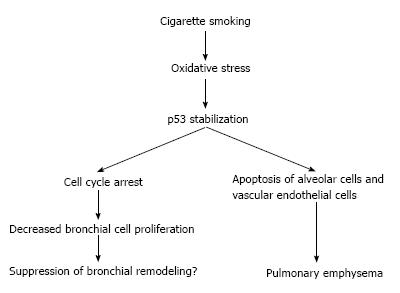
Both higher rates of apoptosis[27,28] and increased alveolar cell proliferation[29] have been reported in the human emphysematous lung, suggesting that a high cell turnover and perhaps impaired regeneration processes of the lung cell may be critical in the pathogenesis of emphysema. In emphysema patients, cigarette smoke will cause exposure of large amounts of free radicals including superoxide (O2-), hydroxyl radical and hydrogen peroxide (H2O2)[30]. Even after cessation of smoking, oxidative stress comes from activated macrophages and neutrophils, which continue to be present in emphysematous lung[31,32]. Probably related to the increased oxidative stress[33], increased level of p53 have been reported in emphysematous lung tissue and the p53 protein expression is significantly higher in lung tissue from patients with emphysema secondary to smoking when compared with tissue from smokers without emphysema or non-smokers[24,34,35], suggesting a possible direct relationship between p53 expression and oxidative stress from smoking.
It can be hypothesized that the susceptibility of smokers to develop COPD is at least in part related to polymorphisms in genes coding for p53 and p53 related proteins. Lee et al[36] reported that polymorphisms in the p53 codon 72 (rs2279744) and the p21 codon 31 (rs1801270) were significantly associated with the occurrence of smoking-related COPD in Taiwan Chinese patients. The p53 codon72 encodes either a proline or arginine residue and resides in a proline-rich region which is important for the function of p53, especially its ability to induce apoptosis. Some studies suggest that cells from individuals with the proline allele of p53 codon72 will undergo less apoptosis in response to genotoxic stress compared with individuals with the arginine allele of p53 codon72[37,38].
The second component of COPD: airway narrowing due to chronic bronchitis may also be affected by the p53 pathway through the induction of bronchial cell apoptosis and/or proliferation in response to the oxidative stress from smoking[39,40]. p53-dependent cell death is caspase dependent[41], whereas p21 does not induce cell death on its own[42]. Although p21 regulates cell-cycle arrest in response to p53 activation[43], previous reports on p21 polymorphism showed conflicting reports linking p21 overexpression to both cancer suppressive as well as promoting effects[44]. Thus, each of these observations remains controversial, especially in the pulmonary emphysema due to lung cell apoptosis (Figure 2).
Epithelial repair processes play an important pathogenic role in initiating and maintaining the airway inflammation and remodeling of asthma. Epithelial loss has been described as a typical pathologic feature of asthma[45,46]. Several studies on lung cells and in animal suggested that increased presence of apoptotic cells in the asthmatic bronchus contributes to the pathogenesis of chronic inflammation and remodeling[45]. Defects in apoptotic cell uptake by alveolar macrophages (defective efferocytosis) could contribute to the chronic inflammation of asthmatic lungs[47]. The p53 pathway could be involved in the persistent presence of apoptosis cells in the asthmatic bronchus. Saccucci et al[48], showed by polymorphism analysis that asthmatic children exhibited a higher frequency of the arginine genotype in p53 codon 72 than the proline genotype. This result is in line with a previous report showing the increased presence of apoptosis cells in the asthmatic bronchus, since the arginine genotype of p53 codon 72 is associated with a higher induction of apoptosis compared to the proline genotype[37,38]. Interestingly, the polymorphism pattern in asthma is completely opposite from the pattern in COPD[36].
Mast cells play a central role in the allergic asthmatic response. Activation of mast cells via cross-linking of allergen-specific IgE induces degranulation and release of mediators, particularly histamine and lipid mediators, chemokines, and cytokines that evoke the different symptoms of the asthmatic response[49]. Interestingly, Suzuki et al[50], reported that p53 suppresses IgE mediated mast cell activation through NF-κB mediated cytokine production. Another study showed that p53 plays a pivotal role in mast cell survival via mTOR pathways[51]. These results suggest that p53 activation by genotoxic stress in the bronchus from asthmatic patients may prevent progression of bronchial remodeling by inhibiting mast cell and IgE mediated inflammation. Together these results may imply that increased p53 activity may promote disease susceptibility but, at the same time, attenuate the progression of bronchial inflammation and/or remodeling. Longitudinal follow-up of patients having different p53 gene pathways will be necessary to confirm the genomic role on the disease.
Pulmonary hypertension is defined clinically as a mean pulmonary artery pressure of greater than 25 mmHg. In most mammals, chronic hypoxia causes chronic pulmonary hypertension. Hypoxic environment is known as one of the key factors to induce pulmonary vascular remodeling and secondary pulmonary hypertension[52]. According to a WHO statement in 1996, approximately 140 million people were living at high altitudes more than 2500m and some of residents were living at altitudes more than 4000 m. After a couple of weeks living at such high altitude, lowlanders manifest secondary pulmonary hypertension, which cannot recover completely by oxygenation[53], indicating manifestation of chronic hypoxia causes chronic pulmonary hypertension and pulmonary vascular remodeling[54]. Remodeling of small pulmonary vessels by proliferation of pulmonary vascular cells features secondary pulmonary hypertension[55-57]. These phenomena indicate enhancement of pulmonary vascular cell proliferation induced by hypoxic environment make a considerable contribution to the progression of hypoxic pulmonary vascular remodeling and chronichypoxicpul monaryhypertension (CHPH).
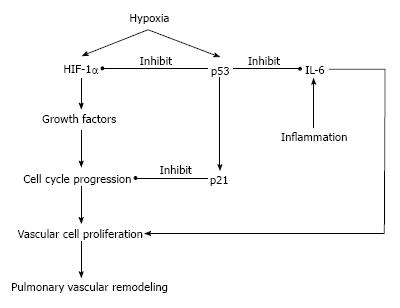
Induction of the p53 induces the expression of CDK inhibitor p21, which plays a critical role in the proliferation of pulmonary arterial smooth muscle cells[43,58]. The p21 has been thought to a pivotal regulative factor of cell cycle in cells exposed to hypoxic environment and oxidative stress[59-61]. There are many cells undergoing apoptosis in tumors with wild-type p53 gene exposed to hypoxic environment, whereas tumors with mutational inactivated p53 gene have reduced levels of apoptosis in hypoxic regions[62]. Embryo fibroblasts from p53 knockout mouse are relatively resistant to apoptosis induced by hypoxia, and have better proliferative properties compared to the cells with wild-type p53 gene[63]. Hypoxic p53 accumulation is closely correlated with HIF-1α, known as a transcriptional factor which plays a central role in the control of angiogenesis during hypoxia[64,65]. Previously, we reported that hypoxic p53 accumulation is relevant to HIF-1α expression in lung tissue and pulmonary vascular cells[22,66]. It was shown that chronic hypoxia was associated with increases in p53 and p21 expression in wild-type mice, whereas the mutation of the p53 gene attenuated the hypoxia induced p21 expression and resulted in more severe vascular remodeling of small pulmonary arteries[22]. Furthermore, Mouraret et al[67], reported that the activation of p53 by nutlin-3a, known as inducer of p53 protein by inhibition of p53-MDM2 binding, prevented CHPH in mice[67]. These findings suggest that under hypoxic environment, induced p53 and the p53 signaling pathway play a role as a negative feedback loop to attenuate excessive proliferation of vascular cells and pulmonary arterial remodeling, and serve as a central role as a regulator of hypoxic vascular remodeling of small pulmonary artery.
p53 mutations or defects in cancer cells can enhance interleukin 6 (IL-6) expression[68]. IL-6 overexpression is known to change the balance between pro- and anti-apoptotic proteins, which can promote vascular remodeling and pulmonary hypertension[69]. We previously showed that the endothelial cell IL-6 expression in pulmonary arteries was increased in a non-hypoxic animal model of pulmonary hypertension[70]. The results could be explained by activation of a IL-6/STAT3/HIF-1α signaling axis, as was previously described by Nilsson et al[71], or involvement of NF-κB and HIF-1α[72]. Recently, Dickinson et al[73], showed that down-regulation of early growth response protein 1 (Egr-1) resulted in a reduction of pulmonary vascular proliferation and increased apoptosis with decreases of IL-6 and p53 expression in rats[73]. The Egr-1 regulation of p53 and IL-6 in vascular remodeling could be another of potential target of CHPH treatment in future (Figure 3).
In normal cells, the expressed level of p53 is very low, and the biochemical effects of p53 are thought to be latent. In response to DNA damage, oxidative stress and hypoxia, p53 can become stabilized and activates p53 pathways. Previously, the activation of p53 was believed to be primarily involved in the prevention of propagation of cells with potentially dangerous genetic lesions[74], as a guardian of genome. However, a number of studies have suggested that the effects of p53, including arresting cell cycle and triggering apoptotic cell death, are also involved in lung development and remodeling. Because hypoxia and oxidative stress are environmental factors closely related with CHPH in highlanders, and COPD, asthma and asthma COPD overlap syndrome in smokers, the impact of p53 activity on pulmonary disease has been shown to be much larger. Genomic analysis including genome-wide association studies (GWAS) is a common tool for the analysis of disease susceptibility and profiles in pulmonary diseases[75] and has shown its use in the detection of disease responsible genes and disease specific features. Such studies have shown interesting data pertaining to p53 polymorphisms, but the data is still conflicting at times. The regulatory actions p53 with respect to proliferation and apoptosis are complex and so are the processes involved in lung and vascular remodeling, where numerous interactions exist between vascular endothelial cells, smooth muscle cells, fibroblast and epithelial cells. For instance, hypoxic-ischemic states could release mitogenic factors from pulmonary vascular endothelial cells and adventitial fibroblasts around smooth muscle cells, resulting pulmonary vascular smooth muscle proliferation. Conversely, pulmonary vascular endothelial cells damaged by hypoxia may be able to decrease the expression of suppressors of smooth muscle proliferation. Furthermore, hypoxic proliferation of lung fibroblasts may lead to the secretion of matrix proteins that play a critical role for the smooth muscle cells proliferation[76]. The two characteristic features of COPD, pulmonary emphysema and chronic bronchitis, may come about through the interactions between cigarette smoke exposure and different p53 genotypes. We assume that a deeper understanding of pathophysiologic mechanisms including characterization of genotypes and phenotypes will make therapeutic advances and improved diagnosis.
We wish to thank Professor Norbert F Voelkel, Virginia Commonwealth University, Richmond, VA, United States for his critical reading of this manuscript.
P- Reviewer: Komócsi A S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Kastan MB, Onyekwere O, Sidransky D, Vogelstein B, Craig RW. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991;51:6304-6311. [PubMed] |
| 2. | Dulić V, Kaufmann WK, Wilson SJ, Tlsty TD, Lees E, Harper JW, Elledge SJ, Reed SI. p53-dependent inhibition of cyclin-dependent kinase activities in human fibroblasts during radiation-induced G1 arrest. Cell. 1994;76:1013-1023. [PubMed] |
| 4. | Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5100] [Cited by in RCA: 5144] [Article Influence: 197.8] [Reference Citation Analysis (0)] |
| 6. | Zhou BB, Elledge SJ. The DNA damage response: putting checkpoints in perspective. Nature. 2000;408:433-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2293] [Cited by in RCA: 2364] [Article Influence: 90.9] [Reference Citation Analysis (0)] |
| 7. | Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432:316-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1939] [Cited by in RCA: 2063] [Article Influence: 93.8] [Reference Citation Analysis (0)] |
| 8. | Peterson CL, Côté J. Cellular machineries for chromosomal DNA repair. Genes Dev. 2004;18:602-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 216] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 9. | Bond GL, Hu W, Bond EE, Robins H, Lutzker SG, Arva NC, Bargonetti J, Bartel F, Taubert H, Wuerl P. A single nucleotide polymorphism in the MDM2 promoter attenuates the p53 tumor suppressor pathway and accelerates tumor formation in humans. Cell. 2004;119:591-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 926] [Cited by in RCA: 989] [Article Influence: 47.1] [Reference Citation Analysis (0)] |
| 10. | Schäfer G, Cramer T, Suske G, Kemmner W, Wiedenmann B, Höcker M. Oxidative stress regulates vascular endothelial growth factor-A gene transcription through Sp1- and Sp3-dependent activation of two proximal GC-rich promoter elements. J Biol Chem. 2003;278:8190-8198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 159] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 11. | Voelkel NF, Gomez-Arroyo J, Mizuno S. COPD/emphysema: The vascular story. Pulm Circ. 2011;1:320-326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 51] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 12. | Voelkel NF, Mizuno S, Bogaard HJ. The role of hypoxia in pulmonary vascular diseases: a perspective. Am J Physiol Lung Cell Mol Physiol. 2013;304:L457-L465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Stiewe T. The p53 family in differentiation and tumorigenesis. Nat Rev Cancer. 2007;7:165-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 230] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 14. | Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Butel JS, Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3399] [Cited by in RCA: 3412] [Article Influence: 100.4] [Reference Citation Analysis (0)] |
| 15. | Gottlieb E, Haffner R, King A, Asher G, Gruss P, Lonai P, Oren M. Transgenic mouse model for studying the transcriptional activity of the p53 protein: age- and tissue-dependent changes in radiation-induced activation during embryogenesis. EMBO J. 1997;16:1381-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 141] [Article Influence: 4.9] [Reference Citation Analysis (11)] |
| 16. | Wu G, Nomoto S, Hoque MO, Dracheva T, Osada M, Lee CC, Dong SM, Guo Z, Benoit N, Cohen Y. DeltaNp63alpha and TAp63alpha regulate transcription of genes with distinct biological functions in cancer and development. Cancer Res. 2003;63:2351-2357. [PubMed] |
| 17. | Brosh R, Shalgi R, Liran A, Landan G, Korotayev K, Nguyen GH, Enerly E, Johnsen H, Buganim Y, Solomon H. p53-Repressed miRNAs are involved with E2F in a feed-forward loop promoting proliferation. Mol Syst Biol. 2008;4:229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 117] [Cited by in RCA: 122] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 18. | Hermeking H. p53 enters the microRNA world. Cancer Cell. 2007;12:414-418. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 412] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 19. | Kloosterman WP, Plasterk RH. The diverse functions of microRNAs in animal development and disease. Dev Cell. 2006;11:441-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1552] [Cited by in RCA: 1641] [Article Influence: 82.1] [Reference Citation Analysis (0)] |
| 20. | Bushati N, Cohen SM. microRNA functions. Annu Rev Cell Dev Biol. 2007;23:175-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2105] [Cited by in RCA: 2387] [Article Influence: 125.6] [Reference Citation Analysis (1)] |
| 21. | Tarasov V, Jung P, Verdoodt B, Lodygin D, Epanchintsev A, Menssen A, Meister G, Hermeking H. Differential regulation of microRNAs by p53 revealed by massively parallel sequencing: miR-34a is a p53 target that induces apoptosis and G1-arrest. Cell Cycle. 2007;6:1586-1593. [PubMed] |
| 22. | Mizuno S, Bogaard HJ, Kraskauskas D, Alhussaini A, Gomez-Arroyo J, Voelkel NF, Ishizaki T. p53 Gene deficiency promotes hypoxia-induced pulmonary hypertension and vascular remodeling in mice. Am J Physiol Lung Cell Mol Physiol. 2011;300:L753-L761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 112] [Article Influence: 7.5] [Reference Citation Analysis (1)] |
| 23. | Mizuno S, Yasuo M, Bogaard HJ, Kraskauskas D, Natarajan R, Voelkel NF. Inhibition of histone deacetylase causes emphysema. Am J Physiol Lung Cell Mol Physiol. 2011;300:L402-L413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 24. | Mizuno S, Bogaard HJ, Gomez-Arroyo J, Alhussaini A, Kraskauskas D, Cool CD, Voelkel NF. MicroRNA-199a-5p is associated with hypoxia-inducible factor-1α expression in lungs from patients with COPD. Chest. 2012;142:663-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 87] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 25. | Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet. 2004;364:709-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 778] [Cited by in RCA: 859] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 26. | Stolk J, Stoel BC. Lung densitometry to assess progression of emphysema in chronic obstructive pulmonary disease: time to apply in the clinic? Am J Respir Crit Care Med. 2011;183:1578-1580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Kasahara Y, Tuder RM, Taraseviciene-Stewart L, Le Cras TD, Abman S, Hirth PK, Waltenberger J, Voelkel NF. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest. 2000;106:1311-1319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 795] [Cited by in RCA: 840] [Article Influence: 32.3] [Reference Citation Analysis (0)] |
| 28. | Imai K, Mercer BA, Schulman LL, Sonett JR, D’Armiento JM. Correlation of lung surface area to apoptosis and proliferation in human emphysema. Eur Respir J. 2005;25:250-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 166] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 29. | Yokohori N, Aoshiba K, Nagai A. Increased levels of cell death and proliferation in alveolar wall cells in patients with pulmonary emphysema. Chest. 2004;125:626-632. [PubMed] |
| 30. | Pryor WA. Cigarette smoke radicals and the role of free radicals in chemical carcinogenicity. Environ Health Perspect. 1997;105 Suppl 4:875-882. [PubMed] |
| 31. | Gwinn MR, Vallyathan V. Respiratory burst: role in signal transduction in alveolar macrophages. J Toxicol Environ Health B Crit Rev. 2006;9:27-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 106] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 32. | Dahlgren C, Karlsson A. Respiratory burst in human neutrophils. J Immunol Methods. 1999;232:3-14. [PubMed] |
| 33. | Roos WP, Kaina B. DNA damage-induced cell death by apoptosis. Trends Mol Med. 2006;12:440-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1024] [Cited by in RCA: 1150] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 34. | Siganaki M, Koutsopoulos AV, Neofytou E, Vlachaki E, Psarrou M, Soulitzis N, Pentilas N, Schiza S, Siafakas NM, Tzortzaki EG. Deregulation of apoptosis mediators’ p53 and bcl2 in lung tissue of COPD patients. Respir Res. 2010;11:46. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | Morissette MC, Vachon-Beaudoin G, Parent J, Chakir J, Milot J. Increased p53 level, Bax/Bcl-x(L) ratio, and TRAIL receptor expression in human emphysema. Am J Respir Crit Care Med. 2008;178:240-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 36. | Lee YL, Chen W, Tsai WK, Lee JC, Chiou HL, Shih CM, Wang YC. Polymorphisms of p53 and p21 genes in chronic obstructive pulmonary disease. J Lab Clin Med. 2006;147:228-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 37. | Walker KK, Levine AJ. Identification of a novel p53 functional domain that is necessary for efficient growth suppression. Proc Natl Acad Sci USA. 1996;93:15335-15340. [PubMed] |
| 38. | Sakamuro D, Sabbatini P, White E, Prendergast GC. The polyproline region of p53 is required to activate apoptosis but not growth arrest. Oncogene. 1997;15:887-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 214] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 39. | Shenberger JS, Dixon PS. Oxygen induces S-phase growth arrest and increases p53 and p21(WAF1/CIP1) expression in human bronchial smooth-muscle cells. Am J Respir Cell Mol Biol. 1999;21:395-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 61] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 40. | Bian T, Gibbs JD, Örvell C, Imani F. Respiratory syncytial virus matrix protein induces lung epithelial cell cycle arrest through a p53 dependent pathway. PLoS One. 2012;7:e38052. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 41. | Damico R, Simms T, Kim BS, Tekeste Z, Amankwan H, Damarla M, Hassoun PM. p53 mediates cigarette smoke-induced apoptosis of pulmonary endothelial cells: inhibitory effects of macrophage migration inhibitor factor. Am J Respir Cell Mol Biol. 2011;44:323-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 42. | Almond JB, Cohen GM. The proteasome: a novel target for cancer chemotherapy. Leukemia. 2002;16:433-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 404] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 43. | Mizuno S, Kadowaki M, Demura Y, Ameshima S, Miyamori I, Ishizaki T. p42/44 Mitogen-activated protein kinase regulated by p53 and nitric oxide in human pulmonary arterial smooth muscle cells. Am J Respir Cell Mol Biol. 2004;31:184-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 44. | Grochola LF, Zeron-Medina J, Mériaux S, Bond GL. Single-nucleotide polymorphisms in the p53 signaling pathway. Cold Spring Harb Perspect Biol. 2010;2:a001032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 45. | Walsh GM. Defective apoptotic cell clearance in asthma and COPD--a new drug target for statins? Trends Pharmacol Sci. 2008;29:6-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 46. | Zhou C, Yin G, Liu J, Liu X, Zhao S. Epithelial apoptosis and loss in airways of children with asthma. J Asthma. 2011;48:358-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 47. | Huynh ML, Malcolm KC, Kotaru C, Tilstra JA, Westcott JY, Fadok VA, Wenzel SE. Defective apoptotic cell phagocytosis attenuates prostaglandin E2 and 15-hydroxyeicosatetraenoic acid in severe asthma alveolar macrophages. Am J Respir Crit Care Med. 2005;172:972-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 116] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 48. | Saccucci P, Verrotti A, Giannini C, Verini M, Chiarelli F, Neri A, Magrini A. p53 Codon 72 Genetic Polymorphism in Asthmatic Children: Evidence of Interaction With Acid Phosphatase Locus 1. Allergy Asthma Immunol Res. 2014;6:252-256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 49. | Cockcroft DW, Ruffin RE, Dolovich J, Hargreave FE. Allergen-induced increase in non-allergic bronchial reactivity. Clin Allergy. 1977;7:503-513. [PubMed] |
| 50. | Suzuki K, Murphy SH, Xia Y, Yokota M, Nakagomi D, Liu F, Verma IM, Nakajima H. Tumor suppressor p53 functions as a negative regulator in IgE-mediated mast cell activation. PLoS One. 2011;6:e25412. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 51. | Shin J, Pan H, Zhong XP. Regulation of mast cell survival and function by tuberous sclerosis complex 1. Blood. 2012;119:3306-3314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 52. | Cogo A, Napolitano G, Michoud MC, Barbon DR, Ward M, Martin JG. Effects of hypoxia on rat airway smooth muscle cell proliferation. J Appl Physiol (1985). 2003;94:1403-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 53. | Groves BM, Reeves JT, Sutton JR, Wagner PD, Cymerman A, Malconian MK, Rock PB, Young PM, Houston CS. Operation Everest II: elevated high-altitude pulmonary resistance unresponsive to oxygen. J Appl Physiol (1985). 1987;63:521-530. [PubMed] |
| 54. | Hainsworth R, Drinkhill MJ. Cardiovascular adjustments for life at high altitude. Respir Physiol Neurobiol. 2007;158:204-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 89] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 55. | Biernacki W, Flenley DC, Muir AL, MacNee W. Pulmonary hypertension and right ventricular function in patients with COPD. Chest. 1988;94:1169-1175. [PubMed] |
| 56. | MacNee W. Pathophysiology of cor pulmonale in chronic obstructive pulmonary disease. Part One. Am J Respir Crit Care Med. 1994;150:833-852. [PubMed] |
| 57. | Semmens M, Reid L. Pulmonary arterial muscularity and right ventricular hypertrophy in chronic bronchitis and emphysema. Br J Dis Chest. 1974;68:253-263. [PubMed] |
| 58. | O'Reilly MA, Staversky RJ, Watkins RH, Reed CK, de Mesy Jensen KL, Finkelstein JN, Keng PC. The cyclin-dependent kinase inhibitor p21 protects the lung from oxidative stress. Am J Respir Cell Mol Biol. 2001;24:703-710. [PubMed] |
| 59. | Adachi S, Ito H, Tamamori-Adachi M, Ono Y, Nozato T, Abe S, Ikeda Ma F, Hiroe M. Cyclin A/cdk2 activation is involved in hypoxia-induced apoptosis in cardiomyocytes. Circ Res. 2001;88:408-414. [PubMed] |
| 60. | McGrath-Morrow SA, Stahl J. Growth arrest in A549 cells during hyperoxic stress is associated with decreased cyclin B1 and increased p21(Waf1/Cip1/Sdi1) levels. Biochim Biophys Acta. 2001;1538:90-97. [PubMed] |
| 61. | Roy S, Khanna S, Bickerstaff AA, Subramanian SV, Atalay M, Bierl M, Pendyala S, Levy D, Sharma N, Venojarvi M. Oxygen sensing by primary cardiac fibroblasts: a key role of p21(Waf1/Cip1/Sdi1). Circ Res. 2003;92:264-271. [PubMed] |
| 62. | Graeber TG, Osmanian C, Jacks T, Housman DE, Koch CJ, Lowe SW, Giaccia AJ. Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature. 1996;379:88-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1702] [Cited by in RCA: 1663] [Article Influence: 55.4] [Reference Citation Analysis (8)] |
| 63. | Yu J, Wang Z, Kinzler KW, Vogelstein B, Zhang L. PUMA mediates the apoptotic response to p53 in colorectal cancer cells. Proc Natl Acad Sci USA. 2003;100:1931-1936. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 478] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 64. | An WG, Kanekal M, Simon MC, Maltepe E, Blagosklonny MV, Neckers LM. Stabilization of wild-type p53 by hypoxia-inducible factor 1alpha. Nature. 1998;392:405-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 611] [Cited by in RCA: 634] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 65. | Sano M, Minamino T, Toko H, Miyauchi H, Orimo M, Qin Y, Akazawa H, Tateno K, Kayama Y, Harada M. p53-induced inhibition of Hif-1 causes cardiac dysfunction during pressure overload. Nature. 2007;446:444-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 691] [Cited by in RCA: 758] [Article Influence: 39.9] [Reference Citation Analysis (0)] |
| 66. | Yasuo M, Mizuno S, Kraskauskas D, Bogaard HJ, Natarajan R, Cool CD, Zamora M, Voelkel NF. Hypoxia inducible factor-1α in human emphysema lung tissue. Eur Respir J. 2011;37:775-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 67. | Mouraret N, Marcos E, Abid S, Gary-Bobo G, Saker M, Houssaini A, Dubois-Rande JL, Boyer L, Boczkowski J, Derumeaux G. Activation of lung p53 by Nutlin-3a prevents and reverses experimental pulmonary hypertension. Circulation. 2013;127:1664-1676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 68. | Jin S, Mutvei AP, Chivukula IV, Andersson ER, Ramsköld D, Sandberg R, Lee KL, Kronqvist P, Mamaeva V, Ostling P. Non-canonical Notch signaling activates IL-6/JAK/STAT signaling in breast tumor cells and is controlled by p53 and IKKα/IKKβ. Oncogene. 2013;32:4892-4902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 118] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 69. | Steiner MK, Syrkina OL, Kolliputi N, Mark EJ, Hales CA, Waxman AB. Interleukin-6 overexpression induces pulmonary hypertension. Circ Res. 2009;104:236-244, 28p following 244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 546] [Cited by in RCA: 506] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 70. | Mizuno S, Farkas L, Al Husseini A, Farkas D, Gomez-Arroyo J, Kraskauskas D, Nicolls MR, Cool CD, Bogaard HJ, Voelkel NF. Severe pulmonary arterial hypertension induced by SU5416 and ovalbumin immunization. Am J Respir Cell Mol Biol. 2012;47:679-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 71. | Nilsson CL, Dillon R, Devakumar A, Shi SD, Greig M, Rogers JC, Krastins B, Rosenblatt M, Kilmer G, Major M. Quantitative phosphoproteomic analysis of the STAT3/IL-6/HIF1alpha signaling network: an initial study in GSC11 glioblastoma stem cells. J Proteome Res. 2010;9:430-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 72. | Lee SH, Lee YJ, Han HJ. Effect of arachidonic acid on hypoxia-induced IL-6 production in mouse ES cells: Involvement of MAPKs, NF-kappaB, and HIF-1alpha. J Cell Physiol. 2010;222:574-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 73. | Dickinson MG, Kowalski PS, Bartelds B, Borgdorff MA, van der Feen D, Sietsma H, Molema G, Kamps JA, Berger RM. A critical role for Egr-1 during vascular remodelling in pulmonary arterial hypertension. Cardiovasc Res. 2014;103:573-584. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 74. | Lane DP. Cancer. p53, guardian of the genome. Nature. 1992;358:15-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3502] [Cited by in RCA: 3596] [Article Influence: 105.8] [Reference Citation Analysis (0)] |
| 75. | Hardin M, Cho M, McDonald ML, Beaty T, Ramsdell J, Bhatt S, van Beek EJ, Make BJ, Crapo JD, Silverman EK. The clinical and genetic features of COPD-asthma overlap syndrome. Eur Respir J. 2014;44:341-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 196] [Cited by in RCA: 235] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 76. | Stenmark KR, Mecham RP. Cellular and molecular mechanisms of pulmonary vascular remodeling. Annu Rev Physiol. 1997;59:89-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 265] [Article Influence: 9.1] [Reference Citation Analysis (0)] |